First Principles Study of Atomic Oxygen Adsorption on Austenitic Stainless Steels Surfaces: A Theoretical Study
Abstract
1. Introduction
2. Calculation Details
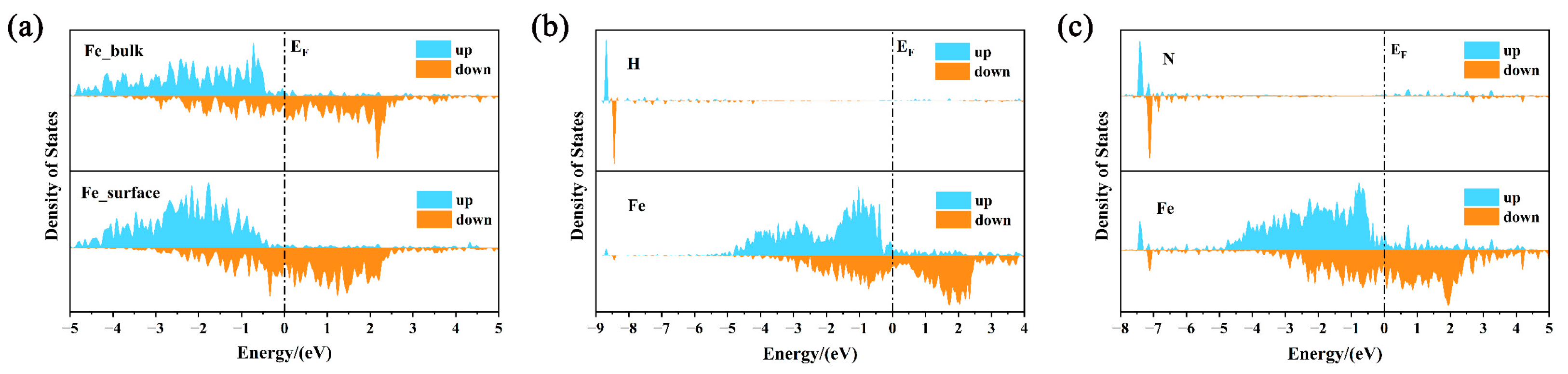
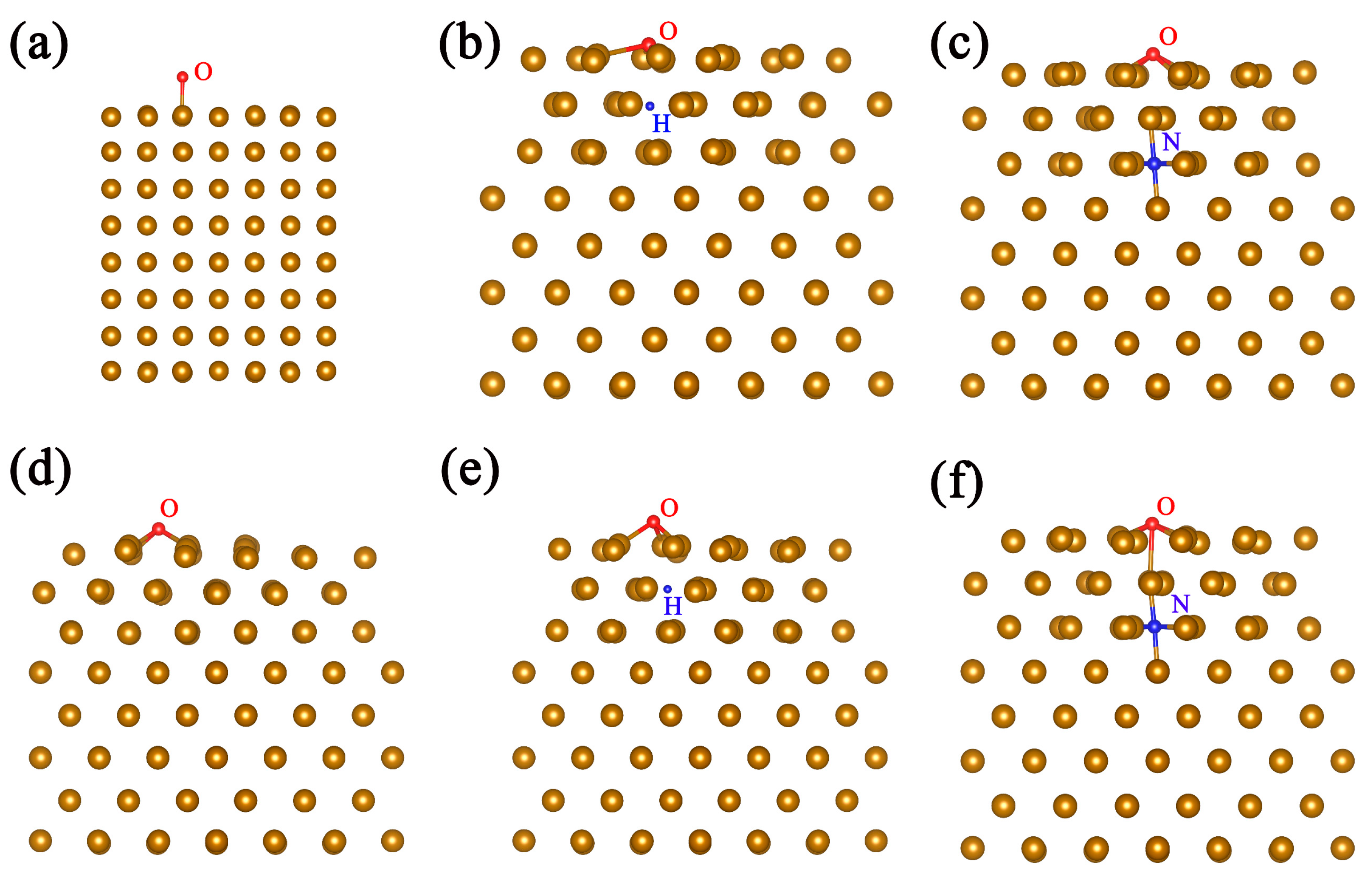
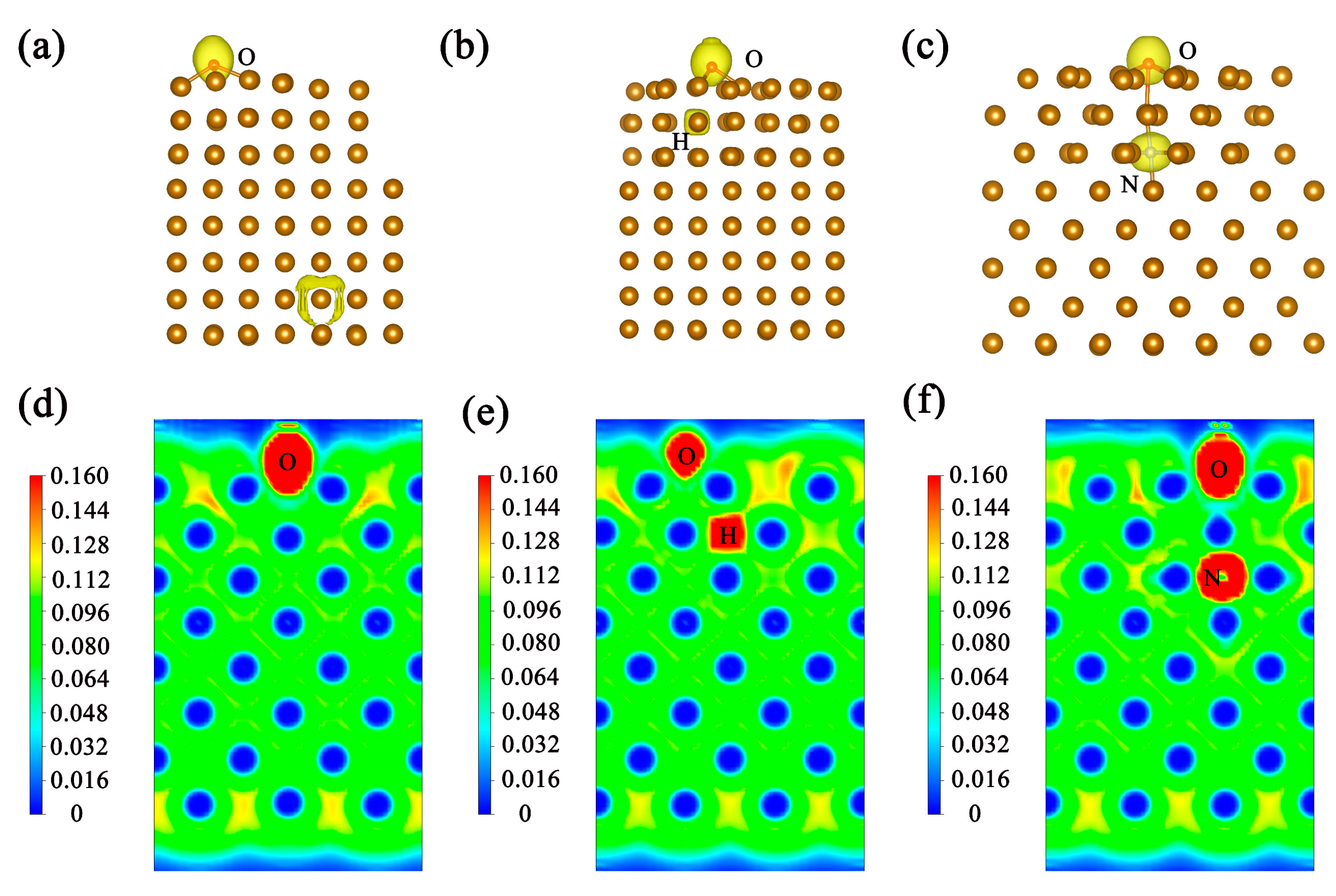
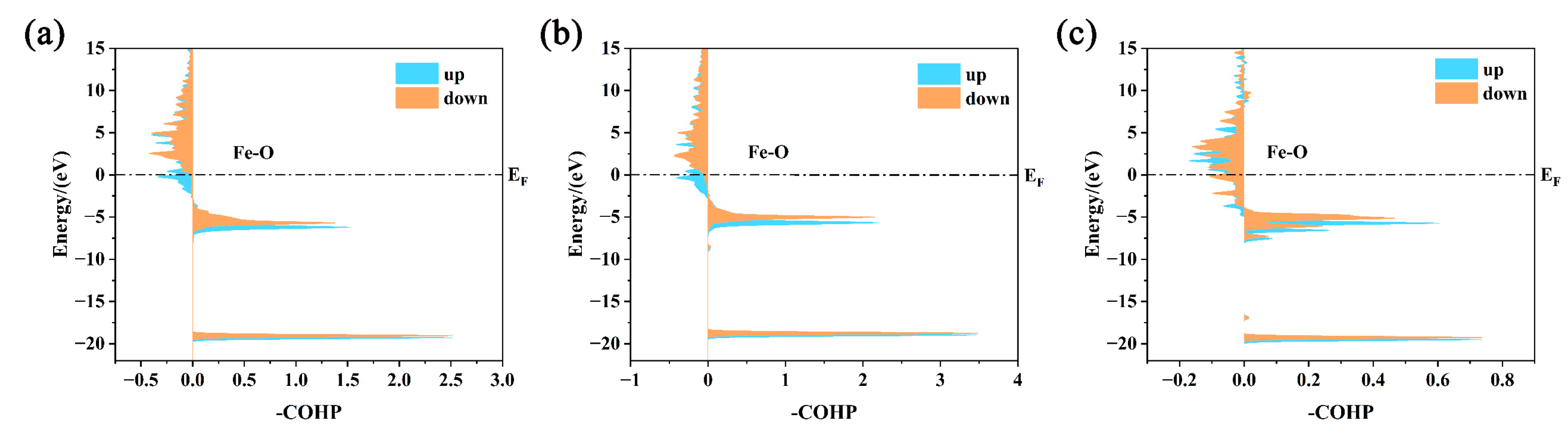
3. Results and Discussion
4. Conclusions
- (1)
- Atomic oxygen can be stably adsorbed on the surface of pure γ-Fe and H/N-containing austenitic because of huge adsorption energies (>6 eV);
- (2)
- Both interstitial atoms H and N can enhance the adsorption of atomic oxygen on the surface, but their mechanism of action is in opposition. Hydrogen enhances adsorption by breaking metal bonds near the surface, while nitrogen enhances adsorption by enhancing structural stability;
- (3)
- The enhancement effect of hydrogen on adsorption energy (−6.7629 eV) is stronger than that of nitrogen (−6.6374 eV), but hydrogen will lead to the decrease of the stability of the system;
- (4)
- The introduction of appropriate nitrogen atoms may contribute to the rapid passivation and stability of the surface passivation film, which is beneficial to the corrosion resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verma, J.; Taiwade, R.V. Effect of welding processes and conditions on the microstructure, mechanical properties and corrosion resistance of duplex stainless steel weldments—A review. J. Manuf. Process. 2017, 25, 134–152. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetič, M.; Zalar, A. Oxidation of AISI 304L stainless steel surface with atomic oxygen. Appl. Surf. Sci. 2002, 200, 94–103. [Google Scholar] [CrossRef]
- Boyer, R.R.; Cotton, J.D.; Mohaghegh, M.; Schafrik, R.E. Materials considerations for aerospace applications. MRS Bull. 2015, 40, 1055–1066. [Google Scholar] [CrossRef]
- Reddy, M.R. Effect of low earth orbit atomic oxygen on spacecraft materials. JMatS 1995, 30, 281–307. [Google Scholar] [CrossRef]
