Recent Progress in Functional Edible Food Packaging Based on Gelatin and Chitosan
Abstract
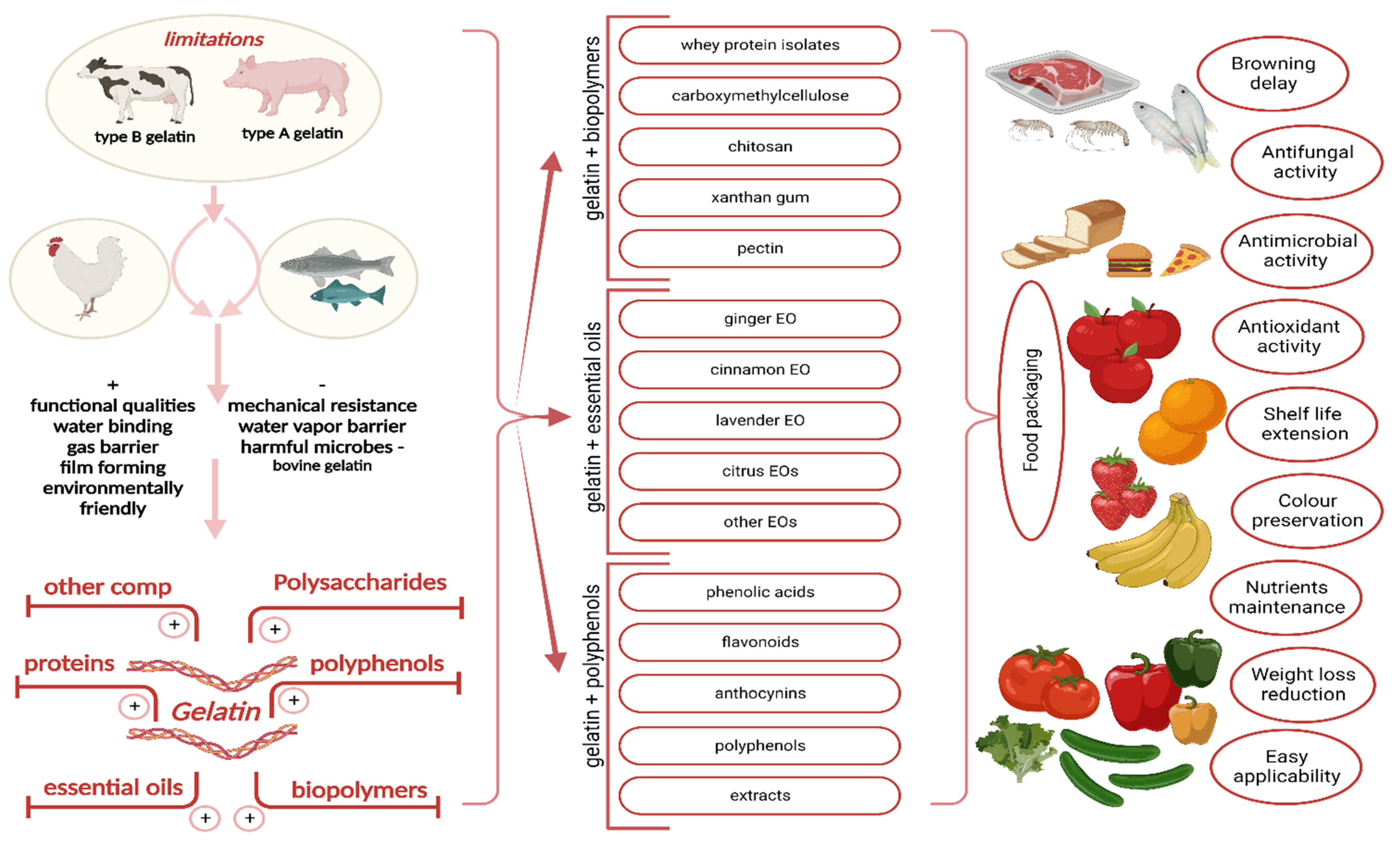
:1. Introduction
2. Gelatin
2.1. Origination of Gelatin
2.1.1. Gelatin Obtained from Mammals
2.1.2. Gelatin Obtained from Poultry
2.1.3. Gelatin Obtained from Aquatic Species
2.2. Gelatin-Based Composites
2.2.1. Combined Gelatin and Other Biopolymers
| Formulation | Physical/Chemical/Mechanical/Biological Characteristics | References |
|---|---|---|
| Gelatin, whey protein isolate | synergistic interaction ↑ gelling properties, EM | [57] |
| Gelatin, soy protein isolate | ↑ mechanical properties when the weight ratio of soy protein isolate: gelatin is 1:3 | [64] |
| Gelatin, soy protein isolate | ↑ TS, EAB, EM, flexibility | [27] |
| Gelatin, chitosan | ↑ mechanical properties ↓ permeability good UV-light protection qualities | [25] |
| Gelatin, CMC | ↑ TS, puncture test of film, thermal stability, WVP, ↓ EAB, opacity, and UV-light penetration of the films | [60] |
| Gelatin, CMC, chitosan | ↓ WVP ↑ biodegradability | [62] |
| Gelatin, CMC, chitosan | ↑ flexibility, EAB, WVP, thickness ↓ TS and puncture force | [63] |
| Gelatin, chitosan, xanthan gum | ↑ thickness, WVP, UV-light protection, thermal stability, ↓ TS, EAB, VIS light transparency | [28] |
| Gelatin, starch | ↑ mechanical strength, water solubility (WS), WVP, thickness ↓ opacityimproved appearance of refrigerated Red Crimson grapes | [26] |
| Gelatin, potato starch | ↑ TS, EM, WVP, melting temperature, UV–VIS light protection ↓ WS, EAB | [61] |
| Gelatin, tapioca starch | ↑ TS, EAB, thickness, WVP, UV-light protection, thermal stability visible light transmission, film transparency | [29] |
| Gelatin, pectin | ↑ thickness, TS, antioxidant, and antibacterial activities ↓ WVP, EAB | [30] |
2.2.2. Combined Gelatin and Polyphenols/Extracts Rich in Polyphenols
| Formulation | Physical/Chemical/Mechanical Characteristics | Biological Properties | Applications | References |
|---|---|---|---|---|
| Gelatin, protocatechuic acid | ↑ thickness, EAB achieved fine look, ↓ light transmittance, TS, WVP | Antioxidant activity (DPPH), antimicrobial activity against E. coli and S. aureus, with high protocatechuic acid amounts. | Beef preservation | [31] |
| Gelatin, epigallocatechin gallate (EGCG) | ↑ bloom strength | Antioxidant activity (DPPH (50%–99%), FRAP (200–662 μg Vc/g)), antimicrobial activity against E. coli and S. aureus | Active packaging | [32] |
| Gelatin, Galla chinensis extract | ↑ gel strength and thermal stability, ↓ swelling of gelatin | Not determined | Packaging | [33] |
| Gelatin, eugenol/β-cyclodextrin emulsion | not determined | Reduced the H2S-producing bacteria, total viable Pseudomonas spp. and Psychrophilic counts, total volatile basic nitrogen, K value, free fatty acids | Chinese Seabass during superchilling storage | [34] |
| Gelatin, mango peel | ↓ WVP, solubility films more rigid and less flexible | Antioxidant activity (DPPH 70%–85%) | Active packaging | [35] |
| Gelatin, green tea extract grape seeds extract gingko leaf extract | ↓ TS, EAB, lowest WVP lowest TS, EAB, ↓ WVP ↓ TS, EAB, WVP | All the films presented antioxidant activity (DPPH) | Active food packaging | [70] |
| Gelatin, Fructus chebulae extract | ↑ gel strength, thermal stability | Not determined | Packaging | [76] |
| Gelatin, chlorogenic acid | not determined | Antioxidant activity (ABTS), antimicrobial activity against E. coli, P. aeruginosa, L. monocytogenes, and S. aureus | Fresh seafood preservation | [65] |
| Gelatin, epigallocatechin gallate | ↑ TS, EM, ↓EAB | Antioxidant activity (DPPH 67%) | Reduce the oxidation of cod-liver oil | [66] |
| Gelatin, green tea powder | ↓ TS, EM, EAB with high amounts of green tea powder | Antioxidant activity (DPPH 77%) | Reduce the oxidation of cod-liver oil | [66] |
| Gelatin, green tea extract | ↑ TS↓ EAB, WS, WVP | Antioxidant activity (DPPH 15%–55%) | Active packaging | [69] |
| Gelatin, rosmarinic acid | ↑ thickness, TS, EAB, light protection, ↓ WS, WVP | Antioxidant activity (DPPH 75%–90%) | Bacon preservation | [67] |
| Gelatin, rosmarinic acid | ↑ EAB, ↓ TS, EM, WVP | Antioxidant activity (ABTS), antimicrobial activity against E. coli and S. aureus | Active packaging | [74] |
| Gelatin, tannic acid | ↑ TS ↓ EAB, WVP, oxygen permeability | Antimicrobial activity against E. coli and S. aureus | Cherry tomatoes, grapes | [68] |
| Gelatin, mangrove extracts | ↑ thickness, EAB, TS ↓ WVP | Antioxidant activity (DPPH 15%–60%), antimicrobial activity against S. aureus, E. coli, Bacillus subtillis, Salmonella sp. | Active packaging | [19] |
| Gelatin, pomegranate peel powder | ↑ thickness, WVP, TS ↓ film solubility, EAB | Antioxidant activity (DPPH 59%–72%, ABTS 48%–80%), antimicrobial activity against S. aureus, L. monocytogenes, and E. coli | Active packaging | [71] |
| Gelatin, haskap berries extract | ↑TS, EAB ↓WVP, WS | Antioxidant activity (DPPH) | Shrimp spoilage | [75] |
| Gelatin, date by-products | ↓water holding capacity, WS color change | Antimicrobial activity against E. coli and S. aureus | Active packaging | [72] |
2.2.3. Combined Gelatin and Essential Oil
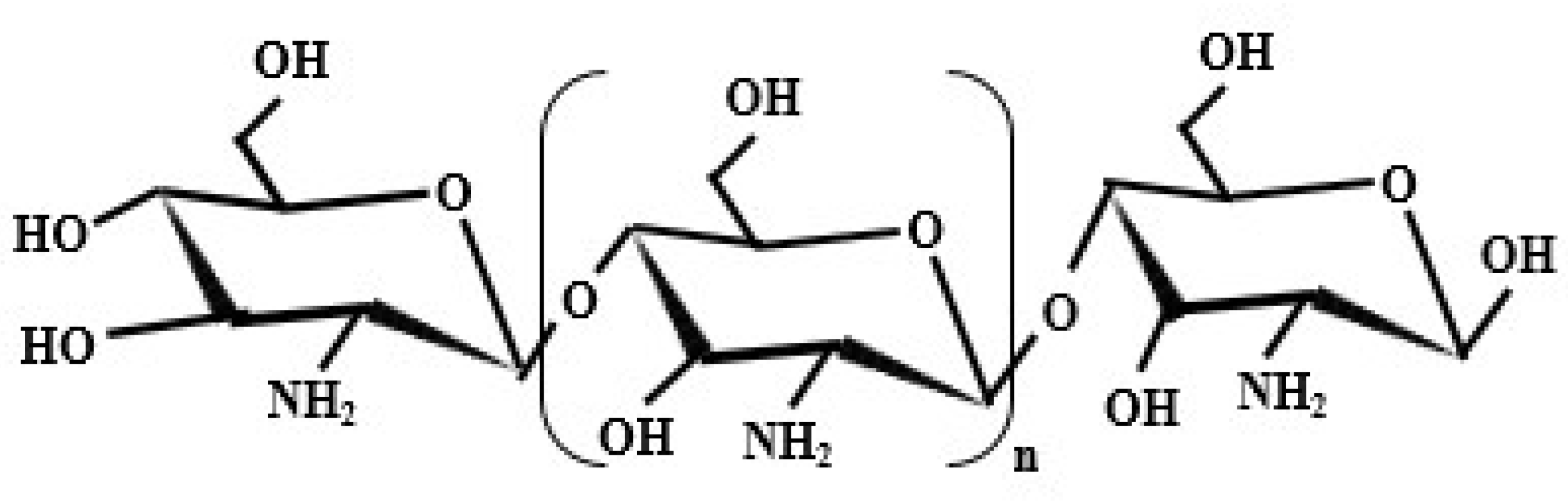
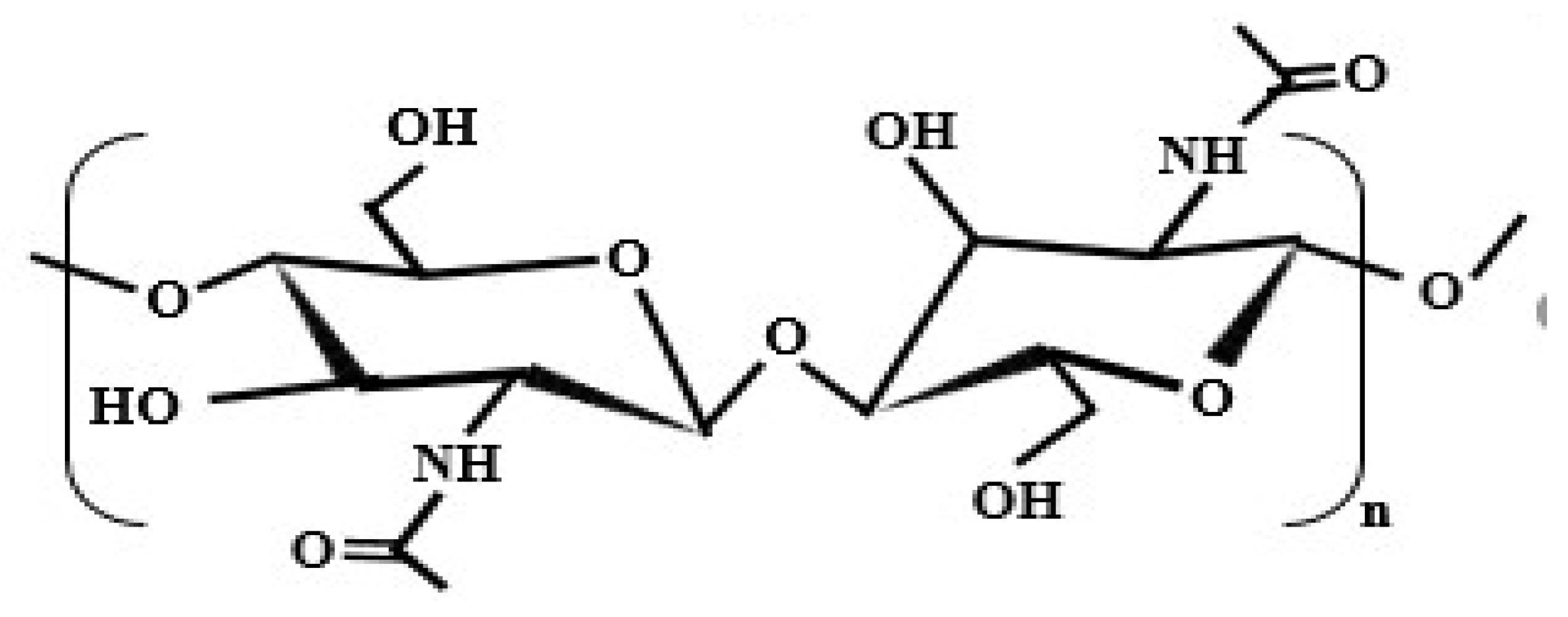
3. Chitosan
3.1. Origination of Chitosan
3.2. Chitosan-Based Composites
3.2.1. Combined Chitosan and Other Biopolymers
3.2.2. Combined Chitosan with Polyphenols/Extracts Rich in Polyphenols
| Formulation | Physical/Chemical/Mechanical Characteristics | Biological Properties | Applications | References |
|---|---|---|---|---|
| Chitosan, propolis extract | ↑ TS, EAB, ↓ WVP, oxygen permeability color changes of the films | Antioxidant activity (DPPH), antimicrobial activity against S. aureus, Salmonella Enteritidis, E. coli, and P. aeruginosa | Active packaging | [103] |
| Chitosan, propolis extract | ↑ thickness, thermal stability, TS ↓ transparency, EAB, WS color change | Antioxidant activity (DPPH (49.8%–94.5%), ABTS (20.3%–83.6%)), antimicrobial activity against Staphylococcus hominis, Pantoea sp., Arthrobacter sp., Erwinia sp., B. cereus, E. coli, S. aureus, Metschnikowia rancensis, Cladosporium sp., Penicillium brevicompactum, Botrytis cinerea, and Alternaria sp. | Active packaging | [104] |
