The Corrosion Behavior of Al-Cu-Li Alloy in NaCl Solution
Abstract
:1. Introduction
2. Experimental Methods
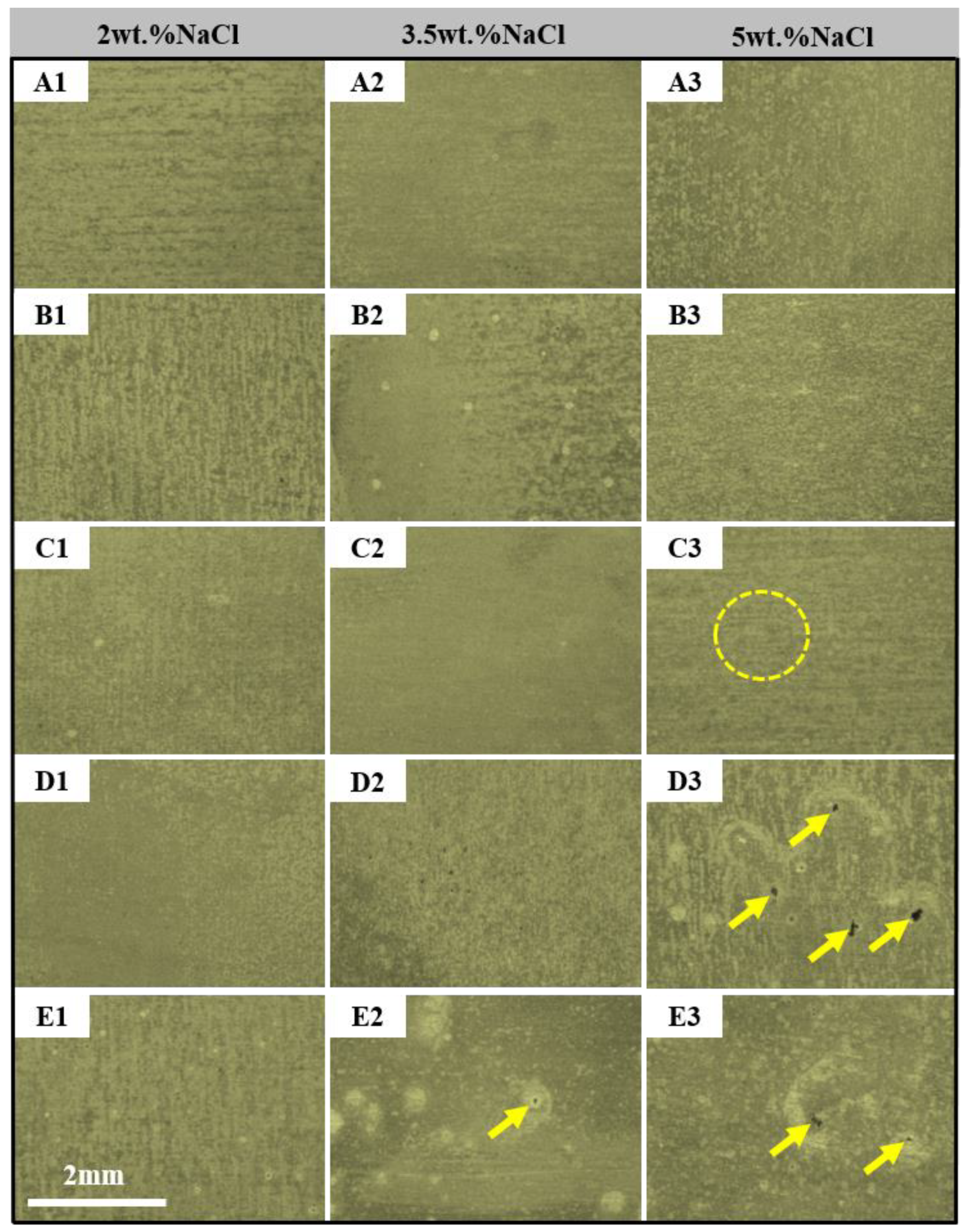
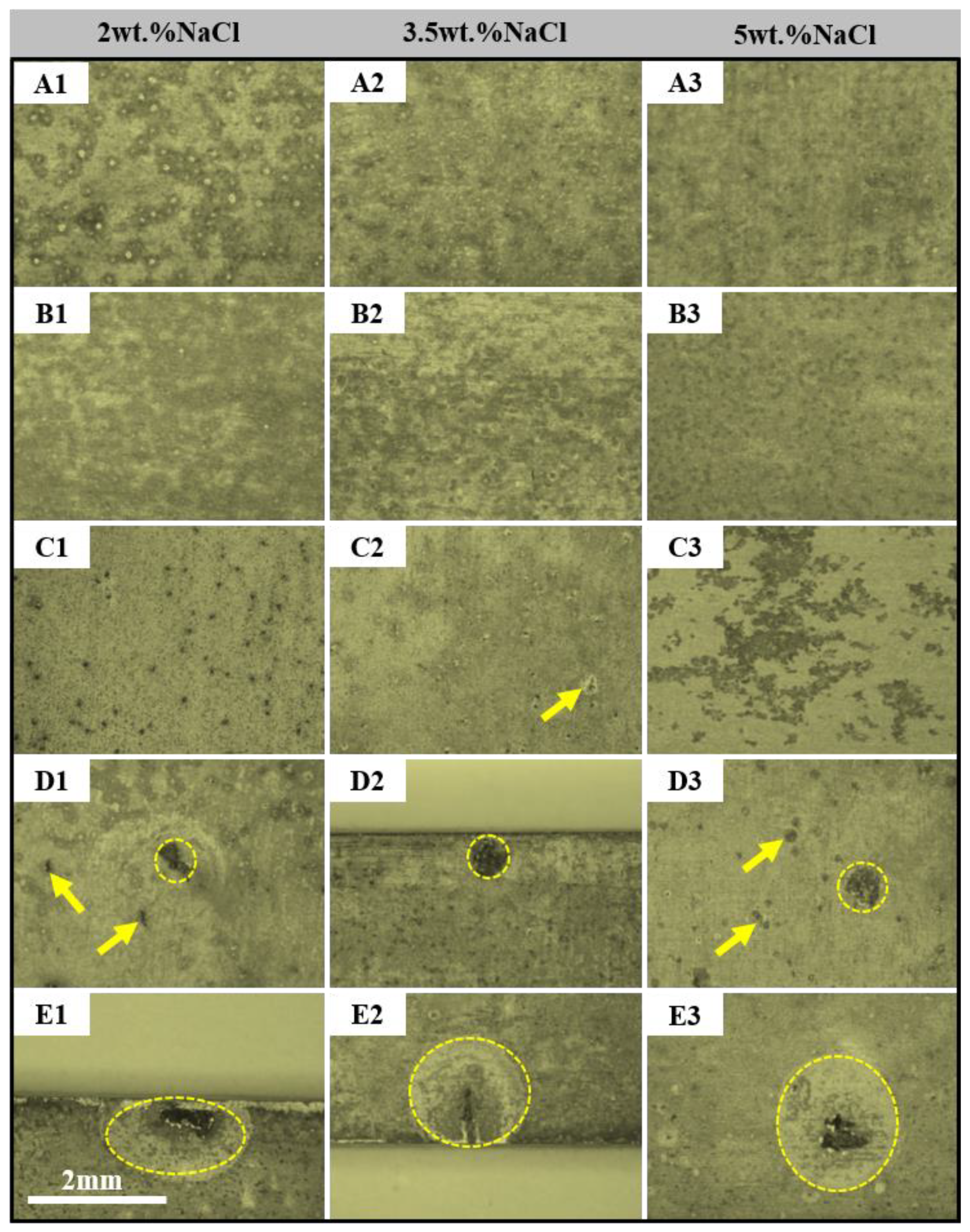
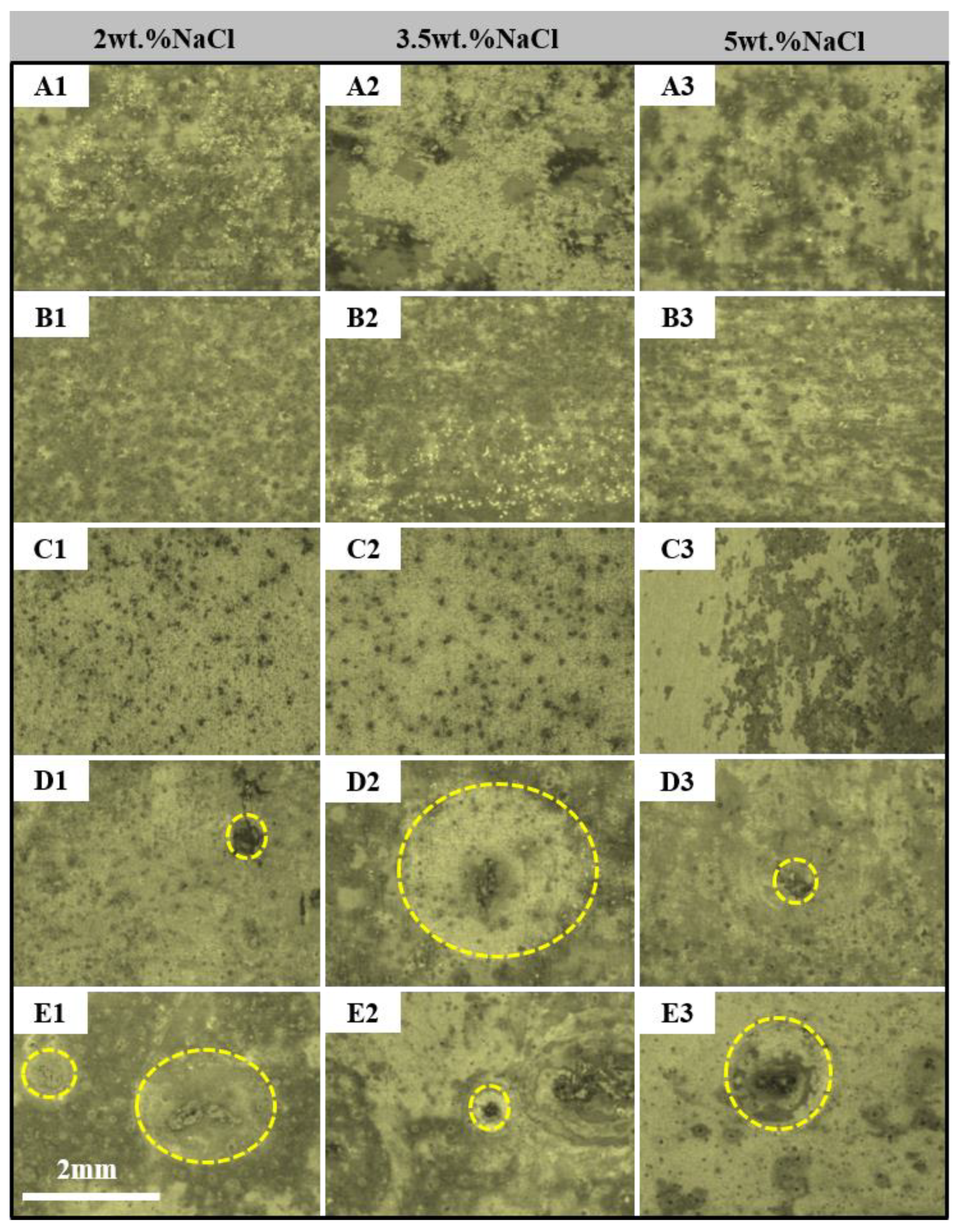
3. Results and Discussion
4. Conclusions
- (1)
