Inhibitory Activity of Chitin, (2-Acetamido-2-Deoxy-Hexopyranose) against Penicillin-Binding Proteins of Staphylococcus aureus
Abstract
:1. Introduction
2. Material and Methods
2.1. Sampling and Systematics of Freshwater Prawn
2.2. Extraction of Chitin
2.2.1. Deproteinization
2.2.2. Decoloration
2.2.3. Demineralization
2.3. Characterization of Chitin
2.3.1. Determination of Yield, Sulfated Ash, and Insoluble Content in Chitin
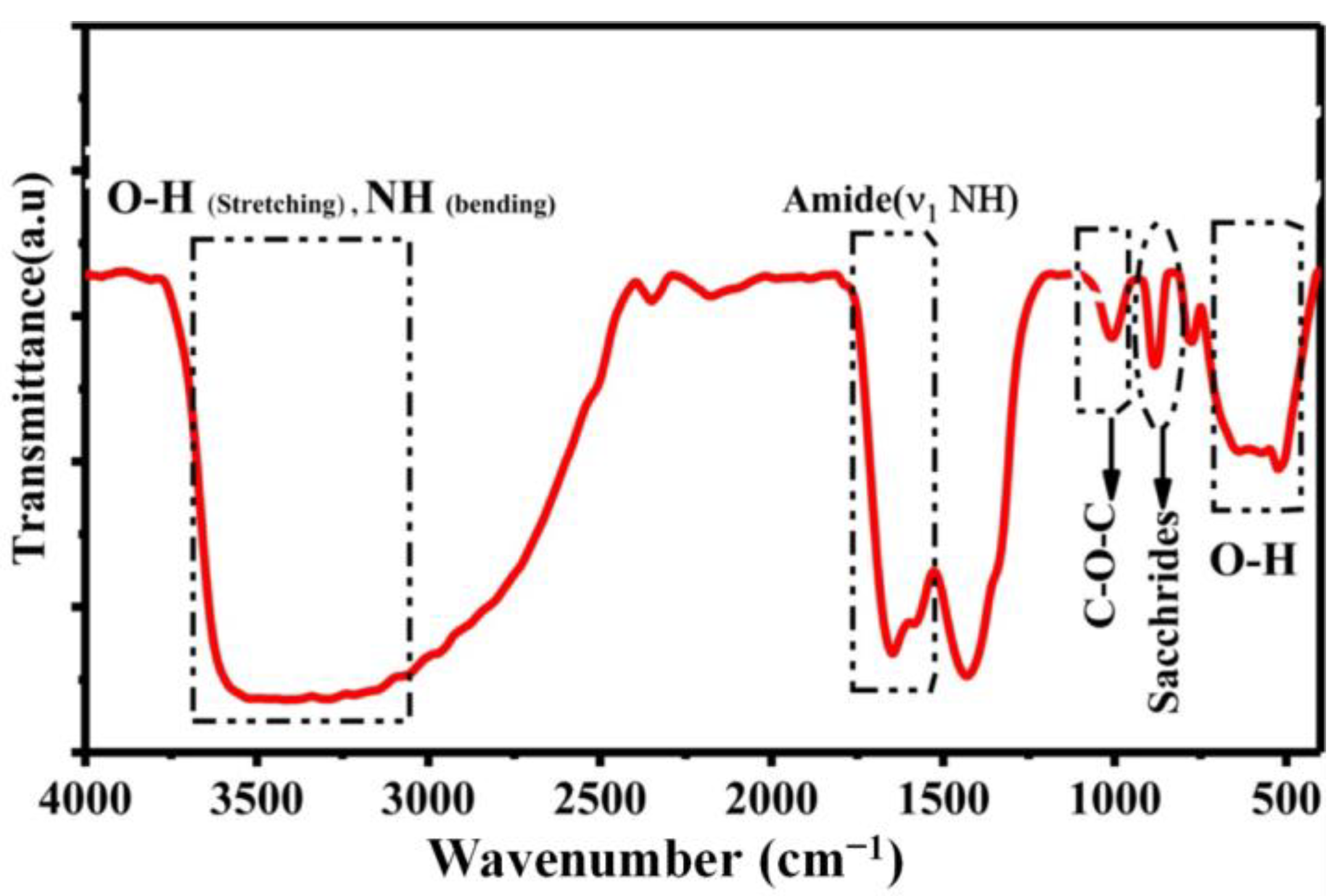
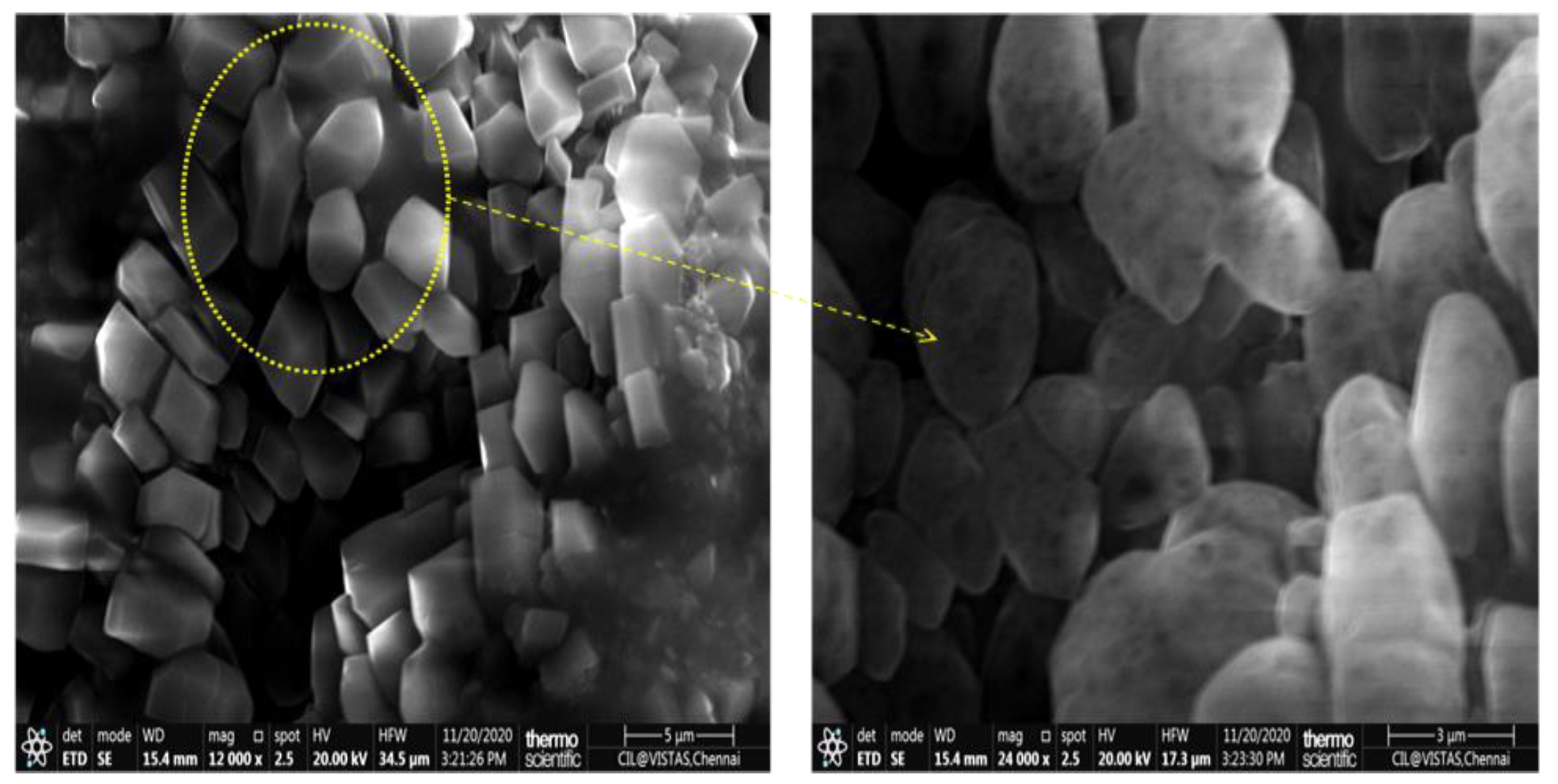
2.3.2. Characterization of Physicochemical Properties
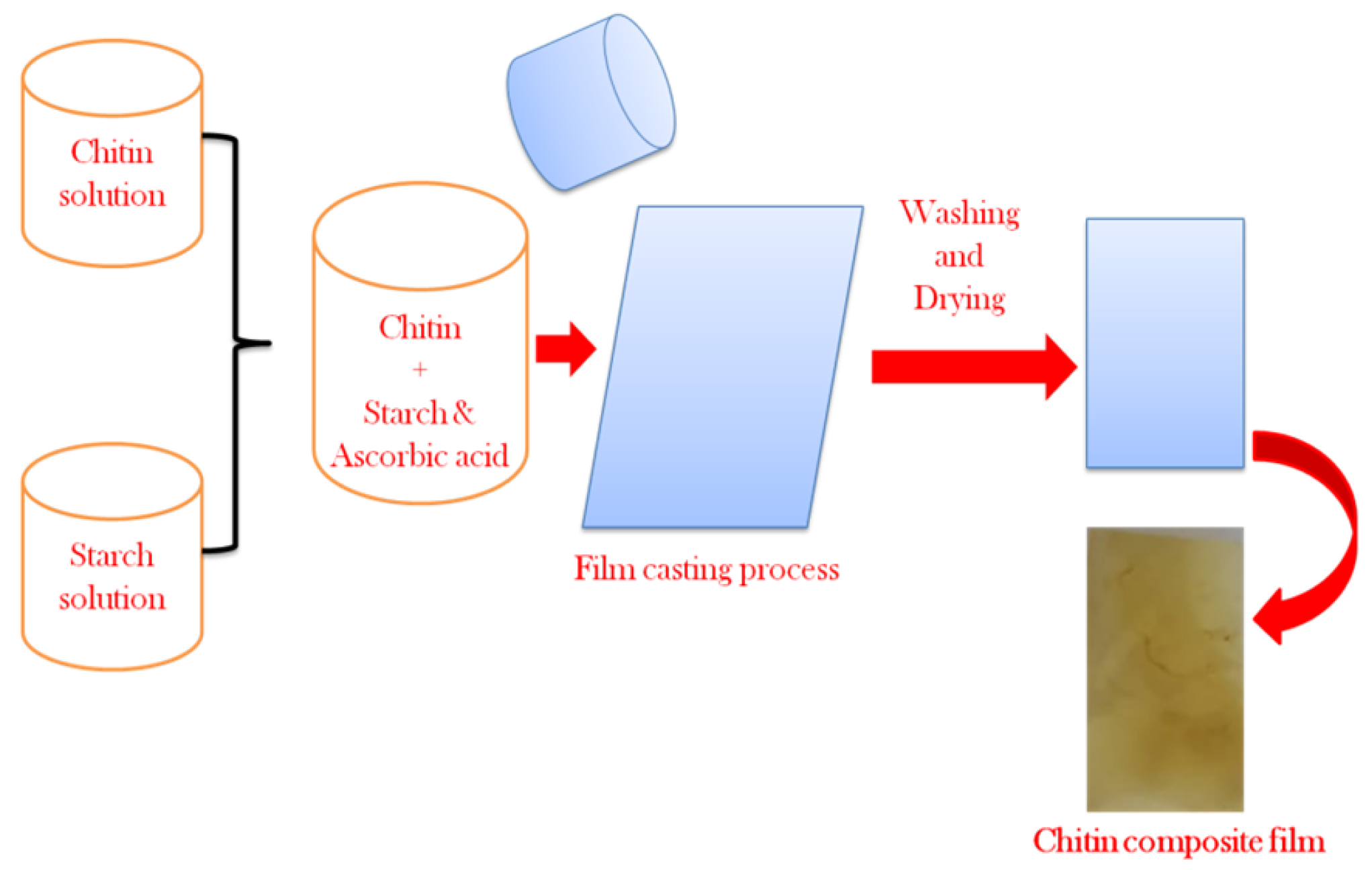
2.4. Preparation of Chitin Composite Film
2.5. Antimicrobial Activity of Chitin Film
2.6. In Silico Inhibitory Mechanisms of Penicillin-Binding Protein (PBP2a) in S. aureus
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiselinova, M.; Velghe, A.; Piers, R.; Verhasselt, B.; Noortgate, N. Methicillin-resistant staphylococcus aureus pneumonia in older people: A diagnostic and therapeutic challenge. Acta Clin. Belg. 2019, 74, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Egbebi, A.O.; Oyama, M.O.; Fadare, O.S. Genetic diversity of antibiotic and plant extract resistant Staphylococcus aureus isolated from hospitalized patients in Ekiti State, Nigeria. Afr. J. Biotechnol. 