Effect of Coating Process on Properties of Two-Component Waterborne Polyurethane Coatings for Wood
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.2.1. Preparation of the Two-Component Polyurethane Wood Coating
- (1)
- Preparation of secondary dispersion of acrylic resin
- (2)
- Mixing of dispersion and curing agent
2.2.2. Coating Amount Tests
- (1)
- Coating amount scheme
- (2)
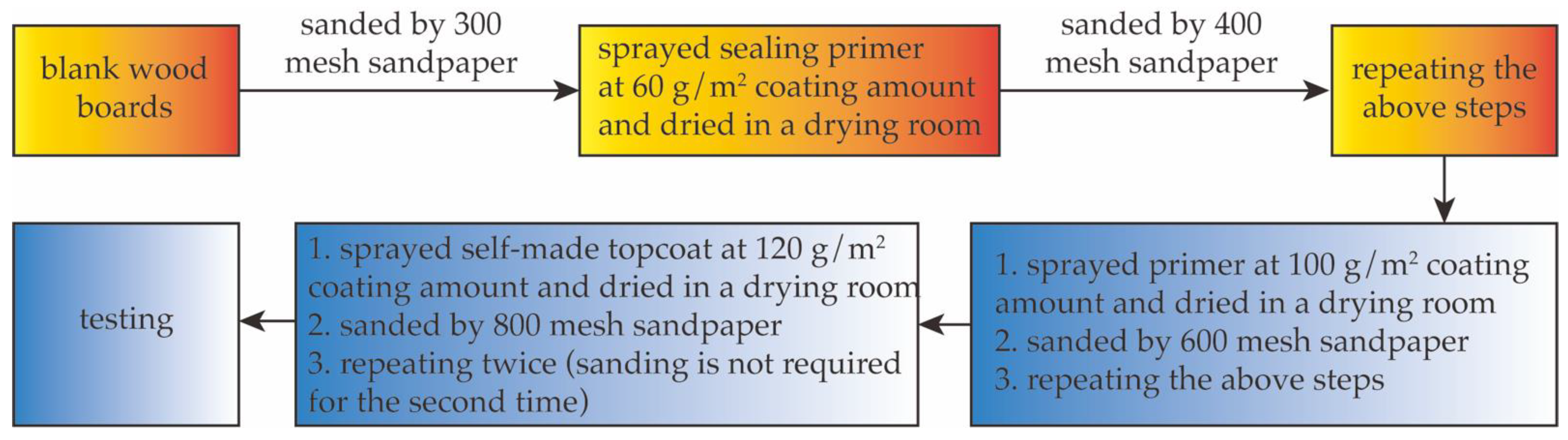
- Coating steps
2.2.3. Test of Spraying Times
2.2.4. Testing and Characterization
3. Results and Discussion
3.1. Characterization of the Hydroxyl-Type Waterborne Acrylic Acid Dispersion
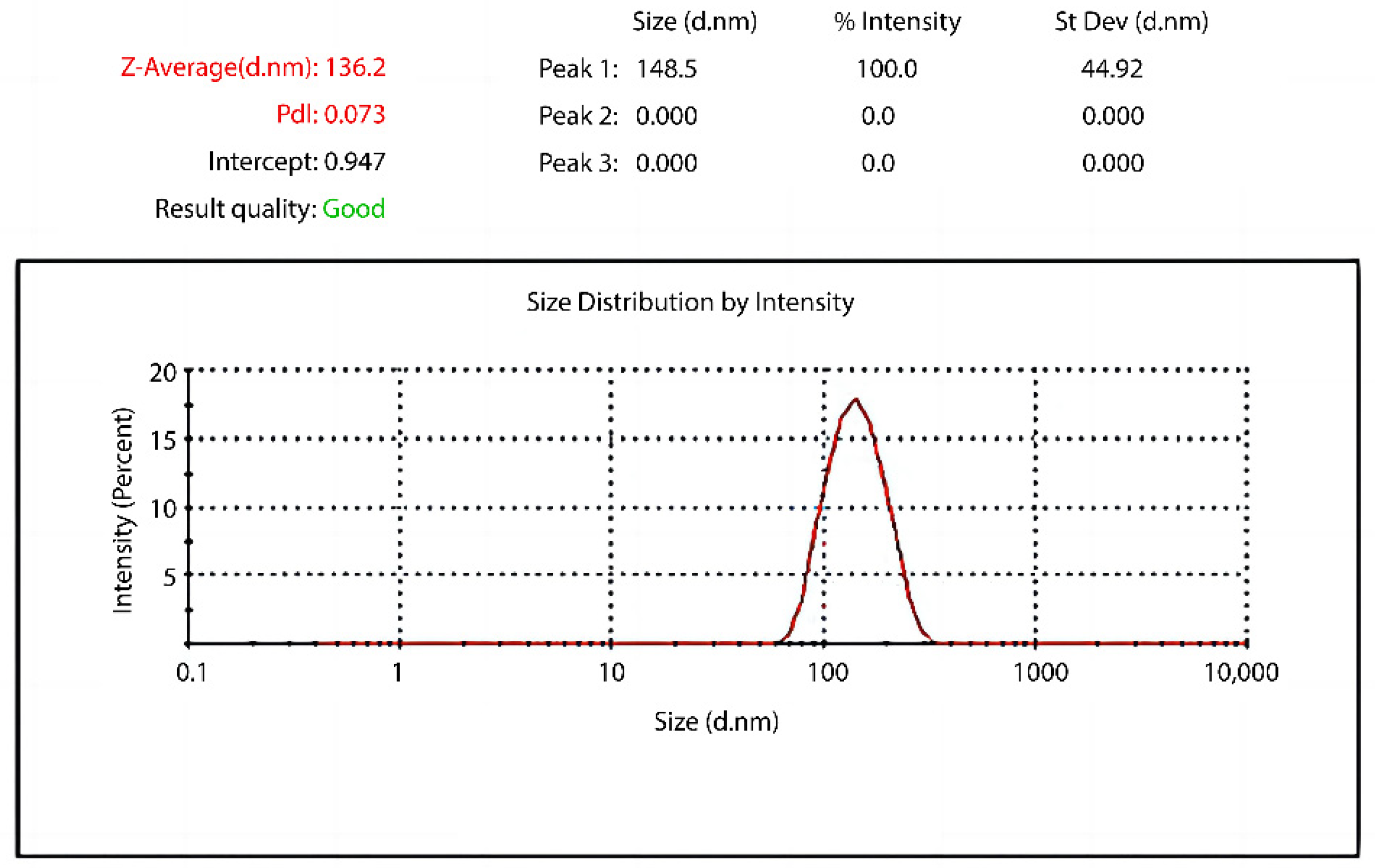
3.1.1. Particle Size Analysis of Hydroxyl-Type Waterborne Acrylic Acid Dispersion
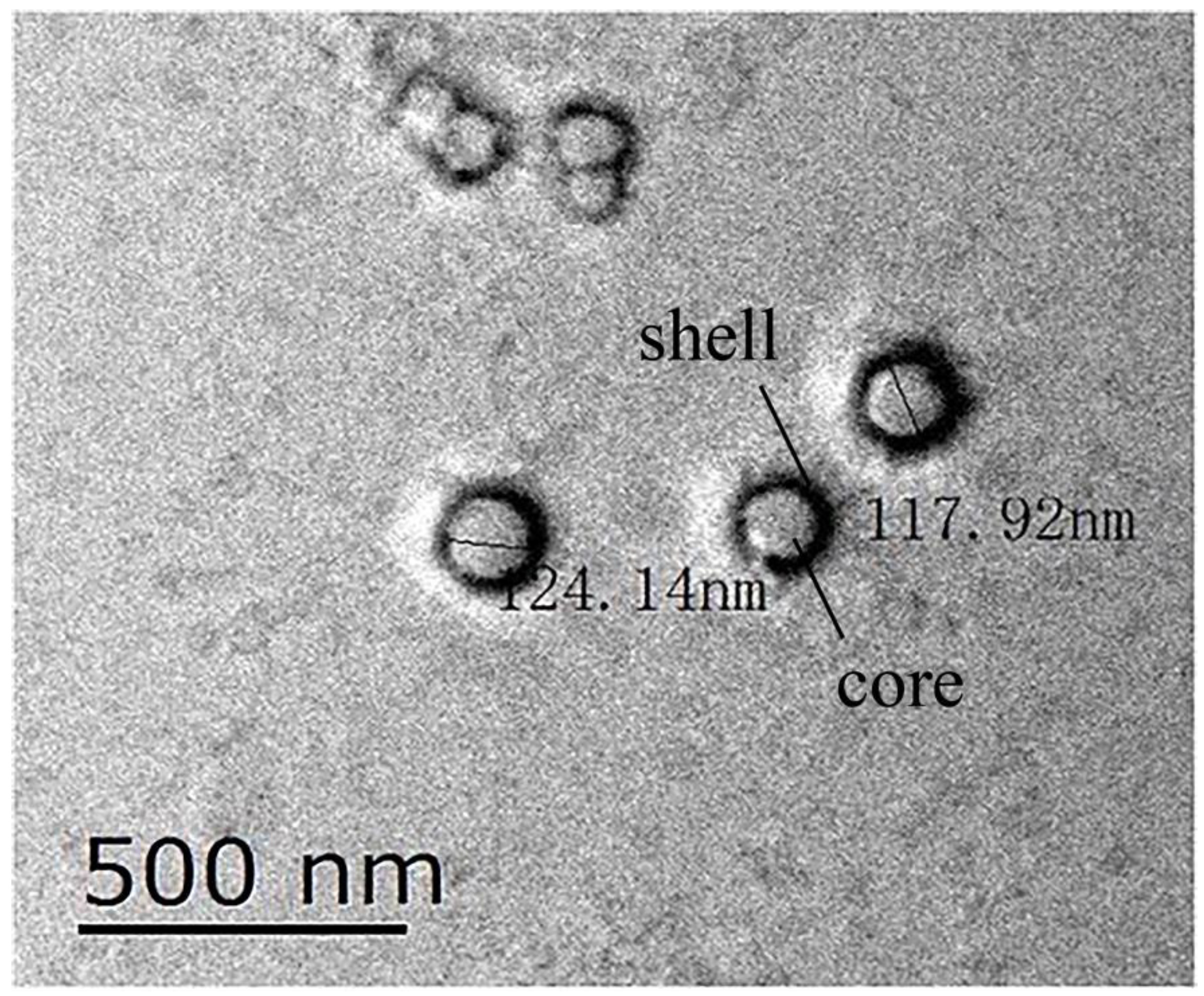
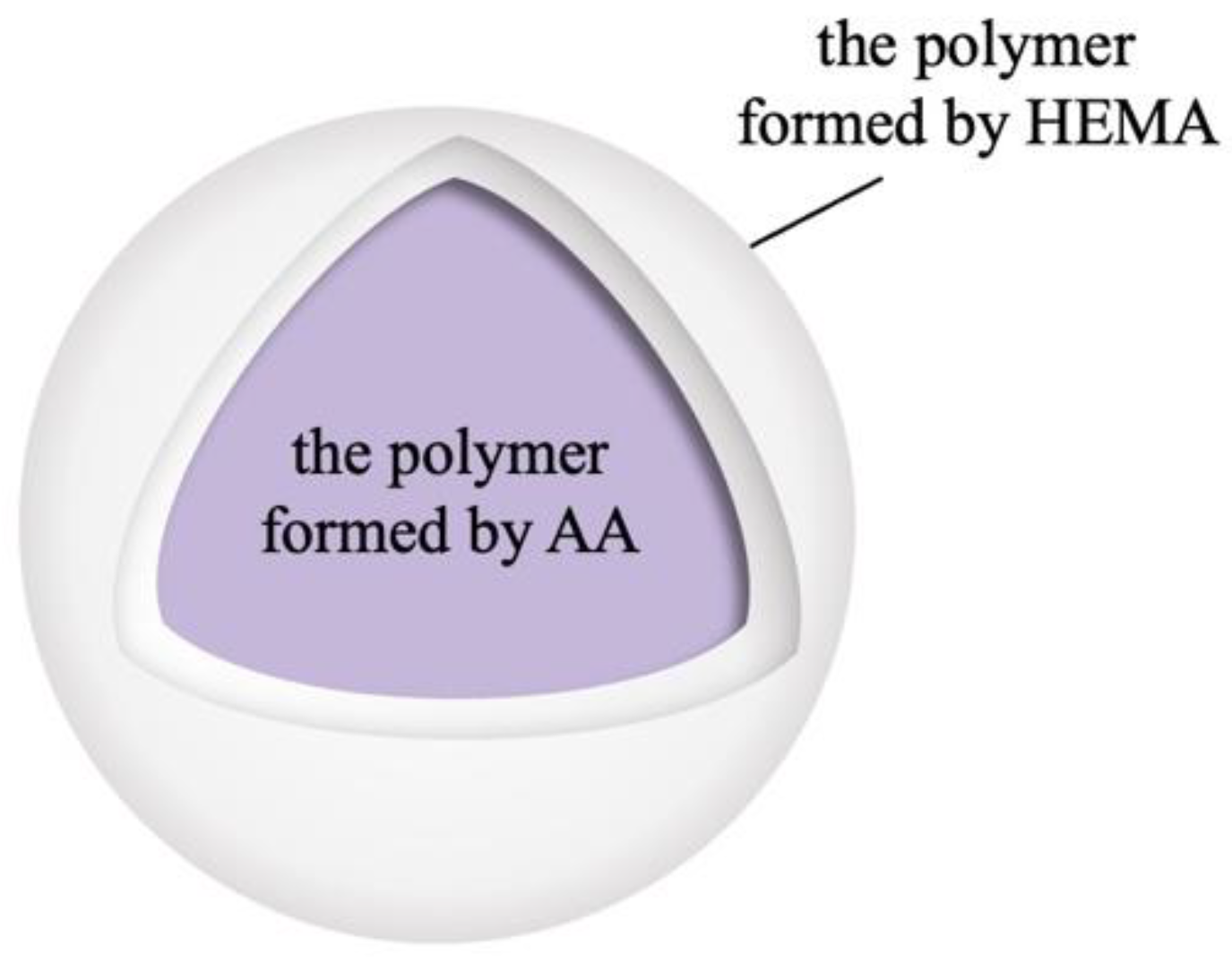
3.1.2. Transmission Electron Microscopy Examination of the Hydroxyl-Type Waterborne Acrylic Acid Dispersion
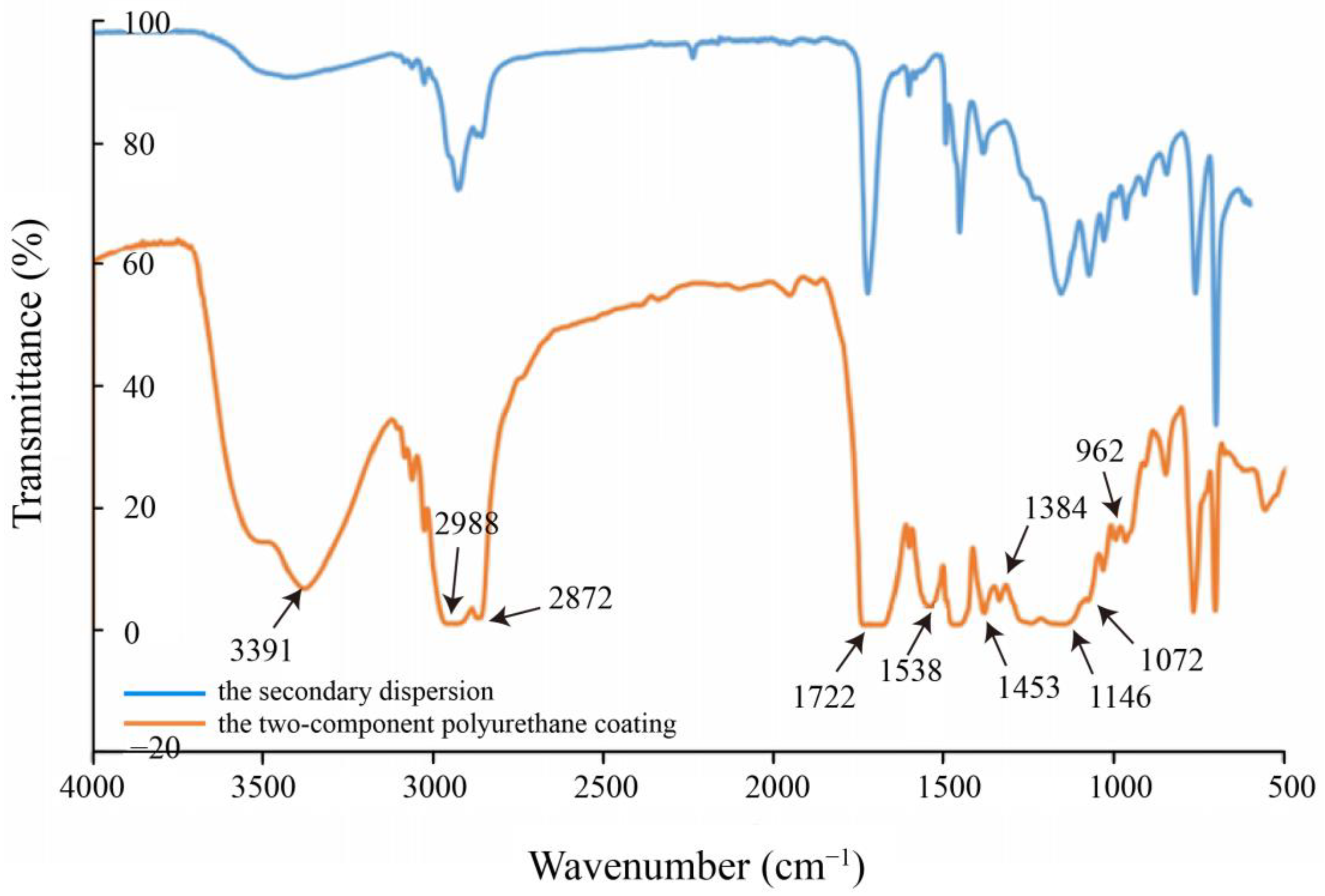
3.1.3. Infrared Spectrum Analysis of Hydroxyl-Type Waterborne Acrylic Acid Dispersion
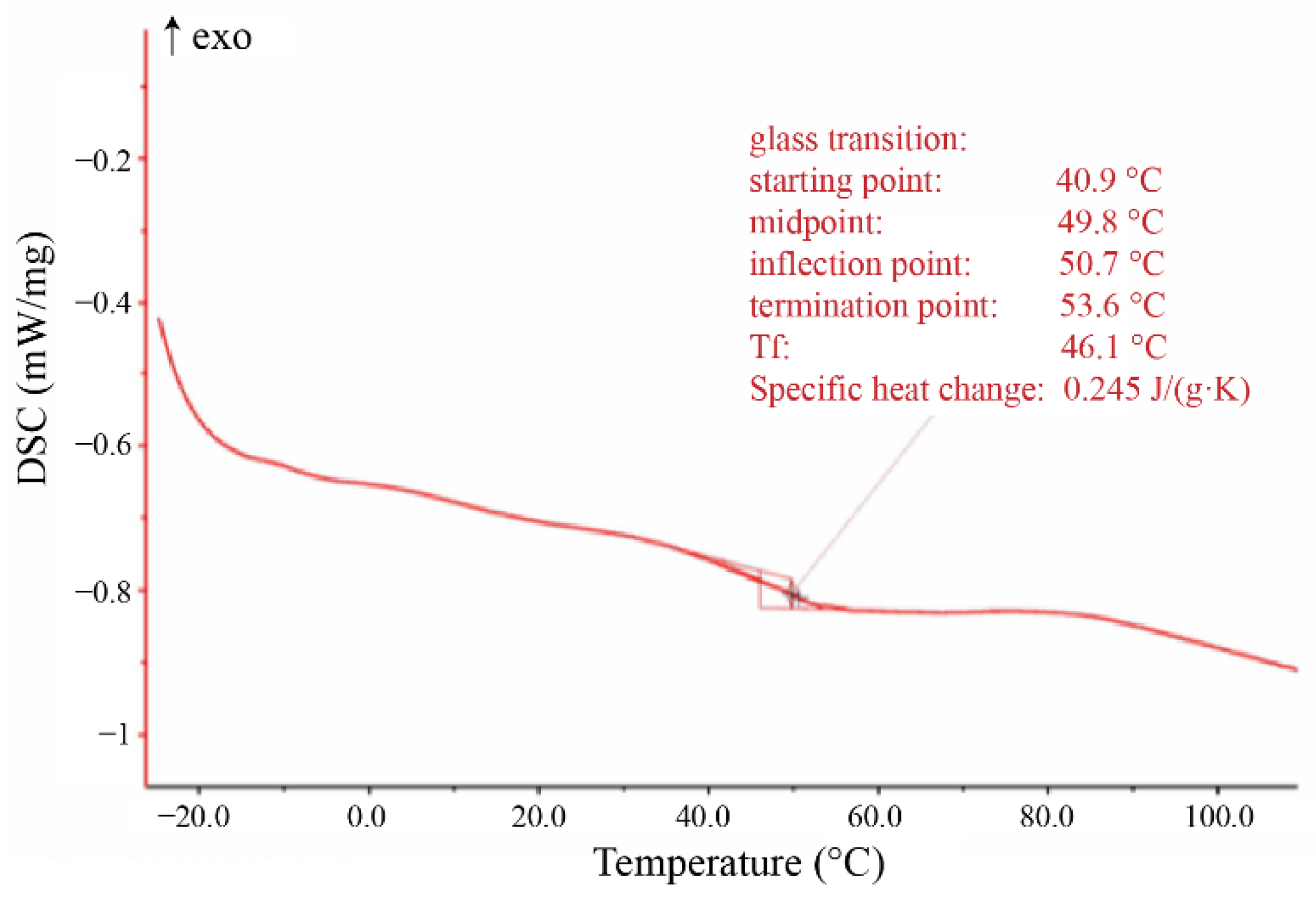
3.1.4. Differential Thermal Analysis of Hydroxyl-Type Waterborne Acrylic Acid Dispersion
3.2. Influence of Different Coating Amount on the Film’s Performance
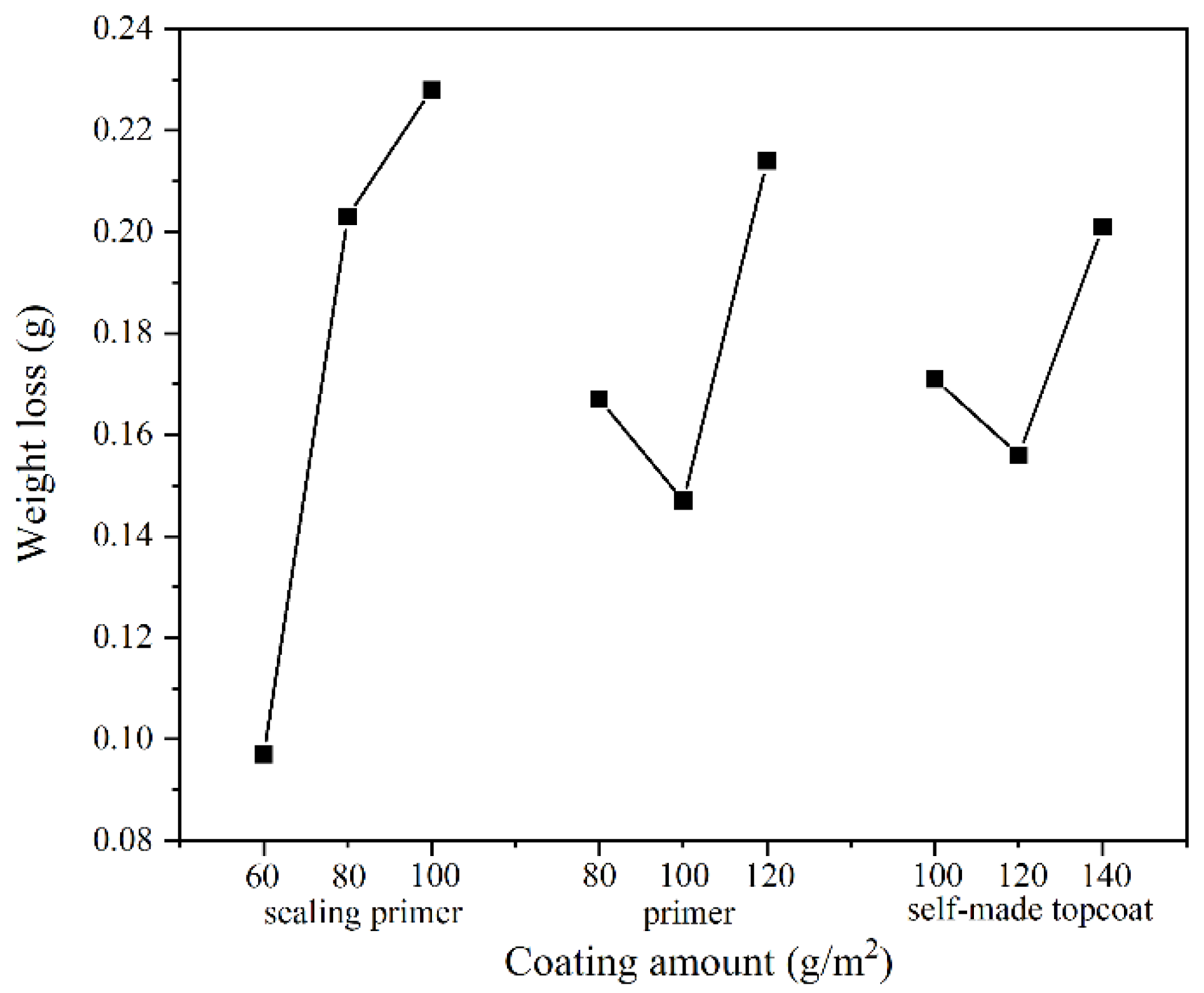
3.2.1. Wear Resistance Analysis
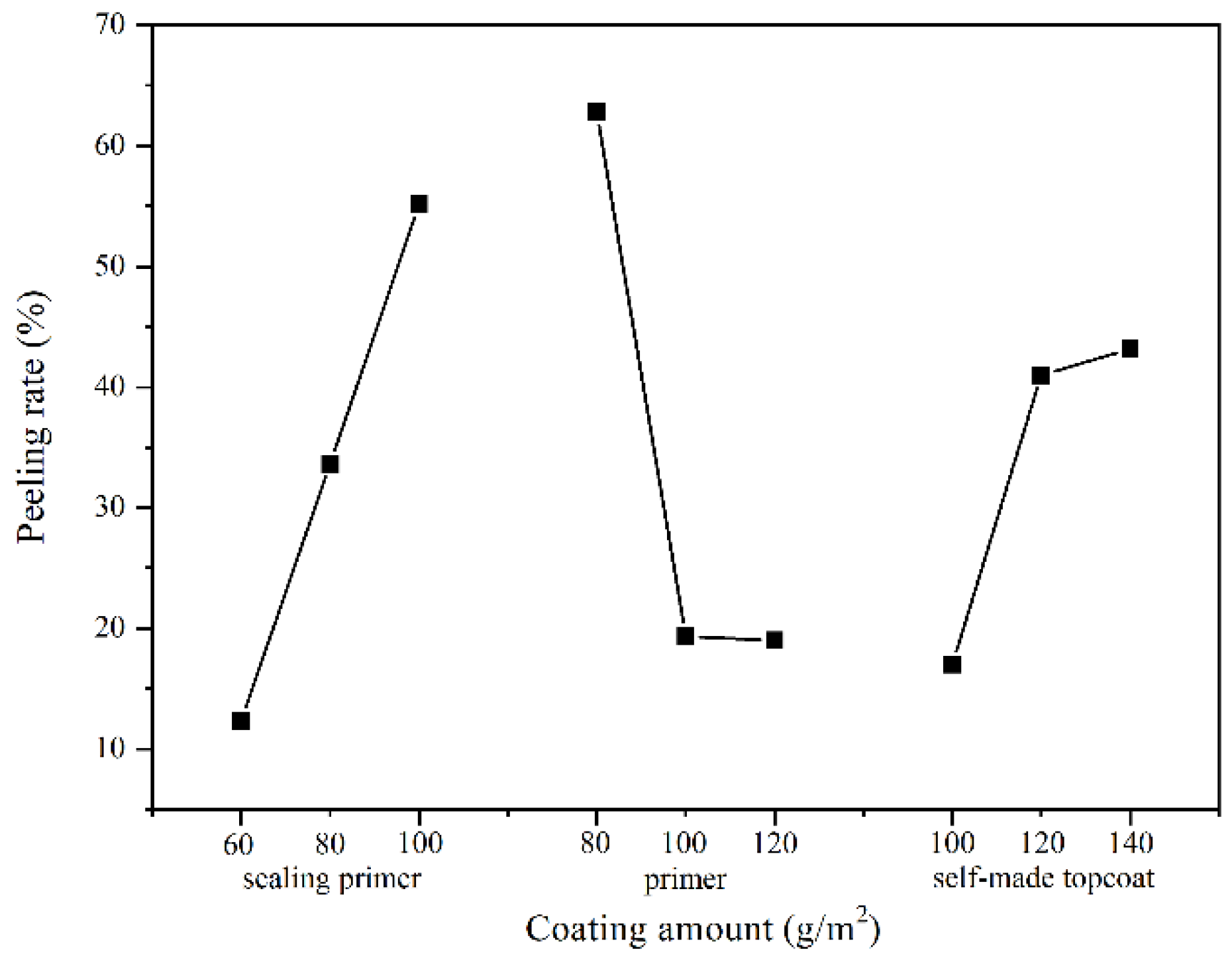
3.2.2. Adhesion Analysis
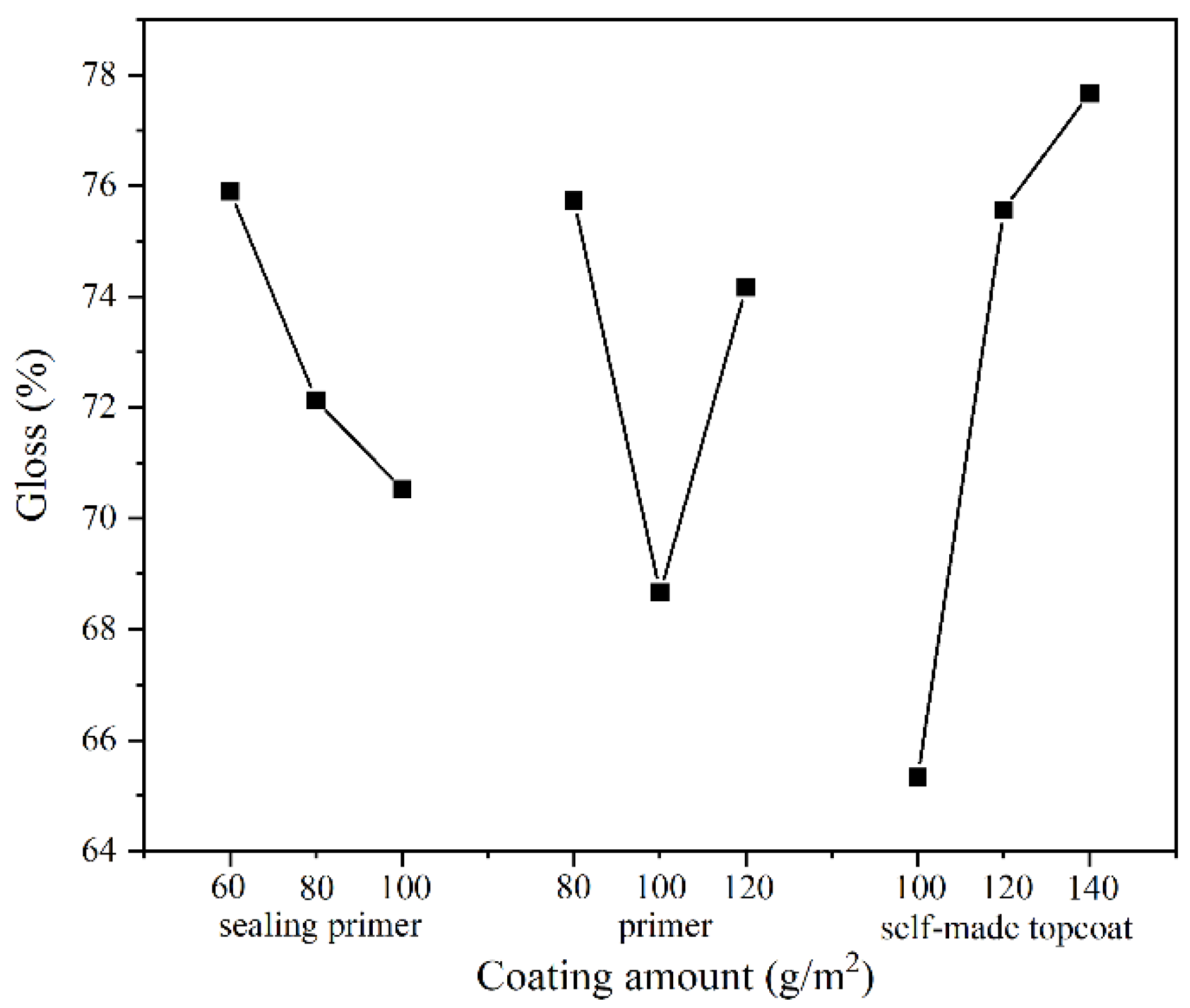
3.2.3. Gloss Analysis
3.3. Process Analysis for the Optimal Coating Thickness
3.4. Effect of Spraying Times on the Performance of Waterborne Two-Component Polyurethane Coating
3.4.1. Adhesion
3.4.2. Gloss
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, Y.; Yan, X. Effect of the Addition of Shellac Self-Healing and Discoloration Microcapsules on the Performance of Coatings Applied on Ebiara Solid Board. Coatings 2022, 12, 1627. [Google Scholar] [CrossRef]
