Deposition and Characterization of Heterostructures Based on Doped Ferrocene for Film-Device Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Remarks
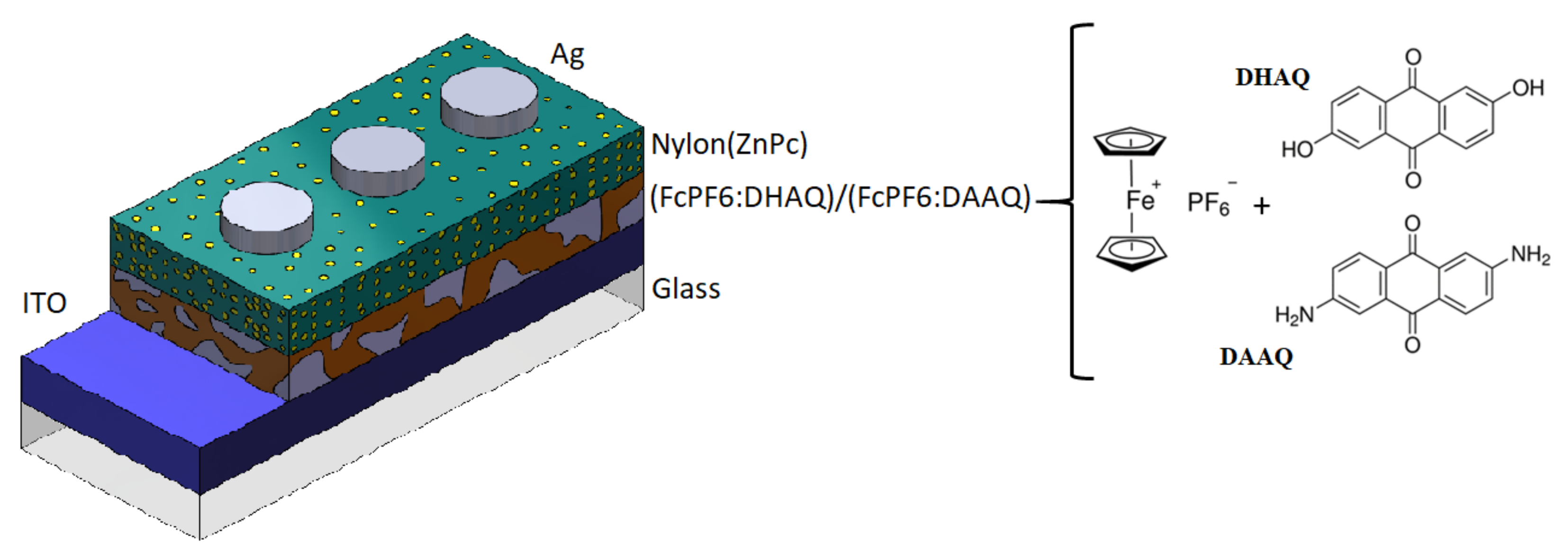
2.2. Heterostructures Assembly and Characterization
3. Results and Discussion
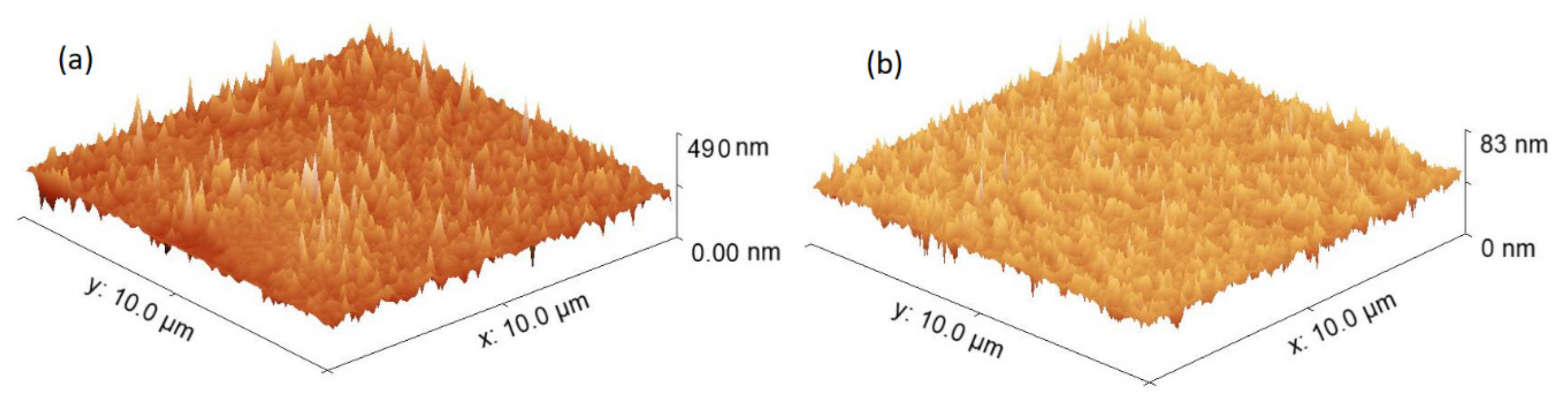
3.1. Morphological and Mechanical Characterization of Ferrocene Heterostructures
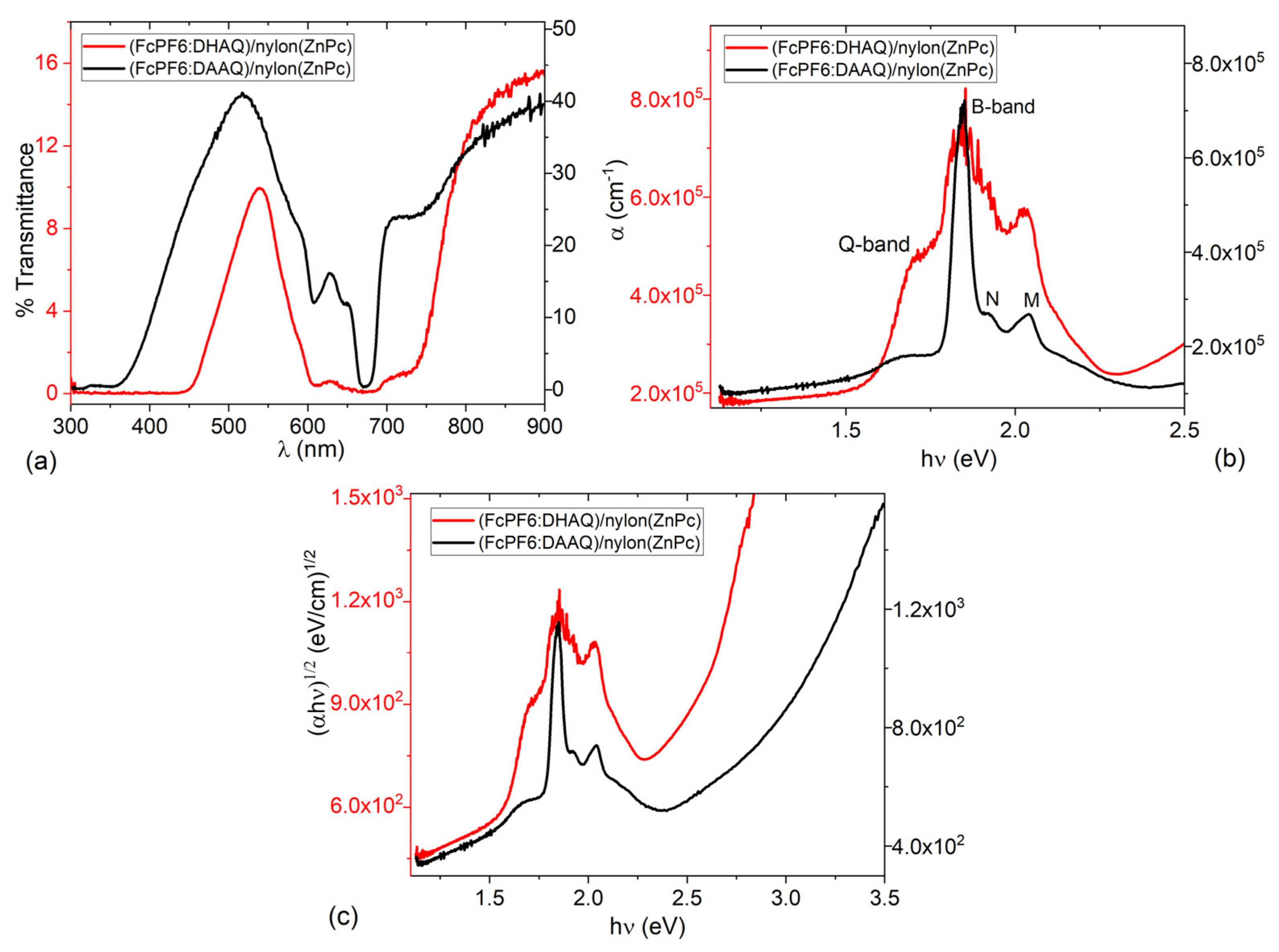
3.2. Evaluation of Optical Properties
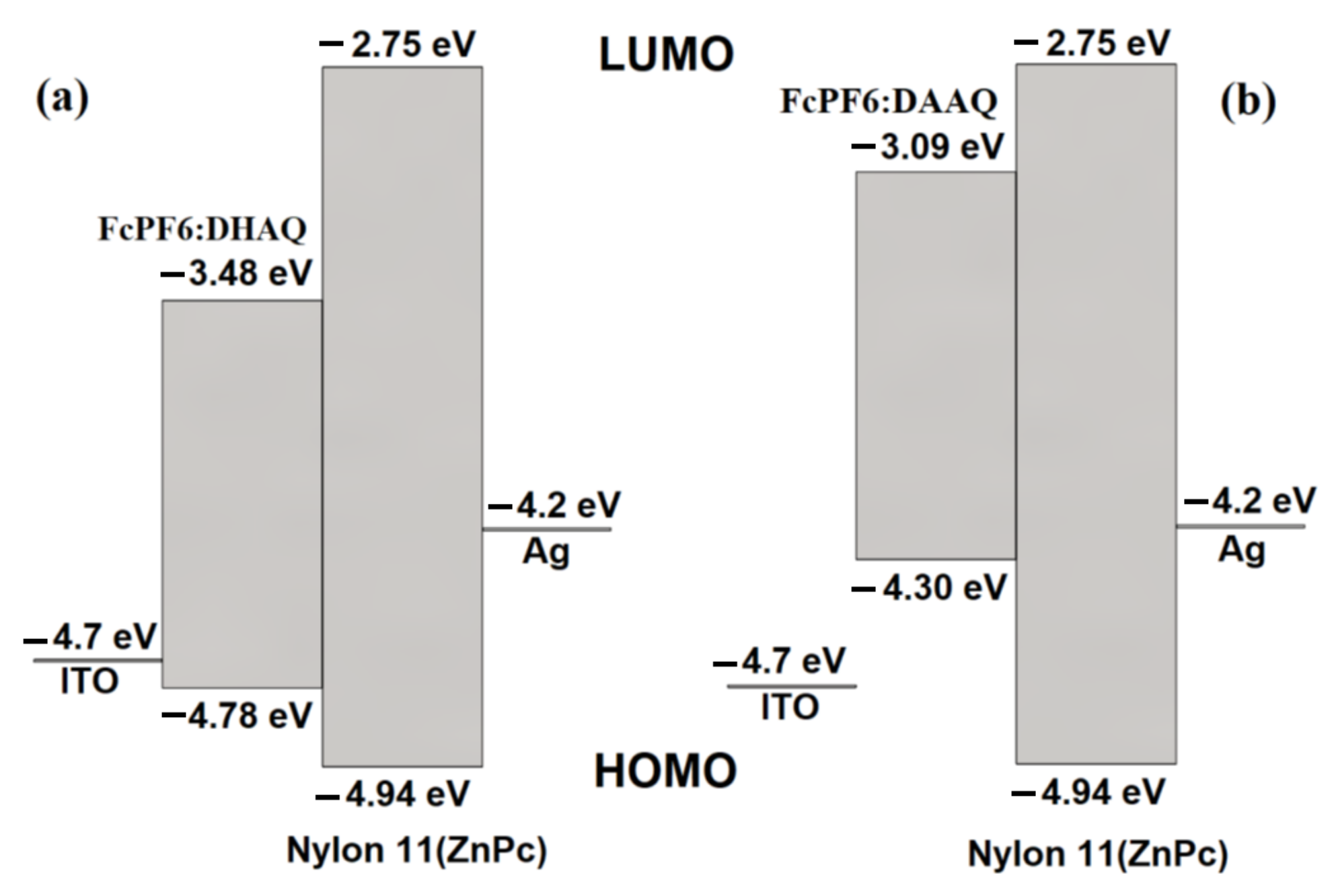
3.3. Fabrication of Thin Film Device and Electrical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peluso, P.; Mamane, V. Ferrocene derivatives with planar chirality and their enantioseparation by liquid-phase techniques. Electrophoresis 2022, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Moss, G.P.; Smith, P.A.S.; Tavernier, D. Glossary of class names of organic compounds and reactivity intermediates based on structure (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67, 1307–1375. [Google Scholar] [CrossRef]
- Astruc, D. Why is ferrocene so exceptional? EurJIC 2017, 2017, 6–29. [Google Scholar] [CrossRef]
- Kealy, T.J.; Pauson, P.L. A new type of organo-iron compound. Nature 1951, 168, 1039–1040. [Google Scholar] [CrossRef]
- Miller, S.A.; Tebboth, J.A.; Tremaine, J.F. 114. Dicyclopentadienyliron. JACS 1952, 632–635. [Google Scholar] [CrossRef]
- Hu, M.-L.; Abbasi-Azad, M.; Habibi, B.; Rouhani, F. Electrochemical Applications of Ferrocene-Based Coordination Polymers. ChemPlusChem 2020, 85, 2397–2418. [Google Scholar] [CrossRef]
- Beitollahi, H.; Khalilzadeh, M.A.; Tajik, S.; Safaei, M.; Zhang, K.; Jang, H.W.; Shokouhimeh, M. Recent Advances in Applications of Voltammetric Sensors Modified with Ferrocene and Its Derivatives. ACS Omega 2022, 5, 2049–2059. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, G.; Rosenblum, M.; Whiting, M.C.; Woodward, R.B. The structure of iron bis-cyclopentadienyl. JACS 1952, 74, 2125–2126. [Google Scholar] [CrossRef]
