Silver-Containing Thin Films on Transparent Polymer Foils for Antimicrobial Applications
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Thin Film Deposition and Characterization
3.1.1. Sample Preparation
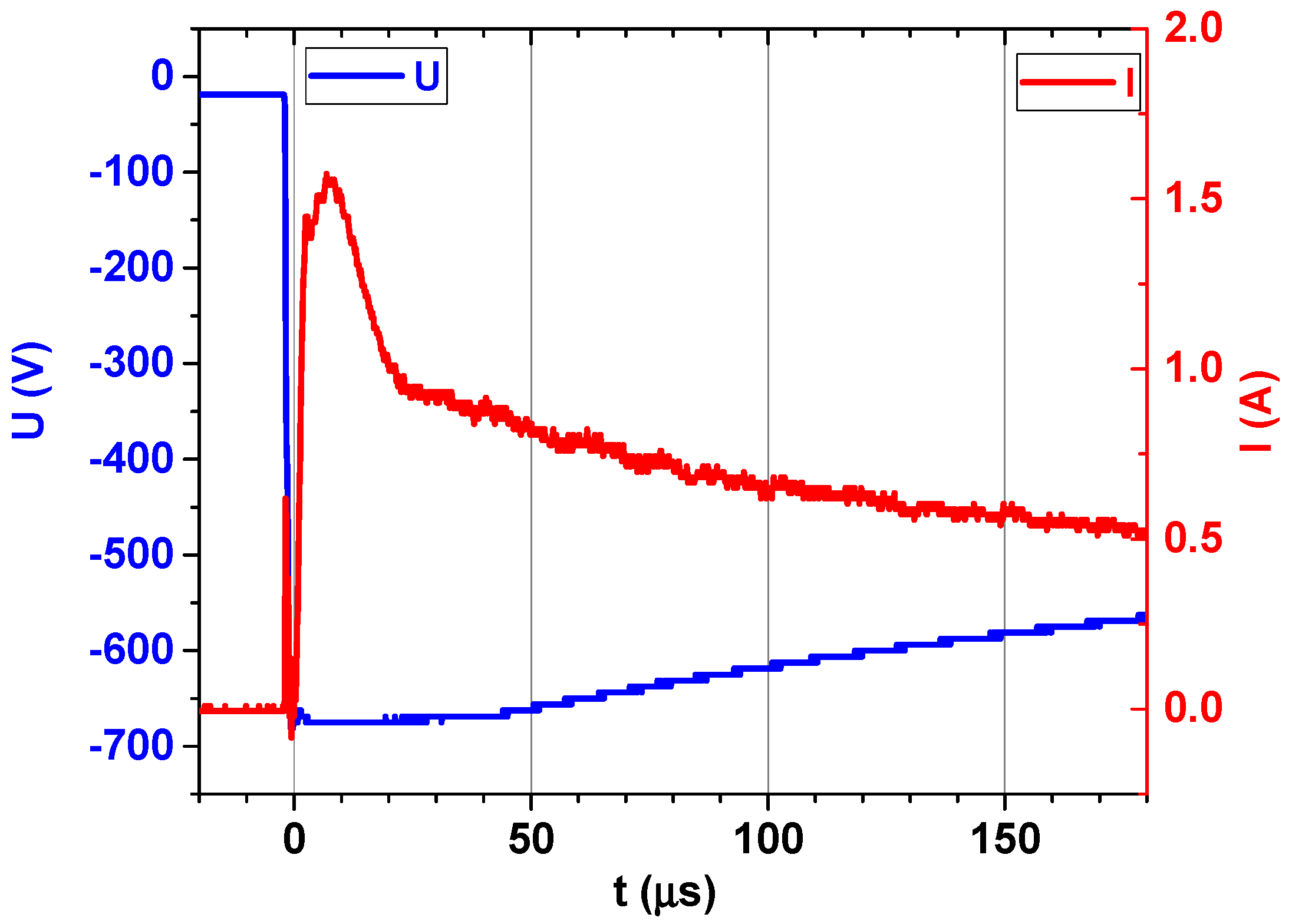
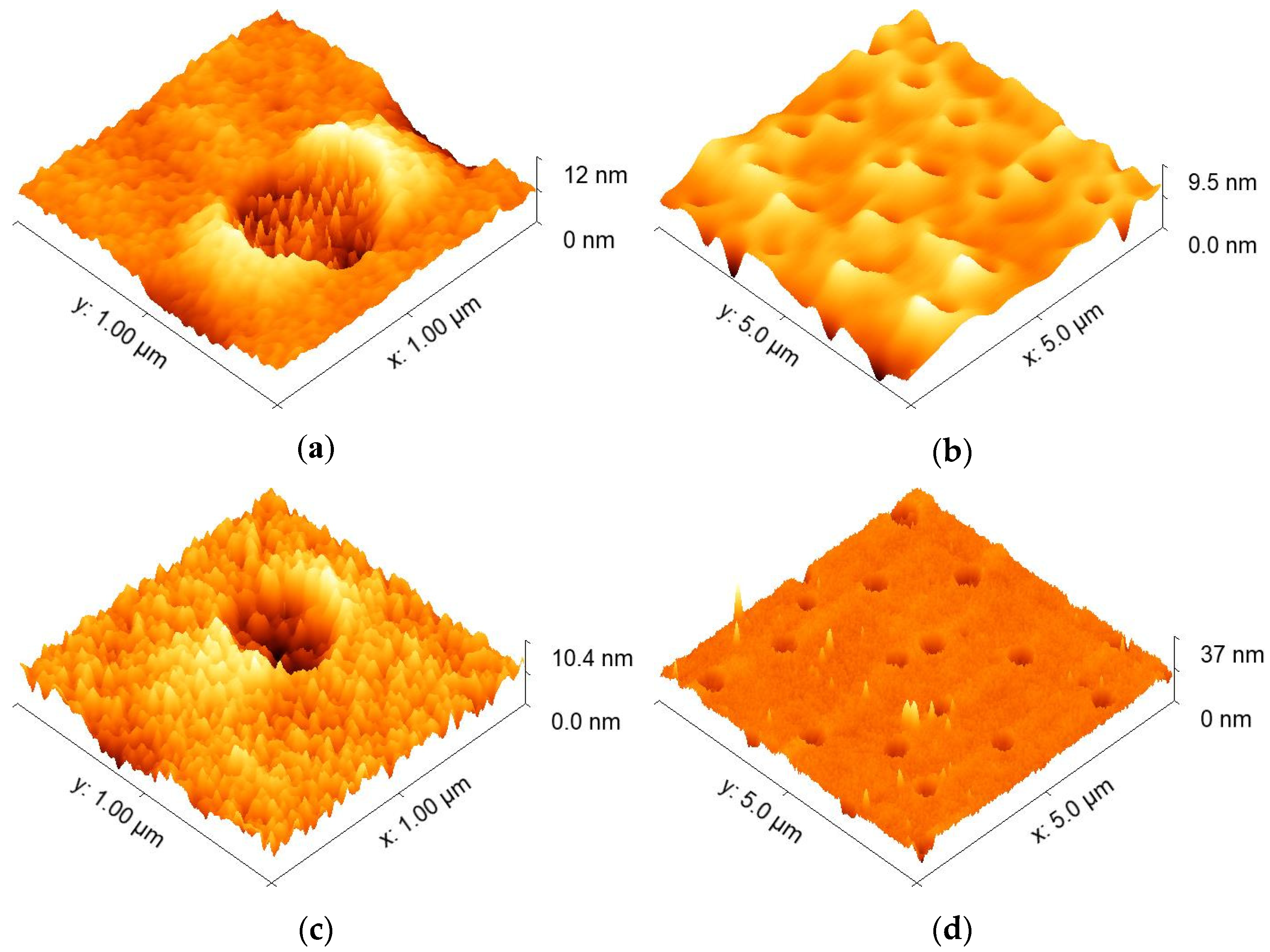
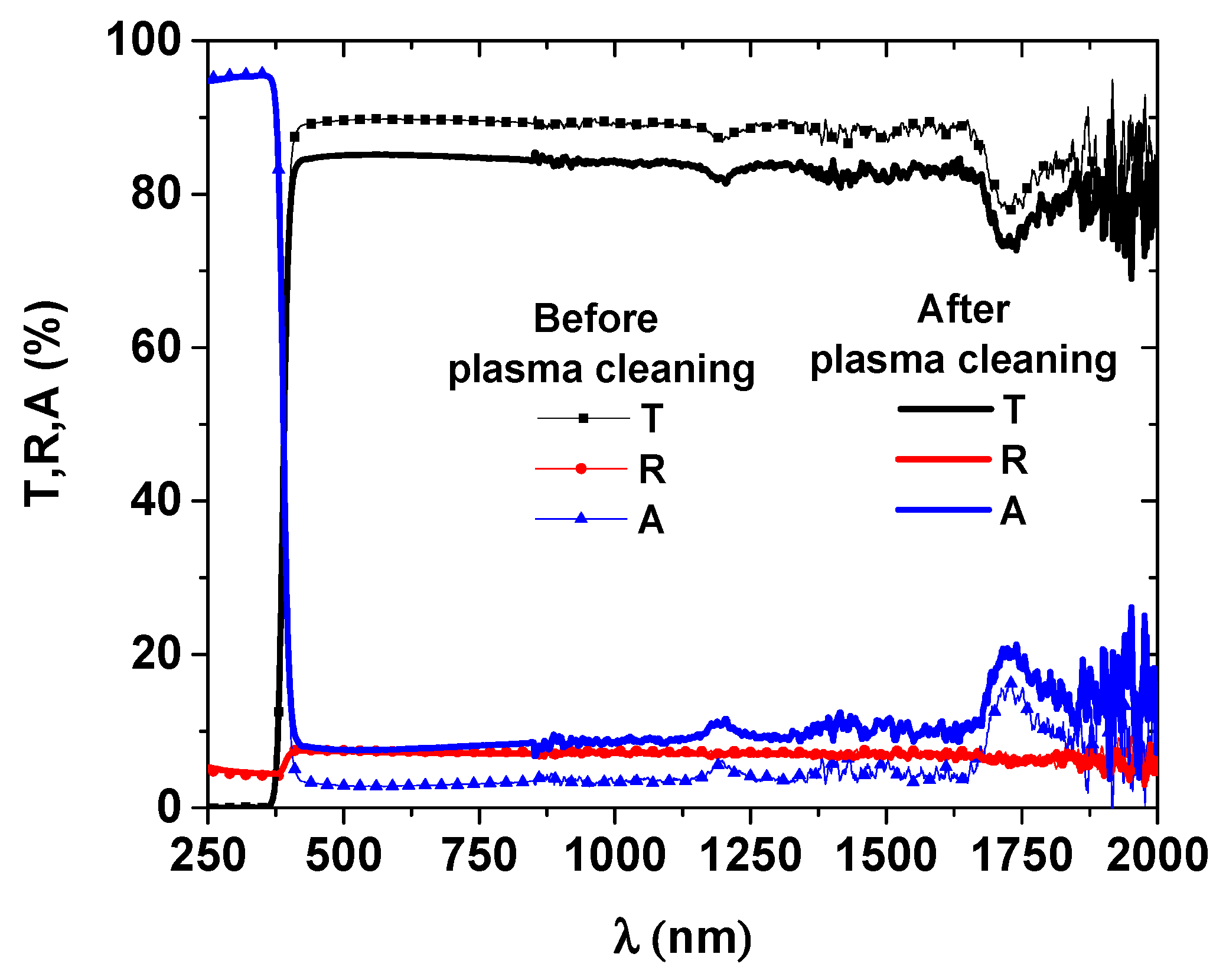
3.1.2. Deposition Process and Physical Characterization
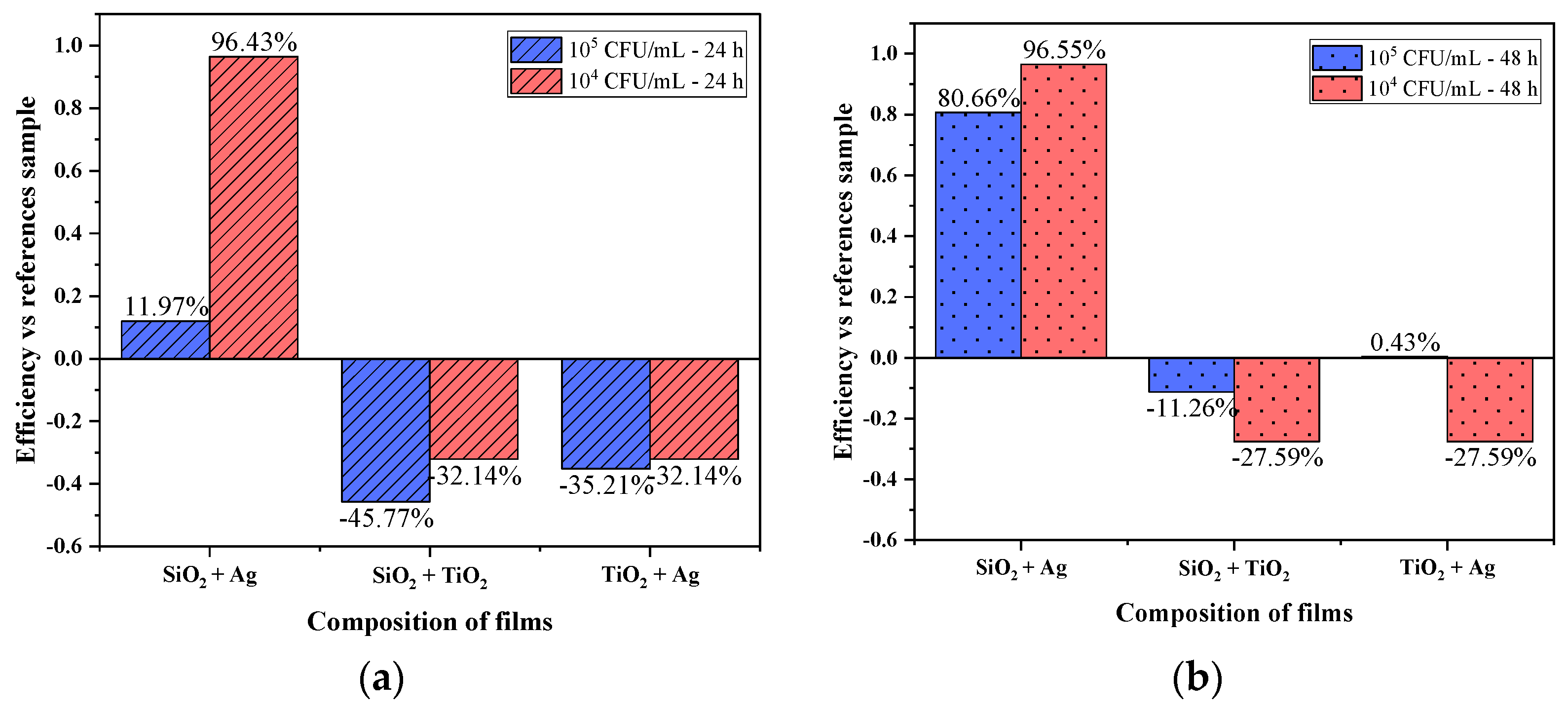
3.2. Antimicrobial Activity
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neely, A.N.; Maley, M.P. Survival of enterococci and staphylococci on hospital fabrics and plastic. J. Clin. Microbiol. 2000, 38, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S.J. Importance of the environment in meticillin-resistant Staphylococcus aureus acquisition: The case for hospital cleaning. Lancet Infect. Dis. 2008, 8, 101–113. [Google Scholar] [CrossRef]
- Martinez, J.A.; Ruthazer, R.; Hansjosten, K.; Barefoot, L.; Snydman, D.R. Role of environmental contamination as a risk factor for acquisition of vancomycin-resistant enterococci in patients treated in a medical intensive care unit. Arch. Intern. Med. 2003, 163, 1905–1912. [Google Scholar] [CrossRef]
- Perez-Gavilan, A.; de Castro, J.V.; Arana, A.; Merino, S.; Retolaza, A.; Alves, S.A.; Francone, A.; Kehagias, N.; Sotomayor-Torres, C.M.; Cocina, D.; et al. Antibacterial activity testing methods for hydrophobic patterned surfaces. Sci. Rep. 2021, 11, 6675. [Google Scholar] [CrossRef]