- Yang, Z.; Han, Y.; Peng, W.; Wang, L.; Yan, X. Effect of Fluorane Microcapsule Content on Properties of Thermochroic Waterborne Topcoat on Tilia europaea. Polymers 2022, 14, 3638. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yan, X.; Peng, W. Preparation of Cellulose Modified Wall Material Microcapsules and Its Effect on the Properties of Wood Paint Coating. Polymers 2022, 14, 3534. [Google Scholar] [CrossRef]
- Pan, P.; Yan, X.; Zhao, W. Effect of Coating Process of Photochromic and Thermochromic Composite Microcapsules on Coating Properties for Basswood. Coatings 2022, 12, 1246. [Google Scholar] [CrossRef]
- Huang, N.; Yan, X.; Han, Y. Preparation of Melamine-Formaldehyde Resin/Rice Husk Powder Coated Epoxy Resin Microcapsules and Effects of Different Microcapsule Contents on the Properties of Waterborne Coatings on Tilia europaea Surface. Coatings 2022, 12, 1213. [Google Scholar] [CrossRef]
- Han, Y.; Yan, X.; Tao, Y. Effect of Transparent, Purple, and Yellow Shellac Microcapsules on Properties of the Coating on Paraberlinia bifoliolata Surface. Polymers 2022, 14, 3304. [Google Scholar] [CrossRef]
- Panda, S.S.; Panda, B.P.; Nayak, S.K.; Mohanty, S. A Review on Waterborne Thermosetting Polyurethane Coatings Based on Castor Oil: Synthesis, Characterization, and Application. Polym. Plast. Technol. Mater. 2018, 57, 500–522. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Xu, L.L.; Zhang, Q.; Gu, Y. Preparation and Corrosion Resistance of gamma-aminopropyltriethoxysilane-TiO2-GO/Waterborne Polyurethane Coating. Int. J. Electrochem. Sci. 2020, 15, 11340–11355. [Google Scholar] [CrossRef]
- Gunesoglu, S.; Cerci, E.; Topalbekiroglu, M. The improved breathability of polyurethane coated cotton fabric via micro-cracking. J. Text. I. 2017, 108, 1815–1821. [Google Scholar] [CrossRef]
- Garcia-Pacios, V.; Jofre-Reche, J.A.; Costa, V.; Colera, M.; Martin-Martinez, J.M. Coatings prepared from waterborne polyurethane dispersions obtained with polycarbonates of 1,6-hexanediol of different molecular weights. Prog. Org. Coat. 2013, 76, 1484–1493. [Google Scholar] [CrossRef]
- Athawale, V.D.; Nimbalkar, R.V. Emulsifyable air drying urethane alkyds. Prog. Org. Coat. 2010, 67, 66–71. [Google Scholar] [CrossRef]