- Chuhmanov, E.P.; Ermolaev, N.L.; Plakhutin, B.N.; Ignatov, S.K. Tris(perfluoroalkyl)germylethynyl derivatives of biphenyl containing ferrocenyl donor group: Structure, spectra, and photoinduced intramolecular electron transfer. Comput. Theor. Chem. 2018, 1123, 50–60. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on ferrocene-based photoinitiating systems. Eur. Polym. 2021, 147, 110328. [Google Scholar] [CrossRef]
- Grecchi, S.; Arnaboldi, S.; Korb, M.; Cirilli, R.; Araneo, S.; Guglielmi, V. Widening the scope of “inherently chiral” electrodes: Enantiodiscrimination of chiral electroactive probes with planar stereogenicity. ChemElectroChem 2020, 7, 3429–3438. [Google Scholar] [CrossRef]
- Mulas, A.; Willener, Y.; Carr-Smith, J.; Joly, K.M.; Male, L.; Moody, C.J. The effect of central and planar chirality on the electrochemical and chiral sensing properties of ferrocenyl urea H-bonding receptors. Dalton Trans. 2015, 44, 7268–7275. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.; Steerteghem, N.V.; Kaur, N.; Ashar, A.Z.; Kaur, P.; Clays, K.; Narayan, K.S.; Singh, K. Multifunctional geometrical isomers of ferrocene-benzo[1,2-b:4,5-b′] difuran-2,6-(3H,7H)-dione adducts: Second-order nonlinear optical behaviour and charge transport in thin film OFET devices. J. Mater. Chem. C 2017, 5, 697–708. [Google Scholar] [CrossRef]
- Dai, L.-X.; Tu, T.; You, S.-L.; Deng, W.-P.; Hou, X.-L. Asymmetric catalysis with chiral ferrocene ligands. Acc. Chem. Res. 2003, 36, 659–667. [Google Scholar] [CrossRef]
- López, R.; Palomo, C. Planar chirality: A mine for catalysis and structure discovery. Angew. Chem. Int. Ed. 2022, 61, e202113504. [Google Scholar] [CrossRef]
- Gibson, V.C.; Long, N.J.; Oxford, P.J.; White, A.J.P.; Williams, D.J. Ferrocene-substituted bis(imino)pyridine iron and cobalt complexes: Toward redox-active catalysts for the polymerization of ethylene. Organometallics 2006, 25, 1932–1939. [Google Scholar] [CrossRef]
- Qiao, L.; Zhou, X.; Li, X.; Du, W.; Yu, A.; Zhang, S. Synthesis and performance of chiral ferrocene modified silica gel for mixed mode chromatography. Talanta 2017, 163, 94–101. [Google Scholar] [CrossRef]