- Graveto, J.M.; Costa, P.J.; Santos, C.I. Cell Phone Usage By Health Personnel: Preventive Strategies to Decrease Risk of Cross Infection In Clinical Context. Texto Contexto Enferm. 2018, 27, e5140016. [Google Scholar] [CrossRef][Green Version]
- Elmanama, A.; Hassona, I.; Marouf, A.; Alshaer, G.; Ghanima, E.A. Microbial Load of Touch Screen Mobile Phones Used by University Students and Healthcare Staff. J. Arab Am. Univ. 2015, 1, 1–18. [Google Scholar] [CrossRef][Green Version]
- Kister, M.P.; Borowska, K.; Jodłowska-Jędrych, B.; Kister, K.A.; Drop, B. The potential role of cell phones in dissemination of bacteria in a healthcare setting. Our Dermatol. Online. 2016, 7, 219–224. [Google Scholar] [CrossRef]
- Anees Ahmad, S.; Sachi Das, S.; Khatoon, A.; Tahir Ansari, M.; Afzal, M.; Saquib Hasnain, M.; Kumar Nayak, A. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Ivanova, T.; Harizanova, A.; Koutzarova, T.; Vertruyen, B. Optical and structural characterization of TiO2 films doped with silver nanoparticles obtained by sol-gel method. Opt. Mater. 2013, 36, 207–213. [Google Scholar] [CrossRef]
- Varghese, S.; Elfakhri, S.; Sheel, D.W.; Sheel, P.; Bolton, F.J.; Foster, H.A. Novel antibacterial silver-silica surface coatings prepared by chemical vapour deposition for infection control. J. Appl. Microbiol. 