Effect of Standoff Distance on Corrosion Resistance of Cold Sprayed Titanium Coatings
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials and Methods
2.2. Solutions
2.3. Electrodes
2.4. Electrochemical Measurements
2.5. Surface Morphologies and Microstructure
2.6. Microhardness
3. Results and Discussion
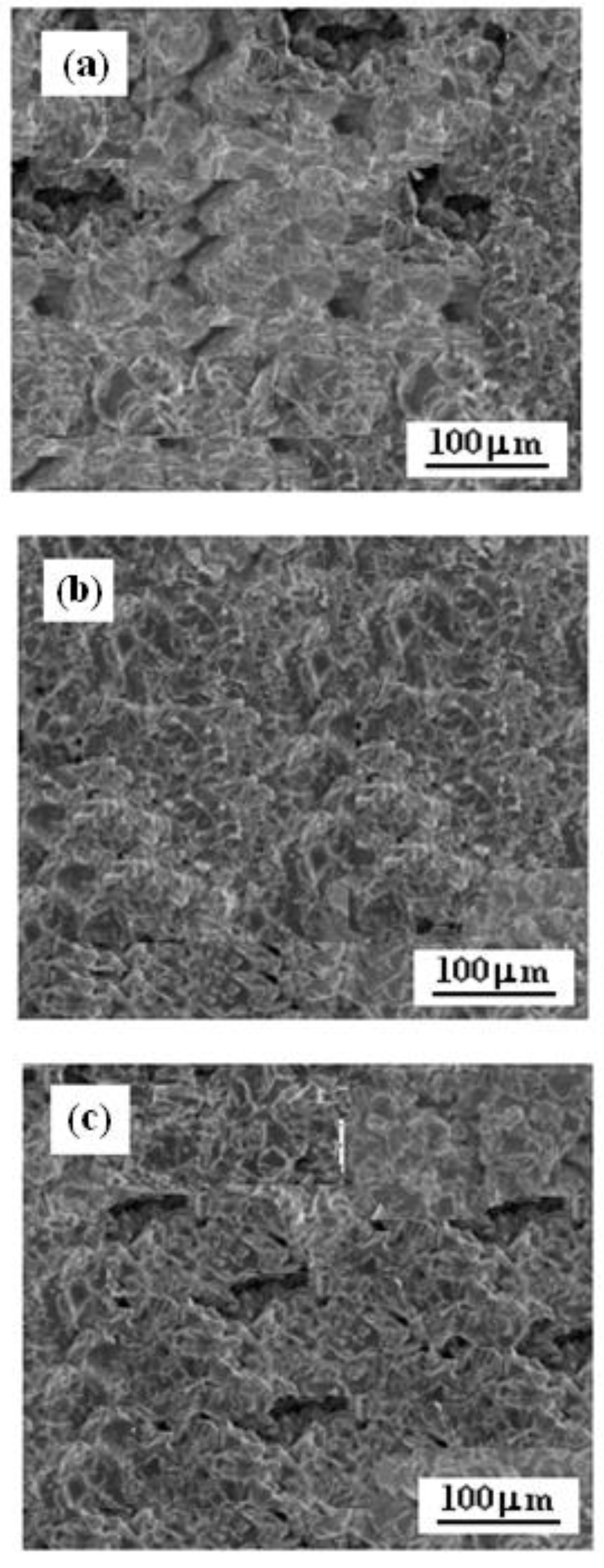
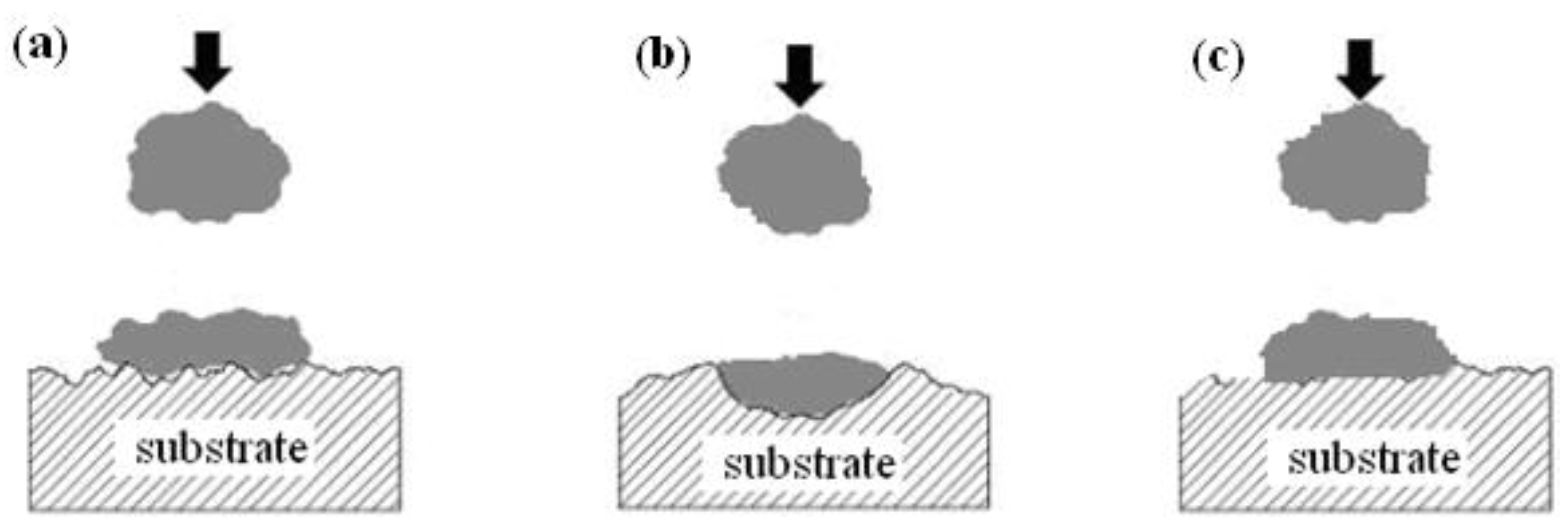
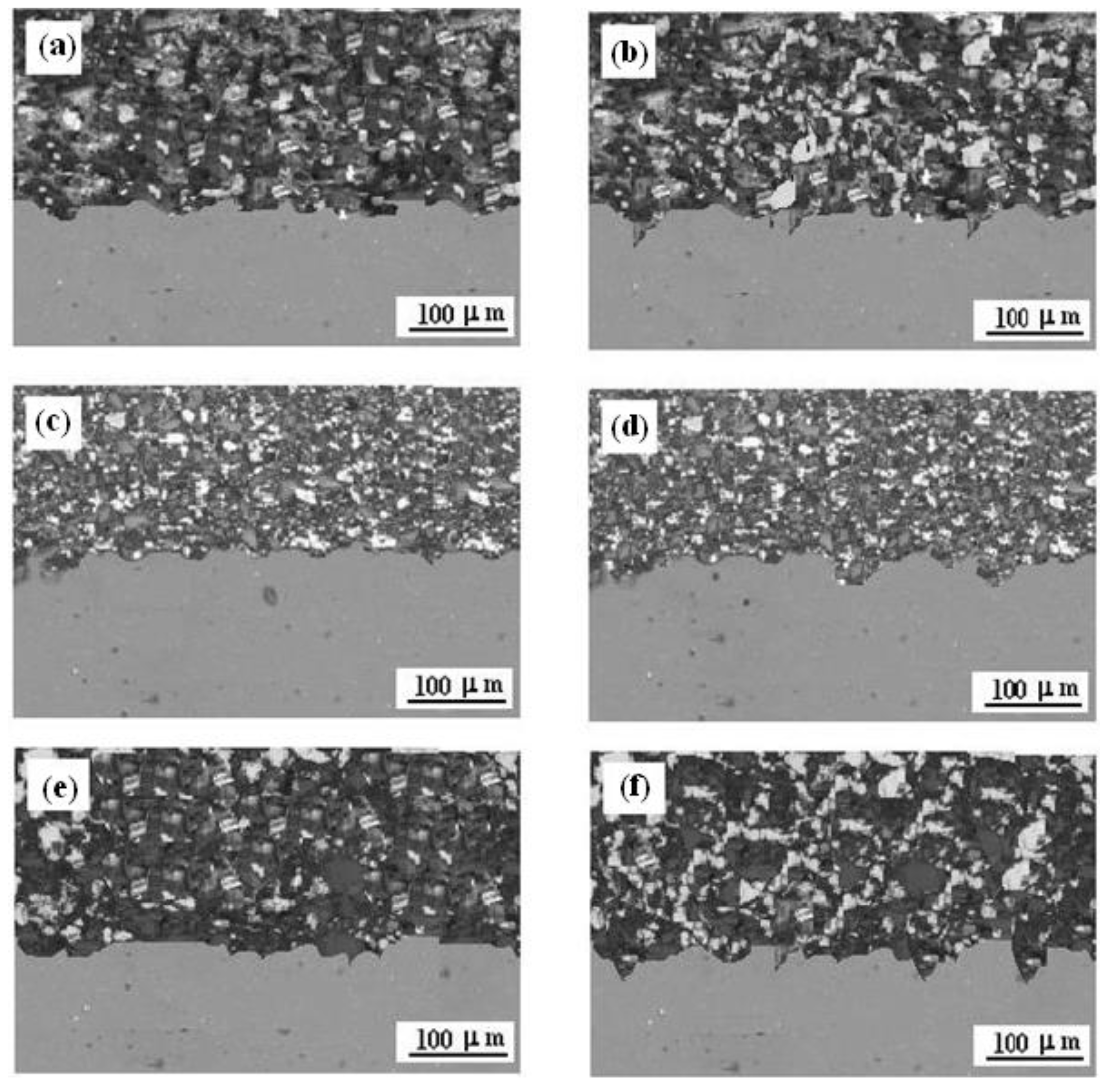