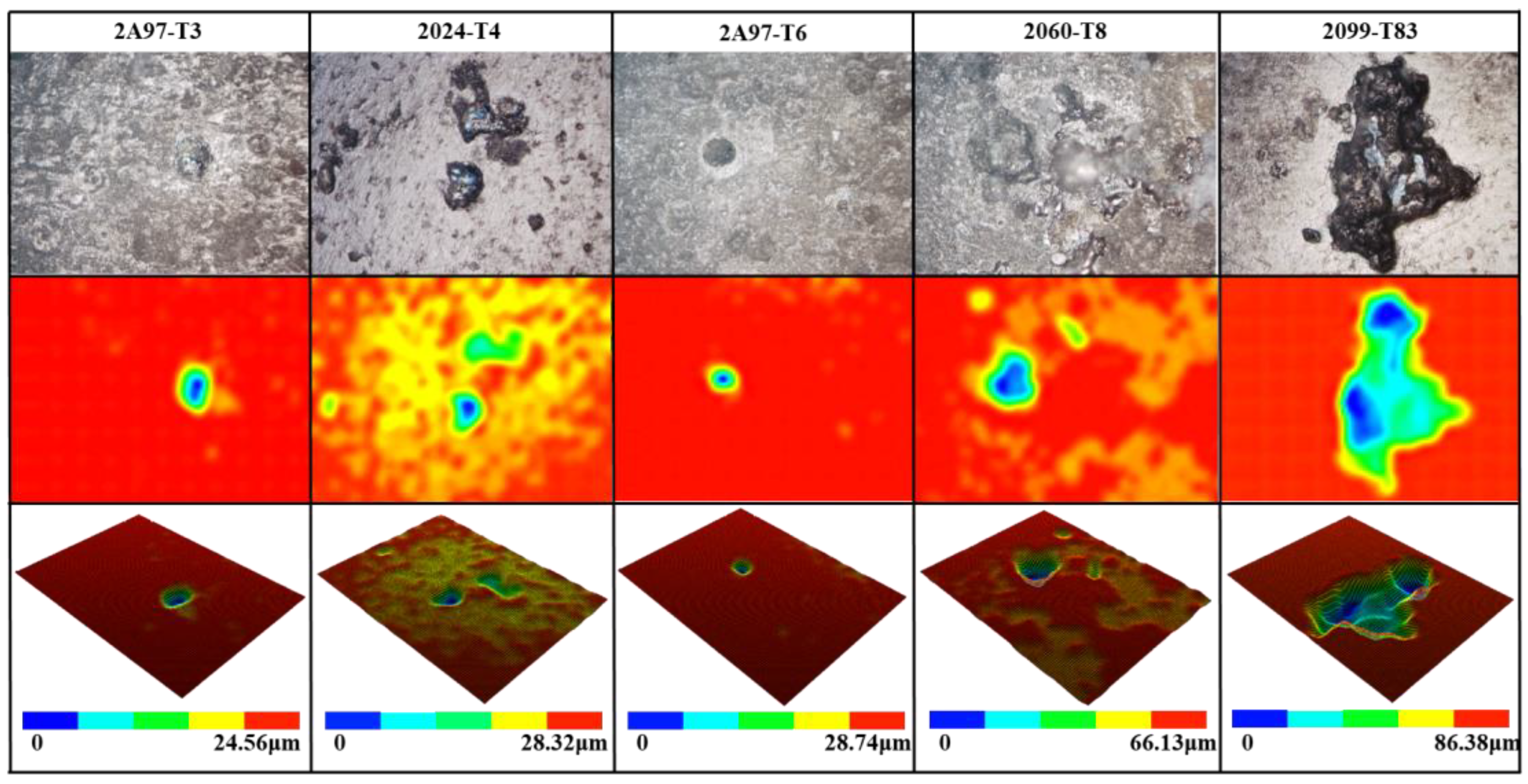
- The pitting corrosion and minor block erosion morphologies are visible on the 2A97 (T3, T6). The corrosion morphology of 2024-T4 is severe localized exfoliation corrosion. Furthermore, there are many deep pits and large areas of exfoliation corrosion on the corrosion surface of the 2060-T8 and 2099-T83.
- (2)
- The corrosion morphology of the sample and the maximum depth of the etching pit are analyzed. It is concluded that the corrosion resistance of each specimen is from strong to weak: 2A97-T3 > 2A97-T6 > 2024-T4 > 2060-T8 > 2099-T83.