- Hubbard, P.J.; Benzie, J.W.; Bakhmutov, V.I.; Blümel, J. Ferrocene adsorbed on silica and activated carbon surfaces: A solid-state NMR study of molecular dynamics and surface interactions. Organometallics 2020, 39, 1080–1091. [Google Scholar] [CrossRef]
- Benecke, J.; Grape, E.S.; Fuẞ, A.; Wöhlbrandt, S.; Engesser, T.A.; Inge, A.K. Polymorphous indium metal–organic frameworks based on a ferrocene linker: Redox activity, porosity, and structural diversity. Inorg. Chem. 2020, 59, 9969–9978. [Google Scholar] [CrossRef]
- Singh, A.; Lumb, I.; Mehra, V.; Kumar, V. Ferrocene-appended pharmacophores: An exciting approach for modulating the biological potential of organic scaffolds. Dalton Trans. 2019, 48, 2840–2860. [Google Scholar] [CrossRef]
- Urbano, A.; Del Hoyo, A.M.; Martinez-Carrion, A.; Carreno, M.C. Asymmetric synthesis and chiroptical properties of enantiopure helical ferrocenes. Org. Lett. 2019, 21, 4623–4627. [Google Scholar] [CrossRef] [PubMed]
- Ravutsov, M.; Dobrikov, G.M.; Dangalov, M.; Nikolova, R.; Dimitrov, V.; Mazzeo, G. 1,2-Disubstituted planar chiral ferrocene derivatives from sulfonamide-directed ortho-lithiation: Synthesis, absolute configuration, and chiroptical properties. Organometallics 2021, 40, 578–590. [Google Scholar] [CrossRef]
- Mazzeo, G.; Pedotti, S.; Longhi, G.; Patti, A.; Abbate, S. Spectroscopic investigation on 1,2-substituted ferrocenes with only planar chirality: How chiroptical data are related to absolute configuration and to substituents. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 272, 121010. [Google Scholar] [CrossRef] [PubMed]
- Moriuchi, T. Helical chirality of ferrocene moieties in cyclic ferrocene-peptide conjugates. Eur. J. Inorg. 2022, 2022, e202100902. [Google Scholar] [CrossRef]
- Kovač, V.; Kodrin, I.; Radošević, K.; Molčanov, K.; Adhikari, B.; Kraatz, H.-B. Oxalamide-bridged ferrocenes: Conformational and gelation properties and in vitro antitumor activity. Organometallics 2022, 41, 920–936. [Google Scholar] [CrossRef]
- Sánchez Vergara, M.E.; Vincent, M.; Rios, C.; Salcedo, R. Doping of Molecular Materials based on Ferrocene and the Study of their Properties as Organic Semiconductors for their Application in Optoelectronic Devices. J. Mol. Struct. 2019, 1193, 365–372. [Google Scholar] [CrossRef]