2019, 18, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Liu, Y.; Smith, D.; Konermann, L.; Siu, K.M.; Golemi-Kotra, D. Diversity of Penicillin-binding Proteins resistance factor FmtA of Staphylococcus aureus. J. Biol. Chem. 2007, 282, 35143–35152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, S.B.; Adlan, T.A.; Khalafalla, N.A.; Abdalla, N.I.; Ali, Z.S.; Munir, K.A.; Elnour, M.A.B. Proteomics and Docking Study Targeting Penicillin-Binding Protein and Penicillin-Binding Protein2a of Methicillin-Resistant Staphylococcus aureus Strain SO-1977 Isolated from Sudan. Evol. Bioinform. 2019, 15, 1176934319864945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Devel. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [Green Version]
- Dutta, J.; Tripathi, S.; Dutta, P.K. Progress in antimicrobial activities of chitin, chitosan and its oligosaccharides: A systematic study needs for food applications. Food Sci. Technol. Int. 2012, 18, 3–34. [Google Scholar] [CrossRef]
- Tareq, A.; Alam, M.; Raza, S.; Sarwar, T.; Alamgir, Z.F.; Chowdhury, Z.; Hossain, S. Comparative Study of Antibacterial Activity of Chitin and Chemically Treated Chitosan Prepared from Shrimp (Macrobrachium Rosenbergii) Shell Waste. J. Virol. Microbiol. 2013, 9, 122–128. [Google Scholar] [CrossRef]
- Richard, J.; Chandran, M.R. A systematic report on the fresh water prawns of the atyid genus Caridina H. Milne Edwards 1837, from Madras (Tamilnadu, India). J. Bombay Nat. Hist. Soc. 1994, 91, 241–259. [Google Scholar]