| Chitosan, gallic acid | ↑ TS (for chitosan:gallic acid ratio 1:0.1, 1:0.5) ↓ EAB and WVP (for chitosan:gallic acid ratio 1:0.1) | Antioxidant activity (DPPH, ABTS), antimicrobial activity against E. coli and L. monocytogenes | Active food packaging | [105] |
| Chitosan, epigallocatechin gallate nanocapsules (with zein) | ↑ TS, EAB, VIS-light protection | Antioxidant activity (DPPH) | Active packaging | [106] |
| Chitosan, ellagic acid | ↑ EAB, WVP ↓ TS, Young’s modulus, UV-light protection good thermal stability | Antioxidant activity (DPPH), antimicrobial activity against P. aeruginosa and S. aureus, prevent photo-oxidation of light-sensitive foods | Active packaging | [107] |
| Chitosan, protocatechuic acid | ↑ thickness, opacity, WS, UV-light barrier ↓ moisture content, WVP, EAB, color change TS increased up to 1% acid incorporation, afterwards decreased | Antioxidant activity (DPPH) | Active packaging | [108] |
| Chitosan, thinned young apple polyphenols | ↑ thickness, density, WS ↓ WVP, TS, EAB, water content | Antioxidant activity (DPPH 68%–92%), antimicrobial activity against E. coli, S aureus, L. monocytogenes, Colletotrichum fructicola, Botryosphaerial dothidea, and Alternaria tenuissima | Active packaging | [123] |
| Chitosan, apple peel polyphenols | ↑ thickness, density, WS, ↓ thermal stability, WVP, TS, EAB, moisture content, transparency color change | Antioxidant activity (DPPH 30%–67%, ABTS 70%–90%), antimicrobial activity against E. coli, B. cereus, S. aureus, and S. typhimurium | Active packaging | [101] |
| Chitosan, proanthocyanidins | ↓ thermal stability | Antioxidant activity (DPPH, ABTS), antimicrobial activity against M. luteus, B. subtilis, E. coli, S. aureus, Proteus vulgaris, and P. aeruginosa | Active packaging | [132] |
| Chitosan, proanthocyanidins | ↑ thickness, opacity, thermal stability, WS, WVP, TS, UV–VIS light barrier ↓ moisture content, EAB, oxygen permeability color change | Antioxidant activity (DPPH), antimicrobial activity against E. coli, Salmonella, S. aureus, and L. monocytogenes | Active packaging | [109] |
| Chitosan, syringic acid | ↑ thickness, density, WS, opacity, TS when the amount of syringic acid was under 0.5% and EAB when the amount of syringic acid was 0.25%, ↓ moisture content, thermal stability and WVP color change | Antimicrobial activity against S. aureus and E. coli | Preservation of quail egg Active packaging | [110] |
| Chitosan, phenolic acids (ferulic acid, caffeic acid, tannic acid, gallic acid) | ↑ TS, EAB, Young’s modulus, thermal stability, WVP color change | Antioxidant activity (DPPH 17%–89%) | Active packaging | [81] |
| Chitosan, curcumin | ↑ TS ↓ EAB, WVP, | Antimicrobial activity against S. aureus and Rhizoctonia solani | Active packaging | [111] |
| Chitosan, carvacrol | ↓ WVP, TS, EAB, thickness and transparency, change color to yellow | Antioxidant activity (FRAP), antimicrobial activity against E. coli and S. aureus | Active packaging | [114] |
| Chitosan, pomegranate peel extract | ↑ thickness, TS ↓ EAB and transparency change color | Antioxidant activity (FRAP), antimicrobial activity against S. aureus | Active packaging | [114] |
| Chitosan, pomegranate peel extract | ↑ EAB ↓ TS, WVP | Antioxidant activity (DPPH 21%–57%) | Active packaging | [119] |
| Chitosan, thyme extract | ↑ TS, EM, opacity decreased: EAB, color change | Antioxidant activity (DPPH) | Active packaging | [115] |
| Chitosan, turmeric extract | ↑ TS, Young’s modulus, WVP, UV–VIS barrier property | Antimicrobial activity against S. aureus and Salmonella | Active packaging | [116] |
| Chitosan, tea extract | ↑ thickness, WS ↓ water content, WVP, TS, EAB | Antioxidant activity (DPPH) | Active packaging | [124] |
| Chitosan, grapefruit seed extract | ↑ thickness, EAB, ↓TS | Antifungal activity | Bread preservation | [120] |
| Chitosan, maqui berry extract (Aristotelia chilensis) | not determined | Antioxidant activity (DPPH, FRAP), antimicrobial activity against Serratia marcescens, Alcaligenes faecalis, Aeromonas hydrophila, Pseudomonas fluorescens, Citrobacter freundii, Achromobacter denitrifican, S. putrefaciens | Active packaging | [131] |
| Chitosan, Lycium barbarum fruit extract | ↑ density ↓ TS, EAB, WVP, WS, moisture content | Antioxidant activity (DPPH) | Active packaging | [125] |
| Chitosan, honeysuckle flower extract (Lonicera japonica Thunb) | ↑ WS, density ↓ WVP, TS, EAB, moisture content | Antioxidant activity (DPPH), antimicrobial activity against E. coli | Active packaging | [126] |
| Chitosan, Berberis crataegina fruit extract | ↑ thickness, EAB ↓ transparency, TS, WS, Young’s modulus | Antioxidant activity (DPPH 86%), antimicrobial activity against E. coli, S. thypmurium, Proteus microbilis, Proteus vulgaris, P. aeruginosa, Enterobacter aerogenes, S. aureus, Streptococcus mutans, Bacillus thuringiensis | Active packaging | [121] |
| Chitosan, Nigella sativa seedcake extract | ↑ thickness, EAB, ↓ moisture content, WVP, TS color change | Antioxidant activity (DPPH, FRAP) | Active packaging | [122] |
| Chitosan, mango leaf extract | ↑ thickness, TS, EM, ↓ moisture content, WS, WVP, EAB | Antioxidant activity (DPPH, FRAP, ABTS) | Cashew nuts preservation | [117] |
| Chitosan, Herba Lophatheri extract from dried leaves of Lophatherum gracile Brongn | ↑ opacity, density, ↓ WS, WVP, moisture content color change, higher oil resistance | Antioxidant activity (DPPH), antimicrobial activity against E. coli and S. aureus | Active packaging | [129] |
| Chitosan, Chinese chive (Allium tuberosum) root extract | ↑ thickness, thermal stability ↓ TS, EAB, WS, WVP, moisture content | Antioxidant activity (DPPH 20%–47%, ABTS 28%–57%), antimicrobial activity against B. cereus, S. aureus, E. coli, and S. typhimurium | Soybean oil packaging | [102] |
| Chitosan, Sonneratia caseolaris (L.) Engl. leaf extract | ↑ light barrier property, WS, WVP ↓ TS, EAB, moisture content change color | Antimicrobial activity against S. aureus and P. aeruginosa | Vietnamese banana preservation | [84] |
| Chitosan, olive leaves extract | ↑ WS, TS, and EAB, ↓ WVP | Antioxidant activity (ABTS), antimicrobial activity against L. monocytogenes and Campylobacter jejuni | Active packaging | [112] |
| Chitosan, blueberry extract by-products | ↑ thickness, WVP ↓ oxygen permeability, water content | Antioxidant activity (DPPH, ABTS, FRAP) | Active packaging | [128] |