- (3)
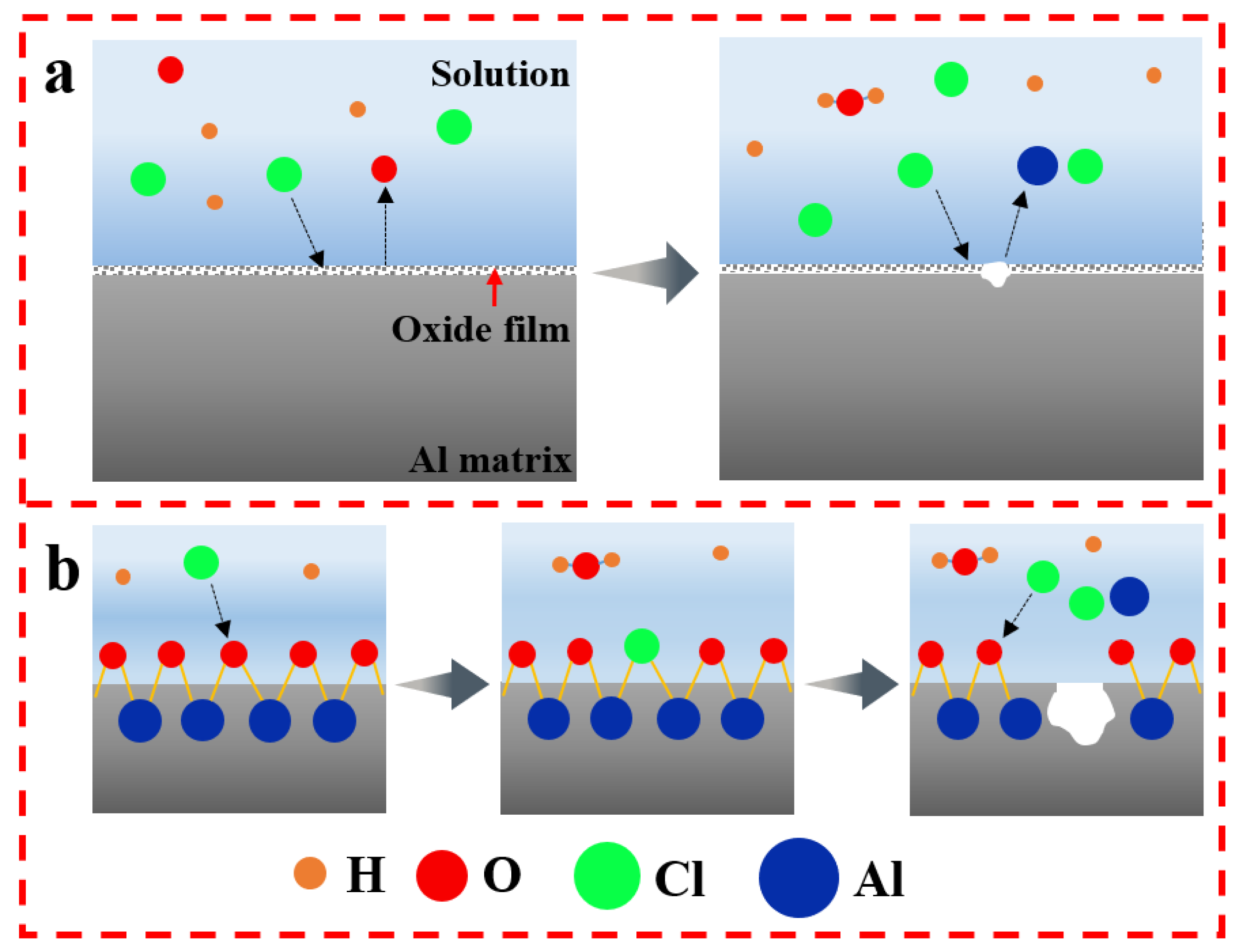
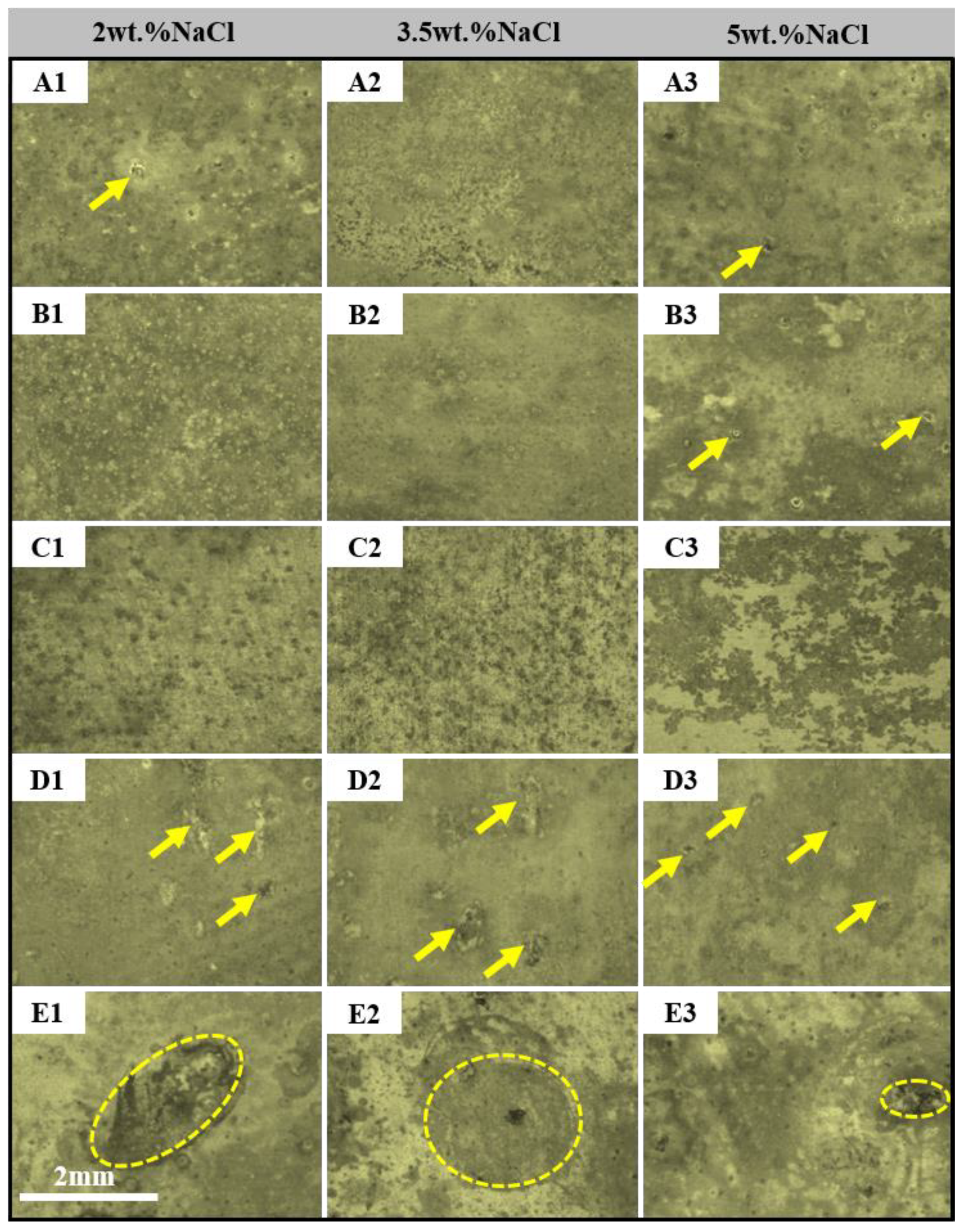
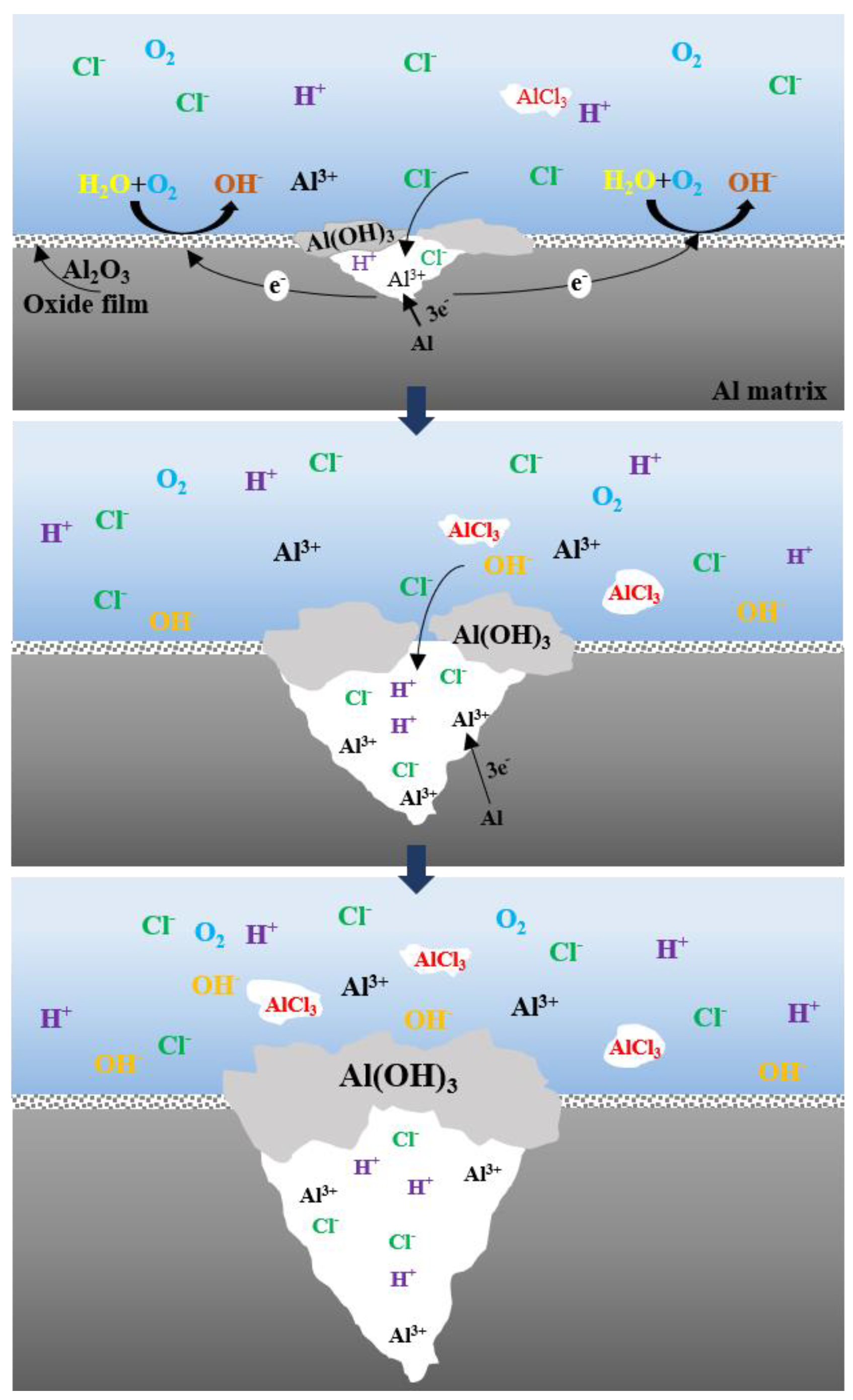
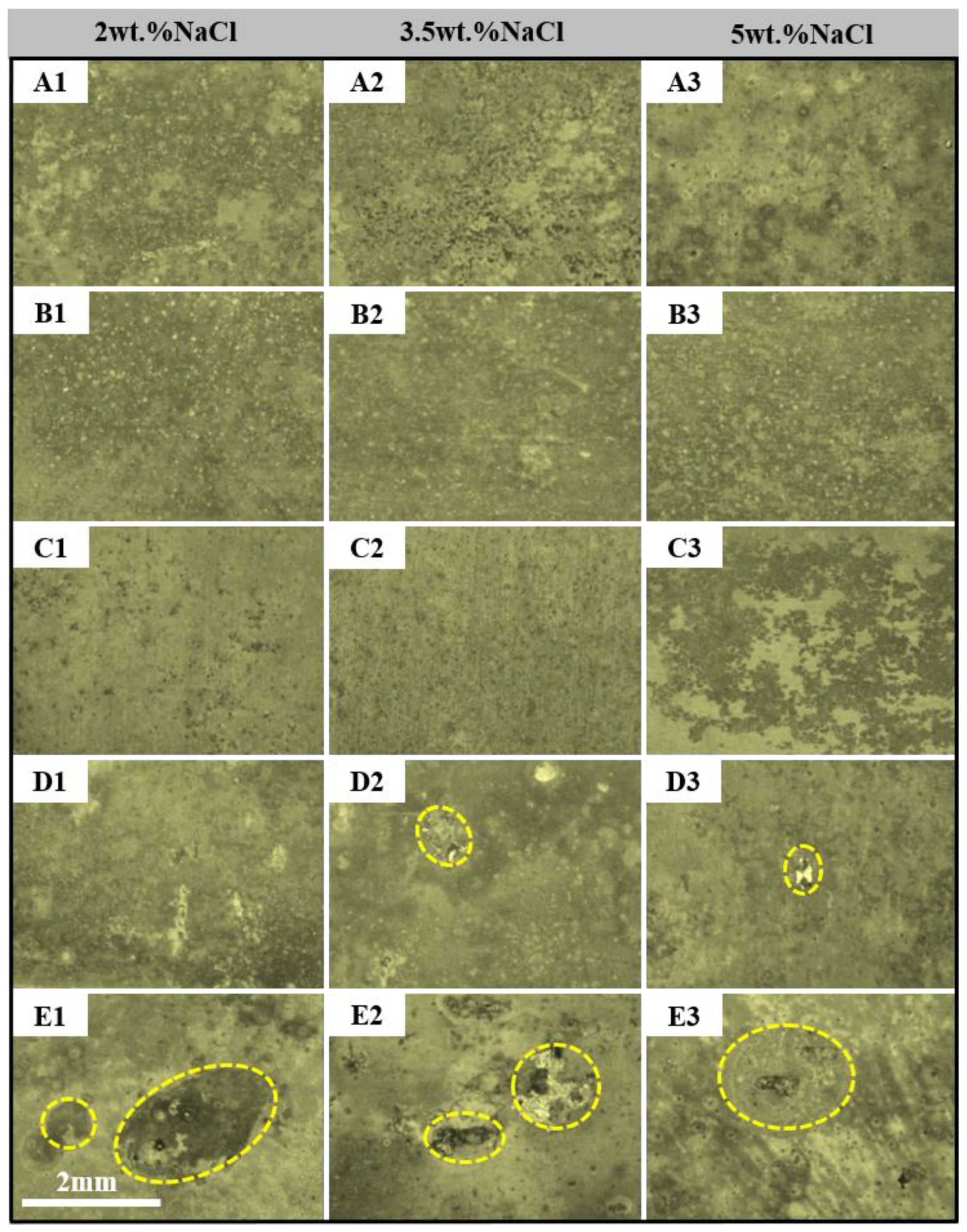
- The dissolution and rupture of the oxide film of the alloys are accelerated with the increase in NaCl solution concentration and the extension of immersion time, so the local corrosion occurs. However, the intermediate products adhere to the alloy, which retards the process of corrosion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rioja, R.J. Fabrication methods to manufacture isotropic Al-Li alloys and products for space and aerospace applications. Mater. Sci. Eng. A 1998, 257, 100–107. [Google Scholar] [CrossRef]
- Khokhlatova, L.B.; Kolobnev, N.I.; Oglodkov, M.S.; Mikhaylov, E.D. Aluminum-lithium alloys for aircraft building. Metallurgist 2012, 56, 336–341. [Google Scholar] [CrossRef]
- Rioja, R.J.; Liu, J. The Evolution of Al-Li Base Products for Aerospace and Space Applications. Metall. Mater. Trans. A 2012, 43, 3325–3337. [Google Scholar] [CrossRef]
- Abd El-Aty, A.; Xu, Y.; Guo, X.; Zhang, S.-H.; Ma, Y.; Chen, D. Strengthening mechanisms, deformation behavior, and anisotropic mechanical properties of Al-Li alloys: A review. J. Adv. Res. 2018, 10, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lu, C.; Wei, C.; Zheng, Z. Effect of aging treatment on microstructures, tensile properties and intergranular corrosion behavior of Al–Cu–Li alloy. Mater. Charact. 2018, 141, 163–168. [Google Scholar] [CrossRef]
- Jia, L.; Ren, X.; Hou, H.; Zhang, Y. Microstructural evolution and superplastic deformation mechanisms of as-rolled 2A97 alloy at low-temperature. Mater. Sci. Eng. A 2019, 759, 19–29. [Google Scholar] [CrossRef]
- Gurao, N.P.; Adesola, A.O.; Odeshi, A.G.; Szpunar, J.A. On the evolution of heterogeneous microstructure and microtexture in impacted aluminum–lithium alloy. J. Alloys Compd. 2013, 578, 183–187. [Google Scholar] [CrossRef]
- Paz Martínez-Viademonte, M.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A Review on Anodizing of Aerospace Aluminum Alloys for Corrosion Protection. Coatings 2020, 10, 1106. [Google Scholar] [CrossRef]
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, M.-H.; Chen, W.; Zhang, S.-H.; Xu, Y. Mechanisms and microstructures of 2A97 Al-Li alloy under the hot forming with synchronous quenching process. Microsc. Res. Tech. 2021, 84, 358–367. [Google Scholar] [CrossRef]