- Mohammed, M.H.; Williams, P.A.; Tverezovskaya, O. Extraction of chitin from prawn shells and conversion to low molecular mass chitosan. Food Hydrocoll. 2013, 31, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Ibitoye, E.B.; Lokman, I.H.; Hezmee, M.N.M.; Goh, Y.M.; Zuki, A.B.Z.; Jimoh, A.A. Extraction and physicochemical characterization of chitin and chitosan isolated from house cricket. Biomed. Mater. 2018, 13, 025009. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz Antonino, R.S.C.; Fook, L.; Paschoal, B.R.; De Oliveira Lima, V.A.; De Farias Rached, R.Í.; Lima, E.P.N.; Lia Fook, M.V. Preparation and characterization of chitosan obtained from shells of shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badawy, M.E.; Rabea, E.I.; Taktak, N.E.; El-Nouby, M.A. The antibacterial activity of chitosan products blended with monoterpenes and their biofilms against plant pathogenic bacteria. Scientifica 2016, 2016, 1796256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadarajah, S.K.; Vijayaraj, R.; Mani, J. Therapeutic significance of Loligo vulgaris (Lamarck, 1798) ink extract: A biomedical approach. Pharmacogn. Research. 2017, 9 (Suppl. 1), S105–S109. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexiblity. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar]
- Jeuniaux, C.; Voss-Foucart, M.; Poulick, M.; Bussers, J. Source of chitin, estimated from new data on chitin biomass and production. In Proceedings of the 4th International Conference on Chitin and Chitosan 1988, Trondheim, Norway, 22–24 August 1988; Elsevier: London, UK; New York, NY, USA, 1988; pp. 3–11. [Google Scholar]