| Chitosan, parsley extract by-products | ↑ thickness, WVP ↓ oxygen permeability, water content | Antioxidant activity (DPPH, ABTS, FRAP) | Active packaging | [128] |
| Chitosan, red grapes extract by-products | ↑ thickness, WVP ↓ oxygen permeability, water content | Antioxidant activity (DPPH, ABTS, FRAP), antimicrobial activity against E. coli | Active packaging | [128] |
| Chitosan, purple-fleshed sweet potato extract | ↑ thickness, WS, WVP when the extract exceeded 5 wt% and TS when the extract was 5 wt% ↓ EAB, WVP when the extract was 5 wt%, TS when the extract exceeded 5 wt%, moisture content and light transmittance | Antioxidant activity (DPPH), color variations of films to pH, pink-red (pH 3.0–6.0), purple-brown (pH 7.0–8.0), and greenish–green (pH 9.0–10.0) | Monitoring food spoilage | [118] |
| Chitosan, purple rice extract | ↑ thickness, EAB, TS, light barrier property, and WVP when the extract exceeded 1 wt% ↓ moisture content, change color | Antioxidant activity (DPPH), pH-sensitive in different buffer solutions | Monitor pork spoilage | [113] |
| Chitosan, black rice extract | ↑ thickness, EAB, light barrier property ↓ moisture content, TS when the extract exceeded 1 wt% change color | Antioxidant activity (DPPH) | Active packaging | [113] |
3.2.3. Combined Chitosan and Essential Oil
| Formulation | Physical/Chemical/Mechanical Characteristics | Biological Properties | Applications | References |
|---|---|---|---|---|
| Chitosan, citronella essential oil | ↑ EAB (low essential oil content), thermal stability ↓ WVP, TS, moisture content | Not determined | Packaging | [135] |
| Chitosan, cedarwood essential oil | ↑ EAB (low essential oil content), thermal stability ↓ WVP, TS, moisture content | Not determined | Packaging | [135] |
| Chitosan, basil essential oil | ↑ thickness, TS, EM ↓ WVP, EAB | Tested for antifungal activity, but the film did not inhibit the growth of A. niger, Botrytis cinerea, and R. stolonifer | Packaging | [138] |
| Chitosan, thyme essential oil | ↑ thickness, TS, EM ↓ WVP, EAB | Tested for antifungal activity, but the film did not inhibit the growth of A. niger, B. cinerea, and R. stolonifer | Packaging | [138] |
| Chitosan, fennel essential oil | ↑ density, thermal stability, and opacity ↓ WS, water swelling, thickness, and moisture content color change | Antioxidant activity (DPPH 68%) | Active packaging | [139] |
| Chitosan, peppermint essential oil | ↑ density, thermal stability, and opacity ↓ WS, water swelling, and thickness color change | Antioxidant activity (DPPH 66%) | Active packaging | [139] |
| Chitosan, Eucalyptus globulus essential oil | ↑ opacity ↓ moisture content, WS | Antioxidant activity (DPPH 23%–43%), antimicrobial activity against E. coli, S. aureus, P. aeruginosa, C. albicans, C. parapsilosis | Active packaging | [140] |
| Chitosan, apricot kernel essential oil | ↑ opacity, TS ↓ moisture content, WS, WVP EAB first increased, and then when the ratio of chitosan:essential oil exceeded 1: 0.5 decreased | Antioxidant activity (DPPH 26%–35%), antimicrobial activity against S. aureus and B. subtillis, antifungal activity against R. stolonifer | Inhibited the growth of fungi on bread, active food packaging | [136] |
| Chitosan, piper betle Linn oil | ↑ UV-light barrier, EAB (at 0.4 and 1% oil incorporation), ↓ thermal stability, TS, EM, and EAB (at 1.2% oil incorporated) | Antioxidant activity (DPPH), antimicrobial activity against S. aureus, E. coli, P. aeruginosa, and S. typhimurium | King orange preservation | [137] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef] [PubMed]
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total Environ. 2019, 651, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef]
- Kumar, V.A.; Hasan, M.; Mangaraj, S.; Pravitha, M.; Verma, D.K.; Srivastav, P.P. Trends in Edible Packaging Films and its Prospective Future in Food: A Review. Appl. Food Res. 2022, 2, 100118. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, N.; Islam, J.; Tahergorabi, R. Marine biopolymers: Applications in food packaging. Processes 2021, 9, 2245. [Google Scholar] [CrossRef]
- Kaur, J.; Rasane, P.; Singh, J.; Kaur, S. Edible Packaging: An Overview. In Edible Food Packaging; Springer Nature: Singapore, 2022; pp. 3–25. ISBN 9789811623837. [Google Scholar]
- Ubeda, S.; Aznar, M.; Rosenmai, A.K.; Vinggaard, A.M.; Nerín, C. Migration studies and toxicity evaluation of cyclic polyesters oligomers from food packaging adhesives. Food Chem. 2020, 311, 125918. [Google Scholar] [CrossRef] [PubMed]
- Samsudin, H.; Auras, R.; Burgess, G.; Dolan, K.; Soto-Valdez, H. Migration of antioxidants from polylactic acid films, a parameter estimation approach: Part I—A model including convective mass transfer coefficient. Food Res. Int. 2018, 105, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Nechita, P.; Roman, M. Review on Polysaccharides Used in Coatings for Food. Coatings 2020, 10, 566. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Gavara, R.; Catalá, R.; Hernández-Muñoz, P. The Potential of Proteins for Producing Food Packaging Materials: A Review. Packag. Technol. Sci. 2016, 29, 203–224. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, Q.; Chu, Y.; Tao, N.; Deng, S.; Wang, L.; Li, L. Application of Gelatin in Food Packaging: A Review. Polymers 2022, 14, 436. [Google Scholar] [CrossRef] [PubMed]
- Díaz-montes, E.; Castro-muñoz, R. Trends in chitosan as a primary biopolymer for functional films and coatings manufacture for food and natural products. Polymers 2021, 13, 767. [Google Scholar] [CrossRef] [PubMed]
- Calinoiu, L.F.; Ştefanescu, B.E.; Pop, I.D.; Muntean, L.; Vodnar, D.C. Chitosan coating applications in probiotic microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef] [Green Version]
- Nair, M.S.; Tomar, M.; Punia, S.; Kukula-Koch, W.; Kumar, M. Enhancing the functionality of chitosan- and alginate-based active edible coatings/films for the preservation of fruits and vegetables: A review. Int. J. Biol. Macromol. 2020, 164, 304–320. [Google Scholar] [CrossRef] [PubMed]