3.1. Surface Morphologies
3.2. Microhardness
3.3. Corrosion Test
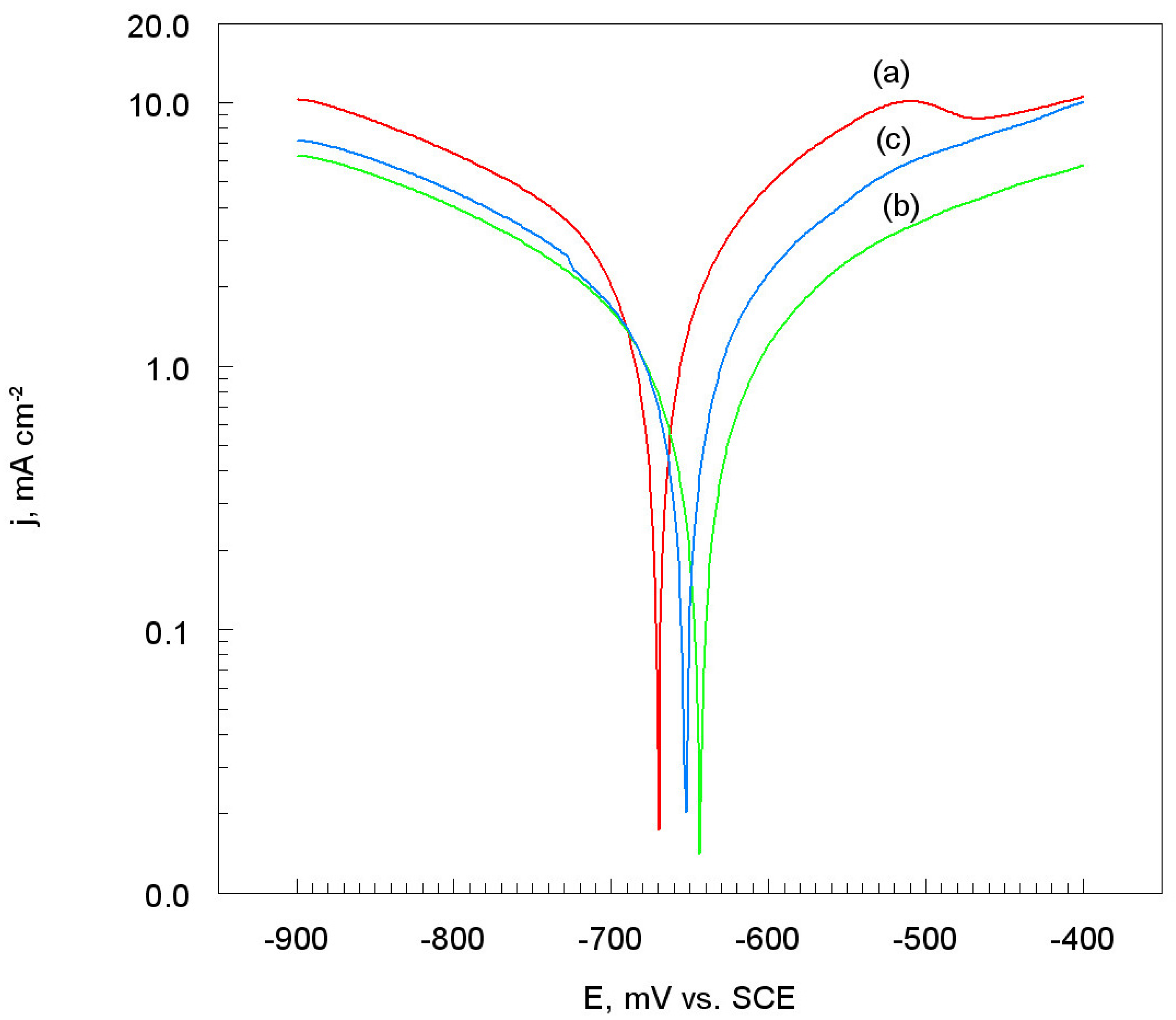
3.3.1. Corrosion Electrochemical Parameters
3.3.2. Polarization Resistance and Corrosion Rate
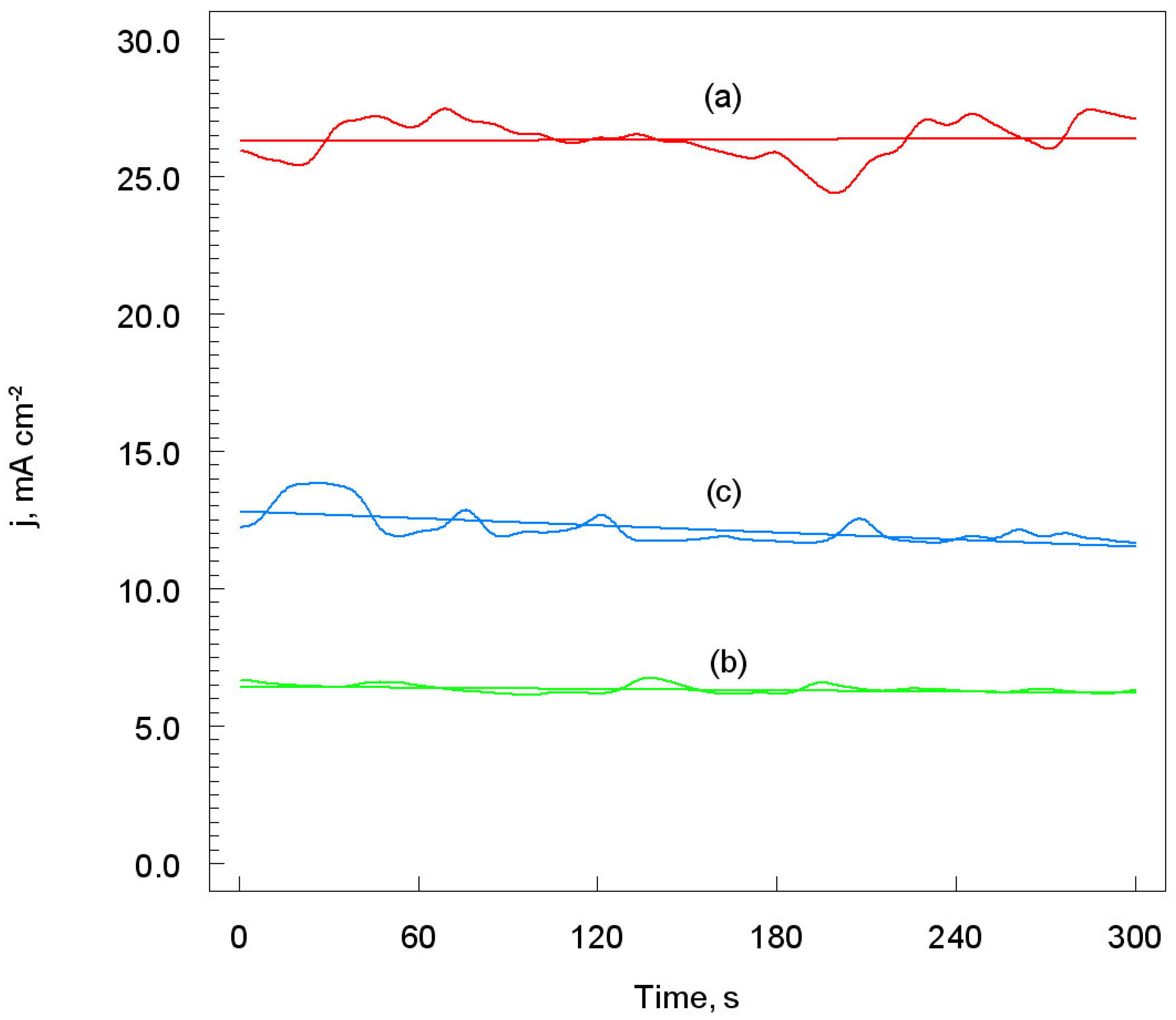
3.3.3. Chronoamperometric Measurements
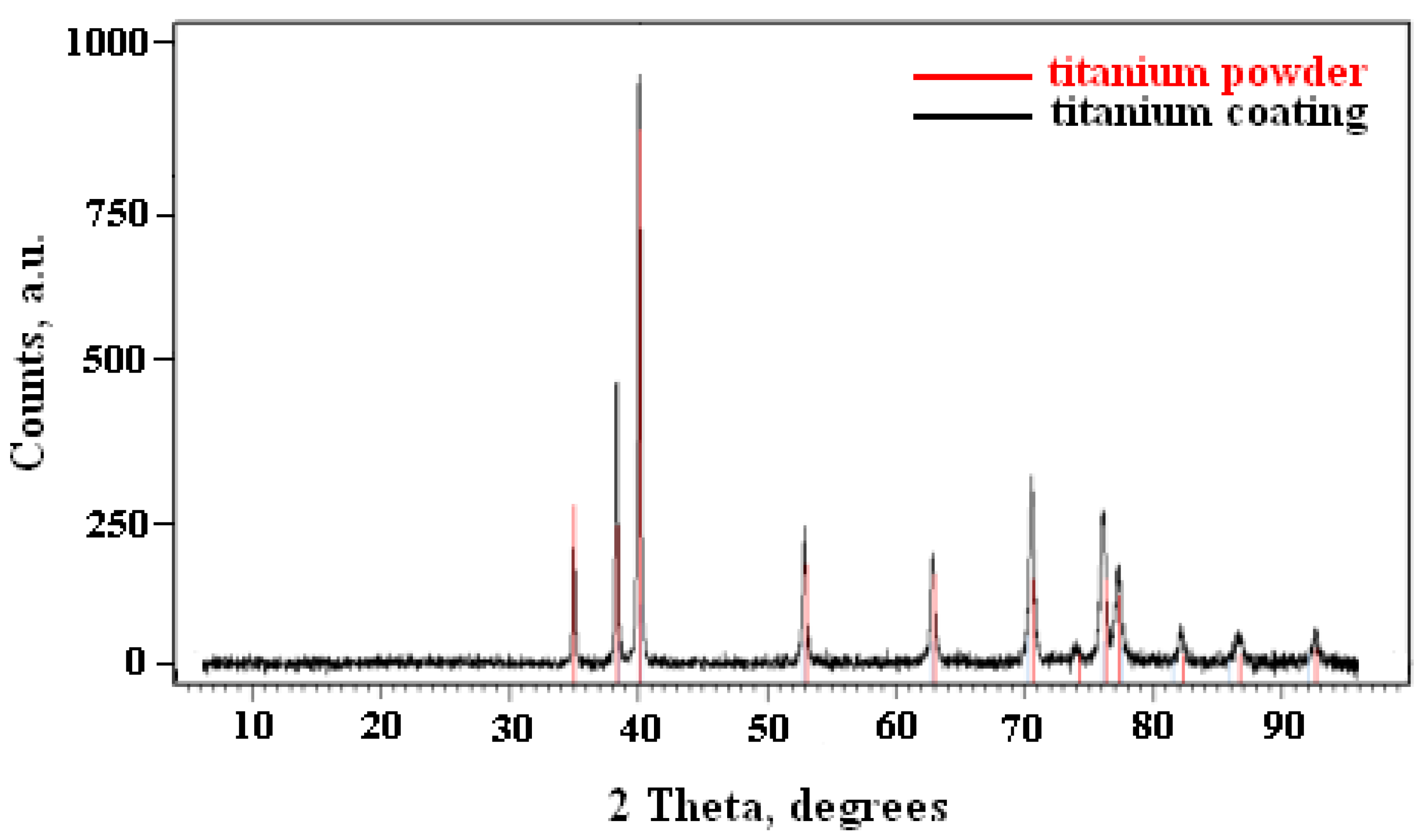
3.4. Microstructure Titanium Coatings
4. Conclusions
- The different standoff distance of the nozzle from the specimen surface (i.e., 20 mm, 70 mm, and 100 mm) in the cold spray process has a significant influence on the properties of the Al7075/Ti coating.
- Titanium coatings adhere well onto Al7075 alloy, and the most homogeneous and smooth surface of the Ti coating was obtained when the nozzle distance from the sample surface was 70 mm.
- The microhardness (HV0.3) of the deposit depend significantly on the nozzle distance. The highest level of HV0.3 value was achieved for deposits obtained with the SoD of 70 mm.
- There were no phase changes in the phase composition of the titanium deposits due to the increased of SoD.
- Corrosion test (electrochemical method) of the titanium coatings onto Al7075 substrate were carried out in acidic chloride solutions.
- The mechanism of electrochemical corrosion of titanium coatings is a multi-stage process, and the main product of the corrosion process was (TiO2)ads. The oxide layer did not protect the materials against the penetration of the aggressive solution.
- The polarization resistance (Rp) of the Ti coatings was the highest, while the corrosion rate (υcorr) was the lowest, for the SoD of 70 mm. Thus, in this case, the exchange of mass and electrical charge between the electrode and the electrolyte solution is significantly impeded.