- Yin, X.; Li, X.Y.; Luo, Y.J. Synthesis and Characterization of Multifunctional Two-Component Waterborne Polyurethane Coatings: Fluorescence, Thermostability and Flame Retardancy. Polymers 2017, 9, 492. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.W.; Lu, Q.L.; Zhu, S.; Pang, R.B.; Shan, W.W. Effect of resins on the salt spray resistance and wet adhesion of two component waterborne polyurethane coating. E-Polym. 2019, 19, 444–452. [Google Scholar] [CrossRef]
- Liu, H.N.; Bi, Z.J.; Wan, Z.; Wang, X.M.; Wan, Y.; Guo, X.X.; Cai, Z.Y. Preparation and Performance Optimization of Two-Component Waterborne Polyurethane Locomotive Coating. Coatings 2020, 10, 4. [Google Scholar] [CrossRef]
- Li, S.Y.; Liu, Z.Y.; Hou, L.J.; Chen, Y.; Xu, T.H. Effect of polyether/polyester polyol ratio on properties of waterborne two-component polyurethane coatings. Prog. Org. Coat. 2020, 141, 105545. [Google Scholar] [CrossRef]
- Wang, R.T.; Li, C.X.; Jiang, Z.H.; Wang, Z.J. Self-Assembly of Amphiphilic Linear-Dendritic Carbosilane Block Surfactant for Waterborne Polyurethane Coating. Polymers 2022, 12, 1318. [Google Scholar] [CrossRef]
- Tiryaki, S.; Bardak, S.; Aydin, A.; Nemli, G. Analysis of volumetric swelling and shrinkage of heat treated woods: Experimental and artificial neural network modeling approach. Maderas. Cienc. Y Tecnol. 2017, 18, 477–492. [Google Scholar] [CrossRef]
- Nowrouzi, Z.; Mohebby, B.; Petric, M.; Ebrahimi, M. Influence of nanoparticles and olive leaf extract in polyacrylate coating on the weathering performance of thermally modified wood. Eur. J. Wood Wood Prod. 2022, 80, 301–311. [Google Scholar] [CrossRef]
- Moradi, M.; Yeganeh, H.; Pazokifard, S. Synthesis and assessment of novel anticorrosive polyurethane coatings containing an amine-functionalized nanoclay additive prepared by the cathodic electrophoretic deposition method. RSC Adv. 2016, 6, 28089–28102. [Google Scholar] [CrossRef]
- GB/T 4893.8-1985; Furniture—Assessment of Wearability of Surface Coatings. Standardization Administration of the People’s Republic of China: Beijing, China, 1985; pp. 1–3. (In Chinese)
