Corrosion Behaviour of an Epoxy Resin Reinforced with Aluminium Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of the Epoxy Coatings
3. Results and Discussion
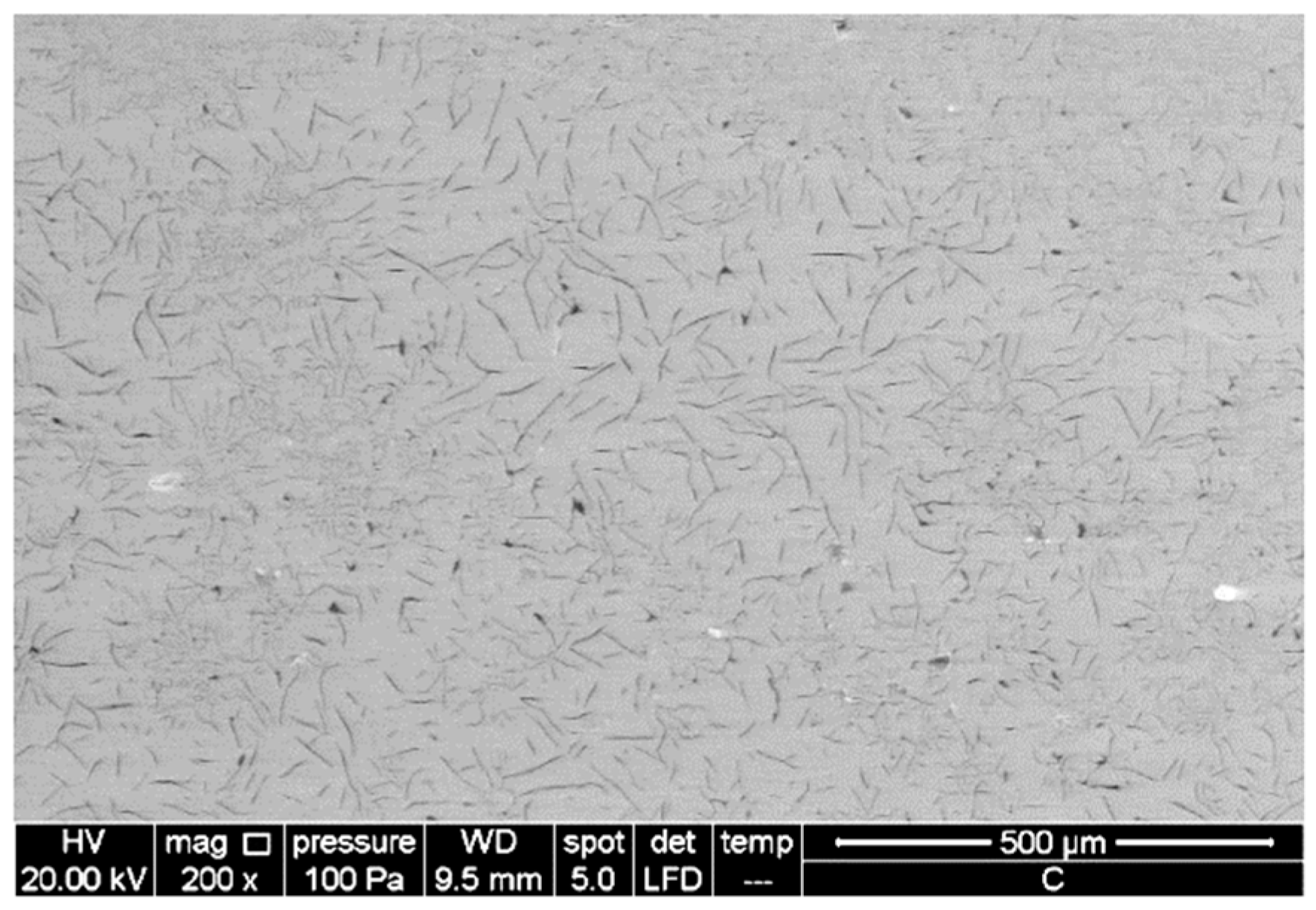
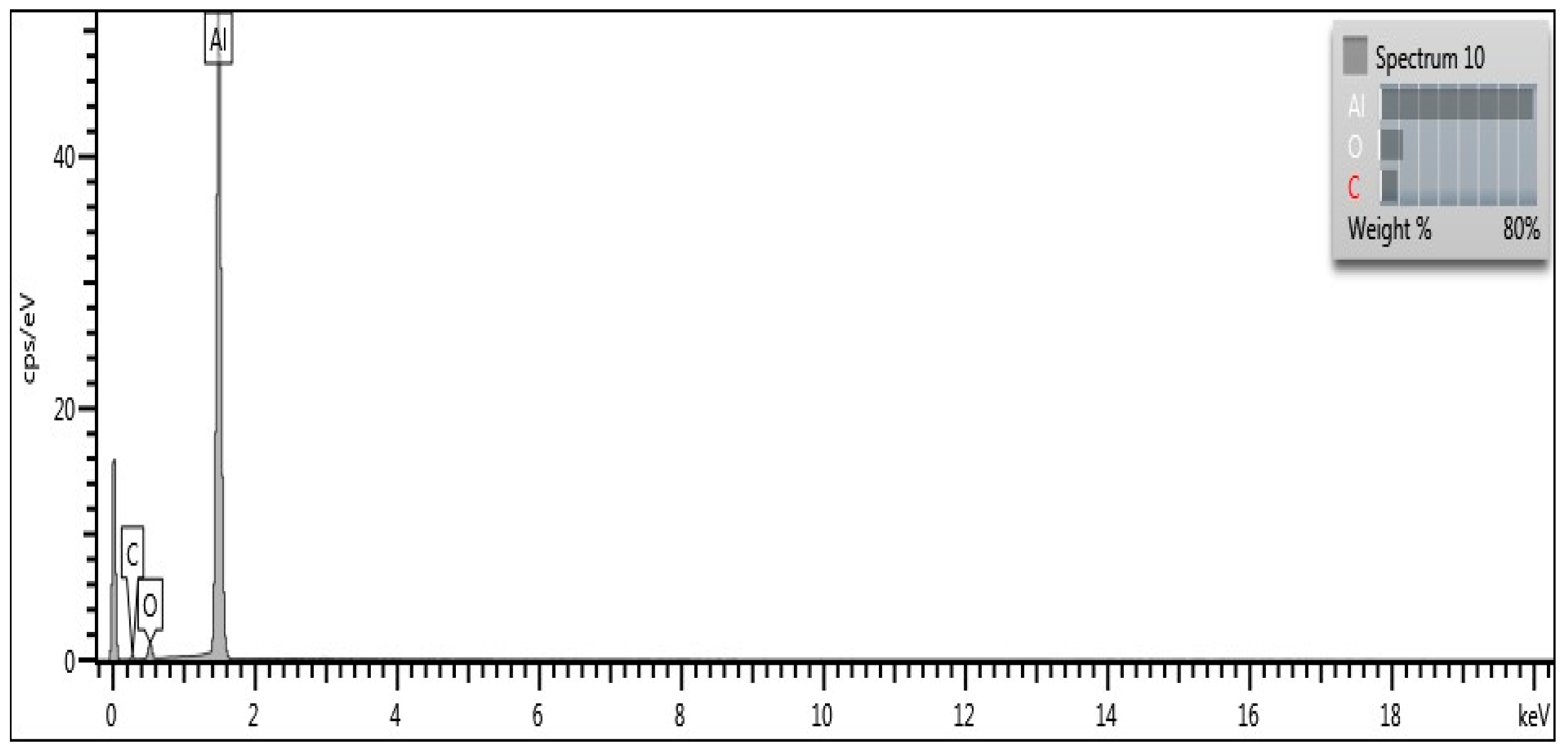
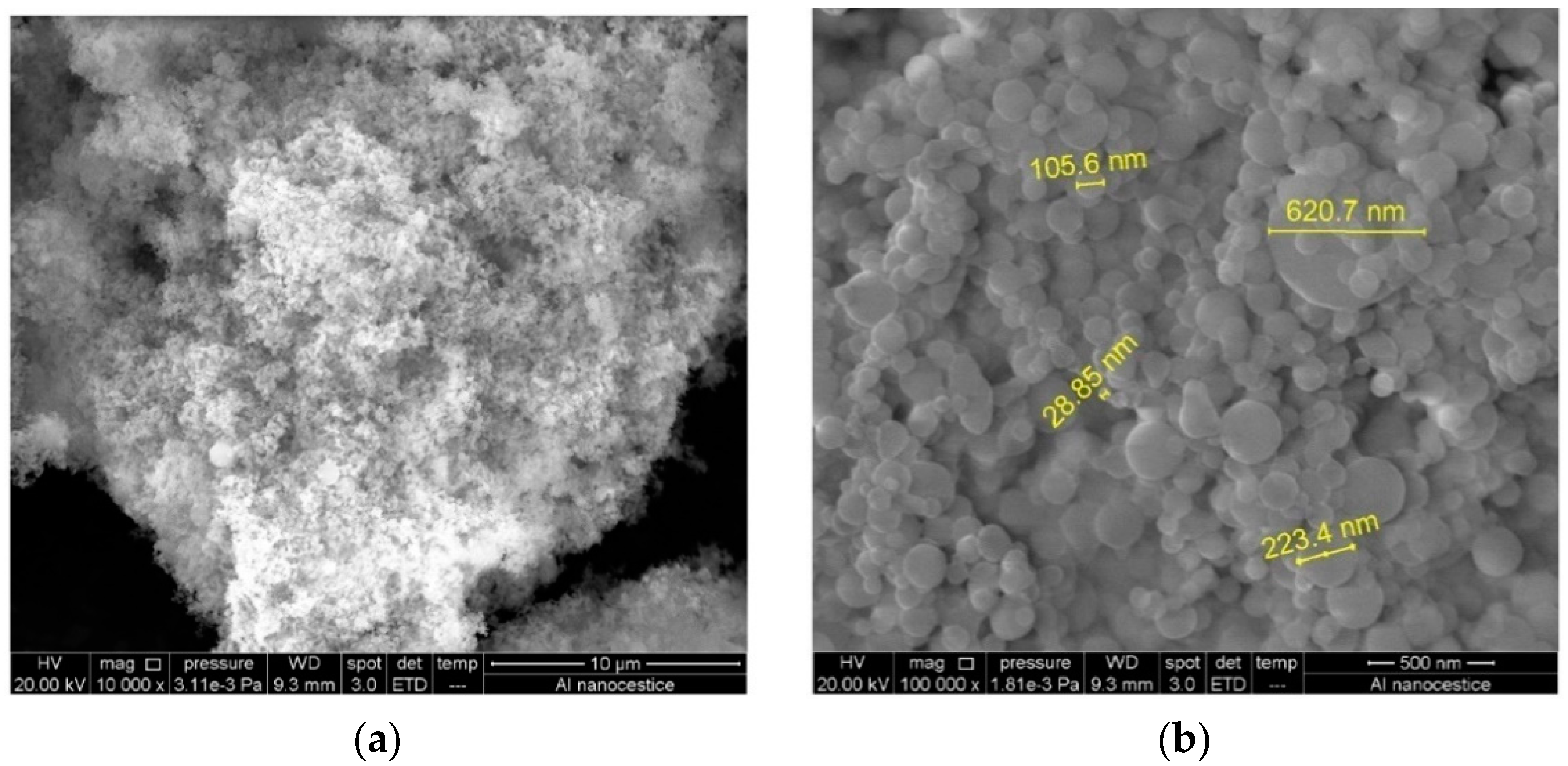
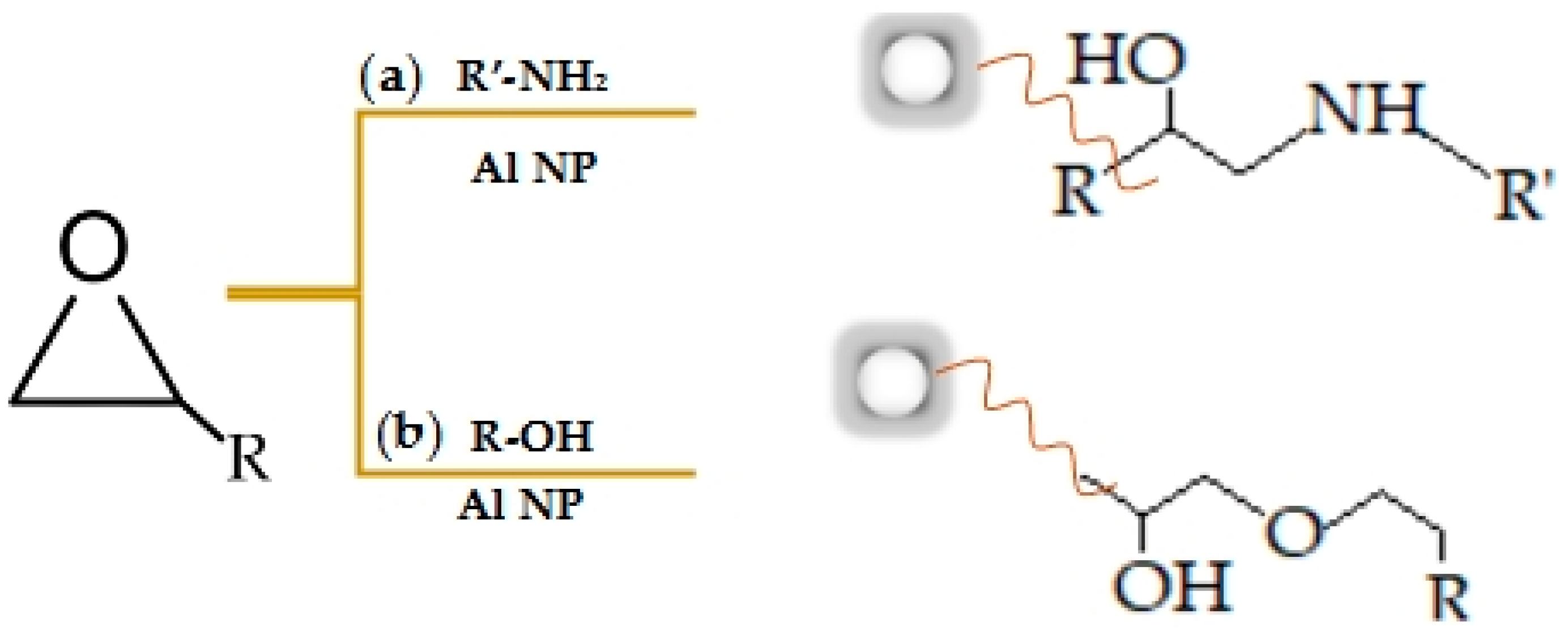
3.1. Characterisation of Aluminium Nanoparticles (Al NP)
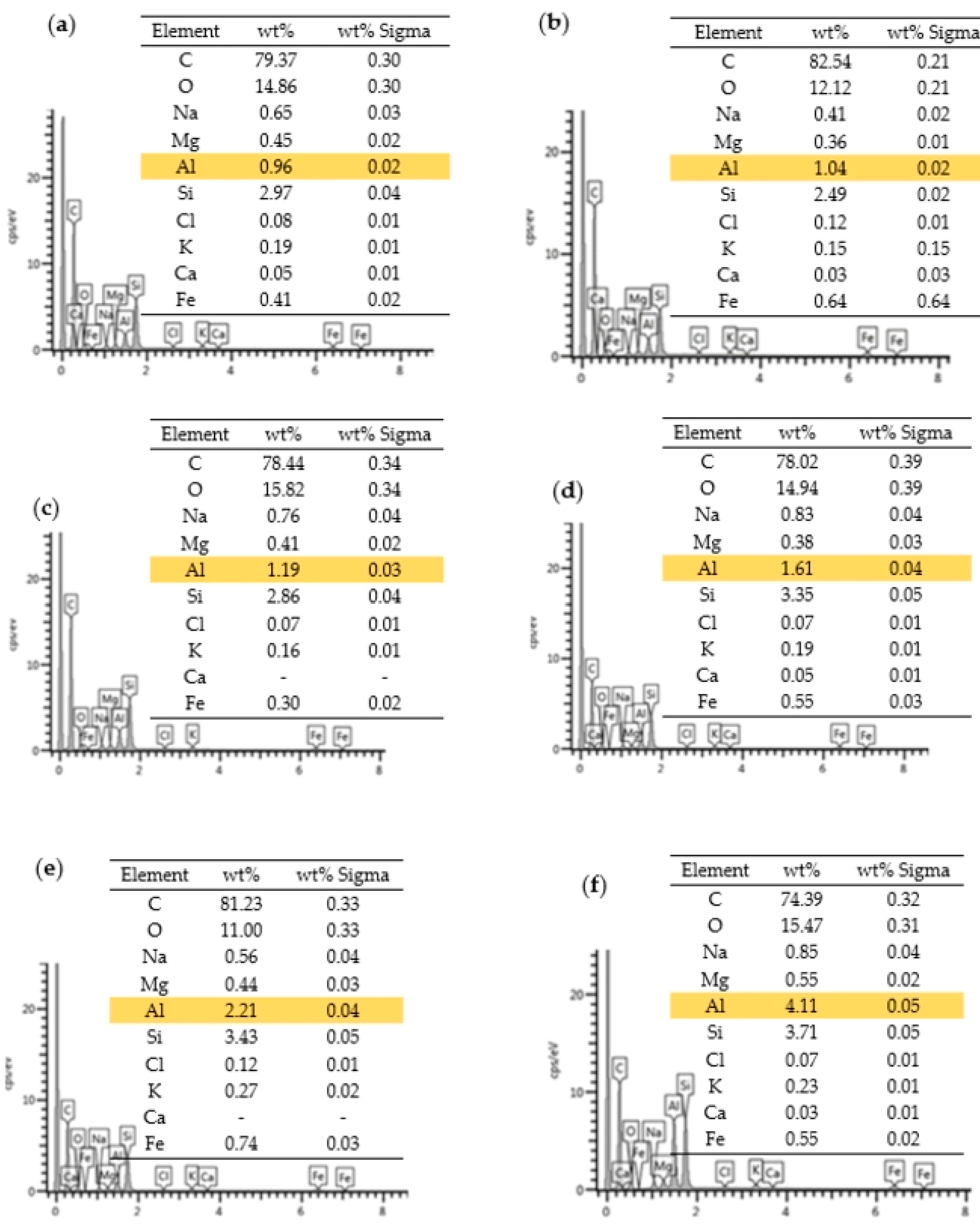
3.2. Characterisation of Nanocomposite
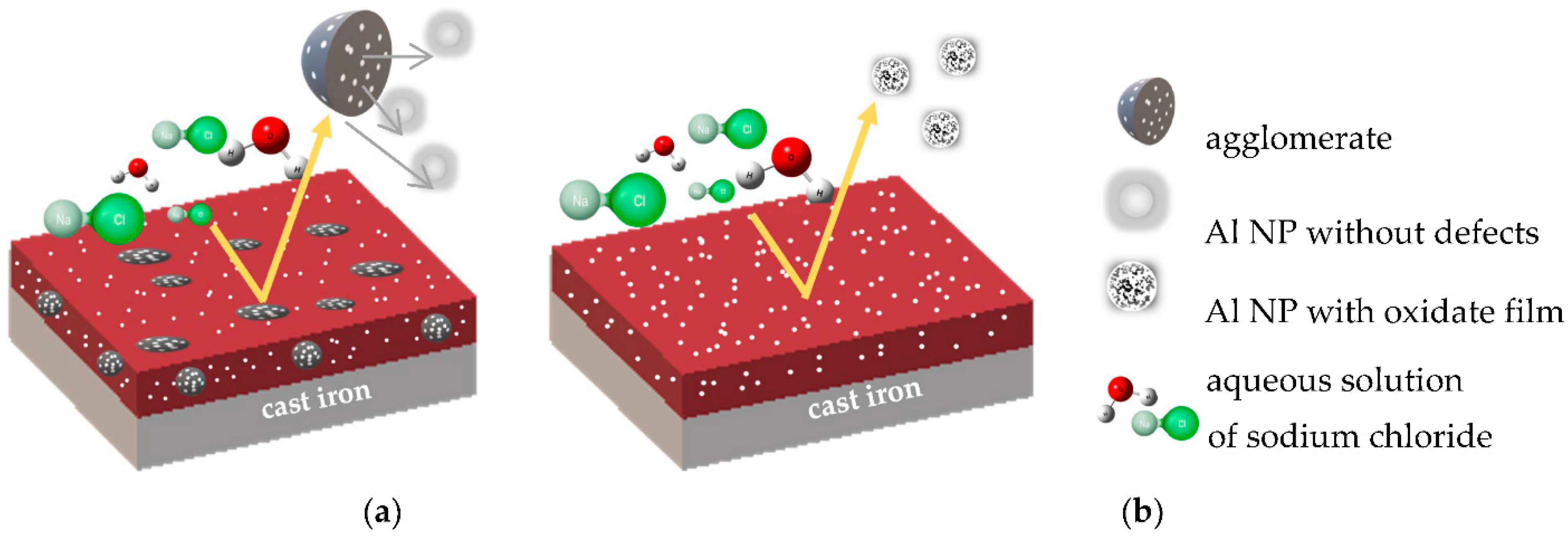
3.3. Physical Properties of Coatings
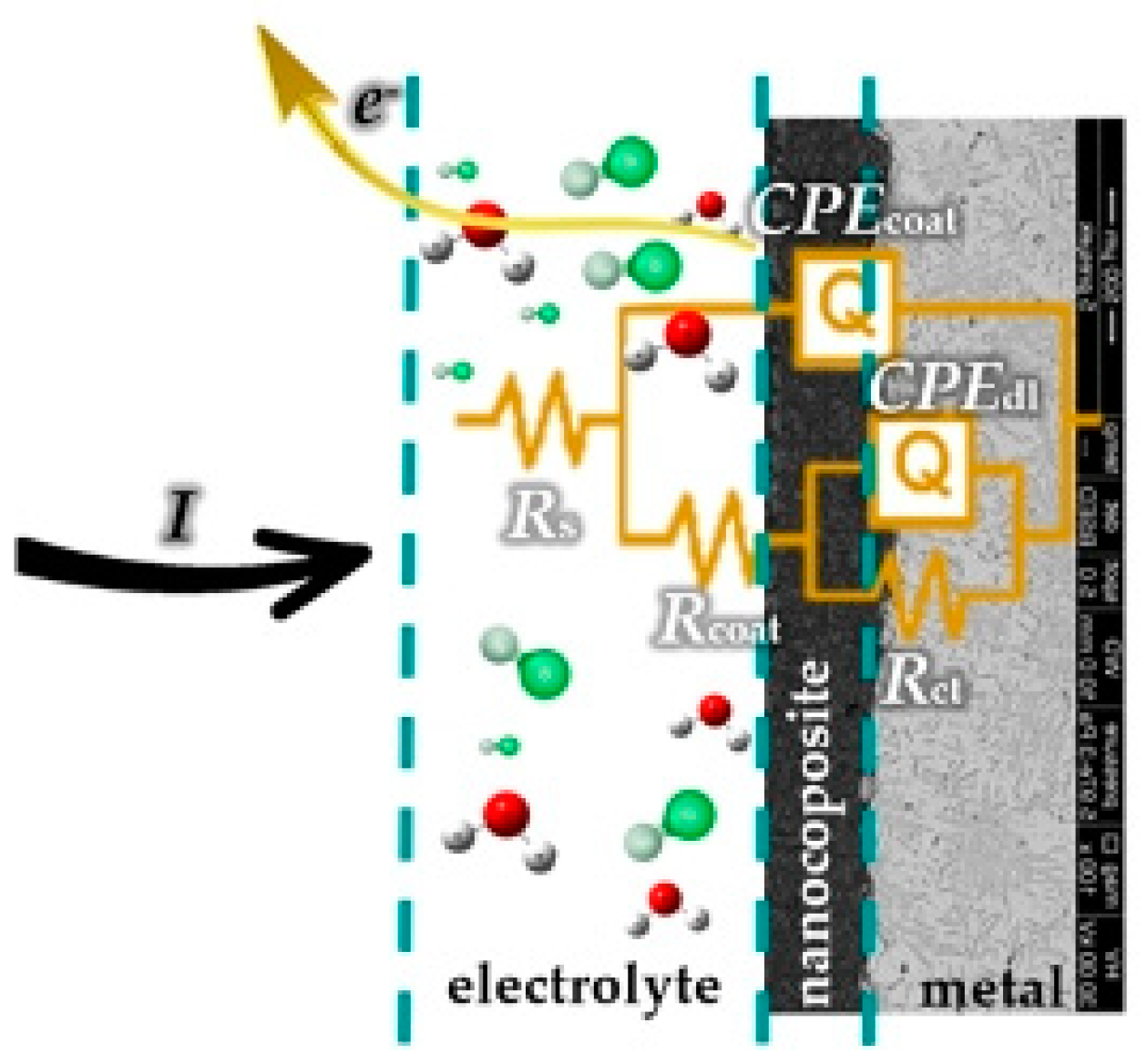
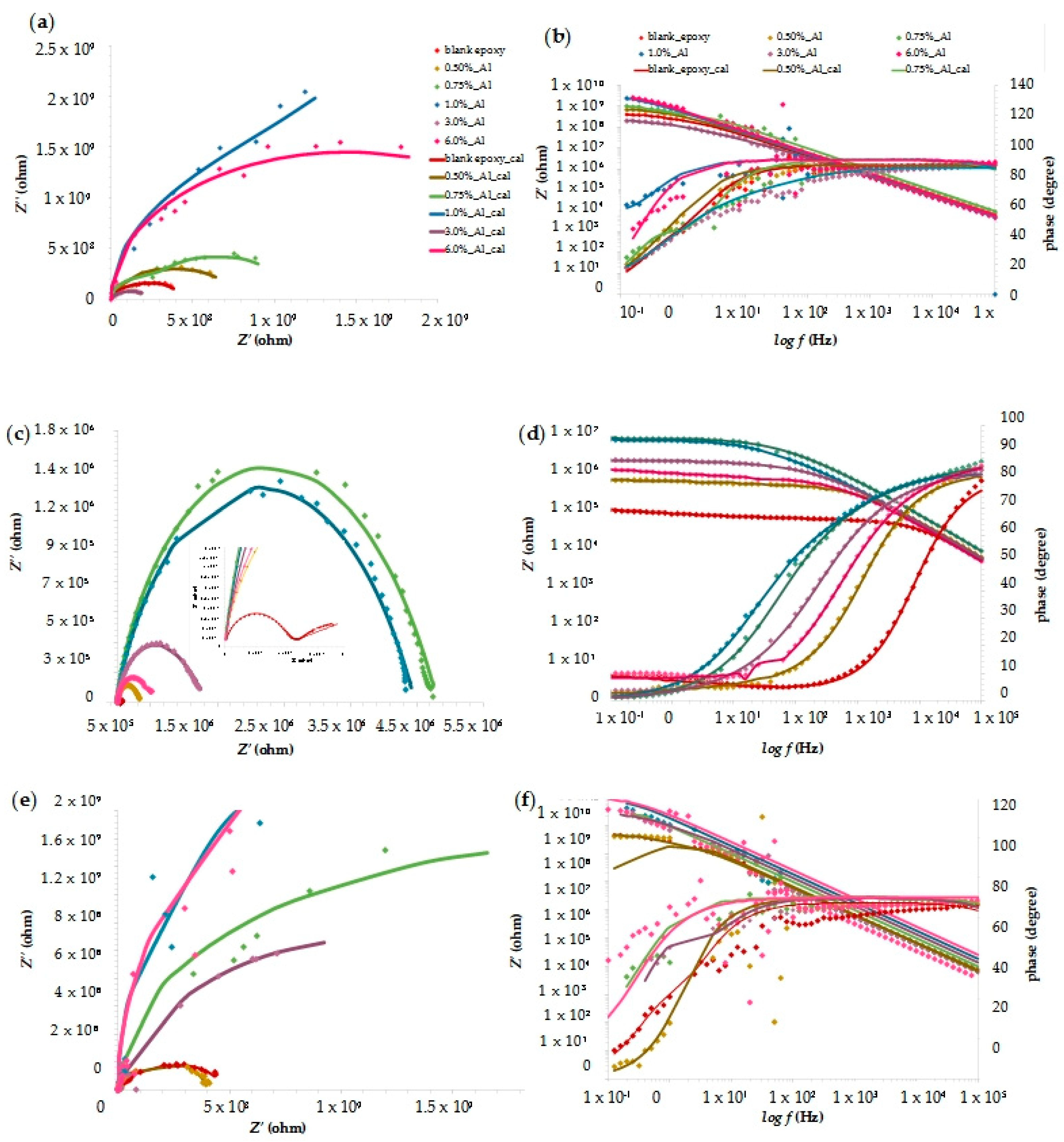
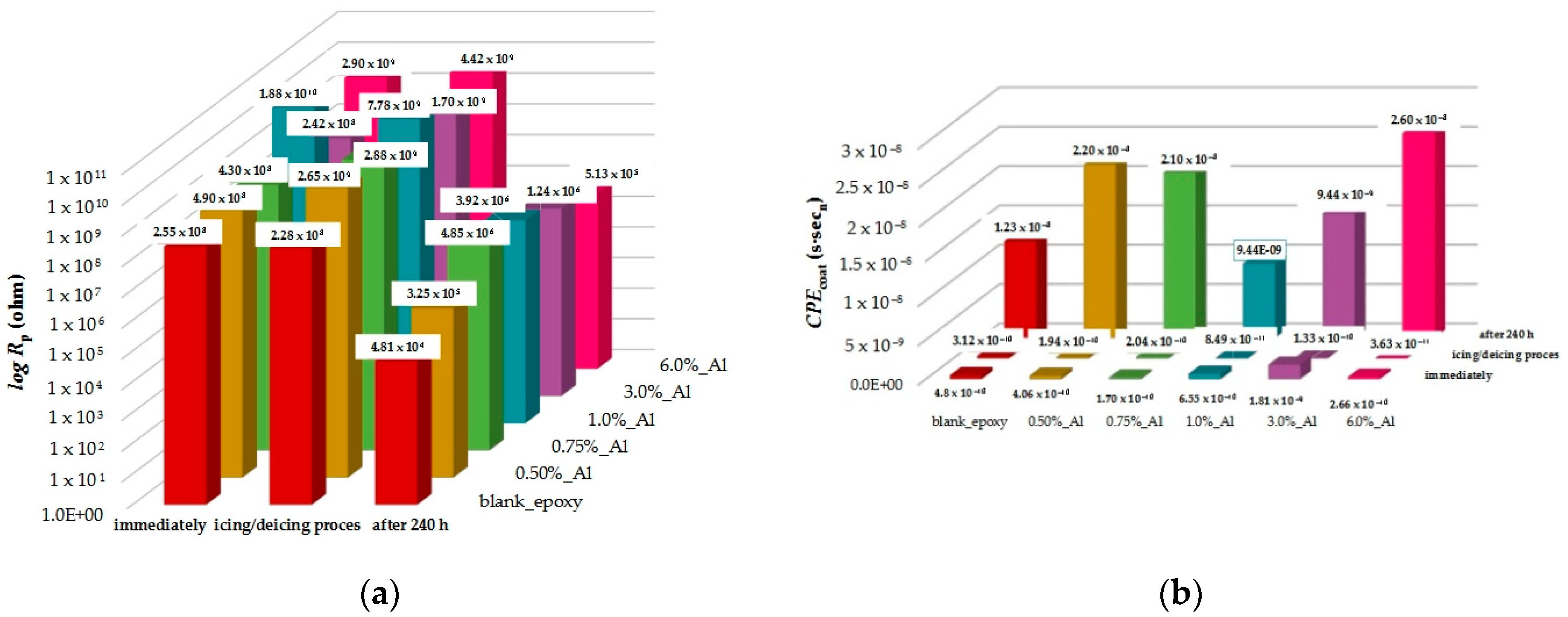
3.4. EIS Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melchers, R.E. Post-perforation external corrosion of cast iron pressurised water mains. Corros. Eng. Sci. Technol. 2017, 52, 541–546. [Google Scholar] [CrossRef]
- Kadhim, L. The Corrosion Behavior and Wear Resistance of Gray Cast Iron. Kufa J. Eng. 2018, 9, 118–132. [Google Scholar] [CrossRef]
- Jur, T.; Middleton, J.; Yurko, A.; Windham, R.; Grey, J. Case Studies in Graphitic Corrosion of Cast Iron Pipe. J. Fail. Anal. Prev. 2021, 21, 376–386. [Google Scholar] [CrossRef]