- The titanium surface on the Al7075 substrate was slightly damaged when exposed to an acid chloride solution, while still protecting the aluminum substrate from the corrosive effects of the environment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Assadi, H.; Gärtner, F.; Stoltenhoff, T.; Kreye, H. Bonding mechanism in cold gas spraying. Acta Mater. 2003, 51, 4379–4394. [Google Scholar] [CrossRef]
- Li, W.-Y.; Gao, W. Some aspects on 3D numerical modeling of high velocity impact of particles in cold spraying by explicit finite element analysis. Appl. Surf. Sci. 2009, 255, 7878–7892. [Google Scholar] [CrossRef]
- Yin, S.; Cavaliere, P.; Aldwell, B.; Jenkins, R.; Liao, H.; Li, W.; Lupoi, R. Cold spray additive manufacturing and repair: Fundamentals and applications. Addit. Manuf. 2018, 21, 628–650. [Google Scholar] [CrossRef]
- Klinkov, S.V.; Fedorovich, V.F.; Rein, M. Cold spray deposition: Significance of particle impact phenomena. Aerosp. Sci. Technol. 2005, 10, 582–591. [Google Scholar] [CrossRef]
- Raletz, F.; Vardelle, M.; Ezo’o, G. Critical particle velocity under cold spray conditions. Surf. Coat. Technol. 2006, 201, 1942–1947. [Google Scholar] [CrossRef]
- Pattison, J.; Khan, A.; O’Neill, W.; Celetto, S. Standoff distance and bow shock phenomena in the cold spray process. Surf. Coat. Technol. 2008, 202, 1443–1454. [Google Scholar] [CrossRef]
- Gnanasekaran, B.; Liu, G.R.; Fu, Y.; Wang, G.; Niu, W.; Lin, T. A Smoothed Particle Hydrodynamics (SPH) procedure for simulating cold spray process – A study using particles. Surf. Coat. Technol. 2019, 377, 24812. [Google Scholar] [CrossRef]
- Hussain, T.; McCartney, D.G.; Shipway, P.H.; Zhang, D. Bonding mechanisms in cold spraying: The contributions of Metallurgical and Mechanical Components. J. Thern. Spray Technol. 2009, 48, 364–379. [Google Scholar] [CrossRef]
- Schmidt, T.; Gartner, E.; Assadi, H.; Kreye, H. Development of a generalized parameter window for cold spray deposition. Acta Mater. 2006, 54, 729–742. [Google Scholar] [CrossRef]
- Li, W.Y.; Liao, H.L.; Li, C.J.; Bang, H.-S.; Coddet, C. Numerical simulation of deformation behavior of Al particles impacting on Al substrate and effect of surface oxide films on interfacial bonding in cold spraying. Appl. Surf. Sci. 2007, 253, 5084–5091. [Google Scholar] [CrossRef]
- Vidaller, M.V.; List, A.; Gaertner, F.; Klassen, T.; Dosta, S.; Guilemany, J.M. Single impact bonding of cold sprayed Ti-6Al-4V powders on different substrates. J. Therm. Spray Technol. 2015, 24, 644–658. [Google Scholar] [CrossRef]
- Bhattiprolu, V.S.; Johnson, K.W.; Ozdemir, O.C.; Crawford, G.A. Influence of feedstock powder and cold spray processing parameters on microstructure and mechanical properties of Ti-6Al-4V cold spray depositions. Surf. Coat. Technol. 2018, 335, 1–12. [Google Scholar] [CrossRef]
- Bhattiprolu, V.S.; Johnson, K.W.; Crawford, G.A. Influence of powder microstructure on the microstructural evolution of as-sprayed and heat treated cold-sprayed Ti-6Al-4V coatings. J. Therm. Spray Technol. 2018, 28, 174–188. [Google Scholar] [CrossRef]