- GB/T 4893.4-1985; Furniture—Assessment of Adhesion of Surface Coatings—Cross Cut. Standardization Administration of the People’s Republic of China: Beijing, China, 1985; pp. 1–5. (In Chinese)
- Pan, P.; Yan, X.; Peng, W. Tung Oil Microcapsules Prepared with Different Emulsifiers and Their Effects on the Properties of Coating Film. Coatings 2022, 12, 1166. [Google Scholar] [CrossRef]
- Han, Y.; Yan, X.; Tao, Y. Effect of Transparent, Purple, and Yellow Shellac Microcapsules on the Optical Properties and Self-Healing Performance of Waterborne Coatings. Coatings 2022, 12, 1056. [Google Scholar] [CrossRef]
- Han, Y.; Yan, X.; Tao, Y. Effect of Number of Impregnations of Microberlinla sp with Microcapsule Emulsion on the Performance of Self-Repairing Coatings on Wood Surfaces. Coatings 2022, 12, 989. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, X. Preparation of Resin-Coated Waterborne Coating Microcapsules and Its Effect on the Properties of Waterborne Coating for Wood Surfaces. Coatings 2022, 12, 1394. [Google Scholar] [CrossRef]
- GB/T 4893.7-1985; Furniture—Assessment of Surface Resistance to Alternation of Heat and Cold. Standardization Administration of the People’s Republic of China: Beijing, China, 1985; pp. 1–2. (In Chinese)
- GB/T 4893.2-2020; Test of Surface Coatings of Furniture—Part 2: Determination of Resistance to Wet Heat. Standardization Administration of the People’s Republic of China: Beijing, China, 2020; pp. 1–12. (In Chinese)
- GB/T 4893.3-2020; Test of Surface Coatings of Furniture—Part 3: Determination of Resistance to Dry Heat. Standardization Administration of the People’s Republic of China: Beijing, China, 2020; pp. 1–12. (In Chinese)
- Kutcherlapati, S.N.R.; Yeole, N.; Gadi, M.R.; Perali, R.S.; Jana, T. RAFT mediated one-pot synthesis of glycopolymer particles with tunable core-shell morphology. Polym. Chem. 2017, 8, 1371–1380. [Google Scholar] [CrossRef]