- Sánchez Vergara, M.E.; Hamui, L.; Barcenas Hernandez, M.F.; Rios, C.; Salcedo, R. Combined experimental and theoretical study of conjugated ferrocene semiconductors and the effect of doping on their opto-electrical and structural properties. J. Mol. Struct. 2022, 1262, 132998. [Google Scholar] [CrossRef]
- Nar, I.; Atsay, A.; Altindal, A.; Hamuryudan, E. o-Carborane, Ferrocene, and Phthalocyanine Triad for High-Mobility Organic Field-Effect Transistors. Inorg. Chem. 2018, 57, 2199–2208. [Google Scholar] [CrossRef]
- Akutsu, H.; Hashimoto, R.; Yamada, J.-I.; Nakatsuji, S.; Turner, S.S.; Nakazawa, Y. Structure and Properties of a BEDT-TTF-Based Organic Conductor with a Ferrocene-Based Magnetic Anion Octamethylferrocenedisulfonate. Eur. J. Inorg. Chem. 2018, 27, 3249–3252. [Google Scholar] [CrossRef] [Green Version]
- Rui, H.; Nanfang, J.; Guofeng, T.; Shengli, Q.; Lei, S.; Xiaodong, W.; Dezhen, W. Flash memory effects and devices based on functional polyimides bearing pendent ferrocene group. Mater. Des. 2018, 139, 298–303. [Google Scholar] [CrossRef]
- Zhai, Z.; Xu, M. All-solution-processed small-molecule solar cells by stripping-transfer method. JMS Mater. Electron. 2020, 31, 5789–5793. [Google Scholar] [CrossRef]
- Heeger, A.J. 25th Anniversary Article: Bulk Heterojunction Solar Cells: Understanding the Mechanism of Operation. Adv. Mater. 2014, 26, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Socol, M.; Preda, N.; Rasoga, O.; Breazu, C.; Stavarache, I.; Stanculescu, F.; Socol, G.; Gherendi, F.; Grumezescu, V.; Popescu-Pelin, G.; et al. Flexible heterostructures based on metal phthalocyanines thin films obtained by MAPLE. Appl. Surf. Sci. 2016, 374, 403–410. [Google Scholar] [CrossRef]
- Pfuetzner, S.; Meiss, J.; Petrich, A.; Riede, M.; Leo, K. Thick C60: ZnPc bulk heterojunction solar cells with improved performance by film deposition on heated substrates. Appl. Phys. Lett. 2009, 94, 253303. [Google Scholar] [CrossRef]