- Hajipour, H.; Abdollah-zadeh, A.; Assadi, H.; Taheri-Nassaj, E.; Jahed, H. Effect of feedstock powder morphology on cold-sprayed titanium dioxide coatings. J. Therm. Spray Technol. 2018, 27, 1542–1550. [Google Scholar] [CrossRef] [Green Version]
- Munagala, V.N.V.; Akinyi, V.; Vo, P.; Chromik, R.R. Influence of powder morphology and microstructure on the cold spray and mechanical properties of Ti-6Al-4V coatings. J. Therm. Spray Technol. 2018, 27, 827–842. [Google Scholar] [CrossRef]
- Munagala, V.N.V.; Bessette, S.; Gauvin, R.; Chromik, R.R. Sliding wear of cold sprayed Ti-6Al-4V coatings: Effect of porosity and normal load. Wear 2020, 450–451, 203268. [Google Scholar] [CrossRef]
- Sirvent, P.; Garrido, M.Á.; Sharp, J.; Rainforth, W.M.; Poza, P. Improving the oscillating wear response of cold sprayed Ti-6Al-4V coatings through a heat treatment. Surf. Coat. Technol. 2020, 399, 126128. [Google Scholar] [CrossRef]
- Wu, K.; Sun, W.; Tan, A.W.-Y.; Marinescu, I.; Liu, E.; Zhou, W. An investigation into microstructure, tribological and mechanical properties of cold sprayed Inconel 625 coatings. Surf. Coat. Technol. 2021, 424, 127660. [Google Scholar] [CrossRef]
- Wu, K.; Chee, S.W.; Sun, W.; Tan, A.W.-Y.; Tan, S.C.; Liu, E.; Zhou, W. Inconel 713C coating by cold spray for surface enhancement of Inconel 718. Metals 2021, 11, 2048. [Google Scholar] [CrossRef]
- Samareh, B.; Stier, O.; Lüthen, V.; Dolatabadi, A. Assessment of cfd modeling via flow visualization in cold spray process. J. Therm. Spray Technol. 2009, 18, 934–943. [Google Scholar] [CrossRef]
- Meyer, M.C.; Yin, S.; Lupoi, R. Particle in-flight velocity and dispersion measurements at increasing particle feed rates in cold spray. J. Therm. Spray Technol. 2016, 26, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.C.; Yin, S.; McDonnell, K.A.; Stier, O.; Lupoi, R. Feed rate effect on particulate acceleration in cold spray under low stagnation pressure conditions. Surf. Coat. Technol. 2016, 304, 237–245. [Google Scholar] [CrossRef]
- Morgan, R.; Fox, P.; Pattison, J.; Sutcliffe, C.; O’Neill, W. Analysis of cold gas dynamically sprayed aluminium deposits. Mater. Lett. 2004, 58, 1317–1320. [Google Scholar] [CrossRef]
- Grujicic, M.; Zhao, C.L.; DeRosset, W.S.; Helfritch, D. Adiabatic shear instability based mechanism for particles/substrate bonding in the cold-gas dynamic-spray process. Mater. Des. 2004, 25, 681–688. [Google Scholar] [CrossRef]
- Żórawski, W.; Molak, R.; Mądry, J.; Sienicki, J.; Góral, A.; Makrenek, M.; Scendo, M.; Dobosz, R. Experimental and numerical investigations of titanium deposition for cold spray additive manufacturing as a function of standoff distance. Materials 2021, 14, 5492. [Google Scholar] [CrossRef] [PubMed]
- Scendo, M.; Zorawski, W.; Staszewska-Samson, K.; Goral, A. Influence of laser treatment on the corrosion resistance of Cr3C2-25(Ni20Cr) cermet coating. Materials 2021, 14, 4078. [Google Scholar] [CrossRef] [PubMed]
