Influence of Pre-Tinning Process on Coating Morphology and Interface Structure of Low Carbon Steel Dipped in Molten 6061 Al Alloy
Abstract
1. Introduction
2. Experimental
2.1. Fluxing and Pre-Tinning Process
2.2. Hot-Dip Process
2.3. Characterization
3. Results and Discussion
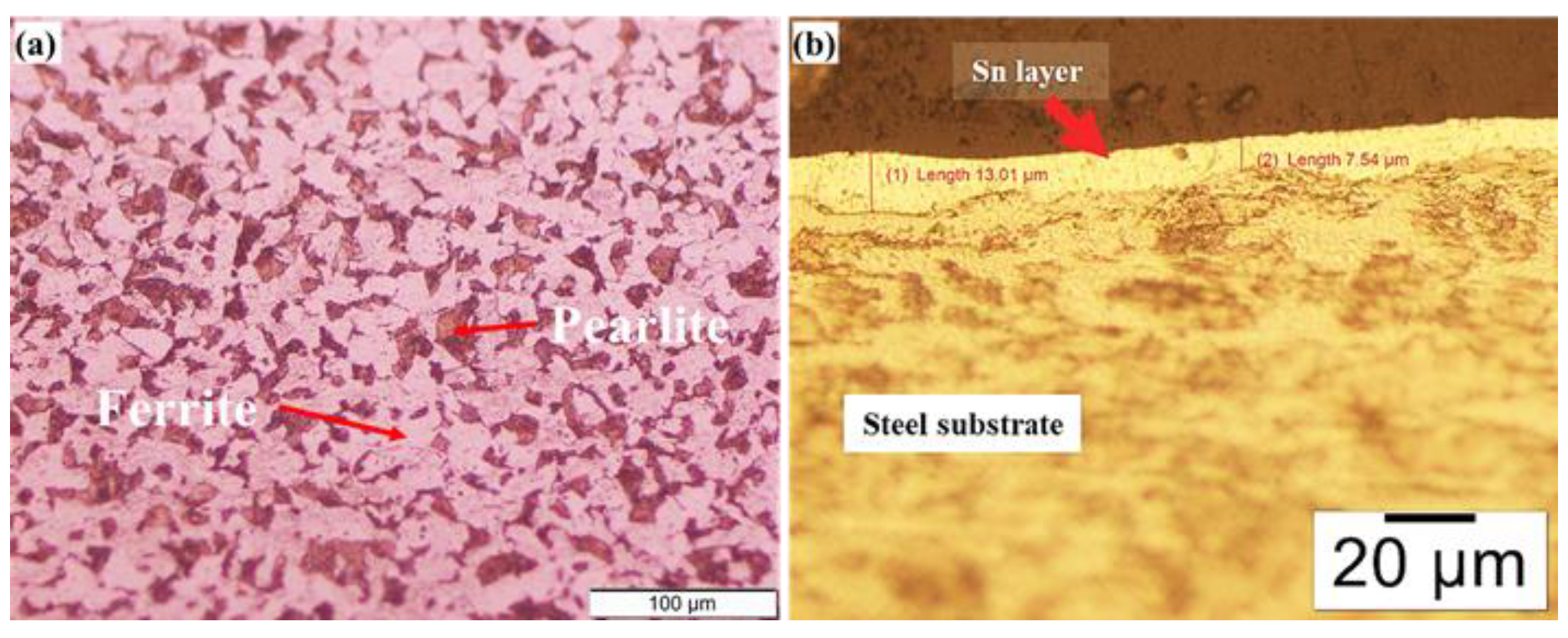
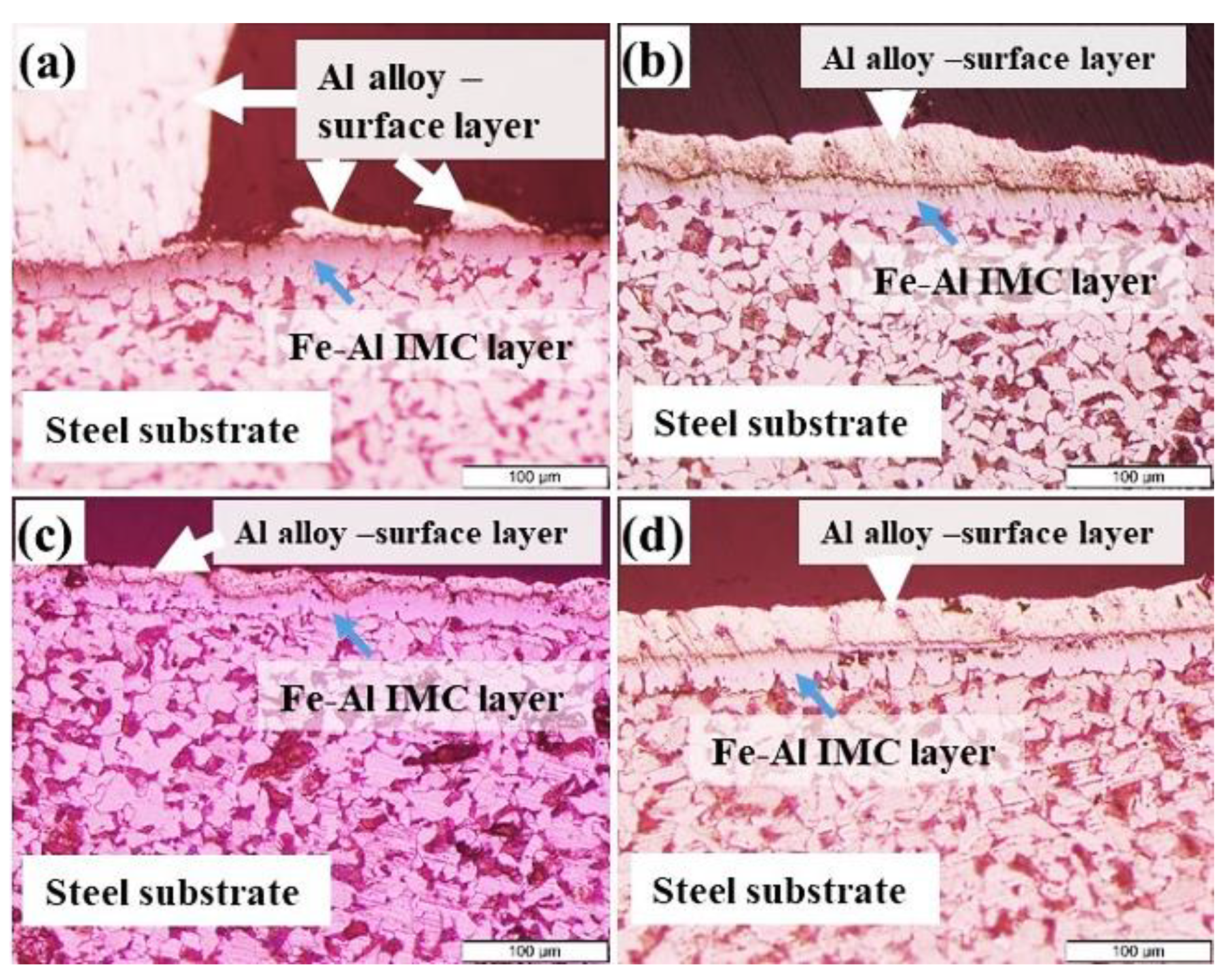
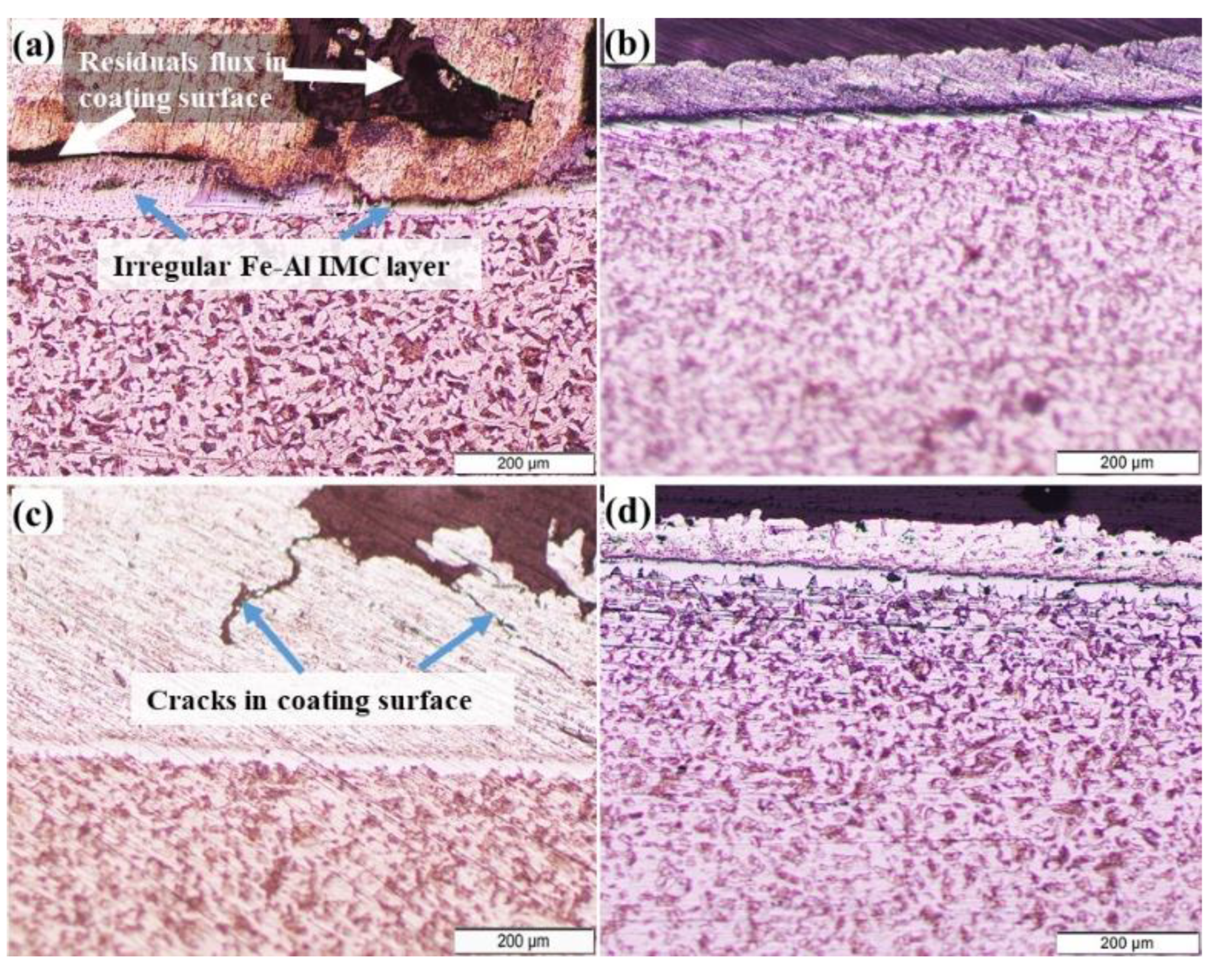
3.1. Microstructural Changes Accompanying Coating Process
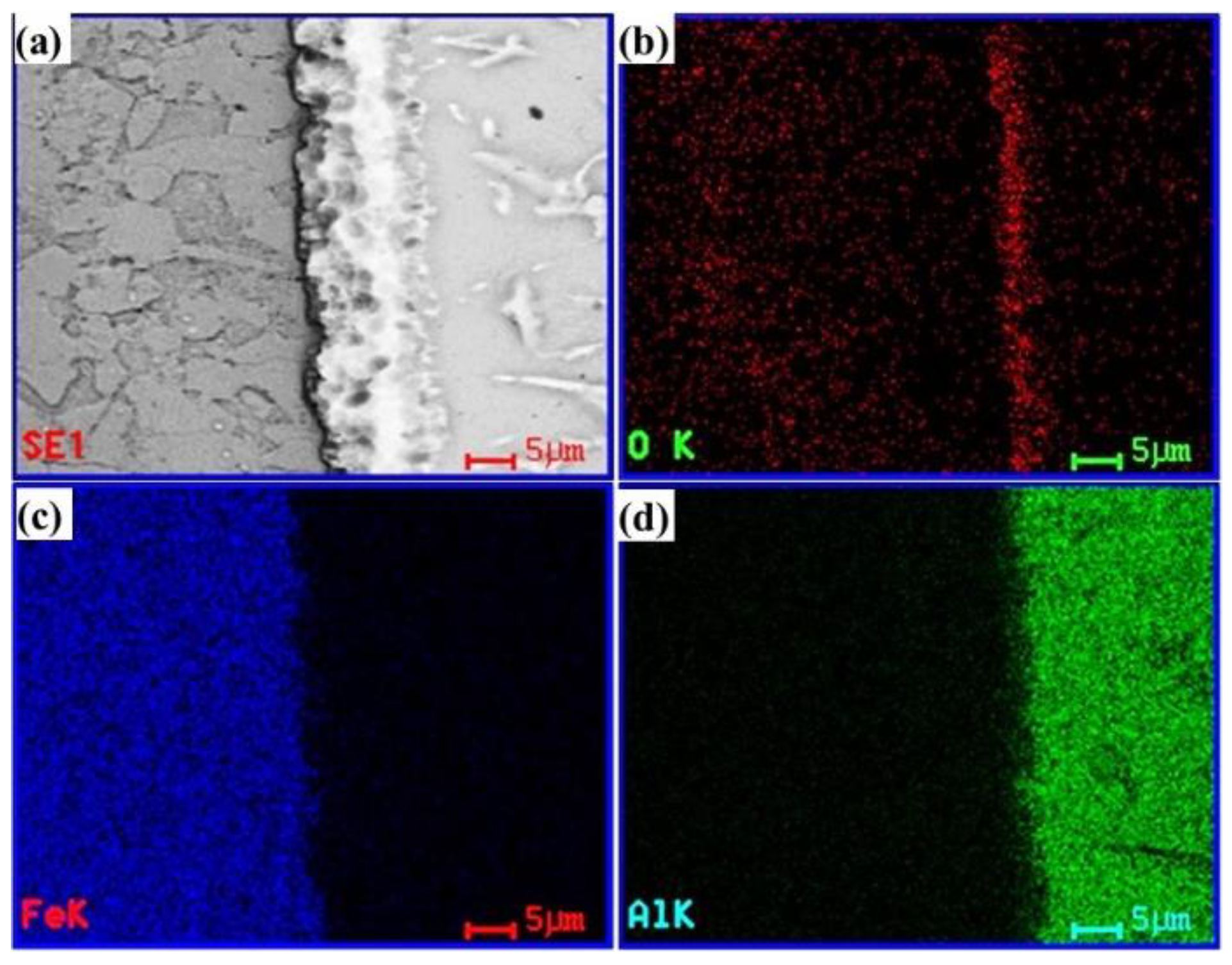
3.2. Mechanism of Tinning-Dipping Processes
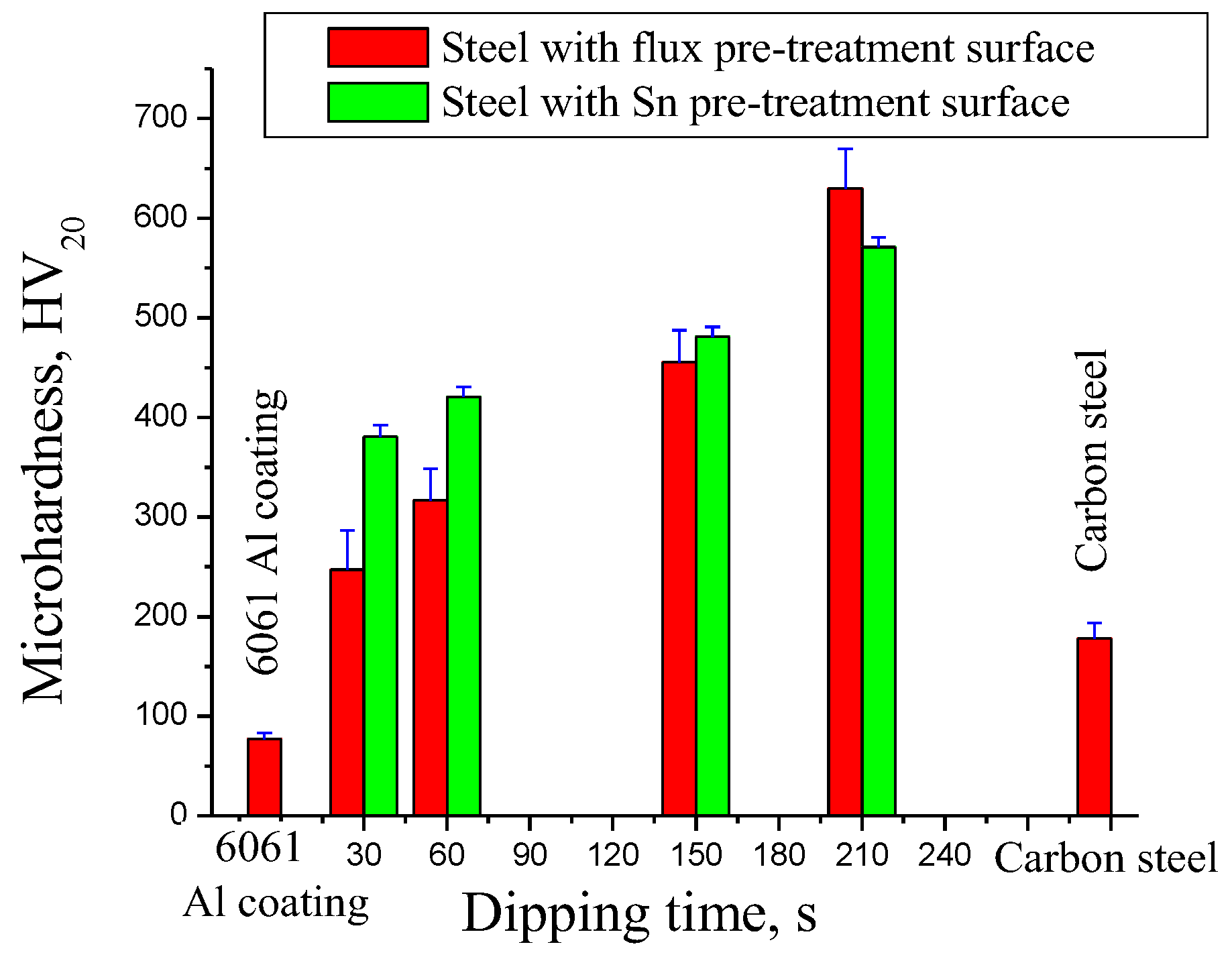
3.3. Interfacial Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dey, P.P.; Modak, P.; Banerjee, P.; Chakrabarti, D.; Seikh, A.H.; Abdo, H.S.; Luqman, M.; Ghosh, M. Studies on the characterization and morphological features of the coating on interstitial free steel dipped in molten Al-Si-Mg alloy at 800 °C. J. Mater. Res. Technol. 2020, 9, 4788–4805. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Wang, C.