- Ho, K.M.; Li, W.Y.; Wong, C.H.; Li, P. Amphiphilic polymeric particles with core-shell nanostructures: Emulsion-based syntheses and potential applications. Colloid Polym. Sci. 2010, 288, 1503–1523. [Google Scholar] [CrossRef]
- Li, B.; Li, S.M.; Liu, J.H.; Yu, M. The heat resistance of a polyurethane coating filled with modified nano-CaCO3. Appl. Surf. Sci. 2014, 315, 241–246. [Google Scholar] [CrossRef]
- Rogina-Car, B.; Kovacevic, S.; Dordevic, S.; Dordevic, D. Influence of Washing and Sterilization on Properties of Polyurethane Coated Fabrics Used in Surgery and for Wrapping Sterile Items. Polymers 2020, 12, 642. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Xiong, J.P.; Tang, Y.M.; Zuo, Y. EIS study on failure process of two polyurethane composite coatings. Prog. Org. Coat. 2010, 69, 7–11. [Google Scholar] [CrossRef]
- Bergamasco, S.; Tamantini, S.; Zikeli, F.; Vinciguerra, V.; Mugnozza, G.S.; Romagnoli, M. Synthesis and Characterizations of Eco-Friendly Organosolv Lignin-Based Polyurethane Coating Films for the Coating Industry. Polymers 2022, 14, 416. [Google Scholar] [CrossRef]
- Zheng, Q.J.; Zhang, Y.F.; Montazerian, M.; Gulbiten, O.; Mauro, J.C.; Zanotto, E.D.; Yue, Y.Z. Understanding Glass through Differential Scanning Calorimetry. Chem. Rev. 2019, 119, 7848–7939. [Google Scholar] [CrossRef]
- Bikiaris, D.; Prinos, J.; Botev, M.; Betchev, C.; Panayiotou, C. Blends of polymers with similar glass transition temperatures: A DMTA and DSC study. J. Appl. Polym. Sci. 2004, 93, 726–735. [Google Scholar] [CrossRef]
- Beckford, S.; Mathurin, L.; Chen, J.Y.; Fleming, R.A.; Zou, M. The effects of polydopamine coated Cu nanoparticles on the tribological properties of polydopamine/PTFE coatings. Tribol. Int. 2016, 103, 87–94. [Google Scholar] [CrossRef]
- Huang, N.; Yan, X.; Zhao, W. Influence of Photochromic Microcapsules on Properties of Waterborne Coating on Wood and Metal Substrates. Coatings 2022, 12, 1750. [Google Scholar] [CrossRef]
- Han, Y.; Yan, X.; Zhao, W. Effect of Thermochromic and Photochromic Microcapsules on the Surface Coating Properties for Metal Substrates. Coatings 2022, 12, 1642. [Google Scholar] [CrossRef]