- Menezes, M.d.L.L.R.; da Rocha Pires, N.; da Cunha, P.L.R.; de Freitas Rosa, M.; de Souza, B.W.S.; de Andrade Feitosa, J.P.; de Souza, M.D.S.M. Effect of tannic acid as crosslinking agent on fish skin gelatin-silver nanocomposite film. Food Packag. Shelf Life 2019, 19, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Nurdiani, R.; Ma’rifah, R.D.A.; Busyro, I.K.; Jaziri, A.A.; Prihanto, A.A.; Firdaus, M.; Talib, R.A.; Huda, N. Physical and functional properties of fish gelatin-based film incorporated with mangrove extracts. PeerJ 2022, 10, e13062. [Google Scholar] [CrossRef] [PubMed]
- Hanani, Z.A.N. Gelatin. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2015; pp. 191–195. ISBN 9780123849533. [Google Scholar]
- Kuan, Y.H.; Nafchi, A.M.; Huda, N.; Ariffin, F.; Karim, A.A. Comparison of physicochemical and functional properties of duck feet and bovine gelatins. J. Sci. Food Agric. 2017, 97, 1663–1671. [Google Scholar] [CrossRef]
- Karayannakidis, P.D.; Zotos, A. Fish Processing By-Products as a Potential Source of Gelatin: A Review. J. Aquat. Food Prod. Technol. 2016, 25, 65–92. [Google Scholar] [CrossRef]
- Ahmad, T.; Ismail, A.; Ahmad, S.A.; Khalil, K.A.; Kumar, Y.; Adeyemi, K.D.; Sazili, A.Q. Recent advances on the role of process variables affecting gelatin yield and characteristics with special reference to enzymatic extraction: A review. Food Hydrocoll. 2017, 63, 85–96. [Google Scholar] [CrossRef]
- Said, N.S.; Sarbon, N.M. Physical and Mechanical Characteristics of Gelatin-Based Films as a Potential Food Packaging Material: A Review. Membranes 2022, 12, 442. [Google Scholar] [CrossRef] [PubMed]
- Fakhreddin Hosseini, S.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Preparation and functional properties of fish gelatin-chitosan blend edible films. Food Chem. 2013, 136, 1490–1495. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible films and coatings based on starch/gelatin: Film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biol. Technol. 2015, 109, 57–64. [Google Scholar] [CrossRef]
- Cao, N.; Fu, Y.; He, J. Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocoll. 2007, 21, 1153–1162. [Google Scholar] [CrossRef]
- Nur Hazirah, M.A.S.P.; Isa, M.I.N.; Sarbon, N.M. Effect of xanthan gum on the physical and mechanical properties of gelatin-carboxymethyl cellulose film blends. Food Packag. Shelf Life 2016, 9, 55–63. [Google Scholar] [CrossRef]
- Loo, C.P.Y.; Sarbon, N.M. Chicken skin gelatin films with tapioca starch. Food Biosci. 2020, 35, 100589. [Google Scholar] [CrossRef]
- Jridi, M.; Abdelhedi, O.; Salem, A.; Kechaou, H.; Nasri, M.; Menchari, Y. Physicochemical, antioxidant and antibacterial properties of fish gelatin-based edible films enriched with orange peel pectin: Wrapping application. Food Hydrocoll. 2020, 103, 105688. [Google Scholar] [CrossRef]
- Zhong, C.; Hou, P.F.; Li, Y.X.; Yang, W.Y.; Shu, M.; Wu, G.P. Characterization, antioxidant and antibacterial activities of gelatin film incorporated with protocatechuic acid and its application on beef preservation. LWT 2021, 151, 112154. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, J.; Yu, H.; Zhang, J.; Yuan, Y.; Shen, X.; Li, C. The effects of EGCG on the mechanical, bioactivities, cross-linking and release properties of gelatin film. Food Chem. 2019, 271, 204–210. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Yang, W.; Xue, C.; Wang, Y.; Dong, J.; Xue, Y. Modification of gelatine with galla chinensis extract, a natural crosslinker. Int. J. Food Prop. 2016, 19, 731–744. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, P.; Fang, S.; Liu, W.; Mei, J.; Xie, J. Preservative effects of gelatin active coating enriched with eugenol emulsion on Chinese seabass (Lateolabrax maculatus) during superchilling (−0.9 °C) storage. Coatings 2019, 9, 489. [Google Scholar] [CrossRef] [Green Version]
- Adilah, A.N.; Jamilah, B.; Noranizan, M.A.; Hanani, Z.A.N. Utilization of mango peel extracts on the biodegradable films for active packaging. Food Packag. Shelf Life 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Li, X.; Tu, Z.C.; Sha, X.M.; Ye, Y.H.; Li, Z.Y. Flavor, antimicrobial activity and physical properties of gelatin film incorporated with of ginger essential oil. J. Food Sci. Technol. 2022, 59, 815–824. [Google Scholar] [CrossRef]
- Yang, S.Y.; Lee, K.Y.; Beak, S.E.; Kim, H.; Song, K. Bin Antimicrobial activity of gelatin films based on duck feet containing cinnamon leaf oil and their applications in packaging of cherry tomatoes. Food Sci. Biotechnol. 2017, 26, 1429–1435. [Google Scholar] [CrossRef]
- Martucci, J.F.; Gende, L.B.; Neira, L.M.; Ruseckaite, R.A. Oregano and lavender essential oils as antioxidant and antimicrobial additives of biogenic gelatin films. Ind. Crops Prod. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Lee, K.Y.; Lee, J.H.; Yang, H.J.; Song, K. Bin Production and characterisation of skate skin gelatin films incorporated with thyme essential oil and their application in chicken tenderloin packaging. Int. J. Food Sci. Technol. 2016, 51, 1465–1472. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils. Food Chem. 2012, 134, 1571–1579. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Physico-chemical properties, morphology and antioxidant activity of film from fish skin gelatin incorporated with root essential oils. J. Food Eng. 2013, 117, 350–360. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S.; Prodpran, T.; Agustini, T.W. Physico-mechanical and antimicrobial properties of gelatin film from the skin of unicorn leatherjacket incorporated with essential oils. Food Hydrocoll. 2012, 28, 189–199. [Google Scholar] [CrossRef]
- Kavoosi, G.; Rahmatollahi, A.; Mohammad Mahdi Dadfar, S.; Mohammadi Purfard, A. Effects of essential oil on the water binding capacity, physico-mechanical properties, antioxidant and antibacterial activity of gelatin films. LWT—Food Sci. Technol. 2014, 57, 556–561. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Pires, C.; Ramos, C.; Batista, I.; Saraiva, J.A.; Nunes, M.L. Characterization of fish protein films incorporated with essential oils of clove, garlic and origanum: Physical, antioxidant and antibacterial properties. LWT—Food Sci. Technol. 2014, 59, 533–539. [Google Scholar] [CrossRef]
- Mihaly Cozmuta, A.; Turila, A.; Apjok, R.; Ciocian, A.; Mihaly Cozmuta, L.; Peter, A.; Nicula, C.; Galić, N.; Benković, T. Preparation and characterization of improved gelatin films incorporating hemp and sage oils. Food Hydrocoll. 2015, 49, 144–155. [Google Scholar] [CrossRef]