2013, 115, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; Elsharkawy, R.G.; Ayad, M.I.; Elgendy, M.Y. Silver nanoparticles deposition on silica, magnetite, and alumina surfaces for effective removal of Allura red from aqueous solutions. J. Solgel Sci. Technol. 2019, 91, 523–538. [Google Scholar] [CrossRef]
- Yin, D.; Liu, Y.; Chen, P.; Meng, G.; Huang, G.; Cai, L.; Zhang, L. Controllable synthesis of silver nanoparticles by the pulsed electrochemical deposition in a forced circulation reactor. Int. J. Electrochem. Sci. 2020, 15, 3469–3478. [Google Scholar] [CrossRef]
- Baptista, A.; Silva, F.J.G.; Porteiro, J.; Míguez, J.L.; Pinto, G.; Fernandes, L. On the Physical Vapour Deposition (PVD): Evolution of Magnetron Sputtering Processes for Industrial Applications. Proc. Procedia Manuf. 2018, 17, 746–757. [Google Scholar] [CrossRef]
- Anders, A. Tutorial: Reactive high power impulse magnetron sputtering (R-HiPIMS). J. Appl. Phys. 2017, 121, 171101. [Google Scholar] [CrossRef]
- Britun, N.; Minea, T.; Konstantinidis, S.; Snyders, R. Plasma diagnostics for understanding the plasma-surface interaction in HiPIMS discharges: A review. J. Phys. D Appl. Phys. 2014, 47, 224001. [Google Scholar] [CrossRef]
- Greczynski, G.; Lu, J.; Jensen, J.; Bolz, S.; Kölker, W.; Schiffers, C.; Lemmer, O.; Greene, J.E.; Hultman, L. A review of metal-ion-flux-controlled growth of metastable TiAlN by HIPIMS/DCMS co-sputtering. Surf. Coat. Technol. 2014, 257, 15–25. [Google Scholar] [CrossRef]