- Ma, L.; Lynch, B.; Wiame, F.; Maurice, V.; Marcus, P. Nanoscale early oxidation mechanisms of model FeCrNi austenitic stainless steel surfaces at room temperature. Corros. Sci. 2021, 190, 109653. [Google Scholar] [CrossRef]
- Massoud, T.; Maurice, V.; Klein, L.H.; Seyeux, A.; Marcus, P. Nanostructure and local properties of oxide layers grown on stainless steel in simulated pressurized water reactor environment. Corros. Sci. 2014, 84, 198–203. [Google Scholar] [CrossRef]
- Ma, L.; Wiame, F.; Maurice, V.; Marcus, P. New insight on early oxidation stages of austenitic stainless steel from in situ XPS analysis on single-crystalline Fe–18Cr–13Ni. Corros. Sci. 2018, 140, 205–216. [Google Scholar] [CrossRef]
- Ma, L.; Pascalidou, E.-M.; Wiame, F.; Zanna, S.; Maurice, V.; Marcus, P. Passivation mechanisms and pre-oxidation effects on model surfaces of FeCrNi austenitic stainless steel. Corros. Sci. 2020, 167, 108483. [Google Scholar] [CrossRef]
- Tan, X.; Zhou, J.; Peng, Y. First-principles study of oxygen adsorption on Fe(110) surface. Appl. Surf. Sci. 2012, 258, 8484–8491. [Google Scholar] [CrossRef]
- Błoński, P.; Kiejna, A.; Hafner, J. Theoretical study of oxygen adsorption at the Fe(110) and (100) surfaces. Surf. Sci. 2005, 590, 88–100. [Google Scholar] [CrossRef]
- Ossowski, T.; Kiejna, A. Oxygen adsorption on Fe(110) surface revisited. Surf. Sci. 2015, 637–638, 35–41. [Google Scholar] [CrossRef]
- Błoński, P.; Kiejna, A.; Hafner, J. Oxygen adsorption on the clean and O-precovered Fe (110) and (100) surfaces. J. Phys. Condens. Matter 2007, 19, 096011. [Google Scholar] [CrossRef]
- Błoński, P.; Kiejna, A.; Hafner, J. Dissociative adsorption of O2 molecules on O-precovered Fe(110) and Fe(100): Density-functional calculations. PhRvB 2008, 77, 155424. [Google Scholar] [CrossRef]
- Busch, M.; Gruyters, M.; Winter, H. FeO(111) formation by exposure of Fe(110) to atomic and molecular oxygen. Surf. Sci. 2006, 600, 2778–2784. [Google Scholar] [CrossRef]