- Scendo, M.; Radek, N.; Trela, J. Influence of laser treatment on the corrosive resistance of WC-Cu coating produced by electrospark deposition. Int. J. Electrochem. Sci. 2013, 8, 9264–9277. [Google Scholar]
- Scendo, M.; Trela, J.; Radek, N. Influence of laser power on the corrosive resistance of WC-Cu coating. Surf. Coat. Technol. 2014, 259, 401–407. [Google Scholar] [CrossRef]
- Scendo, M.; Zorawski, W.; Staszewska, K.; Makrenek, M.; Goral, A. Influence of surface pretreatment on the corrosion resistance of cold spray nickel coatings in acidic chloride solution. J. Mater. Eng. Perform. 2018, 27, 1725–1737. [Google Scholar] [CrossRef]
- Adjdelsztajn, L.; Jodoin, B.; Lavernia, E. Cold gas dynamic spraying of a high temperature Al alloy. Surf. Coat. Technol. 2006, 201, 2109–2116. [Google Scholar] [CrossRef]
- Li, W.-Y.; Zhang, C.; Wang, H.-T.; Guo, X.-P.; Liao, H.L.; Li, C.-J.; Coddet, C. Significant influences of metal reactivity and oxide films at particle surfaces on coating microstructure in cold spraying. Appl. Surf. Sci. 2007, 253, 3557–3562. [Google Scholar] [CrossRef]
- Li, W.-Y.; Zhang, C.; Guo, X.P.; Zhang, G.; Liao, H.L.; Li, C.-J.; Coddet, C. Effect of standoff distance on coating deposition characteristics in cold spraying. Mater. Des. 2008, 29, 297–304. [Google Scholar] [CrossRef]
- Scendo, M.; Zorawski, W.; Goral, A. Influence of Nickel Powders on Corrosion Resistance of Cold Sprayed Coatings on Al7075 Substrate. Metals 2019, 9, 890. [Google Scholar] [CrossRef]

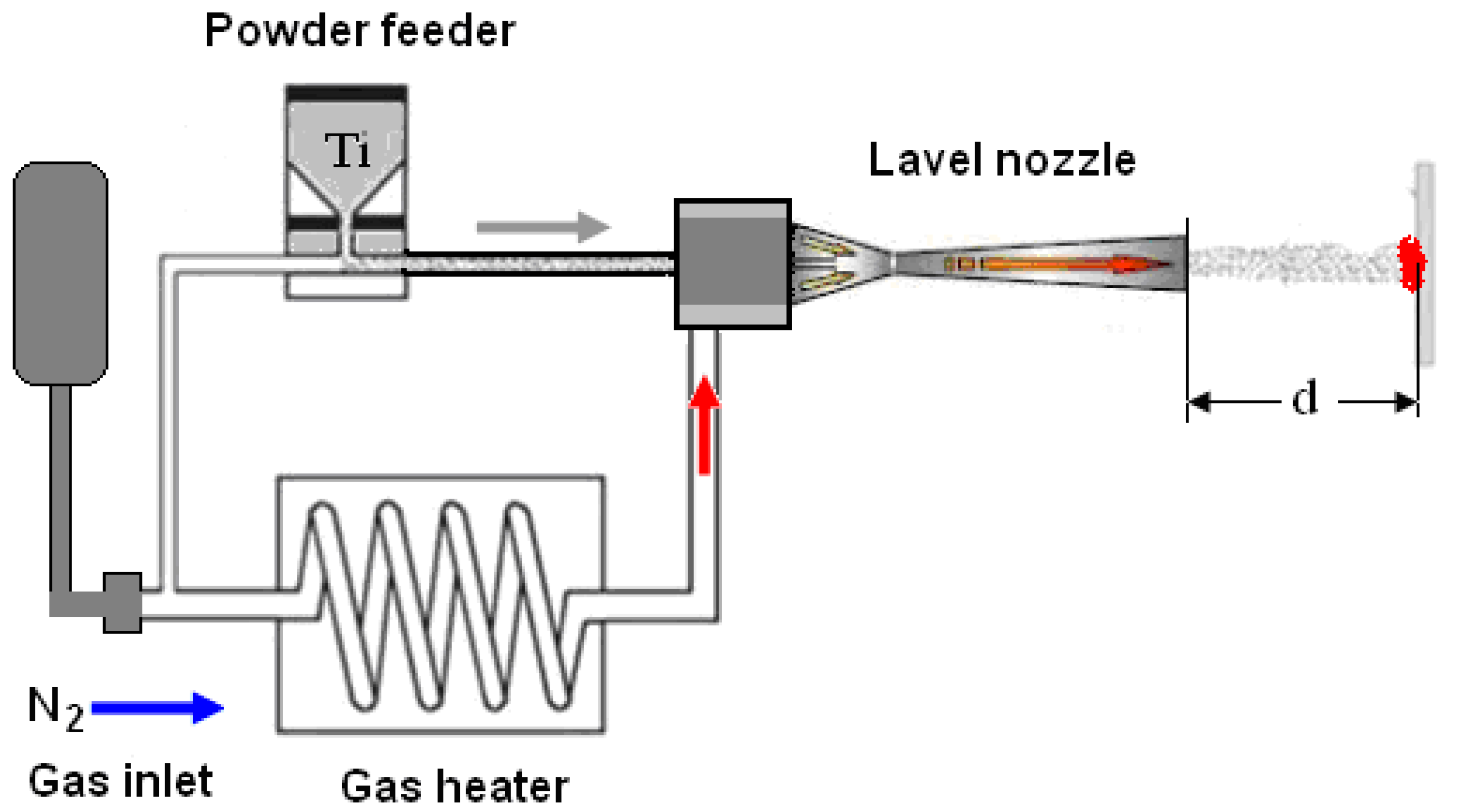

| Sample | Distance d, mm |
|---|---|
| Al7075/Ti-20 | 20 |
| Al7075/Ti-70 | 70 |
| Al7075/Ti-100 | 100 |
| Sample | Microhardness HV0.3 |
|---|---|
| Al7075/Ti-20 | 200 ± 2 |
| Al7075/Ti-70 | 248 ± 1 |
| Al7075/Ti-100 | 218 ± 3 |
| Sample | Ecorr mV vs. SCE | jcorr mA cm−2 | −bc | ba |
|---|---|---|---|---|
| mV dec−1 | ||||
| Al7075/Ti-20 | −670 | 2.80 | 350 | 250 |
| Al7075/Ti-70 | −643 | 1.60 | 340 | 380 |
| Al7075/Ti-100 | −652 | 1.90 | 380 | 300 |
| Sample | Rp kΩ cm2 | vcorr mm y−1 |
|---|---|---|
| Al7075/Ti-20 | 23 | 24.30 |
| Al7075/Ti-70 | 49 | 13.90 |
| Al7075/Ti-100 | 38 | 16.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scendo, M.; Staszewska-Samson, K. Effect of Standoff Distance on Corrosion Resistance of Cold Sprayed Titanium Coatings. Coatings 2022, 12, 1853. https://doi.org/10.3390/coatings12121853
Scendo M, Staszewska-Samson K. Effect of Standoff Distance on Corrosion Resistance of Cold Sprayed Titanium Coatings. Coatings. 2022; 12(12):1853. https://doi.org/10.3390/coatings12121853
Chicago/Turabian StyleScendo, Mieczyslaw, and Katarzyna Staszewska-Samson. 2022. "Effect of Standoff Distance on Corrosion Resistance of Cold Sprayed Titanium Coatings" Coatings 12, no. 12: 1853. https://doi.org/10.3390/coatings12121853
APA StyleScendo, M., & Staszewska-Samson, K. (2022). Effect of Standoff Distance on Corrosion Resistance of Cold Sprayed Titanium Coatings. Coatings, 12(12), 1853. https://doi.org/10.3390/coatings12121853