- Kurita, K. Controlled functionalization of the polysaccharide chitin. Prog. Polym. Sci. 2001, 26, 1921–1971. [Google Scholar] [CrossRef]
- Sharp, R.G. A Review of the Applications of Chitin and Its Derivatives in Agriculture to Modify Plant-Microbial Interactions and Improve Crop Yields. Agronomy 2013, 3, 757–793. [Google Scholar] [CrossRef] [Green Version]
- Nessa, F.; Masum, S.M.; Asaduzzaman, M.; Roy, S.K.; Hossain, M.M.; Jahan, M.S. A process for the preparation of chitin and chitosan from prawn shell waste. Bangladesh J. Sci. Ind. Res. 2010, 45, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Haga, A.; Sekiguchi Hand Hirano, S. Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. Int. J. Biol. Macromol. 2000, 27, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Sajomsang, W.; Gonil, P. Preparation and characterization of chitin from cicada sloughs. Mater. Sci. Eng. C 2010, 30, 357–363. [Google Scholar] [CrossRef]
- Kaya, M.; Erdogan, S.; Mol, A.; Baran, T. Comparison of chitin structures isolated from seven orthoptera species. Int. J. Biol. Macromol. 2015, 72, 797–805. [Google Scholar] [CrossRef]
- Dahmane, E.M.; Taourirte, M.; Eladlani, N.; Rhazi, M. Extraction and characterization of chitin and chitosan from Parapenaeus longirostris from Moroccan local sources. Int. J. Polym. Anal. Charact. 2014, 19, 342–351. [Google Scholar] [CrossRef]
