Hydrogen Isotope Permeation Behavior of AlCrFeTiNb, AlCrMoNbZr and AlCrFeMoTi High-Entropy Alloys Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Coating Preparation
2.2. Deuterium Permeation Experiment and Methods
2.3. Electrochemical Hydrogen Permeation
2.4. Characterization
3. Results and Analysis
3.1. Gas-Driven Permeation Results
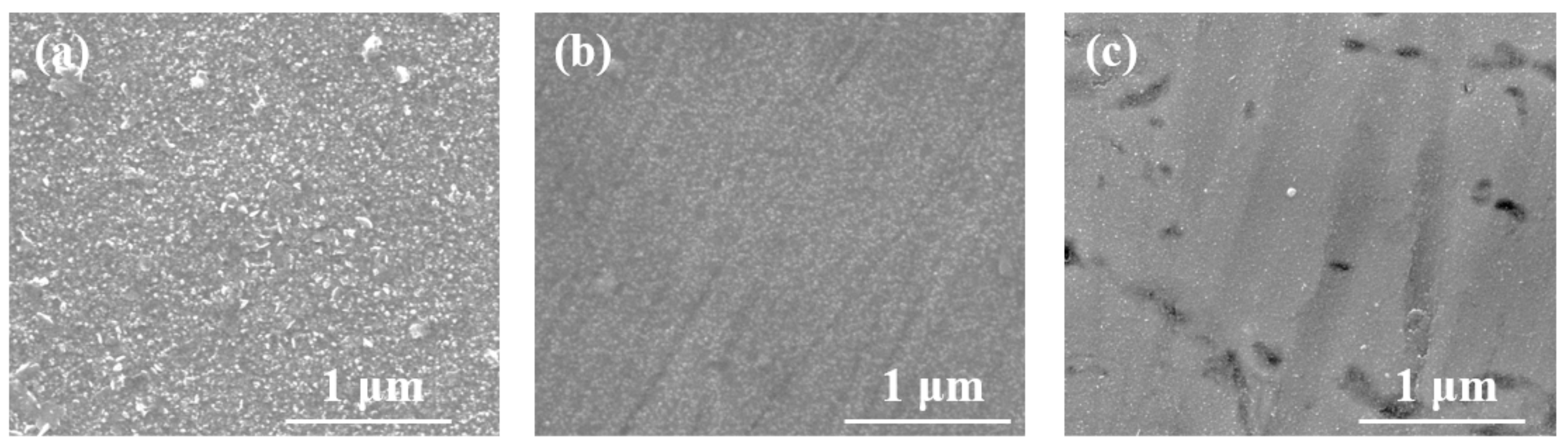
3.2. SEM Analysis
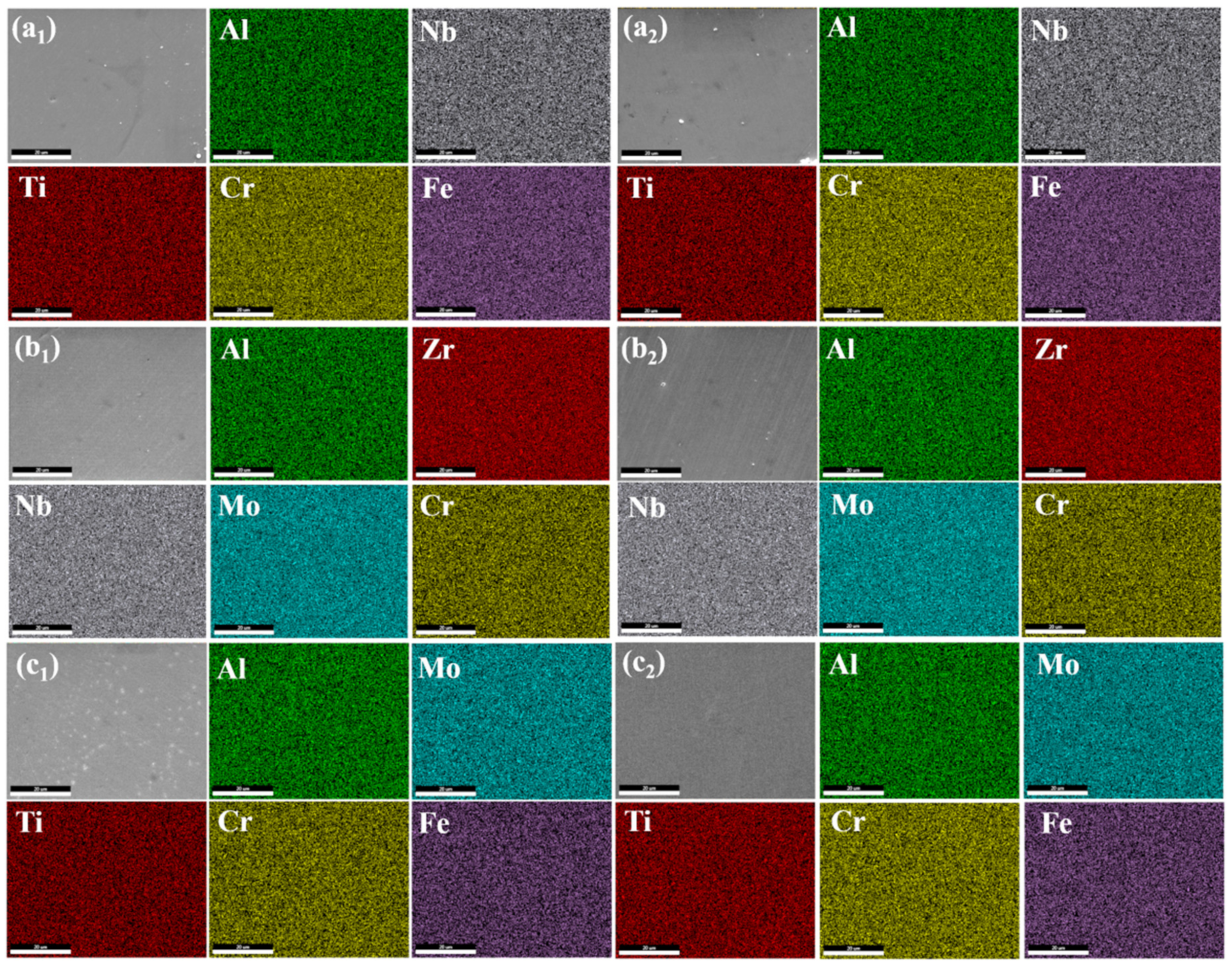
3.3. EDS Analysis
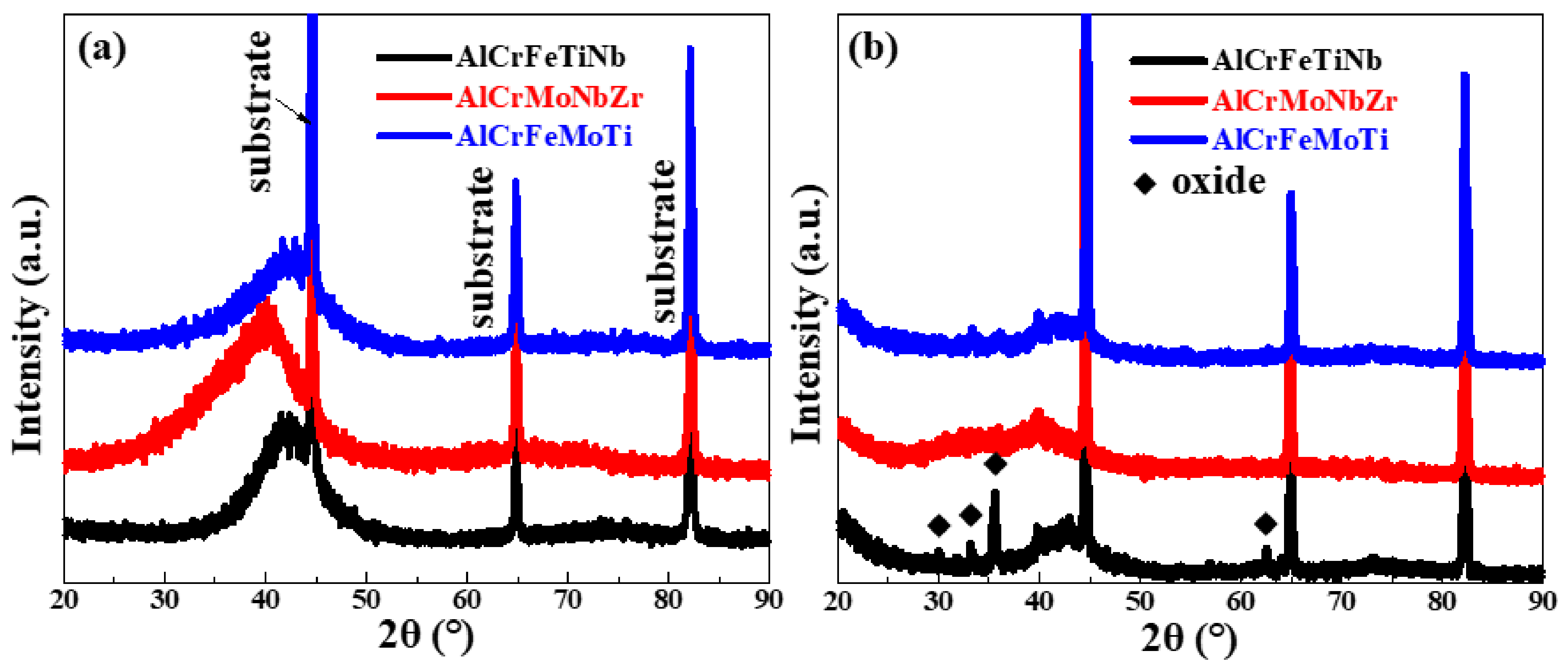
3.4. XRD Analysis
3.5. Electrochemical Hydrogen Permeation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, X.-D.; Xu, Y.-P.; Lu, T.; Zhou, H.-S.; Li, X.-C.; Gao, F.; Luo, G.-N. Influence of irradiation-induced point defects on the dissolution and diffusion properties of hydrogen in α-Al2O3: A first-principles study. Nucl. Fusion 2021, 61, 036004. [Google Scholar] [CrossRef]
- Wang, T.; Pu, J.; Bo, C.; Jian, L. Sol-gel prepared Al2O3 coatings for the application as tritium permeation barrier. Fusion Eng. Des. 2010, 85, 1068–1072. [Google Scholar] [CrossRef]
- Liao, H.B.; Wang, X.Y.; Yang, G.P.; Feng, Y.J.; Wang, P.H.; Feng, K.M. Recent progress of R&D activities on reduced activation ferritic/martensitic steel (CLF-1). Fusion Eng. Des. 2019, 147, 111235. [Google Scholar]
- Matsuda, S.; Katayama, K.; Shimozori, M.; Fukada, S.; Ushida, H.; Nishikawa, M. Hydrogen Permeation Behavior through F82H at High Temperature. Fusion Sci. Technol. 2015, 67, 467–470. [Google Scholar] [CrossRef]
- Wu, M.; Chen, Y.; Peng, J.-Q.; Yan, G.-Q.; Sun, Y.-P.; Zhang, J.-D.; Zhang, S.-L.; Wang, L.-J. Hydrogen permeation resistance and characterization of Si–Al and Si–Zr composite sol oxide coating on surface of zirconium hydride. Rare Met. 2017, 36, 55–60. [Google Scholar] [CrossRef]
- Kim, K.I.; Hong, T.W. Hydrogen permeation of TiN–graphene membrane by hot press sintering (HPS) process. Solid State Ion. 2012, 225, 699–702. [Google Scholar] [CrossRef]