- Yu, J.; Lu, Z.; Xiong, Y.C.; Li, G.A.; Feng, Z.H. Effect of Intermediate Thermomechanical Treatment on Microstructure and Mechanical Properties of 2A97 Al-Li Alloy. J. Mater. Eng. 2021, 49, 130–136. [Google Scholar] [CrossRef]
- Chen, H.; Fu, L.; Liang, P. Microstructure, texture and mechanical properties of friction stir welded butt joints of 2A97 Al Li alloy ultra-thin sheets. J. Alloys Compd. 2017, 692, 155–169. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, M.-H. Effect of Preliminary Tension and Conditions of Artificial Aging on the Microstructure and Properties of Al–Li Alloy 2A97-T3. Met. Sci. Heat Treat. 2021, 63, 47–52. [Google Scholar] [CrossRef]
- Wang, K.P.; Alkire, R.C. Local Chemistry and Growth of Single Corrosion Pits in Aluminum. J. Electrochem. Soc. 1990, 137, 3010–3015. [Google Scholar] [CrossRef]
- Sinyavskii, V.S. Pitting and Stress Corrosions of Aluminum Alloys; Correlation between Them. Prot. Met. 2001, 37, 521–530. [Google Scholar] [CrossRef]
- Del Silva Campos, M.R.; Blawert, C.; Scharnagl, N.; Störmer, M.; Zheludkevich, M.L. Cathodic Protection of Mild Steel Using Aluminium-Based Alloys. Materials 2022, 15, 1301. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, H.; Chen, S.; Wang, D. Ag Nanoparticle-Loaded Hierarchical Superamphiphobic Surface on an Al Substrate with Enhanced Anticorrosion and Antibacterial Properties. J. Phys. Chem. C 2015, 119, 25449–25456. [Google Scholar] [CrossRef]
- Klumpp, R.E.; Donatus, U.; Araujo, J.V.S.; Redígolo, M.M.; Machado, C.d.S.C.; Costa, I. The Effect of Acid Pickling on the Corrosion Behavior of a Cerium Conversion-Coated AA2198-T851 Al-Cu-Li Alloy. J. Mater. Eng. Perform. 2020, 29, 167–174. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Thompson, G.E.; Zhang, X.; Luo, C.; Curioni, M.; Liu, H. Microstructural Modification Arising from Alkaline Etching and Its Effect on Anodizing Behavior of Al-Li-Cu Alloy. J. Electrochem. Soc. 2013, 160, C111–C118. [Google Scholar] [CrossRef]
- Proton, V.; Alexis, J.; Andrieu, E.; Delfosse, J.; Deschamps, A.; de Geuser, F.; Lafont, M.-C.; Blanc, C. The influence of artificial ageing on the corrosion behaviour of a 2050 aluminium–copper–lithium alloy. Corros. Sci. 2014, 80, 494–502. [Google Scholar] [CrossRef]
- Na, J.; Li, J.; Zheng, Z.; Wei, X.; Li, Y. Effect of aging on mechanical properties and localized corrosion behaviors of Al-Cu-Li alloy. Trans. Nonferr. Met. Soc. China 2005, 15, 23–29. [Google Scholar]