-J. Observation of high-temperature phase transformation in the Si-modified aluminide coating on mild steel using EBSD. Mater. Charact. 2010, 61, 467–473. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Wang, C.-J. Microstructural evolution of intermetallic layer in hot-dipped aluminide mild steel with silicon addition. Surf. Coat. Technol. 2011, 205, 4726–4731. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Tsaur, C.C.; Rock, J.C. Rock. Microstructure studies of an aluminide coating on 9Cr-1Mo steel during high temperature oxidation. Surf. Coat. Technol. 2006, 200, 6588. [Google Scholar] [CrossRef]
- Wang, C.J.; Chen, S.M. The high-temperature oxidation behavior of hot-dipping Al–Si coating on low carbon steel. Surf. Coat. Technol. 2006, 200, 6601. [Google Scholar] [CrossRef]
- Richards, R.W.; Jones, R.D.; Clements, P.D.; Clarke, H. Metallurgy of continuous hot dip aluminizing. Int. Mater. Rev. 1994, 39, 191. [Google Scholar] [CrossRef]
- Khaliq, A.; Alghamdi, A.S.; Ramadan, M.; Subhani, T.; Rajhi, W.; Haider, W.; Hasan, M.M. Intermetallic compounds formation during 316L stainless steel reaction with Al–Zn–Si coating alloy. Crystals 2022, 12, 735. [Google Scholar] [CrossRef]
- Dey, P.P.; Modak, P.; Ghosh, A.; Chakrabarti, D.; Banerjee, P.; Ghosh, M. Investigation of phase evolution of Al–Si–Mg coating on hot dipped interstitial-free steel. Results Mater. 2020, 6, 100078. [Google Scholar] [CrossRef]
- Bahadur, A.; Mohanti, O.N. Aluminium diffusion coating on medium carbon steel. Mater. Trans. JIM 1995, 36, 1170–1175. [Google Scholar] [CrossRef]
- Cheng, W.J.; Wang, C.J. EBSD study of crystallographic identification on Fe-Al-Si intermetallic phases in aluminide coatings on mild steels. Adv. Mater. Res. 2009, 79–82, 907–910. [Google Scholar] [CrossRef]
- Ramadan, M.; Alghamdi, A.; Hafez, K.; Subhani, T.; Halim, K.A. Development and optimization of tin/flux mixture for direct tinning and interfacial bonding in aluminum/steel bimetallic compound casting. Materials 2020, 13, 5642. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.; Alghamdi, A.S.; Subhani, T.; Halim, K.S.A. Fabrication and characterization of Sn-based babbitt alloy nanocomposite reinforced with Al2O3 nanoparticles/carbon steel bimetallic material. Materials 2020, 13, 2759. [Google Scholar] [CrossRef]
- Ramadan, M.; Hafez, K.M.; Alghamdi, A.S.; Ayadi, B.; Halim, K.S.A. Correction to: Novel approach for using ductile iron as substrate in bimetallic materials for higher interfacial bonding bearings. Int. J. Met. 2021, 16, 1001. [Google Scholar] [CrossRef]
- Alghamdi, A.S.; Halim, K.S.A.; Amin, M.A.; Alshammari, A.S.; Fathy, N.; Ramadan, M. Interfacial microstructure and corrosion behaviour of mild steel coated with alumina nanoparticles doped tin composite via direct tinning route. Coatings 2021, 11, 1318. [Google Scholar] [CrossRef]
- Halim, K.S.A.; Ramadan, M.; Sherif, E.-S.M.; Hafez, K.M.; Subhani, T.; Fathy, N.; Alghamdi, A.S.; Khedr, M.H. Enhancement of surface and interface properties of low carbon steel by hybrid ZnO and NiO nanoparticles reinforced tin coating. Crystals 2022, 12, 332. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Y.; Hu, Z.; Zhang, X.; Wu, S.; Wang, R.; Zhu, Y. A novel electrodeposition route for fabrication of the superhydrophobic surface with unique self-cleaning, mechanical abrasion and corrosion resistance properties. Chem. Eng. J. 2016, 303, 37–47. [Google Scholar] [CrossRef]
- Huang, J.; Lou, C.; Xu, D.; Lu, X.; Xin, Z.; Zhou, C. Cardanol-based polybenzoxazine superhydrophobic coating with improved corrosion resistance on mild steel. Prog. Org. Coat. 2019, 136, 105191. [Google Scholar] [CrossRef]
- Gines, J.L.; Benítez, G.J.; Egli, W.; Zubimendi, J.L.; Pérez, T. Formation of an Fe–Sn intermetallic layer during the reflow, process after tin plating. Plat. Surf. Finish. 2003, 90, 44–49. [Google Scholar]
- Kumar, K.; Wollants, P.; Delaey, L. Thermodynamic evaluation of Fe–Sn phase diagram. Calphad 1996, 20, 139–149. [Google Scholar] [CrossRef]