- Miraclea, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.-W.; Chen, S.K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Zhang, W.; Liaw, P.K.; Zhang, Y. Science and technology in high-entropy alloys. Sci. China Mater. 2018, 61, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Senkov, O.N.; Semiatin, S.L. Microstructure and Properties of a Refractory High-Entropy Alloy after Cold Working. J. Alloy Compd. 2015, 649, 1110–1123. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, J.; Wu, K.; Liang, X.; Liu, G.; Sun, J. Ultrastrong Al0.1CoCrFeNi high-entropy alloys at small scales: Effects of stacking faults vs. nanotwins. Nanoscale 2018, 10, 13329–13334. [Google Scholar] [CrossRef]
- Wen, L.H.; Kou, H.C.; Li, J.S.; Chang, H.; Xue, X.Y.; Zhou, L. Effect of aging temperature on microstructure and properties of AlCoCrCuFeNi high-entropy alloy. Intermetallics 2009, 17, 266–269. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhý, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The influences of temperature and microstructure on the tensile properties of a CoCrFeMnNi high-entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef] [Green Version]
- Chou, Y.; Wang, Y.; Yeh, J.; Shih, H. Pitting corrosion of the high-entropy alloy Co1.5CrFeNi1.5Ti0.5Mo0.1 in chloride-containing sulphate solutions. Corros. Sci. 2010, 52, 3481–3491. [Google Scholar] [CrossRef]
- Shih, H.C.; Lee, C.P.; Chen, Y.; Wu, C.; Hsu, C.; Yeh, J. Effect of Boron on the Corrosion Properties of Al0.5CoCrCuFeNiBx High Entropy Alloys in 1N Sulfuric Acid. ECS Trans. 2007, 2, 15–33. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, M.; Wang, L.; Liu, C.H.; Chang, H.; Yang, J.J.; Liao, J.L.; Yang, Y.Y.; Liu, N. Interface stability, mechanical and corrosion properties of AlCrMoNbZr/(AlCrMoNbZr)N high-entropy alloy multilayer coatings under helium ion irradiation. Appl. Surf. Sci. 2019, 485, 108–118. [Google Scholar] [CrossRef]