- Liang, W.; Pan, Q.; He, Y.; Li, Y.; Zhou, Y.; Lu, C. Effect of aging on the mechanical properties and corrosion susceptibility of an Al-Cu-Li-Zr alloy containing Sc. Rare Met. 2008, 27, 146–152. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Hashimoto, T.; Lindsay, J.; Ciuca, O.; Luo, C.; Sun, Z.; Tang, Z. The influence of grain structure on the corrosion behaviour of 2A97-T3 Al-Cu-Li alloy. Corros. Sci. 2017, 116, 14–21. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Hashimoto, T.; Liu, B.; Luo, C.; Sun, Z.; Tang, Z.; Lu, F.; Ma, Y. Corrosion behaviour of 2A97-T6 Al-Cu-Li alloy: The influence of non-uniform precipitation. Corros. Sci. 2018, 132, 1–8. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Huang, W.; Thompson, G.E.; Zhang, X.; Luo, C.; Sun, Z. Localized corrosion in AA2099-T83 aluminum–lithium alloy: The role of intermetallic particles. Mater. Chem. Phys. 2015, 161, 201–210. [Google Scholar] [CrossRef]
- Luo, C.; Gao, M.; Sun, Z.; Zhang, X.; Tang, Z.; Zhou, X. FIB-SEM investigation on corrosion propagation of aluminium–lithium alloy in sodium chloride solution. Corros. Eng. Sci. Technol. 2015, 50, 390–396. [Google Scholar] [CrossRef]
- Xu, X.; Hao, M.; Chen, J.; He, W.; Li, G.; Li, K.; Jiao, C.; Zhong, X.L.; Moore, K.L.; Burnett, T.L.; et al. Role of constituent intermetallic phases and precipitates in initiation and propagation of intergranular corrosion of an Al-Li-Cu-Mg alloy. Corros. Sci. 2022, 201, 110294. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, B.; Zhou, X.; Wang, J.; Hashimoto, T.; Luo, C.; Sun, Z.; Tang, Z.; Lu, F. Laser welding introduced segregation and its influence on the corrosion behaviour of Al-Cu-Li alloy. Corros. Sci. 2018, 135, 177–191. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, Y.; Zhou, X.; Hashimoto, T.; Ma, Y. Corrosion behaviour of 2A97-T8 Al-Cu-Li alloy extrusion. J. Alloys Compd. 2022, 898, 162872. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, B.; Zhao, X.; Wang, J.; Luo, C.; Sun, Z.; Tang, Z.; Lu, F. Corrosion behavior of friction stir welded 2A97 Al-Cu-Li alloy. Corrosion 2017, 73, 988–997. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Ma, Y.; Thompson, G.E.; Luo, C.; Sun, Z.; Tang, Z. The propagation of localized corrosion in Al-Cu-Li alloy. Surf. Interface Anal. 2016, 48, 745–749. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Modestov, A.D.; Domantovsky, A.G.; Shiryaev, A.A.; Emelyanenko, K.A.; Dvoretskaya, O.V.; Ganne, A.A. Corrosion behavior of superhydrophobic aluminum alloy in concentrated potassium halide solutions: When the specific anion effect is manifested. Corros. Sci. 2016, 112, 517–527. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Pitting corrosion of aluminum. Corros. Sci. 1999, 41, 1743–1767. [Google Scholar] [CrossRef]