- Gomez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Prodpran, T.; Tanaka, M. Characterization of edible films from skin gelatin of brownstripe red snapper and bigeye snapper. Food Hydrocoll. 2006, 20, 492–501. [Google Scholar] [CrossRef]
- Mhd Sarbon, N.; Badii, F.; Howell, N.K. Preparation and characterisation of chicken skin gelatin as an alternative to mammalian gelatin. Food Hydrocoll. 2013, 30, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Abedinia, A.; Ariffin, F.; Huda, N.; Mohammadi Nafchi, A. Preparation and characterization of a novel biocomposite based on duck feet gelatin as alternative to bovine gelatin. Int. J. Biol. Macromol. 2018, 109, 855–862. [Google Scholar] [CrossRef]
- Rahman, M.N.; Jamalulail, S.A.S.K.A. Extraction, Physicochemical Characterizations and Sensory. Borneo Sci. 2012, 30, 1–13. [Google Scholar]
- Nik Muhammad, N.A.; Huda, N.; Karim, A.A.; Mohammadi Nafchi, A. Effects of acid type extraction on characterization and sensory profile of duck feet gelatin: Towards finding bovine gelatin alternative. J. Food Meas. Charact. 2018, 12, 480–486. [Google Scholar] [CrossRef]
- Ninan, G.; Joseph, J.; Aliyamveettil, Z.A. A comparative study on the physical, chemical and functional properties of carp skin and mammalian gelatins. J. Food Sci. Technol. 2014, 51, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Extraction and physico-chemical characterisation of Nile perch (Lates niloticus) skin and bone gelatin. Food Hydrocoll. 2004, 18, 581–592. [Google Scholar] [CrossRef]
- da Trindade Alfaro, A.; Balbinot, E.; Weber, C.I.; Tonial, I.B.; Machado-Lunkes, A. Fish Gelatin: Characteristics, Functional Properties, Applications and Future Potentials. Food Eng. Rev. 2015, 7, 33–44. [Google Scholar] [CrossRef]
- Howell, N.K. Elucidation of protein protein interactions in gels and foams. In Gums and Stabilizers for the Food Industry 7; Oxford University Press: Wales, UK, 1994; pp. 77–89. [Google Scholar]
- Sarbon, N.M.; Badii, F.; Howell, N.K. The effect of chicken skin gelatin and whey protein interactions on rheological and thermal properties. Food Hydrocoll. 2015, 45, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Ngarize, S.; Adams, A.; Howell, N. A comparative study of heat and high pressure induced gels of whey and egg albumen proteins and their binary mixtures. Food Hydrocoll. 2005, 19, 984–996. [Google Scholar] [CrossRef]
- Kehoe, J.J.; Foegeding, E.A. The characteristics of heat-induced aggregates formed by mixtures of β-lactoglobulin and β-casein. Food Hydrocoll. 2014, 39, 264–271. [Google Scholar] [CrossRef]
- Nazmi, N.N.; Isa, M.I.N.; Sarbon, N.M. Preparation and characterization of chicken skin gelatin/CMC composite film as compared to bovine gelatin film. Food Biosci. 2017, 19, 149–155. [Google Scholar] [CrossRef]
- Alias, S.A.; Mhd Sarbon, N. Rheological, physical, and mechanical properties of chicken skin gelatin films incorporated with potato starch. NPJ Sci. Food 2019, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Jahit, I.S.; Nazmi, N.N.M.; Isa, M.I.N.; Sarbon, N.M. Preparation and physical properties of gelatin/CMC/chitosan composite films as affected by drying temperature. Int. Food Res. J. 2016, 23, 1068–1074. [Google Scholar]
- Bakry, N.F.; Isa, M.I.N.; Sarbon, N.M. Effect of sorbitol at different concentrations on the functional properties of gelatin/carboxymethyl cellulose (CMC)/chitosan composite films. Int. Food Res. J. 2017, 24, 1753–1762. [Google Scholar]
- Fatyasari Nata, I.; Irawan, C.; Ramadhan, L.; Rizky Ramadhani, M. Influence of soy protein isolate on gelatin-based edible film properties. MATEC Web Conf. 2018, 156, 01014. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.; Wu, C.; Wu, T.; Yu, H.; Yang, S.; Hu, Y. Preparation and characterisation of Chlorogenic acid-gelatin: A type of biologically active film for coating preservation. Food Chem. 2017, 221, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Tammineni, N.; Ünlü, G.; Rasco, B.; Powers, J.; Sablani, S.; Nindo, C. Trout-Skin Gelatin-Based Edible Films Containing Phenolic Antioxidants: Effect on Physical Properties and Oxidative Stability of Cod-Liver Oil Model Food. J. Food Sci. 2012, 77, E342–E347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, L.; Yu, Y.; Zhou, H.; Guo, T.; Dai, H.; Zhang, Y. Physico-mechanical and antioxidant properties of gelatin film from rabbit skin incorporated with rosemary acid. Food Packag. Shelf Life 2019, 19, 121–130. [Google Scholar] [CrossRef]
- Halim, A.L.A.; Kamari, A.; Phillip, E. Chitosan, gelatin and methylcellulose films incorporated with tannic acid for food packaging. Int. J. Biol. Macromol. 2018, 120, 1119–1126. [Google Scholar] [CrossRef]
- Wu, J.; Chen, S.; Ge, S.; Miao, J.; Li, J.; Zhang, Q. Preparation, properties and antioxidant activity of an active film from silver carp (Hypophthalmichthys molitrix) skin gelatin incorporated with green tea extract. Food Hydrocoll. 2013, 32, 42–51. [Google Scholar] [CrossRef]
- Li, J.H.; Miao, J.; Wu, J.L.; Chen, S.F.; Zhang, Q.Q. Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- Hanani, Z.A.N.; Yee, F.C.; Nor-Khaizura, M.A.R. Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll. 2019, 89, 253–259. [Google Scholar] [CrossRef]
- Bessaleh, S.; Jebahi, S.; Abbassi, R.; Ben Belgecem, S.; Faraz, A. Bioactive Gelatin-based Date By-Product for Packaging Applications: Physico-Chemical and Biological Characterization. J. Mater. Appl. 2022, 11, 10–16. [Google Scholar] [CrossRef]
- Luo, Q.; Hossen, M.A.; Zeng, Y.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Gelatin-based composite films and their application in food packaging: A review. J. Food Eng. 2022, 313, 110762. [Google Scholar] [CrossRef]
- Ge, L.; Zhu, M.; Li, X.; Xu, Y.; Ma, X.; Shi, R.; Li, D.; Mu, C. Development of active rosmarinic acid-gelatin biodegradable films with antioxidant and long-term antibacterial activities. Food Hydrocoll. 2018, 83, 308–316. [Google Scholar] [CrossRef]