- Yan, X.; Han, Y.; Yin, T. Coating Process Optimization and Self-Healing Performance Evaluation of Shellac Microcapsules Coated with Melamine/Rice Husk Powder. Appl. Sci. 2021, 11, 8373. [Google Scholar] [CrossRef]

| Experimental Materials | Molecular Mass (g/mol) | CAS | Manufacturer |
|---|---|---|---|
| Methyl methacrylate (MMA) | 100.116 | 80-62-6 | Jilin Shenhua Group Zhangjiagang Chemical Industry Co., Ltd., Suzhou, China |
| 2-hydroxyethyl methacrylate (HEMA) | 130.1418 | 868-77-9 | Suzhou Senfida Chemical Co., Ltd., Suzhou, China |
| Butyl acrylate (BA) | 128.169 | 141-32-2 | Shandong Xiaoqing New Chemical Co., Ltd., Jinan, China |
| Styrene (ST) | 104.15 | 100-42-5 | Wanhua Chemical Group Co., Ltd., Yantai, China |
| Acrylic acid (AA) | 72.063 | 79-10-7 | Wanhua Chemical Group Co., Ltd., Yantai, China |
| 2-ethylhexyl acrylate (EHA) | 184.28 | 103-11-7 | Suzhou Senfida Chemical Co., Ltd., Suzhou, China |
| Butyl methacrylate (BMA) | 142.196 | 97-88-1 | Jinan Yuanxiang Chemical Co., Ltd., Jinan, China |
| N,N-Dimethylethanolamine (DMEA) | 89.136 | 108-01-0 | Xindian chemical materials Co., Ltd., Shanghai, China |
| Propylene glycol butyl ether (PNB) | 238.3211 | 29387-86-8 | Guangzhou Kangyang Chemical Co., Ltd., Guangzhou, China |
| Acrylic phosphate | 167.034021 | - | Suzhou Senfida Chemical Co., Ltd., Suzhou, China |
| Di-tert-butyl peroxide (DTBP) | 146.227 | 110-05-4 | Shandong Shengqi New Material Co., Ltd., Jinan, China |
| 2,2,3,4,4,4-hexafluorobutyl methacrylate | 250.14 | 36405-47-7 | Hubei Zhenbo Chemical Co., Ltd., Wuhan, China |
| Ammonia | - | - | Jining Anping Chemical Co., Ltd., Jining, China |
| Bayhydur 304 isocyanate curing agent | - | - | Kostron (Shanghai) Investment Co., Ltd., Shanghai, China |
| TEGO-842 (defoamer) | - | - | Kostron (Shanghai) Investment Co., Ltd., Shanghai, China |
| Propylene glycol methyl ether acetate (PMA) | - | - | Nanjing Xingsha Chemical Co., Ltd., Nanjing, China |
| RM2020 | - | - | Nantong Yongle Chemical Co., Ltd., Nantong, China |