- Zeyada, H.M.; El-Nahass, M.M.; El-Menyawy, E.M.; El-Sawah, A.S. Electrical and photovoltaic characteristics of indium phthalocyanine chloride/p-Si solar cell. Synth. Met. 2015, 207, 46–53. [Google Scholar] [CrossRef]
- Noguchi, T.; Gotoh, K.; Yamaguchi, Y. Novel method to disperse ultrafine metal particles into polymer. J. Mater. Sci. Lett. 1991, 10, 477–479. [Google Scholar] [CrossRef]
- Akamatsu, K.; Deki, S. Characterization and optical properties of gold nanoparticles dispersed in nylon 11 thin films. J. Mater. Chem. 1997, 7, 1773–1777. [Google Scholar] [CrossRef]
- Catania, F.; Souza Olivera, H.; Lugoda, P.; Catarella, G.; Munzenrieder, N. Thin-film electronics on active substrates: Review of materials, technologies, and applications. Phys. D Appl. Phys. 2022, 55, 323002. [Google Scholar] [CrossRef]
- Zhu, L.; He, G.; Lv, J.; Fortunato, E.; Martins, R. Fully solution-induced high performance indium oxide thin film transistors with ZrOx high-k gate dielectrics. RSC Adv. 2018, 8, 16788–16799. [Google Scholar] [CrossRef] [Green Version]
- Aslan, H.; Kurtay, G.; Korkmaz, A.; Çetin, A.; Öktemer, A. Combined experimental and theoretical study of the novel ferrocene, derivatives and examining the effect of E-Z steroisomerism on their optical properties. Opt. Mater. 2019, 89, 452–459. [Google Scholar] [CrossRef]
- Lv, X.; Huang, C.; Tameev, A.; Qian, L.; Zhu, R.; Katin, K.; Maslov, M.; Nekrasov, A.; Zhang, C. Electrochemical polymerization process and excellent electrochromic properties of ferrocene-functionalized polytriphenylamine derivative. Dyes Pig. 2019, 163, 433–440. [Google Scholar] [CrossRef]
- Yuan, Y.; Yan, J.-F.; Lin, D.-Q.; Mao, B.-W.; Yuan, Y.-F. Ferrocene-alkynyl conjugated molecular wires: Synthesis, characterization, and conductance properties. Chem. Eur. J. 2018, 24, 3545–3555. [Google Scholar] [CrossRef]
- Xiang, J.; Li, X.; Ma, Y.; Zhao, Q.; Ho, C.-L.; Wong, W.-Y. Efficient flash memory devices based on non-conjugated ferrocene-containing copolymers. J. Mater. Chem. C 2018, 6, 11348–11355. [Google Scholar] [CrossRef]