- Das, N.K.; Suzuki, K.; Takeda, Y.; Ogawa, K.; Shoji, T. Quantum chemical molecular dynamics study of stress corrosion cracking behavior for fcc Fe and Fe–Cr surfaces. Corros. Sci. 2008, 50, 1701–1706. [Google Scholar] [CrossRef]
- Das, N.K.; Suzuki, K.; Ogawa, K.; Shoji, T. Early stage SCC initiation analysis of fcc Fe–Cr–Ni ternary alloy at 288 °C: A quantum chemical molecular dynamics approach. Corros. Sci. 2009, 51, 908–913. [Google Scholar] [CrossRef]
- Haynes, W. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2012; Volume 94. [Google Scholar]
- Tang, J.E.; Halvarsson, M.; Asteman, H.; Svensson, J.E. Microstructure of Oxidised 304L Steel and the Effects of Surface Roughness on Oxidation Behaviour. In High Temperature Corrosion and Protection of Materials; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2001. [Google Scholar]
- Ishikawa, Y.; Yoshimura, T. Importance of the surface oxide layer in the reduction of outgassing from stainless steels. J. Vac. Sci. Technol. A 1995, 13, 1847–1852. [Google Scholar] [CrossRef]
- Betz, G.; Wehner, G.K.; Toth, L.; Joshi, A. Composition-vs-depth profiles obtained with Auger electron spectroscopy of air-oxidized stainless-steel surfaces. J. Appl. Phys. 1974, 45, 5312–5316. [Google Scholar] [CrossRef]
- Mathieu, H.J.; Landolt, D. An investigation of thin oxide films thermally grown in situ on Fe–24Cr and Fe–24Cr–11Mo by auger electron spectroscopy and X-ray photoelectron spectroscopy. Corros. Sci. 1986, 26, 547–559. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetic, M.; Drenik, A.; Hauptman, N.; Balat-Pichelin, M. High temperature oxidation of stainless steel AISI316L in air plasma. Appl. Surf. Sci. 2008, 255, 1759–1765. [Google Scholar] [CrossRef]