- Khodaei, P.; Shabani-Nooshabadi, M.; Behpour, M. Epoxy-Based nanocomposite coating reinforced by a zeolite complex: Its anticorrosion properties on mild steel in 3.5 wt % NaCl media. Prog. Org. Coat. 2019, 136, 105254. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Hu, W. Preparation and corrosion behavior of Cu-8-HQ@HNTs/epoxy coating. Prog. Org. Coat. 2020, 139, 105434. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, T.; Ge, Y.; Feng, L.; Feng, H.; Li, P. Multifunctional AgO/epoxy nanocomposites with enhanced mechanical, anticorrosion and bactericidal properties. Prog. Org. Coat. 2021, 152, 106130. [Google Scholar] [CrossRef]
- Guo, D.; Xie, G.; Luo, J. Mechanical properties of nanoparticles: Basics and applications. J. Phys. D. Appl. Phys. 2014, 47, 013001. [Google Scholar] [CrossRef]
- Yuan, H.; Qi, F.; Zhao, N.; Wan, P.; Zhang, B.; Xiong, H.; Liao, B.; Quyang, X. Graphene oxide decorated with titanium nanoparticles to reinforce the anti-corrosion performance of epoxy coating. Coatings 2020, 10, 129. [Google Scholar] [CrossRef]
- Yao, H.; Li, L.; Li, W.; Qi, D.; Fu, W.; Wang, N. Application of nanomaterials in waterborne coatings: A review. Resour. Chem. Mater. 2022, 3, 2772–4433. [Google Scholar] [CrossRef]