- Kimura, Y.; Mashiyama, Y.; Maruyama, H.; Kawabata, Y.; Kijima, T.; Fujimori, A. Characterization of Molecular Arrangement of Long-chain Ferrocenyl Derivatives Having Asymmetric Carbon by Method of Organized Molecular Films and Formation of Its Helical Nanofibers. Chem. Sel. 2021, 6, 2198–2209. [Google Scholar] [CrossRef]
- Alharbi, S.R.; Darwish, A.A.A.; Al Garni, S.E.; El Saeedy, H.I.; Abd El-Rahman, K.F. Influence of thickness and annealing on linear and nonlinear optical properties of manganese (III) chloride tetraphenyl porphine (MnTPPCl) organic thin films. Inf. Phy. Technol. 2016, 78, 77–83. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Ammar, A.H.; Farag, A.A.M.; Atta, A.A.; El-Zaida, E.F.M. Effect of heat treatment on morphological, structural and optical properties of CoMTPP thin films. Solid State Sci. 2011, 13, 596. [Google Scholar] [CrossRef]
- Mane, R.K.; Ajalkar, B.D.; Bhosale, P.N. Electrical and optical properties of bismuth sulphotelluride [Bi2(S1−xTex)3] thin films prepared by arrested precipitation technique (APT). Mater. Chem. Phys. 2003, 82, 534–537. [Google Scholar] [CrossRef]
- Pal, U.; Samanta, D.; Ghori, S.; Chaudhuri, A.K.J. Optical Constants of Vacuum-Evaporated Polycrystalline Cadmium Selenide Thin Films. Appl. Phys. 1993, 74, 6368. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; El-Deeb, A.F.; Metwally, H.S.; Hassanien, A.M. Influence of annealing on the optical properties of 5,10,15,20-tetraphenyl-21H, 23H-porphine iron (III) chloride thin films. Mater. Chem. Phys. 2011, 125, 247–251. [Google Scholar] [CrossRef]
- Tsiper, E.V.; Soos, Z.G.; Gao, W.; Kahn, A. Charge redistribution and polarization energy of organic molecular crystals. Chem. Phys. Lett. 2002, 360, 47. [Google Scholar] [CrossRef]
- Zhokhavets, U.; Goldhahn, R.; Gobsch, G.; Schliefke, W. Dielectric function and one-dimensional description of the absorption of poly(3-octylthiophene). Synth. Met. 2003, 138, 491. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, X.; Tian, B.; Qi, Y.; Lai, X.; Jin, L. Synthesis, electrochemical and spectroelectrochemical properties of carbazole derivatives with ferrocene groups. J. Electroanal. Chem. 2017, 788, 29–37. [Google Scholar] [CrossRef]