- Cho, B.; Choi, E.; Chung, S.; Kim, K.; Kang, T.; Park, C.; Kim, B. A novel Cr2O3 thin film on stainless steel with high sorption resistance. Surf. Sci. 1999, 439, L799–L802. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, D.; Hu, M.; Hu, Q. Cr2O3/C composite coatings on stainless steel 304 as bipolar plate for proton exchange membrane fuel cell. Energy 2014, 76, 816–821. [Google Scholar] [CrossRef]
- Adams, R.O. A review of the stainless steel surface. J. Vac. Sci. Technol. A 1983, 1, 12–18. [Google Scholar] [CrossRef]
- Strehblow, H.-H. Passivity of Metals Studied by Surface Analytical Methods, a Review. Electrochim. Acta 2016, 212, 630–648. [Google Scholar] [CrossRef]
- Maurice, V.; Marcus, P. Progress in corrosion science at atomic and nanometric scales. Prog. Mater. Sci. 2018, 95, 132–171. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Odaka, K. Reduction of outgassing from stainless surfaces by surface oxidation. Vacuu 1990, 41, 1995–1997. [Google Scholar] [CrossRef]
- Kocijan, A.; Donik, Č.; Jenko, M. Electrochemical and XPS studies of the passive film formed on stainless steels in borate buffer and chloride solutions. Corros. Sci. 2007, 49, 2083–2098. [Google Scholar] [CrossRef]
- Shibagaki, S.; Koga, A.; Shirakawa, Y.; Onishi, H.; Yokokawa, H.; Tanaka, J. Chemical reaction path for thin film oxidation of stainless steel. Thin Solid Film. 1997, 303, 101–106. [Google Scholar] [CrossRef]
- Mandrino, D.; Godec, M.; Torkar, M.; Jenko, M. Study of oxide protective layers on stainless steel by AES, EDS and XPS. Surf. Interface Anal. 2008, 40, 285–289. [Google Scholar] [CrossRef]
- Donik, Č.; Kocijan, A.; Grant, J.T.; Jenko, M.; Drenik, A.; Pihlar, B. XPS study of duplex stainless steel oxidized by oxygen atoms. Corros. Sci. 2009, 51, 827–832. [Google Scholar] [CrossRef]
- Borchers, C.; Michler, T.; Pundt, A. Effect of Hydrogen on the Mechanical Properties of Stainless Steels. Adv. Eng. Mater. 2008, 10, 11–23. [Google Scholar] [CrossRef]