- Zaid, B.; Saidi, D.; Benzaid, A.; Hadji, S. Effects of pH and chloride concentration on pitting corrosion of AA6061 aluminum alloy. Corros. Sci. 2008, 50, 1841–1847. [Google Scholar] [CrossRef]
- Soltis, J. Passivity breakdown, pit initiation and propagation of pits in metallic materials—Review. Corros. Sci. 2015, 90, 5–22. [Google Scholar] [CrossRef]
- Li, J.F.; Li, C.X.; Peng, Z.W.; Chen, W.J.; Zheng, Z.Q. Corrosion mechanism associated with T1 and T2 precipitates of Al–Cu–Li alloys in NaCl solution. J. Alloys Compd. 2008, 460, 688–693. [Google Scholar] [CrossRef]
- Liu, Y.; Colin, F.; Skeldon, P.; Thompson, G.E.; Zhou, X.; Habazaki, H.; Shimizu, K. Enrichment factors for copper in aluminium alloys following chemical and electrochemical surface treatments. Corros. Sci. 2003, 45, 1539–1544. [Google Scholar] [CrossRef]
- Hashimoto, T.; Zhou, X.; Skeldon, P.; Thompson, G.E. Structure of the Copper–Enriched Layer Introduced by Anodic Oxidation of Copper-Containing Aluminium Alloy. Electrochim. Acta 2015, 179, 394–401. [Google Scholar] [CrossRef]
- Davoodi, A.; Pan, J.; Leygraf, C.; Norgren, S. Probing of local dissolution of Al-alloys in chloride solutions by AFM and SECM. Appl. Surf. Sci. 2006, 252, 5499–5503. [Google Scholar] [CrossRef]
- Ryl, J.; Wysocka, J.; Jarzynka, M.; Zielinski, A.; Orlikowski, J.; Darowicki, K. Effect of native air-formed oxidation on the corrosion behavior of AA 7075 aluminum alloys. Corros. Sci. 2014, 87, 150–155. [Google Scholar] [CrossRef]
- Al-Moubaraki, A.H.; Al-Rushud, H.H. The Red Sea as a Corrosive Environment: Corrosion Rates and Corrosion Mechanism of Aluminum Alloys 7075, 2024, and 6061. Int. J. Corros. 2018, 2018, 2381287. [Google Scholar] [CrossRef]
- Curioni, M.; Saenz de Miera, M.; Skeldon, P.; Thompson, G.E.; Ferguson, J. Macroscopic and Local Filming Behavior of AA2024 T3 Aluminum Alloy during Anodizing in Sulfuric Acid Electrolyte. J. Electrochem. Soc. 2008, 155, C387. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Thompson, G.E.; Curioni, M.; Zhong, X.; Koroleva, E.; Skeldon, P.; Thomson, P.; Fowles, M. Discontinuities in the porous anodic film formed on AA2099-T8 aluminium alloy. Corros. Sci. 2011, 53, 4141–4151. [Google Scholar] [CrossRef]
- Crossland, A.C.; Habazaki, H.; Shimizu, K.; Skeldon, P.; Thompson, G.E.; Wood, G.C.; Zhou, X.; Smith, C. Residual flaws due to formation of oxygen bubbles in anodic alumina. Corros. Sci. 1999, 41, 1945–1954. [Google Scholar] [CrossRef]