- Xavier, J.R. Electrochemical, mechanical and adhesive properties of surface modified NiO-epoxy nanocomposite coatings on mild steel. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2019, 260, 114639. [Google Scholar] [CrossRef]
- Xue, L.; Xu, L.; Li, Q. Effect of nano Al pigment on the anticorrosive performance of waterborne epoxy coatings. J. Mater. Sci. Technol. 2007, 23, 563–567. [Google Scholar]
- Bello, S.A.; Agunsoye, J.O.; Adebisi, J.A.; Hassan, S.B. Effect of aluminium particles on mechanical and morphological properties of epoxy nanocomposites. Acta Period. Technol. 2017, 48, 25–38. [Google Scholar] [CrossRef]
- Penna, M.O.; Silva, A.A.; Rosário, F.F.; De Souza, C.S.; Soares, B.G. Organophilic nano-alumina for superhydrophobic epoxy coatings. Mater. Chem. Phys. 2020, 255, 123543. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, F.C.; Nie, M.; Zhao, S.; Lin, J.; Han, E.H. Influence of Nano-Al Concentrates on the Corrosion Resistance of Epoxy Coatings. J. Mater. Sci. Technol. 2013, 29, 353–358. [Google Scholar] [CrossRef]
- Niroumandrad, S.; Rostami, M.; Ramezanzadeh, B. Effects of combined surface treatments of aluminium nanoparticle on its corrosion resistance before and after inclusion into an epoxy coating. Prog. Org. Coat. 2016, 101, 486–501. [Google Scholar] [CrossRef]