- Kumar, R.; Münstedt, H. Silver ion release from antimicrobial polyamide/silver composites. Biomaterials 2005, 26, 2081–2088. [Google Scholar] [CrossRef]
- ISO. Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces; ISO 22196; ISO: Geneva, Switzerland, 2011. [Google Scholar]
- Al-Sharqi, A.; Apun, K.; Vincent, M.; Kanakaraju, D.; Bilung, L.M. Enhancement of the antibacterial efficiency of silver nanoparticles against gram-positive and gram-negative bacteria using blue laser light. Int. J. Photoenergy 2019, 2019, 2528490. [Google Scholar] [CrossRef]
- Jalili, S.; Hajakbari, F.; Hojabri, A. Effect of silver thickness on structural, optical and morphological properties of nanocrystalline Ag/NiO thin films. J. Theor. Appl. Phys. 2018, 12, 15–22. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. 2011, 21, 433–446. [Google Scholar] [CrossRef]
- Navabpour, P.; Ostovarpour, S.; Hampshire, J.; Kelly, P.; Verran, J.; Cooke, K. The effect of process parameters on the structure, photocatalytic and self-cleaning properties of TiO2 and Ag-TiO2 coatings deposited using reactive magnetron sputtering. Thin Solid Films 2014, 571, 75–83. [Google Scholar] [CrossRef]
- Adochite, R.C.; Munteanu, D.; Torrell, M.; Cunha, L.; Alves, E.; Barradas, N.P.; Cavaleiro, A.; Riviere, J.P.; Le Bourhis, E.; Eyidi, D.; et al. The influence of annealing treatments on the properties of Ag:TiO2 nanocomposite films prepared by magnetron sputtering. Appl. Surf. Sci. 2012, 258, 4028–4034. [Google Scholar] [CrossRef]