| Waterborne styrene acrylic sealing primer | - | - | Lanzhou Ketian Water Technology Co., Ltd., Lanzhou, China |
| Waterborne acrylic primer | - | - | Hebei Chenyang Waterborne Coatings Co., Ltd., Baoding, China |
| Pinus strobus boards | - | - | Xuzhou Zhonghao Furniture Co., Ltd., Xuzhou, China |
| Sample | Sealing Primer Amount (g/m2) | Primer Amount (g/m2) | Self-Made Topcoat Amount (g/m2) |
|---|---|---|---|
| 1 | 60 | 80 | 100 |
| 2 | 60 | 100 | 120 |
| 3 | 60 | 120 | 140 |
| 4 | 80 | 80 | 120 |
| 5 | 80 | 100 | 140 |
| 6 | 80 | 120 | 100 |
| 7 | 100 | 80 | 140 |
| 8 | 100 | 100 | 100 |
| 9 | 100 | 120 | 120 |
| Sample | Spraying Times (Times) | ||
|---|---|---|---|
| Sealing Primer | Primer | Self-Made Topcoat | |
| 1′ | 1 | 1 | 1 |
| 2′ | 1 | 1 | 2 |
| 3′ | 1 | 2 | 1 |
| 4′ | 1 | 2 | 2 |
| 5′ | 2 | 1 | 1 |
| 6′ | 2 | 1 | 2 |
| 7′ | 2 | 2 | 1 |
| 8′ | 2 | 2 | 2 |
| Variance Source | Coating Amount of the Sealing Primer | Coating Amount of Primer | Coating Amount of Self-Made Topcoat | Error |
|---|---|---|---|---|
| SS | 0.029 | 0.007 | 0.003 | 0.03 |
| df | 2 | 2 | 2 | 2 |
| F-ratio | 1.000 | 0.241 | 0.103 | |
| Fcrit | 19.000 | 19.000 | 19.000 | |
| Significance | No significant effect | |||
| Variance Source | Coating Amount of the Sealing Primer | Coating Amount of Primer | Coating Amount of Self-Made Topcoat | Error |
|---|---|---|---|---|
| SS | 2752.056 | 3813.722 | 1265.389 | 2752.06 |
| df | 2 | 2 | 2 | 2 |
| F-ratio | 1.000 | 1.386 | 0.460 | |
| Fcrit | 19.000 | 19.000 | 19.000 | |
| Significance | No significant effect | |||
| Variance Source | Coating Amount of the Sealing Primer | Coating Amount of the Primer | Coating Amount of the Self-Made Topcoat | Error |
|---|---|---|---|---|
| SS | 45.549 | 82.642 | 261.242 | 45.55 |
| df | 2 | 2 | 2 | 2 |
| F-ratio | 1.000 | 1.814 | 5.735 | |
| Fcrit | 19.000 | 19.000 | 19.000 | |
| Significance | No significant effect | |||
| Variance Source | Peeling Rate (%) | Weight Loss of the Film at 500 Revolutions (g) | Cold and Hot Temperature Difference Resistance | Water Resistance (Grade) | Heat Resistance (Grade) | Gloss (%) |