- Xia, S.Q.; Yang, X.; Yang, T.; Liu, S.; Zhang, Y. Irradiation Resistance in AlxCoCrFeNi High Entropy Alloys. J. Miner. 2015, 67, 2340–2344. [Google Scholar]
- Egami, T.; Guo, W.; Rack, P.D.; Nagase, T. Irradiation Resistance of Multicomponent Alloys. Metall. Mater. Trans. A 2014, 45, 180–183. [Google Scholar] [CrossRef]
- Lu, C.Y.; Niu, L.L.; Chen, N.J.; Jin, K.; Yang, T.N.; Xiu, P.Y.; Zhang, Y.W.; Gao, F.; Bei, H.B.; Shi, S.; et al. Enhancing Radiation Tolerance by Controlling defect Mobility and Migration Pathways In_multicomponent Single-phase Alloys. Nat. Commun. 2016, 7, 13564. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, R.; Yang, Z.; Liu, C.; Chang, H.; Yang, J.; Liao, J.; Yang, Y.; Liu, N. Preparation, Structure, and Properties of an AlCrMoNbZr High-entropy Alloy Coating for Accident-tolerant Fuel Cladding. Surf. Coat. Technol. 2018, 347, 13–19. [Google Scholar] [CrossRef]
- Nagase, T.; Rack, P.D.; Noh, J.H.; Egami, T. In-situ Tem Observation of Structural Changes in Nano-crystalline_cocrcufeni Multicomponent High-entropy Alloy (HEA) Under Fast-electron Irradiation by High Voltage Electron Microscopy (HVEM). Intermetallics 2015, 59, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Liu, B.; Liu, Y.; Guo, W.; Fu, A.; Li, L.; Yan, N.; Fang, Q. Microstructure and Mechanical Properties of Particulate Reinforced NbMoCrTiAl High Entropy Based Composite. Entropy 2018, 20, 517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Wang, L.; Luo, X.; Gong, B.; Wang, X.; Yang, J.; Feng, Y. Deuterium permeability of a novel AlCrTaTiZr high entropy alloy coating. Mater. Res. Express 2021, 8, 056401. [Google Scholar] [CrossRef]
- Devanathan, M.A.V.; Stachurski, Z. The Adsorption and Diffusion of Electrolytic Hydrogen in Palladium. Proc. R. Soc. London Ser. A Math. Phys. Sci. 1962, 270, 90–102. [Google Scholar]
- Engels, J.; Houben, A.; Rasinski, M.; Linsmeier, C. Hydrogen Saturation and Permeation Barrier Performance of Yttriumoxide Coatings. Fusion Eng. Des. 2017, 124, 1140–1143. [Google Scholar] [CrossRef]