- ISO 945-1; Microstructure of cast itons—Part 1: Graphite classification by visual analysis. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 868; Plastic and ebonite—Determination of indentation hardness by means of a durometer (Shore hardness). International Organization for Standardization: Geneva, Switzerland, 2003.
- Long, J.; Li, C.; Li, Y. Enhancement of Mechanical and Bond Properties of Epoxy Adhesives Modified by SiO2 Nanoparticles with Active Groups. Polymers 2022, 14, 2052. [Google Scholar] [CrossRef]
- Fu, X.; Shen, Z.; Chen, X.; Lin, J.; Cao, H. Influence of element penetration region on adhesion and corrosion performance of Ni-base coatings. Coatings 2020, 10, 895. [Google Scholar] [CrossRef]
- Nascimento Silva, M.; Kassab, E.; Ginoble Pandoli, O.; Leite de Oliveira, J.; Pereira Quintela, J.; Bott, I.S. Corrosion behaviour of an epoxy paint reinforced with carbon nanoparticles. Corros. Eng. Sci. Technol. 2020, 55, 603–608. [Google Scholar] [CrossRef]
- Alam, M.A.; Samad, U.A.; Sherif, E.-S.M.; Poulose, A.M.; Mohammed, J.A.; Alharthi, N.; Saeed, M.; Al-Zahran, S.M. Influence of SiO2 content and exposure periods on the anticorrosion behavior of epoxy nanocomposite coatings. Coatings 2020, 10, 118. [Google Scholar] [CrossRef]
- Njoku, D.I.; Cui, M.; Xiao, H.; Shang, B.; Li, Y. Understanding the anticorrosive protective mechanisms of modified epoxy coatings with improved barrier, active and self-healing functionalities: EIS and spectroscopic techniques. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Margarit-Mattos, I.C.P. EIS and organic coatings performance: Revisiting some key points. Electrochim. Acta. 2020, 354, 136725. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; Alam, M.A.; Al-Zahrani, S.M. Fabrication of different protective coatings and studying their mechanical properties and corrosion behavior in sodium chloride solutions. Int. J. Electrochem. Sci. 2015, 10, 373–387. [Google Scholar]
- Sculy, J.R.; Silverman, D.C.; Kendig, M.W. Electrochemical Impedance: Analysis and Interpretation; ASTM Publication: Philadelphia, PA, USA, 1993. [Google Scholar]
- Liu, X.; Xiong, J.; Lv, Y.; Zuo, Y. Study on corrosion electrochemical behavior of several different coating systems by EIS. Prog. Org. Coat. 2009, 64, 497–503. [Google Scholar] [CrossRef]