- Povea, M.B.; Monal, W.A.; Cauich-Rodríguez, J.V.; Pat, A.M.; Rivero, N.B.; Covas, C.P. Interpenetrated chitosan-poly (acrylic acid-co-acrylamide) hydrogels. Synthesis, characterization and sustained protein release studies. Mater. Sci. Appl. 2011, 2, 509–520. [Google Scholar] [CrossRef] [Green Version]
- Bautista-Banos, S.; Romanazzi, G.; Jiménez-Aparicio, A. Chitosan in the Preservation of Agricultural Commodities; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Eddya, M.; Tbib, B.; Khalil, E.H. A comparison of chitosan properties after extraction from shrimp shells by diluted and concentrated acids. Heliyon 2020, 6, e03486. [Google Scholar] [CrossRef] [Green Version]
- Qu, X.; Wirsen, A.; Albertsson, A.C. Effect of lactic/glycolic acid side chains on the thermal degradation kinetics of chitosan derivatives. Polymer 2000, 41, 4841–4847. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrieres, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Kumari, S.; Rath, P.; Kumar, A.S.H.; Tiwari, T.N. Extraction and characterization of chitin and chitosan from fishery waste by chemical method. Environ. Technol. Innov. 2015, 3, 77–85. [Google Scholar] [CrossRef]
- Gbenebor, O.P.; Adeosun, S.O.; Lawal, G.I.; Jun, S.; Olaleye, S.A. Acetylation, crystalline and morphological properties of structural polysaccharide from shrimp exoskeleton. Eng. Sci. Technol. Int. J. 2017, 20, 1155–1165. [Google Scholar] [CrossRef]