- Whiteman, M.B.; Troiano, A.R. Hydrogen Embrittlement Of Austenitic Stainless Steel. Corrosion 2013, 21, 53–56. [Google Scholar] [CrossRef]
- Byrnes, M.L.G.; Grujicic, M.; Owen, W.S. Nitrogen strengthening of a stable austenitic stainless steel. Acta Metall. 1987, 35, 1853–1862. [Google Scholar] [CrossRef]
- Reed, R.P. Nitrogen in austenitic stainless steels. JOM 1989, 41, 16–21. [Google Scholar] [CrossRef]
- Ye, F.; Wang, Y.; Xu, F.; Ke, T. Effects of Interstitial Nitrogen Atoms on Atomic Oxygen Adsorption on Fe (001) Surface from Ab Initio Calculations. Mater. Sci. Forum 2017, 898, 849–855. [Google Scholar] [CrossRef]
- Grabke, H.J. The Role of Nitrogen in the Corrosion of Iron and Steels. ISIJ Int. 1996, 36, 777–786. [Google Scholar] [CrossRef]
- Jargelius-Pettersson, R.f.a. Electrochemical investigation of the influence of nitrogen alloying on pitting corrosion of austenitic stainless steels. Corros. Sci. 1999, 41, 1639–1664. [Google Scholar] [CrossRef]
- Baba, H.; Kodama, T.; Katada, Y. Role of nitrogen on the corrosion behavior of austenitic stainless steels. Corros. Sci. 2002, 44, 2393–2407. [Google Scholar] [CrossRef]
- Lei, M.K.; Zhu, X.M. Role of Nitrogen in Pitting Corrosion Resistance of a High-Nitrogen Face-Centered-Cubic Phase Formed on Austenitic Stainless Steel. J. Electrochem. Soc. 2005, 152, B291. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y. Unusual structural and electronic properties of porous silicene and germanene: Insights from first-principles calculations. Nanoscale Res. Lett. 2015, 10, 13. [Google Scholar] [CrossRef]
- Bai, H.; Zhu, Y.; Qiao, W.; Huang, Y. Structures, stabilities and electronic properties of graphdiyne nanoribbons. RSC Adv. 2011, 1, 768–775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. PhRvB 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. PhRvB 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. Crystal Orbital Hamilton Population (COHP) Analysis As Projected from Plane-Wave Basis Sets. J. Phys. Chem. A 2011, 115, 5461–5466. [Google Scholar] [CrossRef]