- Chikada, T.; Suzuki, A.; Kobayashi, T.; Maier, H.; Terai, T.; Muroga, T. Microstructure Change and Deuterium Permeation Behavior of Erbium Oxide Coating. J. Nucl. Mater. 2011, 417, 1241–1244. [Google Scholar] [CrossRef]
- Endoh, R.; Nakamura, K.; Fujita, H.; Matsunaga, M.; Kimura, K.; Riesch, J.; Hishinuma, Y.; Chikada, T. Deuterium Permeation Behavior Through Yttria-stabilized Zirconia Coating_fabricated By Magnetron Sputtering. Fusion Eng. Des. 2020, 157, 111769. [Google Scholar] [CrossRef]
- Huang, H.; Zheng, J.; Ding, S.; Wang, W.; Zhang, H. Effect of natural oxide film on the deuterium permeation behavior of 430 stainless steel. Fusion Eng. Des. 2020, 152, 111469. [Google Scholar] [CrossRef]
- Takeda, T.; Iwatsuki, J.; Inagaki, Y. Permeability of Hydrogen and Deuterium of Hastelloy Xr. J. Nucl. Mater. 2004, 326, 47–58. [Google Scholar] [CrossRef]
- Levchuk, D.; Koch, F.; Maier, H.; Bolt, H. Deuterium Permeation Through Eurofer_and A-alumina Coated Eurofer. J. Nucl. Mater. 2004, 328, 103–106. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, C.; Yang, J.; Zhang, W.; He, L.; Zhang, R.; Yang, H.; Wang, J.; Long, J.; Chang, H. Mechanical and High-temperature Corrosion Properties of AlTiCrNiTa High Entropy Alloy Coating Prepared by Magnetron Sputtering for Accident-tolerant Fuel Cladding. Surf. Coat. Technol. 2021, 417, 127228. [Google Scholar] [CrossRef]




| Samples | Elements Contents | ||||||
|---|---|---|---|---|---|---|---|
| Al | Cr | Nb | Ti | Fe | O | ||
| AlCrFeTiNb | As-deposited | 7.86 | 20.94 | 20.18 | 13.97 | 36.83 | - |
| After permeation test | 6.67 | 16.73 | 16.66 | 11.64 | 29.99 | 18.31 | |
| AlCrMoNbZr | As-deposited | 6.71 | 17.24 | 26.46 | 26.97 | 22.61 | - |
| After permeation test | 6.99 | 17.50 | 25.99 | 27.41 | 22.12 | - | |
| AlCrFeMoTi | As-deposited | 10.90 | 19.94 | 30.38 | 14.81 | 23.97 | - |
| After permeation test | 9.90 | 20.30 | 29.90 | 15.95 | 23.95 | - | |