- Okumu, J.; Dahmen, C.; Sprafke, A.N.; Luysberg, M.; Von Plessen, G.; Wuttig, M. Photochromic silver nanoparticles fabricated by sputter deposition. J. Appl. Phys. 2005, 97, 094305. [Google Scholar] [CrossRef]
- Xu, G.; Tazawa, M.; Jin, P.; Nakao, S.; Yoshimura, K. Wavelength tuning of surface plasmon resonance using dielectric layers on silver island films. Appl. Phys. Lett. 2003, 82, 3811–3813. [Google Scholar] [CrossRef]
- Sarkheil, M.; Sourinejad, I.; Mirbakhsh, M.; Kordestani, D.; Johari, S.A. Application of silver nanoparticles immobilized on TEPA-Den-SiO2 as water filter media for bacterial disinfection in culture of Penaeid shrimp larvae. Aquac. Eng. 2016, 74, 17–29. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G. Potent antibacterial activities of Ag/TiO2 nanocomposite powders synthesized by a one-pot sol-gel method. Environ. Sci. Technol. 2009, 43, 2905–2910. [Google Scholar] [CrossRef]
- Adams, L.K.; Lyon, D.Y.; McIntosh, A.; Alvarez, P.J.J. Comparative toxicity of nano-scale TiO2, SiO2 and ZnO water suspensions. Water Sci. Technol 2006, 54, 327–334. [Google Scholar] [CrossRef]