- Liu, J.; Yong, H.; Liu, Y.; Qin, Y.; Kan, J.; Liu, J. Preparation and characterization of active and intelligent films based on fish gelatin and haskap berries (Lonicera caerulea L.) extract. Food Packag. Shelf Life 2019, 22, 100417. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Z. Effects of gelatin-polyphenol and gelatin–genipin cross-linking on the structure of gelatin hydrogels. Int. J. Food Prop. 2018, 20, S2822–S2832. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In vitro antibacterial and antioxidant properties of chitosan edible films incorporated with Thymus moroderi or Thymus piperella essential oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Y.; Cao, X.; Yao, F.; Shi, L.; Liu, Y. A Film of Chitosan Blended with Ginseng Residue Polysaccharides as an Antioxidant Packaging for Prolonging the Shelf Life of Fresh-Cut Melon. Coatings 2022, 12, 468. [Google Scholar] [CrossRef]
- Kaczmarek-Szczepańska, B.; Zasada, L.; Grabska-Zielińska, S. The Physicochemical, Antioxidant, and Color Properties of Thin Films Based on Chitosan Modified by Different Phenolic Acids. Coatings 2022, 12, 126. [Google Scholar] [CrossRef]
- Salgado-Cruz, M.d.l.P.; Salgado-Cruz, J.; García-Hernández, A.B.; Calderón-Domínguez, G.; Gómez-Viquez, H.; Oliver-Espinoza, R.; Fernández-Martínez, M.C.; Yáñez-Fernández, J. Chitosan as a coating for biocontrol in postharvest products: A bibliometric review. Membranes 2021, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.d.l.L.; Caballero, A.H. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.T.; Thi Dao, U.T.; Thi Bui, Q.P.; Bach, G.L.; Ha Thuc, C.N.; Ha Thuc, H. Enhanced antimicrobial activities and physiochemical properties of edible film based on chitosan incorporated with Sonneratia caseolaris (L.) Engl. leaf extract. Prog. Org. Coatings 2020, 140, 105487. [Google Scholar] [CrossRef]
- Tokatlı, K.; Demirdöven, A. Effects of chitosan edible film coatings on the physicochemical and microbiological qualities of sweet cherry (Prunus avium L.). Sci. Hortic. (Amst.) 2020, 259, 108656. [Google Scholar] [CrossRef]
- Hu, W.; Sarengaowa; Feng, K. Effect of Edible Coating on the Quality and Antioxidant Enzymatic Activity of Postharvest Sweet Cherry (Prunus avium L.) during Storage. Coatings 2022, 12, 581. [Google Scholar] [CrossRef]
- Bigi, F.; Haghighi, H.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Characterization of chitosan-hydroxypropyl methylcellulose blend films enriched with nettle or sage leaf extract for active food packaging applications. Food Hydrocoll. 2021, 120, 106979. [Google Scholar] [CrossRef]
- Kumari, S.; Kishor, R. Chitin and chitosan: Origin, properties, and applications. In Handbook of Chitin and Chitosan; Volume 1: Preparation and Properties; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–33. ISBN 9780128179703. [Google Scholar]
- Lisitsyn, A.; Semenova, A.; Nasonova, V.; Polishchuk, E.; Revutskaya, N.; Kozyrev, I.; Kotenkova, E. Approaches in animal proteins and natural polysaccharides application for food packaging: Edible film production and quality estimation. Polymers 2021, 13, 1592. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdou, E.S.; Nagy, K.S.A.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Sagheer, F.A.A.; Al-Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-García, M.; Reyes-Basurto, A.; García-Almendárez, B.E.; Hernández-Hernández, E.; Calderón-Domínguez, G.; Rossi-Márquez, G.; Regalado-González, C. Modified starch-chitosan edible films: Physicochemical and mechanical characterization. Coatings 2017, 7, 224. [Google Scholar] [CrossRef] [Green Version]
- Kaya, M.; Akyuz, L.; Sargin, I.; Mujtaba, M.; Salaberria, A.M.; Labidi, J.; Cakmak, Y.S.; Koc, B.; Baran, T.; Ceter, T. Incorporation of sporopollenin enhances acid–base durability, hydrophobicity, and mechanical, antifungal and antioxidant properties of chitosan films. J. Ind. Eng. Chem. 2017, 47, 236–245. [Google Scholar] [CrossRef]
- Younis, H.G.R.; Zhao, G. Physicochemical properties of the edible films from the blends of high methoxyl apple pectin and chitosan. Int. J. Biol. Macromol. 2019, 131, 1057–1066. [Google Scholar] [CrossRef]
- Costa, S.M.; Ferreira, D.P.; Teixeira, P.; Ballesteros, L.F.; Teixeira, J.A.; Fangueiro, R. Active natural-based films for food packaging applications: The combined effect of chitosan and nanocellulose. Int. J. Biol. Macromol. 2021, 177, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Azaza, Y.B.; Hamdi, M.; Charmette, C.; Jridi, M.; Li, S.; Nasri, M.; Nasri, R. Development and characterization of active packaging films based on chitosan and sardinella protein isolate: Effects on the quality and the shelf life of shrimps. Food Packag. Shelf Life 2022, 31, 100796. [Google Scholar] [CrossRef]
- Lan, W.; He, L.; Liu, Y. Preparation and properties of sodium carboxymethyl cellulose/sodium alginate/chitosan composite film. Coatings 2018, 8, 291. [Google Scholar] [CrossRef] [Green Version]
- Mao, H.; Wei, C.; Gong, Y.; Wang, S.; Ding, W. Mechanical and water-resistant properties of eco-friendly chitosan membrane reinforced with cellulose nanocrystals. Polymers 2019, 11, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Lagnika, C.; Luo, H.; Dai, Z.; Nie, M.; Hashim, M.M.; Liu, C.; Song, J.; Li, D. Chitosan-based biodegradable active food packaging film containing Chinese chive (Allium tuberosum) root extract for food application. Int. J. Biol. Macromol. 2020, 150, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Siripatrawan, U.; Vitchayakitti, W. Improving functional properties of chitosan films as active food packaging by incorporating with propolis. Food Hydrocoll. 2016, 61, 695–702. [Google Scholar] [CrossRef]
- De Carli, C.; Aylanc, V.; Mouffok, K.M.; Santamaria-Echart, A.; Barreiro, F.; Tomás, A.; Pereira, C.; Rodrigues, P.; Vilas-Boas, M.; Falcão, S.I. Production of chitosan-based biodegradable active films using bio-waste enriched with polyphenol propolis extract envisaging food packaging applications. Int. J. Biol. Macromol. 2022, 213, 486–497. [Google Scholar] [CrossRef]