| Sample | Hydrogen Diffusivity (cm2/s) | |
|---|---|---|
| AlCrFeTiNb | 10,686 | 3.89918 × 10−8 |
| AlCrMoNbZr | 22,780 | 1.82909 × 10−8 |
| AlCrFeMoTi | 2846 | 1.46404 × 10−7 |
| Samples | Elements Contents | ||||||
|---|---|---|---|---|---|---|---|
| Al | Cr | Nb | Ti | Fe | Al | ||
| AlCrFeTiNb | As-deposited | 7.86 | 20.18 | 13.97 | 20.94 | 36.83 | 7.86 |
| After permeation test | 7.62 | 20.67 | 13.84 | 20.89 | 36.97 | 7.62 | |
| AlCrMoNbZr | As-deposited | 6.71 | 22.61 | 26.46 | 26.97 | 17.24 | 6.71 |
| After permeation test | 6.53 | 22.77 | 25.02 | 27.94 | 17.75 | 6.53 | |
| AlCrFeMoTi | As-deposited | 10.90 | 14.81 | 23.97 | 30.38 | 19.94 | 10.90 |
| After permeation test | 0.57 | 0.14 | 89.17 | 0.43 | 9.80 | 0.57 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Z.; Wang, L.; Zhang, W.; Yang, J.; Feng, Y.; Yang, J.; Li, H.; Yin, H.; Zhang, L.; Wang, X. Hydrogen Isotope Permeation Behavior of AlCrFeTiNb, AlCrMoNbZr and AlCrFeMoTi High-Entropy Alloys Coatings. Coatings 2022, 12, 171. https://doi.org/10.3390/coatings12020171
Hong Z, Wang L, Zhang W, Yang J, Feng Y, Yang J, Li H, Yin H, Zhang L, Wang X. Hydrogen Isotope Permeation Behavior of AlCrFeTiNb, AlCrMoNbZr and AlCrFeMoTi High-Entropy Alloys Coatings. Coatings. 2022; 12(2):171. https://doi.org/10.3390/coatings12020171
Chicago/Turabian StyleHong, Zhihao, Long Wang, Wei Zhang, Jian Yang, Yongjin Feng, Jijun Yang, Haoxiang Li, Huaqiang Yin, Long Zhang, and Xiaoyu Wang. 2022. "Hydrogen Isotope Permeation Behavior of AlCrFeTiNb, AlCrMoNbZr and AlCrFeMoTi High-Entropy Alloys Coatings" Coatings 12, no. 2: 171. https://doi.org/10.3390/coatings12020171
APA StyleHong, Z., Wang, L., Zhang, W., Yang, J., Feng, Y., Yang, J., Li, H., Yin, H., Zhang, L., & Wang, X. (2022). Hydrogen Isotope Permeation Behavior of AlCrFeTiNb, AlCrMoNbZr and AlCrFeMoTi High-Entropy Alloys Coatings. Coatings, 12(2), 171. https://doi.org/10.3390/coatings12020171