- Chilkoor, G.; Sarder, R.; Islam, J.; Arun Kumar, K.E.; Ratnayake, I.; Star, S.; Bharat, K.J.; Sereda, G.; Koratkar, N.; Meyyappan, M.; et al. Maleic anhydride-functionalized graphene nanofillers render epoxy coatings highly resistant to corrosion and microbial attack. Carbon N. Y. 2020, 159, 586–597. [Google Scholar] [CrossRef]
- Feichtenschlager, B.; Pabisch, S.; Svehla, J.; Peterlik, H.; Sajjad, M.; Koch, T.; Kickelbick, G. Epoxy Resin Nanocomposites: The Influence of Interface Modification on the Dispersion Structure—A Small-Angle-X-ray-Scattering Study. Surfaces 2020, 3, 664–682. [Google Scholar] [CrossRef]
- Schmidt, R.G.; Bell, J.P. Epoxy Adhesion to Metals. Adv. Polym. 1986, 75, 33–71. [Google Scholar]
- Vargel, C. Corrosion of Aluminium, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Karlsson, P.; Palmqvist, A.E.C.; Holmberg, K. Surface modification for aluminium pigment inhibition. Adv. Colloid Interface Sci. 2006, 128–130, 121–134. [Google Scholar] [CrossRef]
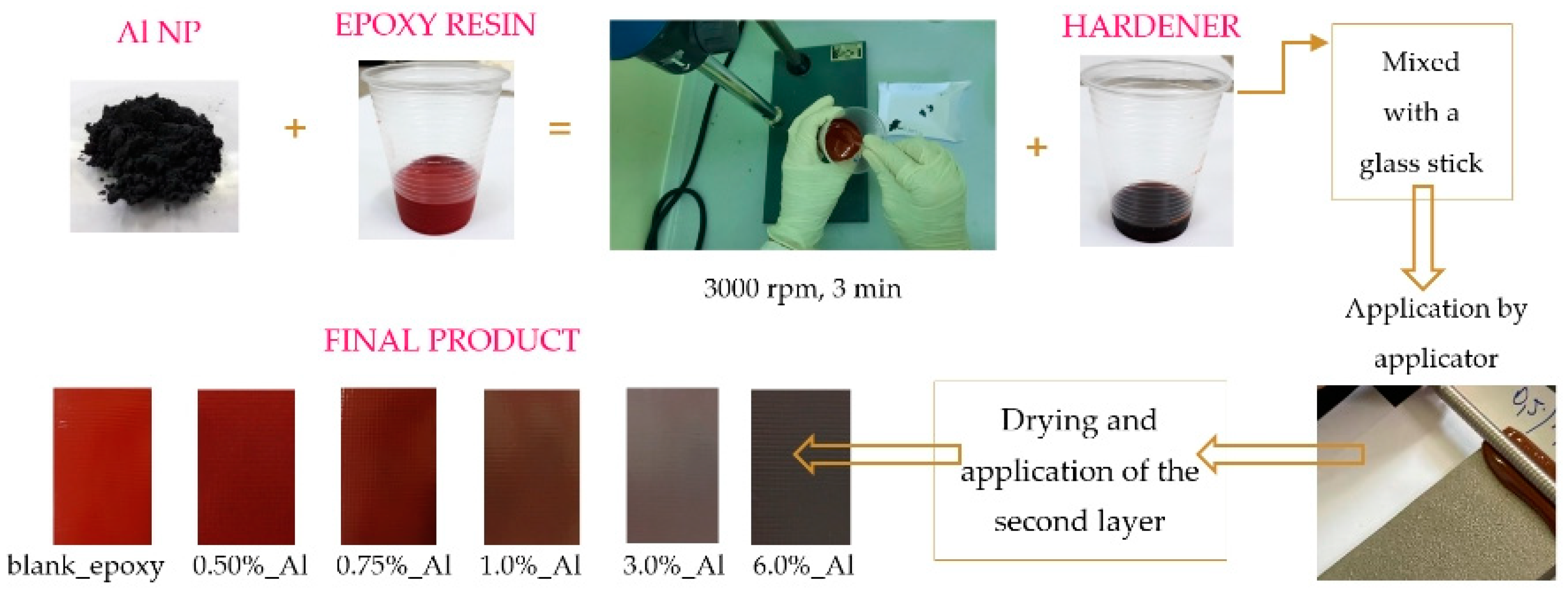
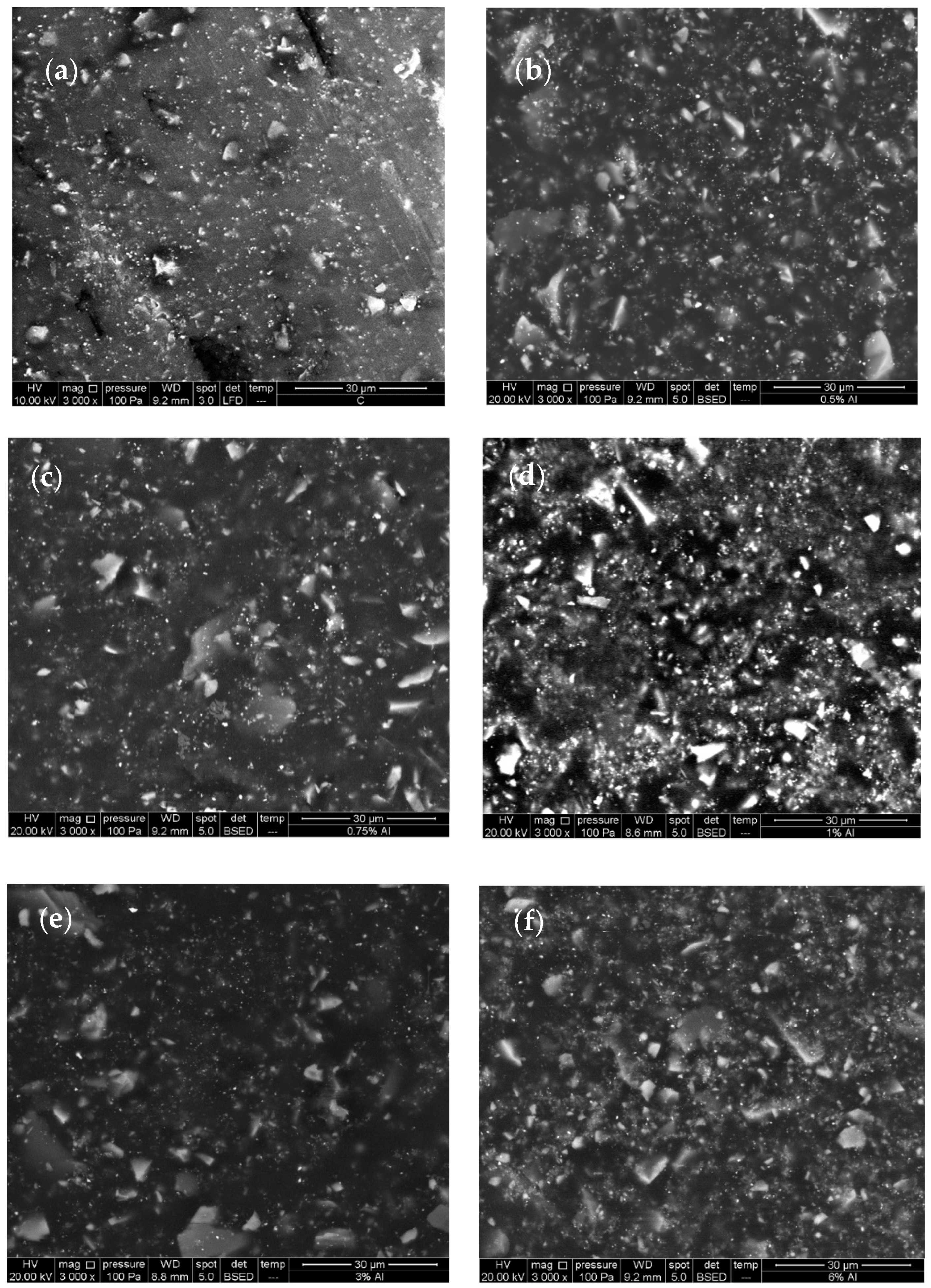
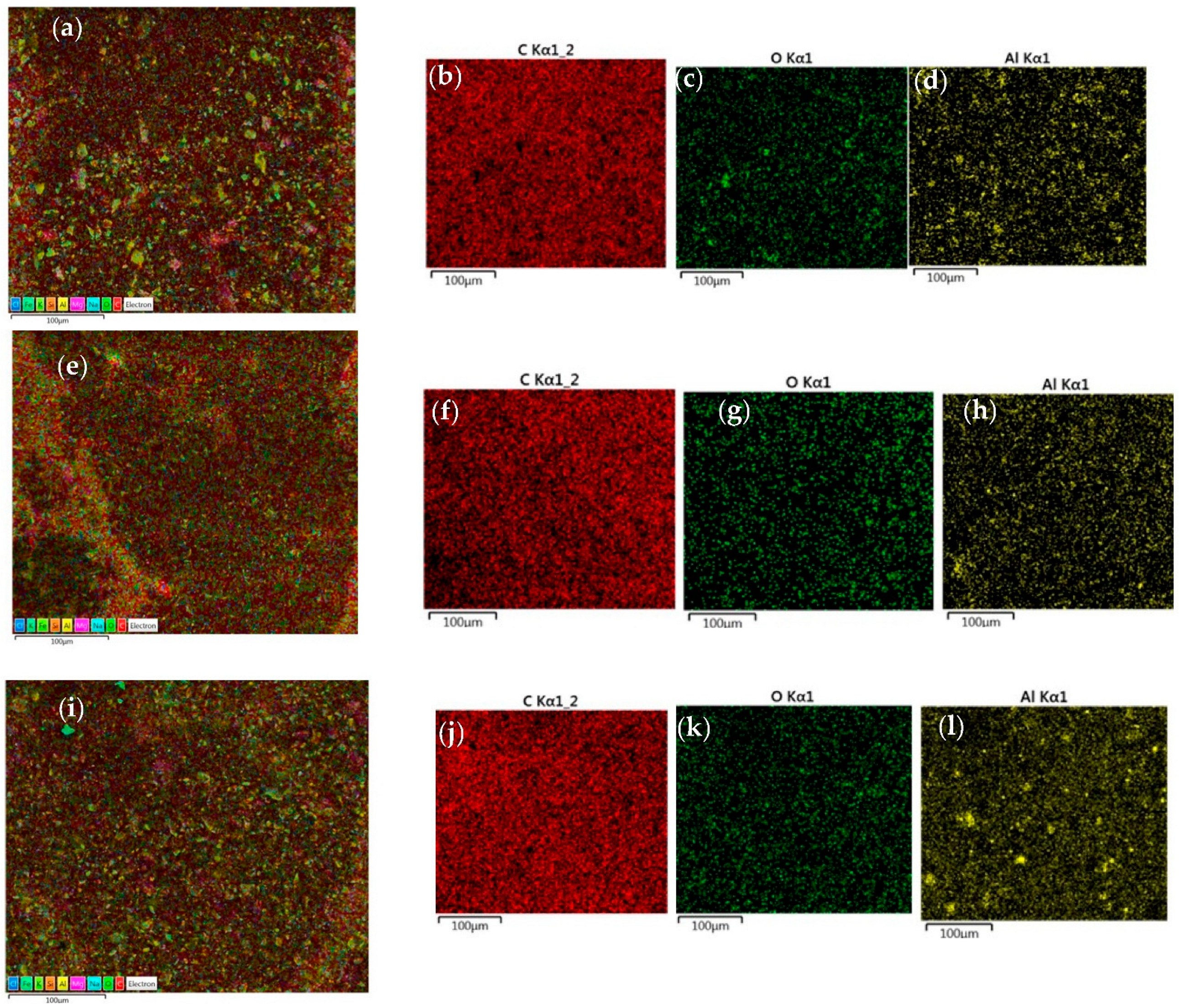
| Sample | Epoxy Resin (g) | Hardener (g) | Al NP (%) | Thickness (μm) |
|---|---|---|---|---|
| blank_epoxy | 30 | 7.5 | 0 | 207.2 |