- Amjed, N.; Bhatti, I.A.; Zia, K.M.; Iqbal, J.; Jamil, Y. Synthesis and characterization of stable and biological active chitin-based polyurethane elastomers. Int. J. Biol. Macromol. 2019, 154, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Yang, Q. Extraction and characterization of chitin from the beetle Holotrichia parallela Motschulsky. Molecules 2012, 17, 4604–4611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyroba, E.; Suski, S.; Miller, K.; Bartosiewicz, R. Biomedical and agricultural applications of energy dispersive X-ray spectroscopy in electron microscopy. Cell. Mol. Biol. Lett. 2015, 20, 488–509. [Google Scholar] [CrossRef]
- Velásquez, C.L.; Albornoz, J.S.; Barrios, E.M. Viscosimetric studies of chitosan nitrate and chitosan chlorhydrate in acid free NaCl aqueous solution. E-Polymer 2008, 8, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Verghese, R.J.; Mathew, S.K.; David, A. Antimicrobial activity of Vitamin C demonstrated on uropathogenic Escherichia coli and Klebsiella pneumoniae. J. Curr. Res. Sci. Med. 2017, 3, 88. [Google Scholar]
- Vijayaraj, R.; Sri Kumaran, N.; Altaff, K.; Ramadevi, S.; Sherlin Rosita, A. In Silico Pharmacokinetics and Molecular Docking of Novel Bioactive Compound (11-Methoxy-2-Methyltridecane-4-Ol) for Inhibiting Carbohydrates Hydrolyzing Enzyme. J. Biol. Act. Prod. Nat. 2019, 9, 445–456. [Google Scholar] [CrossRef]

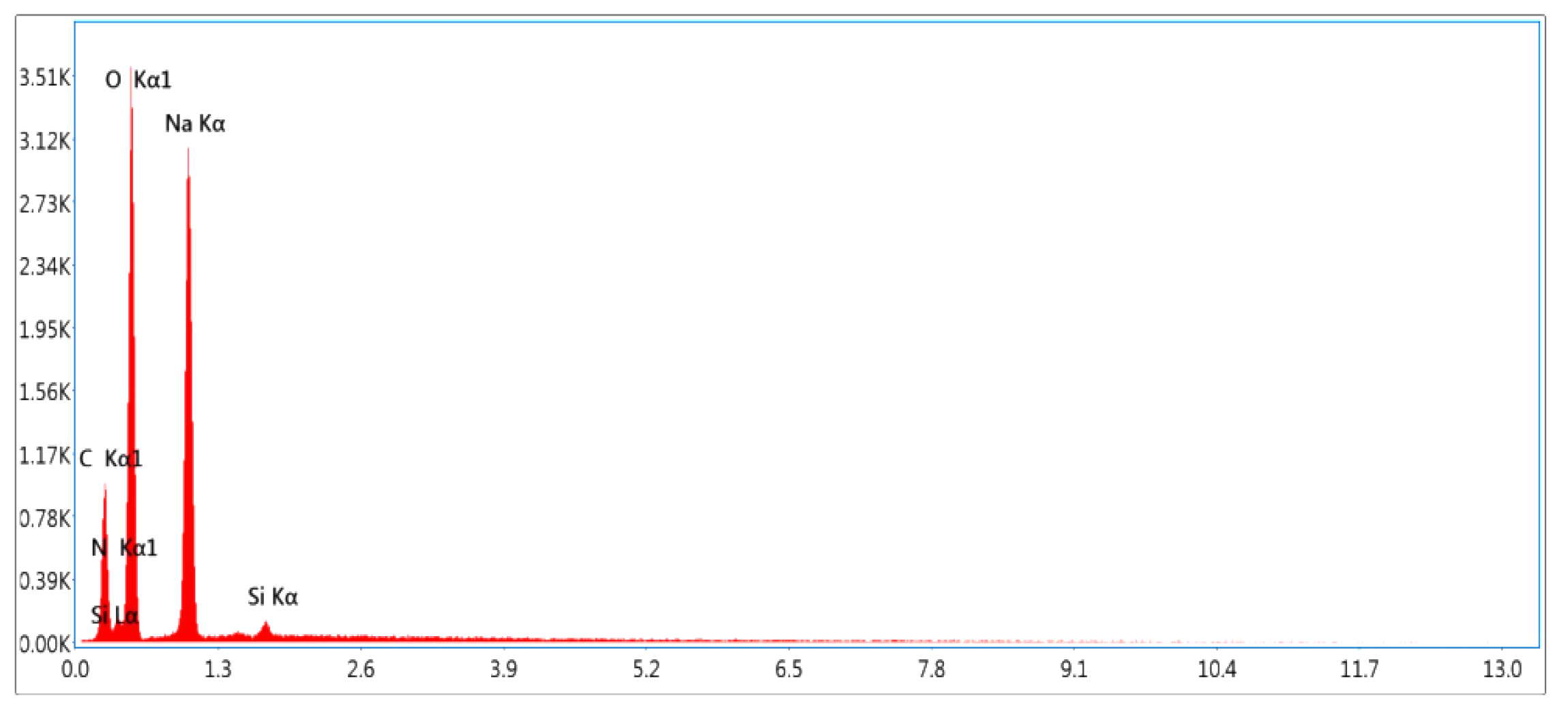

| Elements | Weight (%) |
|---|---|
| Carbon (C) | 20.9 |
| Nitrogen (N) | 3.8 |
| Oxygen (O) | 42.2 |
| Sodium (Na) | 32.1 |
| Silicon (Si) | 0.9 |