- Scharber, M.C.; Sariciftci, N.S. Low Band Gap Conjugated Semiconducting Polymers. Adv. Mater. Technol. 2021, 6, 2000857. [Google Scholar] [CrossRef]
- Farag, A.A.M.; Sawsan, M.S.H.; Mohamed, E.M. Spectral-optical-electrical-thermal properties of deposited thin films of nano sized calcium (II)-8 hidroxi-5,7-dinitroquinolate complex. Spectrochim. Acta Mol. Biomol. Spectrosc. 2011, 82, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Cahen, D.; Kahn, A.; Umbach, E. Energies of molecular interfaces. Mater. Today 2005, 8, 32–41. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Atta, A.A.; El-Sayed, H.E.A.; El-Zaidia, E.F.M. Structural and optical properties of thermal evaporated magnesium phthalocyanine (MgPc) thin films. Appl. Surf. Sci. 2008, 254, 2458–2465. [Google Scholar] [CrossRef]
- Hassan, A.K.; Gouldt, R.D. The interpretation of current density-voltage and activation energy measurements on freshly prepared and heat-treated nickel phthalocyanine thin films. Int. J. Electron. 1993, 74, 59–65. [Google Scholar] [CrossRef]
- Sánchez-Vergara, M.E.; Medrano-Gallardo, D.; Vera-Estrada, I.L.; Jiménez-Sandoval, O. Optical absorption and electrical properties of MPc (M=Fe, Cu, Zn)-TCNQ interfaces for optoelectronic applications. J. Phys. Chem. Solids 2018, 115, 373–380. [Google Scholar] [CrossRef]
- Dongol, M.; El-Nahass, M.M.; El-Denglawey, A.; Abuelwafa, A.A.; Soga, T. Alternating current characterization of nano-Pt(II) octaethylporphyrin (PtOEP) thin film as a new organic semiconductor. Chin. Phys. B 2016, 25, 067201. [Google Scholar] [CrossRef]
- Dumm, M.; Lunkenheimer, P.; Loid, A.; Assmann, B.; Homborg, H.; Fulde, P. Charge transport in lithium phthalocyanine. J. Chem. Phys. 1996, 104, 5048–5053. [Google Scholar] [CrossRef]
- Gabriel, C.; Gabriel, S.; Grant, E.H.; Halstead, B.S.J.; Michael, D.; Mingos, P. Dielectric parameters relevant to microwave dielectric heating. Chem. Soc. Rev. 1998, 27, 213–224. [Google Scholar] [CrossRef]
- Simon, J.; Andrè, J.J.; Skoulios, A. Molecular materials. I: Generalities. New J. Chem. 1986, 10, 295–311. [Google Scholar]