| 0.50%_Al | 30 | 7.5 | 0.50 | 228.1 |
| 0.75%_Al | 30 | 7.5 | 0.75 | 235.4 |
| 1.0%_Al | 30 | 7.5 | 1.0 | 229.7 |
| 3.0%_Al | 30 | 7.5 | 3.0 | 212.3 |
| 6.0%_Al | 30 | 7.5 | 6.0 | 226.7 |
| Sample | m (Al NP), g | EDS(Al), % |
|---|---|---|
| blank epoxy | 0.0000 | 0.96 |
| 0.50%_Al | 0.1884 | 1.04 |
| 0.75%_Al | 0.2915 | 1.19 |
| 1.0%_Al | 0.3794 | 1.61 |
| 3.0%_Al | 1.1598 | 2.21 |
| 6.0%_Al | 2.3936 | 4.11 |
| Samples | Blank-Epoxy | 0.50%_Al | 0.75%_Al | 1.0%_Al | 3.0%_Al | 6.0%_Al |
|---|---|---|---|---|---|---|
| non-exposed samples | RAL 3013 | RAL 8015 | RAL 8016 | RAL 8017 | 8019 | RAL 7015 |
| icing/deicing process | RAL 3013 | RAL 8015 | RAL 8016 | RAL 8017 | 8019 | RAL 7015 |
| Samples | Blank-Epoxy | 0.50%_Al | 0.75%_Al | 1.0%_Al | 3.0%_Al | 6.0%_Al |
|---|---|---|---|---|---|---|
| non-exposed samples | 83.4 | 83.0 | 83.6 | 83.4 | 84.4 | 83.5 |
| icing/deicing process | 82.0 | 83.6 | 80.6 | 80.8 | 82.8 | 83.2 |
| Samples/MPa | Blank-Epoxy | 0.50%_Al | 0.75%_Al | 1.0%_Al | 3.0%_Al | 6.0%_Al |
|---|---|---|---|---|---|---|
| non-exposed samples | 8.34 | 13.00 | 15.56 | - | 13.97 | 12.14 |
| 8.31 | 13.24 | 14.49 | - | 13.45 | 10.93 | |
| icing/deicing process | 9.53 | 14.01 | 14.89 | - | 12.84 | 10.57 |