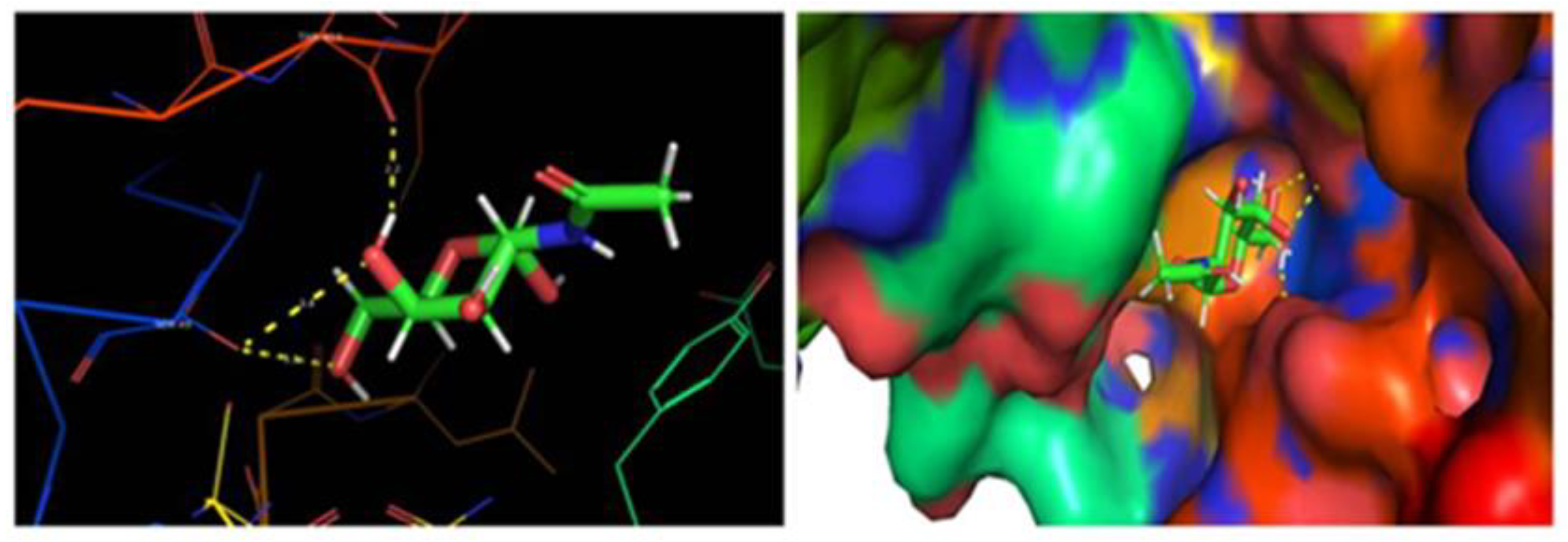
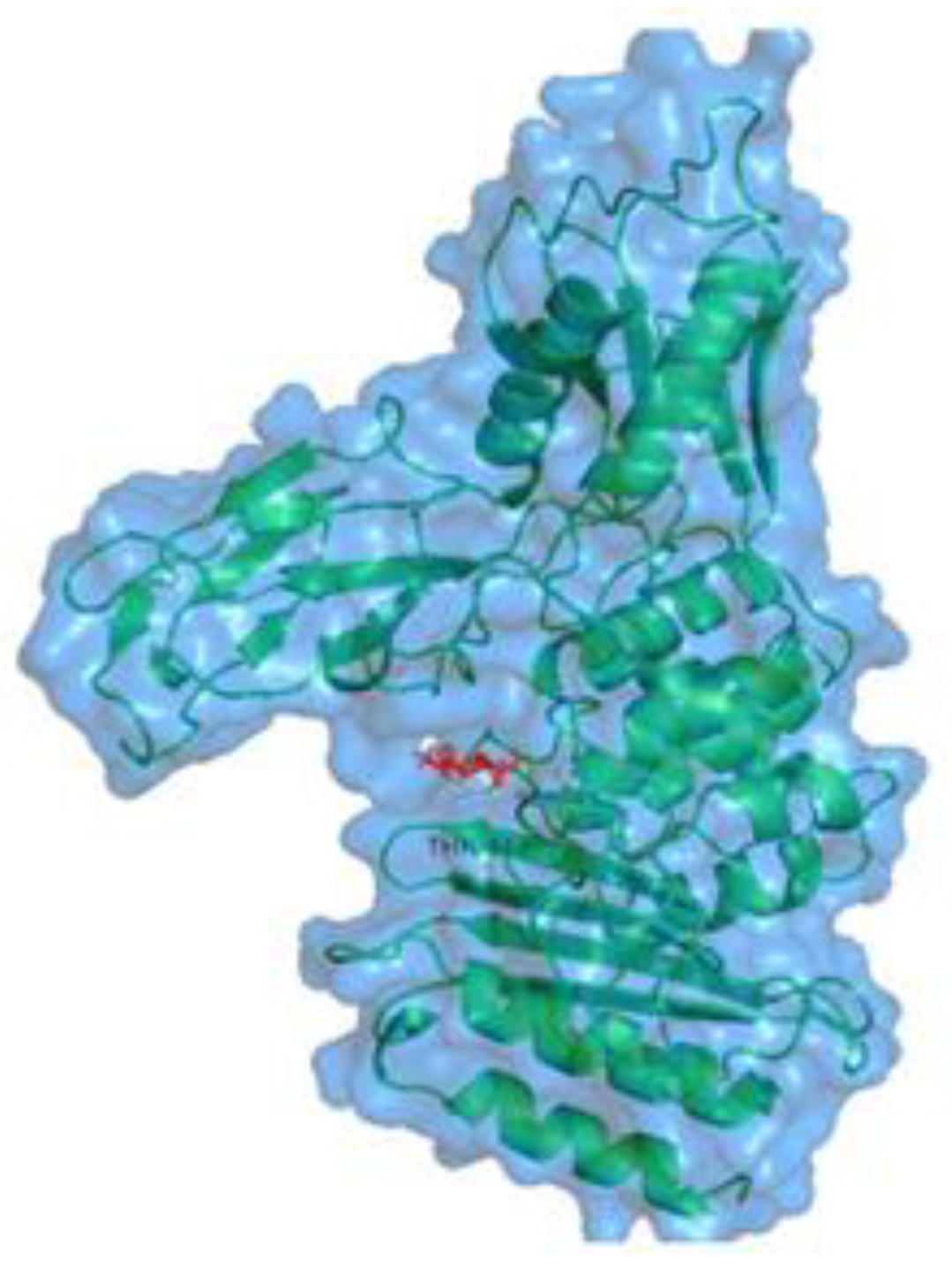
| Binding Energy (kcal/mol) | Ligand Efficiency (kcal/mol) | Inhibitory Constant (Ki) | Hydrogen Bond Interaction |
|---|---|---|---|
| −5.01044 | −0.38 | 239.81 | SER 49 THR 413 |
| Diff Coeff | MLogP | Mw | M_NO | HBDH |
|---|---|---|---|---|
| 0.953 | −2.294 | 221.211 | 7 | 5 |
| Properties | Model Name | Predicted Value |
|---|---|---|
| Absorption | Water solubility | −1.214 mlog mol/L |
| CaCO2 permeability | −0.217 log papp in 10−6 cm/s | |
| Intestinal absorption(human) | 26.824% absorbed | |
| Skin permeability | −3.137 log kp | |
| P glycoprotein substrate | No | |
| P glycoprotein inhibitor I | No | |
| P glycoprotein inhibitor II | No | |
| Distribution | VDss (human) | −0.126 log L/kg |
| Fraction unbound (human) | 0.86 FU | |
| BBB permeability | −0.664 Log(BB) | |
| CNS permeability | −4.043 PS | |
| Metabolism | CYP2D6 substrate | No |
| CYP3A4 substrate | No | |
| CYP1A2 inhibitor | No | |
| CYP2C19 inhibitor | No | |
| CYP2C9 inhibitor | No | |
| CYP2D6 inhibitor | No | |
| CYP3A4 inhibitor | No | |
| Excretion | Total clearance | 0.711 log ml/min/kg |
| Renal OCT2 | No |
| AMES toxicity | No |
| Max tolerated dose (human) | 2.179 log mg/kg/day |
| hERG I inhibitor | No |
| hERG II inhibitor | No |
| Oral rat acute toxicity (LD50) | 1.729 mol/kg |
| Oral rat chronic toxicity (LOAEL) | 3.565 log mg/kg_bw/day |
| Hepatotoxicity | No |
| Skin sensitization | No |