- Levchuk, I.; Kralova, M.; Rueda-Márquez, J.J.; Moreno-Andrés, J.; Gutiérrez-Alfaro, S.; Dzik, P.; Parola, S.; Sillanpää, M.; Vahala, R.; Manzano, M.A. Antimicrobial activity of printed composite TiO2/SiO2 and TiO2/SiO2/Au thin films under UVA-LED and natural solar radiation. Appl. Catal. 2018, 239, 609–618. [Google Scholar] [CrossRef]
- Ahmad Barudin, N.H.; Sreekantan, S.; Thong, O.M.; Sahgal, G. Antibacterial activity of Ag-TiO2 nanoparticles with various silver contents. Proc. Mater. Sci. Forum 2013, 756, 238–245. [Google Scholar] [CrossRef]
- Zhao, J.X.; Zhang, B.P.; Li, Y.; Yan, L.P.; Wang, S.J. Optical and photocatalytic properties of TiO2/Ag-SiO2 nanocomposite thin films. J. Alloys Compd. 2012, 535, 21–26. [Google Scholar] [CrossRef]
- Suwanchawalit, C.; Wongnawa, S.; Sriprang, P.; Meanha, P. Enhancement of the photocatalytic performance of Ag-modified TiO2 photocatalyst under visible light. Ceram. Int. 2012, 38, 5201–5207. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhai, C.; Zhang, J.; Zhu, D.; Zhao, K.; Chen, Y. Study on the attachment of Escherichia coli to sediment particles at a single-cell level: The effect of particle size. Water 2019, 11, 819. [Google Scholar] [CrossRef]
- Soupir, M.L.; Mostaghimi, S.; Dillaha, T. Attachment of Escherichia coli and Enterococci to Particles in Runoff. J. Environ. Qual. 2010, 39, 1019–1027. [Google Scholar] [CrossRef]
- Jeng, H.C.; England, A.J.; Bradford, H.B. Indicator organisms associated with stormwater suspended particles and estuarine sediment. J. Environ. Sci. Health-Part A Toxic/Hazard. Subst. Environ. Eng. 2005, 40, 779–791. [Google Scholar] [CrossRef]