- Buchheit, R.G. A Compilation of Corrosion Potentials Reported for Intermetallic Phases in Aluminum Alloys. J. Electrochem. Soc. 1995, 142, 3994–3996. [Google Scholar] [CrossRef]
- Birbilis, N.; Buchheit, R.G. Electrochemical Characteristics of Intermetallic Phases in Aluminum Alloys: An Experimental Survey and Discussion. J. Electrochem. Soc. 2005, 152, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Karthik, D.; Jiang, J.; Hu, Y.; Yao, Z. Effect of multiple laser shock peening on microstructure, crystallographic texture and pitting corrosion of Aluminum-Lithium alloy 2060-T8. Surf. Coat. Technol. 2021, 421, 127354. [Google Scholar] [CrossRef]
- Han, B.; Chen, Y.; Tao, W.; Li, H.; Li, L. Microstructural evolution and interfacial crack corrosion behavior of double-sided laser beam welded 2060/2099 Al-Li alloys T-joints. Mater. Des. 2017, 135, 353–365. [Google Scholar] [CrossRef]
- Newman, R. Pitting Corrosion of Metals. Electrochem. Soc. Interface 2010, 19, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Ezuber, H.; El-Houd, A.; El-Shawesh, F. A study on the corrosion behavior of aluminum alloys in seawater. Mater. Des. 2008, 29, 801–805. [Google Scholar] [CrossRef]



| Alloy | Cu | Li | Zn | Mg | Mn | Zr | Fe | Ag | Si | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2A97 | 3.55 | 1.31 | 0.5 | 0.37 | 0.31 | 0.11 | 0.044 | <0.03 | <0.03 | 0.024 | Bal. |
| 2060-T8 | 3.81 | 0.8 | 0.311 | 0.793 | 0.269 | 0.169 | 0.02 | 0.527 | 0.07 | 0.0281 | Bal. |
| 2099-T83 | 2.22 | 1.87 | 0.581 | 0.352 | 0.276 | 0.122 | 0.023 | - | 0.048 | 0.026 | Bal. |
| 2024-T4 | 6.12 | - | 0.086 | 1.15 | 1.24 | - | 1.17 | - | 0.16 | 0.022 | Bal. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhang, P.; Zhao, X.; Rao, S. The Corrosion Behavior of Al-Cu-Li Alloy in NaCl Solution. Coatings 2022, 12, 1899. https://doi.org/10.3390/coatings12121899
Wang Z, Zhang P, Zhao X, Rao S. The Corrosion Behavior of Al-Cu-Li Alloy in NaCl Solution. Coatings. 2022; 12(12):1899. https://doi.org/10.3390/coatings12121899
Chicago/Turabian StyleWang, Ziyu, Peng Zhang, Xinsheng Zhao, and Sixian Rao. 2022. "The Corrosion Behavior of Al-Cu-Li Alloy in NaCl Solution" Coatings 12, no. 12: 1899. https://doi.org/10.3390/coatings12121899
APA StyleWang, Z., Zhang, P., Zhao, X., & Rao, S. (2022). The Corrosion Behavior of Al-Cu-Li Alloy in NaCl Solution. Coatings, 12(12), 1899. https://doi.org/10.3390/coatings12121899