- Simon, J.; Tournillac, F. Molecular materials. II: Towards electronics finalities. New J. Chem. 1997, 11, 383–399. [Google Scholar]
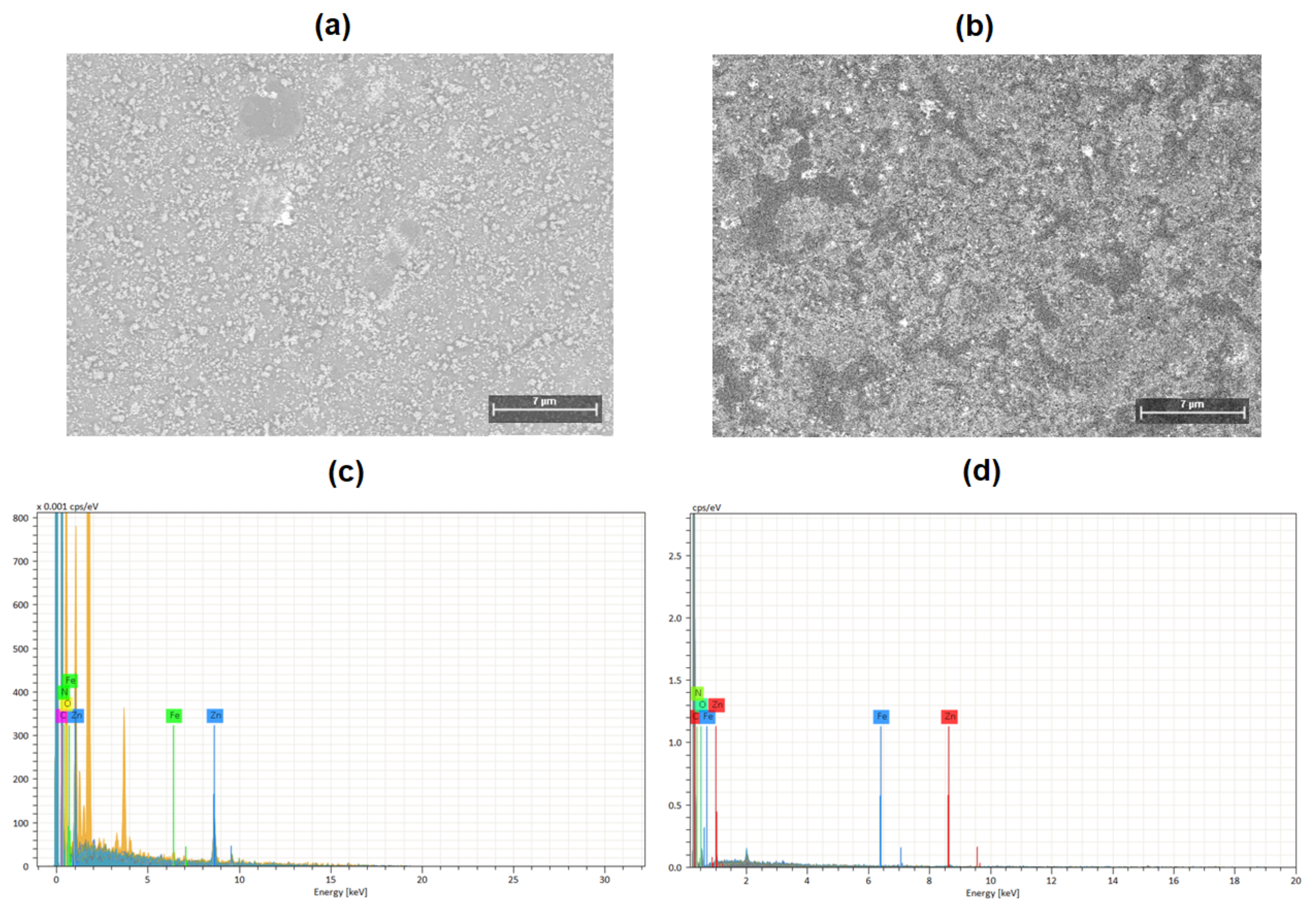

| Sample | C (%) | N (%) | O (%) | Fe (%) | Zn (%) |
|---|---|---|---|---|---|
| FcPF6:DHAQ/nylon(ZnPc) | 67.48 | 10.84 | 13.7 | 0.16 | 7.82 |
| FcPF6:DAAQ/nylon(ZnPc) | 71.45 | 18.03 | 6.75 | 0.03 | 3.73 |
| Sample | RMS (nm) | Ra (nm) | Area (m2) | σmax (Pa) | ε | HK |
|---|---|---|---|---|---|---|
| FcPF6:DHAQ/nylon(ZnPc) | 11.34 | 8.72 | 6.01 × 10−6 | 1.65 × 108 | 0.776 | 0.59 |
| FcPF6:DAAQ/nylon(ZnPc) | 3.83 | 2.83 | 6.12 × 10−6 | 1.62 × 108 | 0.776 | 0.58 |
| Sample | Eg (eV) | Et (eV) | EB (eV) |
|---|---|---|---|
| FcPF6:DHAQ/nylon(ZnPc) | 1.55 | 2.45 | 0.9 |
| FcPF6:DAAQ/nylon(ZnPc) | 1.76 | 2.57 | 0.81 |
| Electrical Parameter | Value |
|---|---|
| μ | 9.27 × 10−8 cm2/Vs |
| p0 | 3.01 × 1023 m−3 |
| Nv | 1.83 × 1025 m−3 |
| P0 | 2.0 × 1040 1/(J m3) |
| 3.93 × 1021 m−3 | |
| σ at 0.1 V | 1.01 × 102 Scm−1 |
| σ at 0.5 V | 8.45 × 101 Scm−1 |
| σ at 1.0 V | 6.78 × 101 Scm−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez Vergara, M.E.; Toledo Dircio, E.; Zubillaga Serrano, R.I. Deposition and Characterization of Heterostructures Based on Doped Ferrocene for Film-Device Applications. Coatings 2022, 12, 1859. https://doi.org/10.3390/coatings12121859
Sánchez Vergara ME, Toledo Dircio E, Zubillaga Serrano RI. Deposition and Characterization of Heterostructures Based on Doped Ferrocene for Film-Device Applications. Coatings. 2022; 12(12):1859. https://doi.org/10.3390/coatings12121859
Chicago/Turabian StyleSánchez Vergara, María Elena, Emiliano Toledo Dircio, and Rafael Imanol Zubillaga Serrano. 2022. "Deposition and Characterization of Heterostructures Based on Doped Ferrocene for Film-Device Applications" Coatings 12, no. 12: 1859. https://doi.org/10.3390/coatings12121859
APA StyleSánchez Vergara, M. E., Toledo Dircio, E., & Zubillaga Serrano, R. I. (2022). Deposition and Characterization of Heterostructures Based on Doped Ferrocene for Film-Device Applications. Coatings, 12(12), 1859. https://doi.org/10.3390/coatings12121859