- Maintz, S.; Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. Analytic projection from plane-wave and PAW wavefunctions and application to chemical-bonding analysis in solids. J. Comput. Chem. 2013, 34, 2557–2567. [Google Scholar] [CrossRef]
- Maintz, S.; Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. LOBSTER: A tool to extract chemical bonding from plane-wave based DFT. J. Comput. Chem. 2016, 37, 1030–1035. [Google Scholar] [CrossRef]
- Dronskowski, R.; Bloechl, P.E. Crystal orbital Hamilton populations (COHP): Energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. 1993, 97, 8617–8624. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO-MO Molecular Wave Functions. IV. Bonding and Antibonding in LCAO and Valence-Bond Theories. J. Chem. Phys. 1955, 23, 2343–2346. [Google Scholar] [CrossRef]
- Turnbull, A.; Hutchings, R.B. Analysis of hydrogen atom transport in a two-phase alloy. Mater. Sci. Eng. A 1994, 177, 161–171. [Google Scholar] [CrossRef]
- Turk, A.; Pu, S.D.; Bombač, D.; Rivera-Díaz-del-Castillo, P.E.J.; Galindo-Nava, E.I. Quantification of hydrogen trapping in multiphase steels: Part II—Effect of austenite morphology. Acta Mater. 2020, 197, 253–268. [Google Scholar] [CrossRef]
- Mingolo, N.; Tschiptschin, A.P.; Pinedo, C.E. On the formation of expanded austenite during plasma nitriding of an AISI 316L austenitic stainless steel. Surf. Coat. Technol. 2006, 201, 4215–4218. [Google Scholar] [CrossRef]
- Xu, X.; Wang, L.; Yu, Z.; Qiang, J.; Hei, Z. Study of microstructure of low-temperature plasma-nitrided AISI 304 stainless steel. MMTA 2000, 31, 1193–1199. [Google Scholar] [CrossRef]
- Oda, K.; Kondo, N.; Shibata, K. X-ray Absorption Fine Structure Analysis of Interstitial (C, N)-Substitutional (Cr) Complexes in Austenitic Stainless Steels. ISIJ Int. 1990, 30, 625–631. [Google Scholar] [CrossRef]