- Wu, C.; Tian, J.; Li, S.; Wu, T.; Hu, Y.; Chen, S.; Sugawara, T.; Ye, X. Structural properties of films and rheology of film-forming solutions of chitosan gallate for food packaging. Carbohydr. Polym. 2016, 146, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yan, H.; Zhang, J.; Dai, W.; Gao, X.; Zhou, Y.; Wan, X.; Puligundla, P. Preparation and characterization of antioxidant edible chitosan films incorporated with epigallocatechin gallate nanocapsules. Carbohydr. Polym. 2017, 171, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Pinto, R.J.B.; Coelho, J.; Domingues, M.R.M.; Daina, S.; Sadocco, P.; Santos, S.A.O.; Freire, C.S.R. Bioactive chitosan/ellagic acid films with UV-light protection for active food packaging. Food Hydrocoll. 2017, 73, 120–128. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Wu, Q.; Gu, Y.; Kan, J.; Jin, C. Effect of protocatechuic acid incorporation on the physical, mechanical, structural and antioxidant properties of chitosan film. Food Hydrocoll. 2017, 73, 90–100. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, X.; Bai, R.; Liu, Y.; Liu, J.; Liu, J. Preparation and characterization of antioxidant and antimicrobial packaging films based on chitosan and proanthocyanidins. Int. J. Biol. Macromol. 2019, 134, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Dang, H.; Liu, L.; Hu, X.; Li, X.; Ma, Z.; Wang, X.; Ren, T. Effect of syringic acid incorporation on the physical, mechanical, structural and antibacterial properties of chitosan film for quail eggs preservation. Int. J. Biol. Macromol. 2019, 141, 876–884. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Y.; Jiang, X.; Wu, J.; Le, X. Molecular interactions, characterization and antimicrobial activity of curcumin-chitosan blend films. Food Hydrocoll. 2016, 52, 564–572. [Google Scholar] [CrossRef]
- Musella, E.; El Ouazzani, I.C.; Mendes, A.R.; Rovera, C.; Farris, S.; Mena, C.; Teixeira, P.; Poças, F. Preparation and characterization of bioactive chitosan-based films incorporated with olive leaves extract for food packaging applications. Coatings 2021, 11, 1339. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J.; Qin, Y.; Bai, R.; Zhang, X.; Liu, J. Antioxidant and pH-sensitive films developed by incorporating purple and black rice extracts into chitosan matrix. Int. J. Biol. Macromol. 2019, 137, 307–316. [Google Scholar] [CrossRef]
- Yuan, G.; Lv, H.; Yang, B.; Chen, X.; Sun, H. Physical properties, antioxidant and antimicrobial activity of chitosan films containing carvacrol and pomegranate peel extract. Molecules 2015, 20, 11034–11045. [Google Scholar] [CrossRef] [Green Version]
- Talón, E.; Trifkovic, K.T.; Nedovic, V.A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant edible films based on chitosan and starch containing polyphenols from thyme extracts. Carbohydr. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Torlak, E.; Akın-Evingür, G.; Özen, İ.; Erim, F.B. Antimicrobial and physical properties of chitosan films incorporated with turmeric extract. Int. J. Biol. Macromol. 2017, 101, 882–888. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Bai, R.; Miao, Z.; Zhang, X.; Liu, J. Development of antioxidant and intelligent pH-sensing packaging films by incorporating purple-fleshed sweet potato extract into chitosan matrix. Food Hydrocoll. 2019, 90, 216–224. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, Z.H.; Qin, Y.Y.; Zhao, T.R.; Cheng, C.S. Characterization of antioxidant chitosan film incorporated with pomegranate peel extract. Adv. Mater. Res. 2013, 706, 24–27. [Google Scholar] [CrossRef]
- Tan, Y.M.; Lim, S.H.; Tay, B.Y.; Lee, M.W.; Thian, E.S. Functional chitosan-based grapefruit seed extract composite films for applications in food packaging technology. Mater. Res. Bull. 2015, 69, 142–146. [Google Scholar] [CrossRef]
- Kaya, M.; Ravikumar, P.; Ilk, S.; Mujtaba, M.; Akyuz, L.; Labidi, J.; Salaberria, A.M.; Cakmak, Y.S.; Erkul, S.K. Production and characterization of chitosan based edible films from Berberis crataegina’s fruit extract and seed oil. Innov. Food Sci. Emerg. Technol. 2018, 45, 287–297. [Google Scholar] [CrossRef]
- Kadam, D.; Shah, N.; Palamthodi, S.; Lele, S.S. An investigation on the effect of polyphenolic extracts of Nigella sativa seedcake on physicochemical properties of chitosan-based films. Carbohydr. Polym. 2018, 192, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Wu, Y.; Li, Y. Development of tea extracts and chitosan composite films for active packaging materials. Int. J. Biol. Macromol. 2013, 59, 282–289. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, F.; Feng, Z.; Fan, X.; Pan, Z.; Zhou, J. Antioxidant activity and physicochemical properties of chitosan films incorporated with Lycium barbarum fruit extract for active food packaging. Int. J. Food Sci. Technol. 2015, 50, 458–464. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Tong, J.; Zhou, J. Physicochemical Properties of Chitosan Films Incorporated with Honeysuckle Flower Extract for Active Food Packaging. J. Food Process Eng. 2017, 40, e12305. [Google Scholar] [CrossRef] [Green Version]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology; A review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Dordevic, S.; Dordevic, D.; Sedlacek, P.; Kalina, M.; Tesikova, K.; Antonic, B.; Tremlova, B.; Treml, J.; Nejezchlebova, M.; Vapenka, L.; et al. Incorporation of natural blueberry, red grapes and parsley extract by-products into the production of chitosan edible films. Polymers 2021, 13, 3388. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, H.; Wang, J.; Jiang, G.; Du, F.; Liu, X. Effects of Herba Lophatheri extract on the physicochemical properties and biological activities of the chitosan film. Int. J. Biol. Macromol. 2019, 133, 51–57. [Google Scholar] [CrossRef]
- Zhu, F. Polysaccharide based films and coatings for food packaging: Effect of added polyphenols. Food Chem. 2021, 359, 129871. [Google Scholar] [CrossRef] [PubMed]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernandez-Lopez, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of antibacterial and antioxidant properties of chitosan edible films incorporated with maqui berry (Aristotelia chilensis). LWT 2015, 64, 1057–1062. [Google Scholar] [CrossRef]
- Jing, Y.; Huang, J.; Yu, X. Preparation, characterization, and functional evaluation of proanthocyanidin-chitosan conjugate. Carbohydr. Polym. 2018, 194, 139–145. [Google Scholar] [CrossRef]
- Shiekh, K.A.; Ngiwngam, K.; Tongdeesoontorn, W. Polysaccharide-Based Active Coatings Incorporated with Bioactive Compounds for Reducing Postharvest Losses of Fresh Fruits. Coatings 2022, 12, 8. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Morsi, R.E.; Fathy, M. Chitosan-Oregano Essential Oil Blends Use as Antimicrobial Packaging Material; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128007235. [Google Scholar]