| 8.98 | 13.58 | 14.47 | - | 13.25 | 13.01 |
| Samples | 0.50%_Al | 0.75%_Al | 1.0%_Al | 3.0%_Al | 6.0%_Al |
|---|---|---|---|---|---|
| immediately | 47.96 | 40.70 | 98.64 | - | 91.21 |
| after 240 h | 85.20 | 99.01 | 98.77 | 96.12 | 90.62 |
| icing/deicing process | 91.40 | 92.08 | 97.07 | 86.59 | 94.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samardžija, M.; Alar, V.; Špada, V.; Stojanović, I. Corrosion Behaviour of an Epoxy Resin Reinforced with Aluminium Nanoparticles. Coatings 2022, 12, 1500. https://doi.org/10.3390/coatings12101500
Samardžija M, Alar V, Špada V, Stojanović I. Corrosion Behaviour of an Epoxy Resin Reinforced with Aluminium Nanoparticles. Coatings. 2022; 12(10):1500. https://doi.org/10.3390/coatings12101500
Chicago/Turabian StyleSamardžija, Marina, Vesna Alar, Vedrana Špada, and Ivan Stojanović. 2022. "Corrosion Behaviour of an Epoxy Resin Reinforced with Aluminium Nanoparticles" Coatings 12, no. 10: 1500. https://doi.org/10.3390/coatings12101500
APA StyleSamardžija, M., Alar, V., Špada, V., & Stojanović, I. (2022). Corrosion Behaviour of an Epoxy Resin Reinforced with Aluminium Nanoparticles. Coatings, 12(10), 1500. https://doi.org/10.3390/coatings12101500