- Li, T.; Chien, S.-C.; Ren, Z.; Windl, W.; Ernst, F.; Frankel, G.S. Understanding the efficacy of concentrated interstitial carbon in enhancing the pitting corrosion resistance of stainless steel. Acta Mater. 2021, 221, 117433. [Google Scholar] [CrossRef]
- Marcus, P.; Bussell, M.E. XPS study of the passive films formed on nitrogen-implanted austenitic stainless steels. Appl. Surf. Sci. 1992, 59, 7–21. [Google Scholar] [CrossRef]
- Murakami, Y.; Kanezaki, T.; Mine, Y.; Matsuoka, S. Hydrogen Embrittlement Mechanism in Fatigue of Austenitic Stainless Steels. MMTA 2008, 39, 1327–1339. [Google Scholar] [CrossRef]
- Tsay, L.W.; Yu, S.C.; Huang, R.T. Effect of austenite instability on the hydrogen-enhanced crack growth of austenitic stainless steels. Corros. Sci. 2007, 49, 2973–2984. [Google Scholar] [CrossRef]
- Barnoush, A.; Vehoff, H. Electrochemical nanoindentation: A new approach to probe hydrogen/deformation interaction. Scr. Mater. 2006, 55, 195–198. [Google Scholar] [CrossRef]
- Berns, H.; Gavriljuk, V.; Shanina, B. Intensive Interstitial Strengthening of Stainless Steels. Adv. Eng. Mater. 2008, 10, 1083–1093. [Google Scholar] [CrossRef]
- Shankar, P.; Sundararaman, D.; Ranganathan, S. Clustering and ordering of nitrogen in nuclear grade 316LN austenitic stainless steel. J. Nucl. Mater. 1998, 254, 1–8. [Google Scholar] [CrossRef]
- Ledbetter, H.M.; Austin, M.W. Effects of carbon and nitrogen on the elastic constants of AISI type 304 stainless steel. MSEng 1985, 70, 143–149. [Google Scholar] [CrossRef]
- Parihar, S.S.; Meyerheim, H.L.; Mohseni, K.; Ostanin, S.; Ernst, A.; Jedrecy, N.; Felici, R.; Kirschner, J. Structure of O/Fe(001)-p(1×1) studied by surface x-ray diffraction. PhRvB 2010, 81, 075428. [Google Scholar] [CrossRef]
- Erley, W.; Ibach, H. Vibrational excitations and structure of oxygen on Fe(110). Solid State Commun. 1981, 37, 937–942. [Google Scholar] [CrossRef]