- Shen, Z.; Kamdem, D.P. Development and characterization of biodegradable chitosan films containing two essential oils. Int. J. Biol. Macromol. 2015, 74, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, R.; Sauraj; Kumar, B.; Deeba, F.; Kulshreshtha, A.; Negi, Y.S. Chitosan films incorporated with Apricot (Prunus armeniaca) kernel essential oil as active food packaging material. Food Hydrocoll. 2018, 85, 158–166. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, T.T.T.; Van Tran, T.; Van Tan, L.; Danh, L.T.; Than, V.T. Development of antibacterial, antioxidant, and uv-barrier chitosan film incorporated with piper betle linn oil as active biodegradable packaging material. Coatings 2021, 11, 351. [Google Scholar] [CrossRef]
- Perdones, Á.; Chiralt, A.; Vargas, M. Properties of film-forming dispersions and films based on chitosan containing basil or thyme essential oil. Food Hydrocoll. 2016, 57, 271–279. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Chi, F.; Tan, Z.; Liu, L. Development and characterization of novel active chitosan films containing fennel and peppermint essential oils. Coatings 2020, 10, 936. [Google Scholar] [CrossRef]
- Hafsa, J.; ali Smach, M.; Khedher, M.R.B.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Physical, antioxidant and antimicrobial properties of chitosan films containing Eucalyptus globulus essential oil. LWT 2016, 68, 356–364. [Google Scholar] [CrossRef]

| Formulation | Physical/Chemical/Mechanical Characteristics | Biological Properties | Applications | References |
|---|---|---|---|---|
| Gelatin, ginger essential oil | ↑ thickness, WVP, EAB, ↓ TS | Antimicrobial activity against E. coli and S. aureus | Antimicrobial active packaging | [36] |
| Gelatin, cinnamon leaf oil | ↓ TS, slightly decreased WVP | Antimicrobial activity against Salmonella typhimurium, E. coli, L. monocytogenes, and S. aureus | Cherry tomatoes | [37] |
| Gelatin, oregano essential oil | Insignificant modification | Antioxidant activity (DPPH 12%–60%, FRAP), antimicrobial activity against E. coli and S. aureus | Food active packaging | [38] |
| Gelatin, lavender essential oil | ↓ WVP, TS | Antioxidant activity (DPPH 1%–9%, FRAP), antimicrobial activity against E. coli and S. aureus | Food active packaging | [38] |
| Gelatin, thyme essential oil | ↑ EAB, ↓ TS, WVP | Antimicrobial activity against L. monocytogenes and E. coli | Chicken tenderloin packaging | [39] |
| Gelatin, citrus essential oils (bergamot, kaffir lime, lemon, lime) | ↑TS, ↓EAB, WVP (glycerol 20%)↑ EAB, ↓ TS (glycerol 30%) | Antioxidant activity (DPPH, ABTS, FRAP) | Active packaging | [40] |
| Gelatin, root essential oils (ginger, turmeric, plai) | ↑ EAB, ↓ TS and WVP | Antioxidant activity (DPPH, ABTS)plai > turmeric > ginger essential oils | Active packaging | [41] |
| Gelatin, Zataria multiflora (thyme-like plant) essential oil | ↑ WVP, EAB, light barrier properties, ↓ TS | Antioxidant activity (ABTS), antimicrobial activity against P. aeruginosa, E. coli, S. aureus, B. subtilis | Antioxidant, antimicrobial active packaging | [43] |
| Gelatin, essential oils (bergamot, lemongrass) | ↓ TS, EAB, WVP (lemongrass), solubility, transparency ↑ heat stability | Antimicrobial activity Lemongrass: E. coli, L. monocytogenes, S. aureus, S. typhimurium Bergamot: L. monocytogenes, S. aureus | Active packaging | [42] |
| Gelatin, essential oils (clove, garlic, origanum) | ↓ thickness, WS, EABslightly decreased WVP | Antioxidant activity (DPPH 38%–72%), antimicrobial activity against Brochothrix thermosphacta, Listeria innocua, L. monocytogenes, Shewanella putrefaciens | Biodegradable food packaging systems | [44] |
| Gelatin, sage essential oil | ↓ WVP, ↑ thickness | Antimicrobial activity against E. coli, S. aureus, L. innocua, Saccharomyces cerevisiae, Penicillium expansum | Fruits, vegetables, and meat packaging | [45] |
| Formulation | Physical/Chemical/Mechanical Characteristics | Biological Properties | Applications | References |
|---|---|---|---|---|
| Chitosan, corn starch | ↑ WS, TS, EAB, ↓ WVP by comparison with corn starch film color change | Not determined | Active packaging | [93] |
| Chitosan, starch | ↑ thickness and WS, ↓ WVP | Antimicrobial activity against L. innocua | Active packaging | [94] |
| Chitosan, sporopollenin | ↓ thickness, light transmittance, ↑ TS, EAB, Young’s modulus successfully incorporate sporopollenin into chitosan, enhanced hydrophobicity of films | Antifungal activity against Aspergillus niger, antioxidant activity | Active packaging | [95] |
| Chitosan, pectin | ↑ thickness, WVP, WS, TS, EAB, Young’s modulus ↓ density and opacity | Not determined | Packaging | [96] |
| Chitosan, nanocellulose | ↑ thermal stability, oxygen barrier properties, thickness, WVP, TS, Young’s modulus, ↓ film’s transparency | Antimicrobial activity against S. aureus, E. coli, and Candida albicans | Chicken meat | [97] |
| Chitosan, Sardinella protein isolate | ↑ thickness, moisture content, opacity, UV–VIS light barrier, WS, ↓ WVP, TS, and EAB, color change | Antioxidant activity (DPPH), antimicrobial activity against S. aureus, Micrococcus luteus, L. monocytogenes, Bacillus cereus, Salmonella enterica, P. aeruginosa, E. coli, Klebsiella pneumoniae | Shrimp packaging | [98] |
| Chitosan, CMC, sodium alginate | The optimal contents of the chitosan, CMC, and sodium alginate for the preparation of this composite film were 1.5%, 0.5%, and 1.5%. ↑ TS, EAB, WVP | Antimicrobial activity against E. coli and S. aureus | Packaging | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ștefănescu, B.E.; Socaciu, C.; Vodnar, D.C. Recent Progress in Functional Edible Food Packaging Based on Gelatin and Chitosan. Coatings 2022, 12, 1815. https://doi.org/10.3390/coatings12121815
Ștefănescu BE, Socaciu C, Vodnar DC. Recent Progress in Functional Edible Food Packaging Based on Gelatin and Chitosan. Coatings. 2022; 12(12):1815. https://doi.org/10.3390/coatings12121815
Chicago/Turabian StyleȘtefănescu, Bianca Eugenia, Carmen Socaciu, and Dan Cristian Vodnar. 2022. "Recent Progress in Functional Edible Food Packaging Based on Gelatin and Chitosan" Coatings 12, no. 12: 1815. https://doi.org/10.3390/coatings12121815
APA StyleȘtefănescu, B. E., Socaciu, C., & Vodnar, D. C. (2022). Recent Progress in Functional Edible Food Packaging Based on Gelatin and Chitosan. Coatings, 12(12), 1815. https://doi.org/10.3390/coatings12121815