|---|---|---|---|---|---|---|
| 1 | 13.0 | 0.052 | Qualified | 2 | 4 | 71.7 |
| 2 | 11.0 | 0.086 | Qualified | 2 | 2 | 75.9 |
| 3 | 13.0 | 0.153 | Qualified | 2 | 4 | 80.1 |
| 4 | 76.0 | 0.167 | Unqualified | 1 | 3 | 75.8 |
| 5 | 17.0 | 0.169 | Qualified | 2 | 4 | 73.2 |
| 6 | 8.0 | 0.274 | Unqualified | 3 | 4 | 67.4 |
| 7 | 99.5 | 0.281 | Qualified | 2 | 5 | 79.7 |
| 8 | 30.0 | 0.187 | Qualified | 2 | 4 | 56.9 |
| 9 | 36.0 | 0.216 | Qualified | 2 | 4 | 75.0 |
| Sample | Spraying Times of Sealing Primer (Times) | Spraying Times of Primer (Times) | Spraying Times of Self-Made Topcoat (Times) | Peeling Rate (%) |
|---|---|---|---|---|
| 1′ | 1 | 1 | 1 | 58.9 |
| 2′ | 1 | 1 | 2 | 14.5 |
| 3′ | 1 | 2 | 1 | 79.4 |
| 4′ | 1 | 2 | 2 | 14.3 |
| 5′ | 2 | 1 | 1 | 11.4 |
| 6′ | 2 | 1 | 2 | 1.7 |
| 7′ | 2 | 2 | 1 | 2.4 |
| 8′ | 2 | 2 | 2 | 0.7 |
| Sample | Spraying Times of Sealing Primer (Times) | Spraying Times of Primer (Times) | Spraying Times of Self-Made Topcoat (Times) | Gloss (%) |
|---|---|---|---|---|
| 1′ | 1 | 1 | 1 | 50.7 |
| 2′ | 1 | 1 | 2 | 48.0 |
| 3′ | 1 | 2 | 1 | 69.0 |
| 4′ | 1 | 2 | 2 | 61.0 |
| 5′ | 2 | 1 | 1 | 62.6 |
| 6′ | 2 | 1 | 2 | 57.8 |
| 7′ | 2 | 2 | 1 | 78.8 |
| 8′ | 2 | 2 | 2 | 56.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Xu, W. Effect of Coating Process on Properties of Two-Component Waterborne Polyurethane Coatings for Wood. Coatings 2022, 12, 1857. https://doi.org/10.3390/coatings12121857
Liu C, Xu W. Effect of Coating Process on Properties of Two-Component Waterborne Polyurethane Coatings for Wood. Coatings. 2022; 12(12):1857. https://doi.org/10.3390/coatings12121857
Chicago/Turabian StyleLiu, Cheng, and Wei Xu. 2022. "Effect of Coating Process on Properties of Two-Component Waterborne Polyurethane Coatings for Wood" Coatings 12, no. 12: 1857. https://doi.org/10.3390/coatings12121857
APA StyleLiu, C., & Xu, W. (2022). Effect of Coating Process on Properties of Two-Component Waterborne Polyurethane Coatings for Wood. Coatings, 12(12), 1857. https://doi.org/10.3390/coatings12121857