- Hodgson, A.; Wight, A.; Worthy, G. The kinetics of O2 dissociative chemisorption on Fe(110). Surf. Sci. 1994, 319, 119–130. [Google Scholar] [CrossRef]
- Freindl, K.; Partyka-Jankowska, E.; Karaś, W.; Zając, M.; Madej, E.; Spiridis, N.; Ślęzak, M.; Ślęzak, T.; Wiśnios, D.; Korecki, J. Oxygen on an Fe monolayer on W(110): From chemisorption to oxidation. Surf. Sci. 2013, 617, 183–191. [Google Scholar] [CrossRef]



| /(eV/atom) | |
|---|---|
| Pure γ-Fe | −4.4515 |
| H-containing Fe | −4.4413 |
| N-containing Fe | −4.4741 |
| /(eV) | /(eV/atom) | Mulliken Charge of Atomic Oxygen/(e) | |
|---|---|---|---|
| Pure γ-Fe with atomic oxygen | −6.1312 | −4.7355 | −0.69 |
| H-containing Fe with atomic oxygen | −6.7629 | −4.7242 | −0.66 |
| N-containing Fe with atomic oxygen | −6.6374 | −4.7483 | −0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Liu, Z.; Feng, Q.; Huang, Z.; Zhu, X.; Xiao, L.; He, J.; Wang, N.; Xu, Y. First Principles Study of Atomic Oxygen Adsorption on Austenitic Stainless Steels Surfaces: A Theoretical Study. Coatings 2023, 13, 455. https://doi.org/10.3390/coatings13020455
Zhu X, Liu Z, Feng Q, Huang Z, Zhu X, Xiao L, He J, Wang N, Xu Y. First Principles Study of Atomic Oxygen Adsorption on Austenitic Stainless Steels Surfaces: A Theoretical Study. Coatings. 2023; 13(2):455. https://doi.org/10.3390/coatings13020455
Chicago/Turabian StyleZhu, Xinghua, Zhou Liu, Qingguo Feng, Zhiyong Huang, Xiaoyang Zhu, Lei Xiao, Jianguo He, Ning Wang, and Yi Xu. 2023. "First Principles Study of Atomic Oxygen Adsorption on Austenitic Stainless Steels Surfaces: A Theoretical Study" Coatings 13, no. 2: 455. https://doi.org/10.3390/coatings13020455
APA StyleZhu, X., Liu, Z., Feng, Q., Huang, Z., Zhu, X., Xiao, L., He, J., Wang, N., & Xu, Y. (2023). First Principles Study of Atomic Oxygen Adsorption on Austenitic Stainless Steels Surfaces: A Theoretical Study. Coatings, 13(2), 455. https://doi.org/10.3390/coatings13020455