- George, I.; Anzil, A.; Servais, P. Quantification of fecal coliform inputs to aquatic systems through soil leaching. Water Res. 2004, 38, 611–618. [Google Scholar] [CrossRef]
- Oliver, D.M.; Clegg, C.D.; Heathwaite, A.L.; Haygarth, P.M. Preferential attachment of Escherichia coli to different particle size fractions of an agricultural grassland soil. Water Air Soil Pollut. 2007, 185, 369–375. [Google Scholar] [CrossRef]
- Ahmed, F.; Awada, C.; Ansari, S.A.; Aljaafari, A.; Alshoaibi, A. Photocatalytic inactivation of Escherichia coli under UV light irradiation using large surface area anatase TiO2 quantum dots. R. Soc. Open Sci. 2019, 6, 191444. [Google Scholar] [CrossRef]
- Benabbou, A.K.; Derriche, Z.; Felix, C.; Lejeune, P.; Guillard, C. Photocatalytic inactivation of Escherischia coli. Appl. Catal. B Environ. 2007, 76, 257–263. [Google Scholar] [CrossRef]

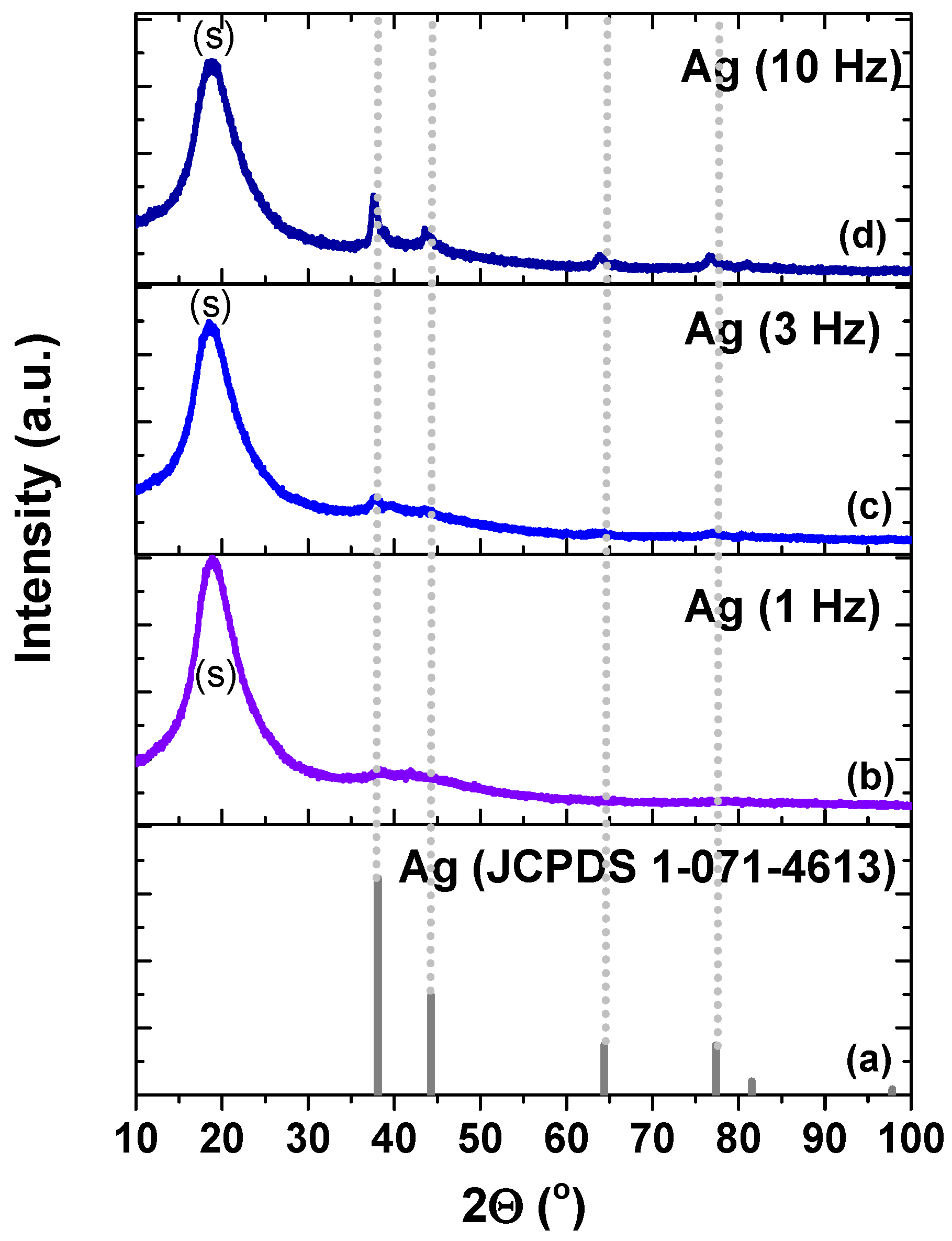
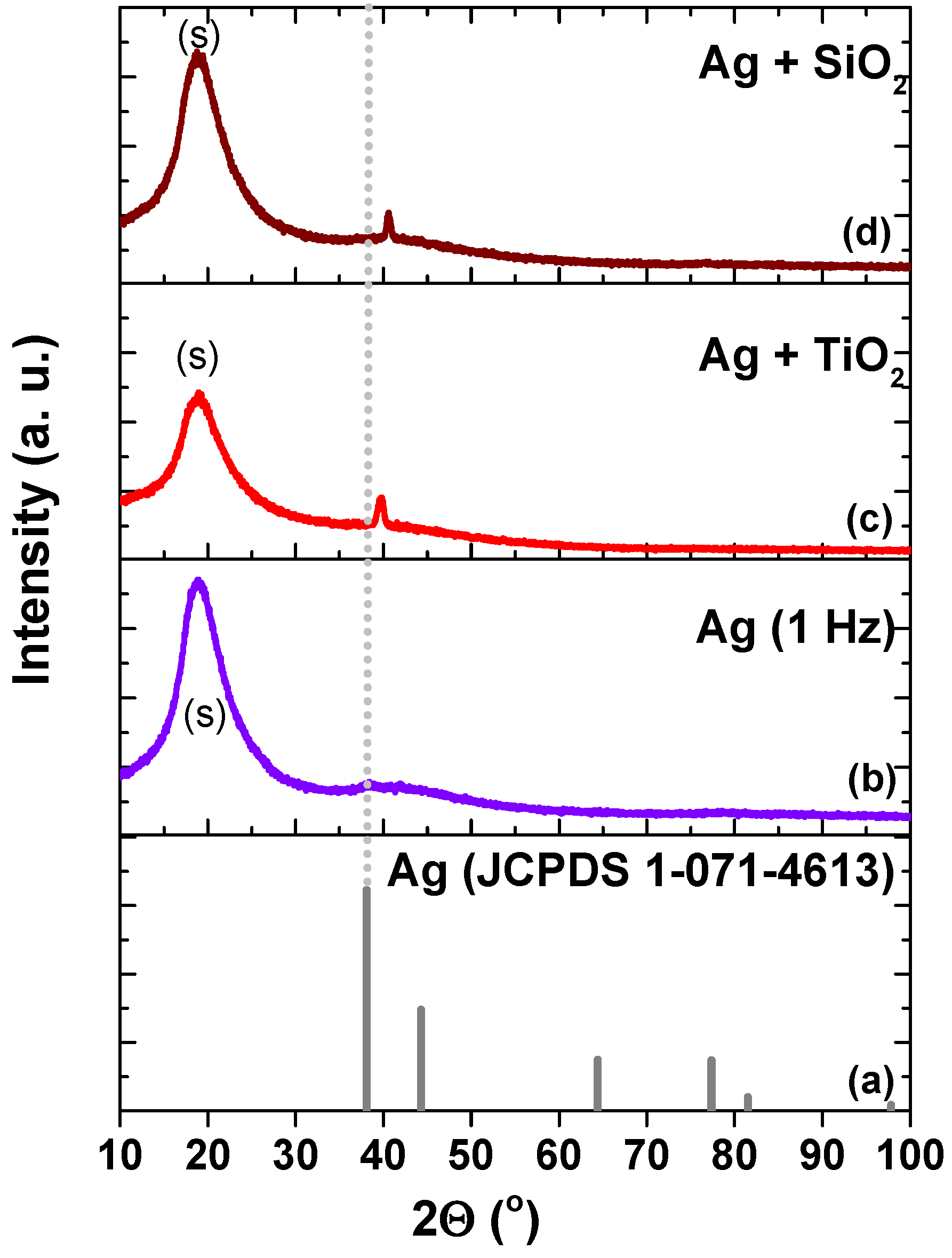
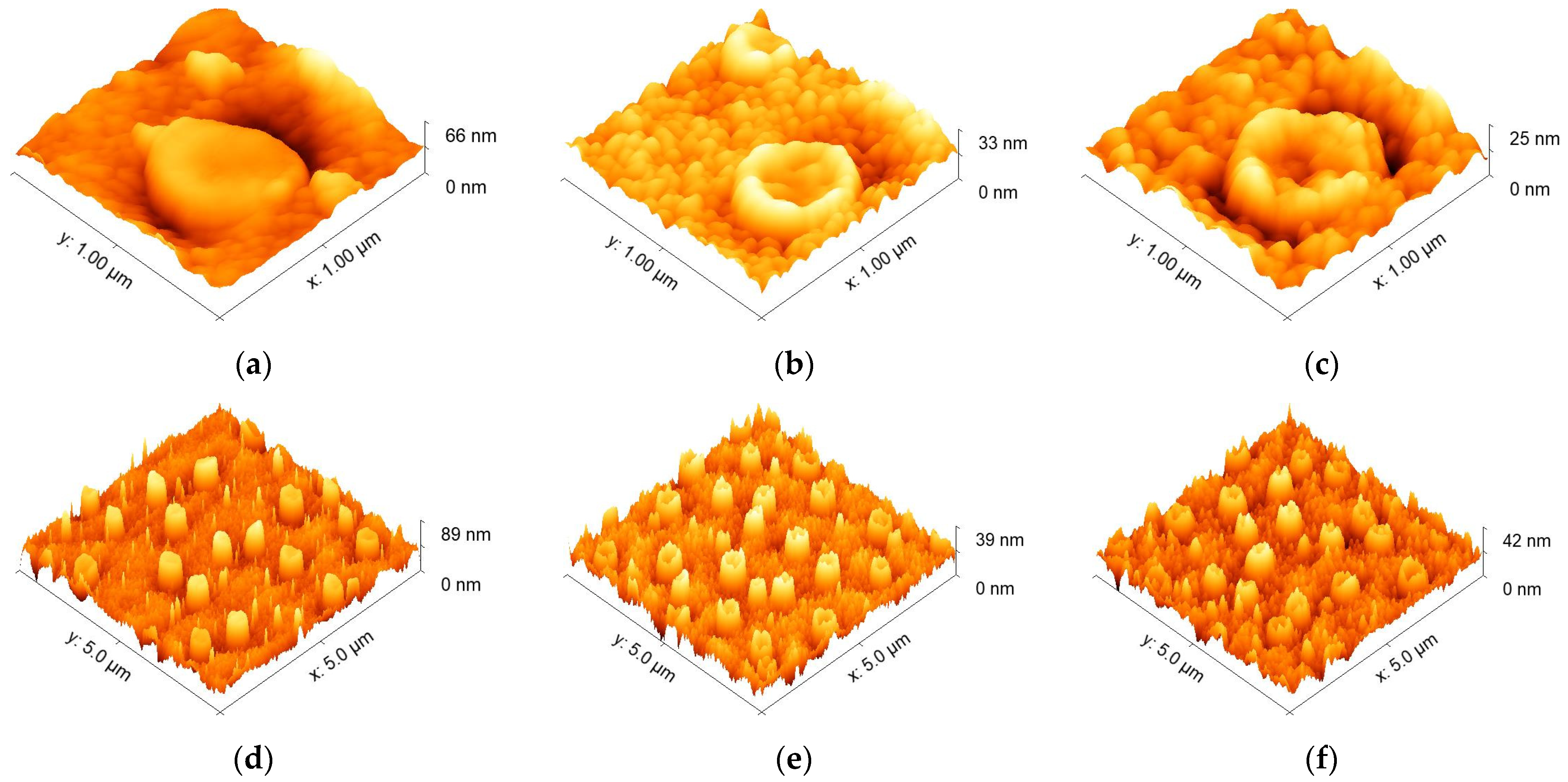
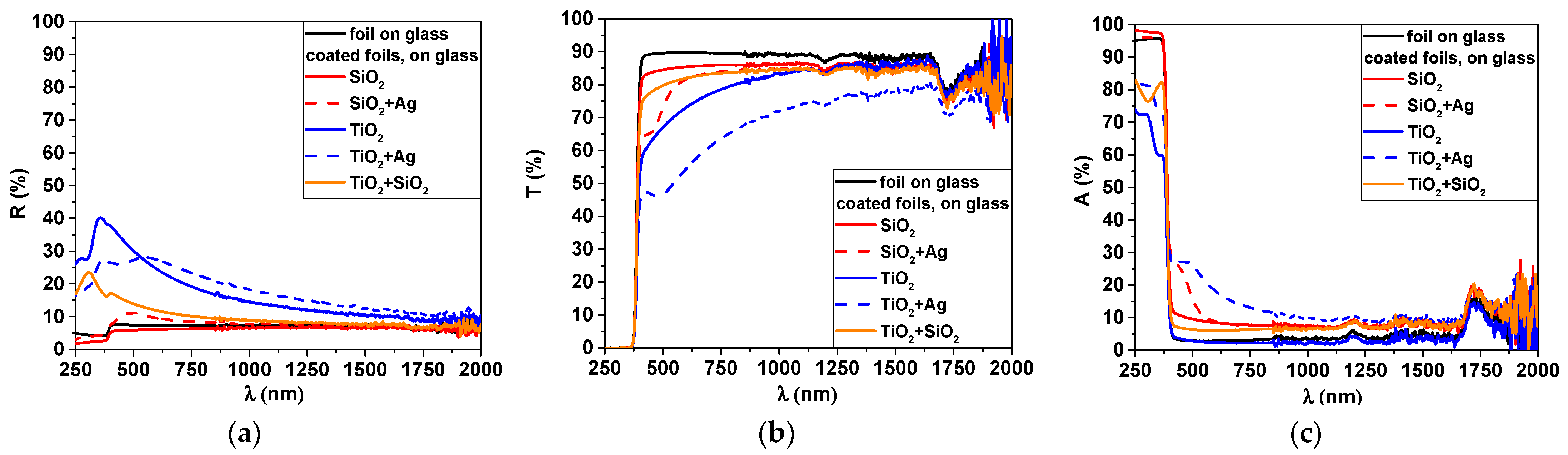
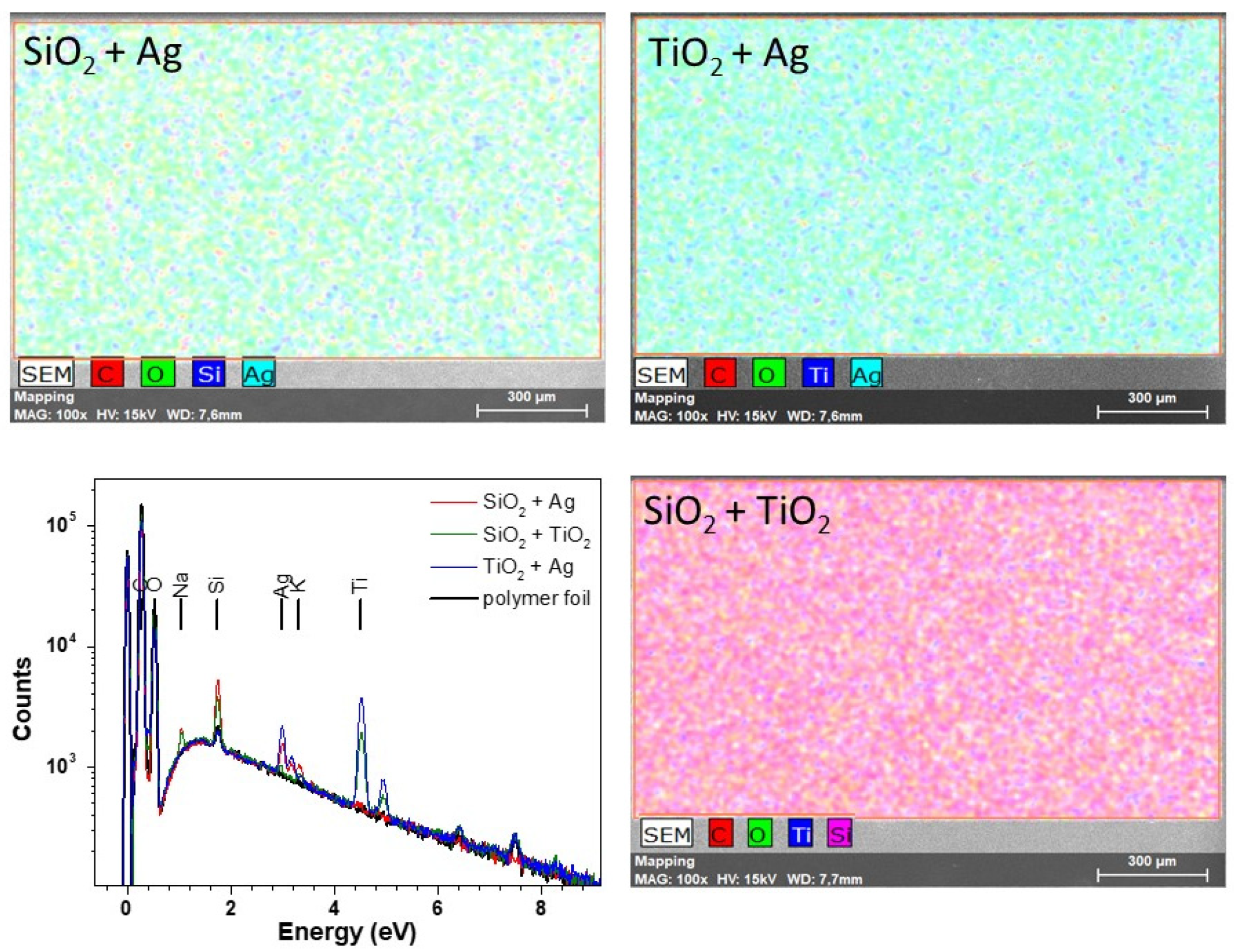
| Sample | Peak Position (111) (°) | Grain Size (nm) |
|---|---|---|
| Ag (JCPDS 01-071-4613) | 38.1 | NA |
| Ag (1 Hz, 30 min) | 38.3 | 1.07 |
| Ag (3 Hz, 30 min) | 37.64 | 1.53 |
| Ag (10 Hz, 30 min) | 37.63 | 9.42 |