| T. pyriformis toxicity | 0.285 log ug/L |
| Minnow toxicity | 5.272 log nM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijayaraj, R.; Altaff, K.; Lakshmanan, G.; Jayaprakashvel, M.; Mickymaray, S.; Gunapriya, R.; Palanisamy, M.; Alothaim, A.S. Inhibitory Activity of Chitin, (2-Acetamido-2-Deoxy-Hexopyranose) against Penicillin-Binding Proteins of Staphylococcus aureus. Coatings 2022, 12, 1854. https://doi.org/10.3390/coatings12121854
Vijayaraj R, Altaff K, Lakshmanan G, Jayaprakashvel M, Mickymaray S, Gunapriya R, Palanisamy M, Alothaim AS. Inhibitory Activity of Chitin, (2-Acetamido-2-Deoxy-Hexopyranose) against Penicillin-Binding Proteins of Staphylococcus aureus. Coatings. 2022; 12(12):1854. https://doi.org/10.3390/coatings12121854
Chicago/Turabian StyleVijayaraj, Radha, Kareem Altaff, Govindan Lakshmanan, Mani Jayaprakashvel, Suresh Mickymaray, Raghunath Gunapriya, Manikandan Palanisamy, and Abdulaziz S. Alothaim. 2022. "Inhibitory Activity of Chitin, (2-Acetamido-2-Deoxy-Hexopyranose) against Penicillin-Binding Proteins of Staphylococcus aureus" Coatings 12, no. 12: 1854. https://doi.org/10.3390/coatings12121854
APA StyleVijayaraj, R., Altaff, K., Lakshmanan, G., Jayaprakashvel, M., Mickymaray, S., Gunapriya, R., Palanisamy, M., & Alothaim, A. S. (2022). Inhibitory Activity of Chitin, (2-Acetamido-2-Deoxy-Hexopyranose) against Penicillin-Binding Proteins of Staphylococcus aureus. Coatings, 12(12), 1854. https://doi.org/10.3390/coatings12121854