| Ag + SiO2 | 40.57 | 16.6 |
| Ag + TiO2 | 39.76 | 12.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitelaru, C.; Parau, A.C.; Kiss, A.E.; Pana, I.; Dinu, M.; Constantin, L.R.; Vladescu, A.; Tonofrei, L.E.; Adochite, C.S.; Costinas, S.; et al. Silver-Containing Thin Films on Transparent Polymer Foils for Antimicrobial Applications. Coatings 2022, 12, 170. https://doi.org/10.3390/coatings12020170
Vitelaru C, Parau AC, Kiss AE, Pana I, Dinu M, Constantin LR, Vladescu A, Tonofrei LE, Adochite CS, Costinas S, et al. Silver-Containing Thin Films on Transparent Polymer Foils for Antimicrobial Applications. Coatings. 2022; 12(2):170. https://doi.org/10.3390/coatings12020170
Chicago/Turabian StyleVitelaru, Catalin, Anca C. Parau, Adrian E. Kiss, Iulian Pana, Mihaela Dinu, Lidia R. Constantin, Alina Vladescu, Lavinia E. Tonofrei, Cristina S. Adochite, Sarah Costinas, and et al. 2022. "Silver-Containing Thin Films on Transparent Polymer Foils for Antimicrobial Applications" Coatings 12, no. 2: 170. https://doi.org/10.3390/coatings12020170
APA StyleVitelaru, C., Parau, A. C., Kiss, A. E., Pana, I., Dinu, M., Constantin, L. R., Vladescu, A., Tonofrei, L. E., Adochite, C. S., Costinas, S., Rogozea, L., Badea, M., & Idomir, M. E. (2022). Silver-Containing Thin Films on Transparent Polymer Foils for Antimicrobial Applications. Coatings, 12(2), 170. https://doi.org/10.3390/coatings12020170






.jpg)