- Ramadan, M.; Khaliq, A.; Hafez, K.M.; Alghamdi, A.S.; Fathy, N.; Harraz, F.A.; Ayadi, B.; Halim, K.S.A. Super bonding strength of Al2O3 nanoparticles reinforced Sn interlayer steel/aluminum bimetal casting. Crystals 2022, 12, 324. [Google Scholar] [CrossRef]
- Durandet, Y.; Strezov, L.; Ebrill, N. Formation of Al–Zn–Si coatings on low carbon steel substrates. Galvatech 1998, 98, 147–152. [Google Scholar]
- Raghavan, V. Al–Fe–Si (aluminum–iron–silicon). J. Phase Equilibria 2002, 23, 184–188. [Google Scholar] [CrossRef]
- Dionisio, P.H.; de Barros, B.A.S., Jr.; Baumvol, I.J.R. Intermetallic phases formed during tin implantation into iron and steels. J. Appl. Phys. 1984, 55, 4219–4224. [Google Scholar] [CrossRef]






| C | Si | Mn | Cu | Cr | Mg | Fe | Al | |
|---|---|---|---|---|---|---|---|---|
| Steel substrate | 0.14 | 0.30 | 0.43 | 0.20 | 0.12 | - | Bal. | - |
| 6061 Aluminum coating alloy | - | 0.72 | 0.10 | 0.20 | 0.05 | 1.01 | 0.53 | Bal. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fathy, N.; Abdel Halim, K.S.; Hafez, K.M.; Ramadan, M.; Ayadi, B.; Alghamdi, A.S.; Ibrahim, K.M. Influence of Pre-Tinning Process on Coating Morphology and Interface Structure of Low Carbon Steel Dipped in Molten 6061 Al Alloy. Coatings 2022, 12, 1499. https://doi.org/10.3390/coatings12101499
Fathy N, Abdel Halim KS, Hafez KM, Ramadan M, Ayadi B, Alghamdi AS, Ibrahim KM. Influence of Pre-Tinning Process on Coating Morphology and Interface Structure of Low Carbon Steel Dipped in Molten 6061 Al Alloy. Coatings. 2022; 12(10):1499. https://doi.org/10.3390/coatings12101499
Chicago/Turabian StyleFathy, Naglaa, Khaled Saad Abdel Halim, K. M. Hafez, Mohamed Ramadan, Badreddine Ayadi, Abdulaziz S. Alghamdi, and Khaled M. Ibrahim. 2022. "Influence of Pre-Tinning Process on Coating Morphology and Interface Structure of Low Carbon Steel Dipped in Molten 6061 Al Alloy" Coatings 12, no. 10: 1499. https://doi.org/10.3390/coatings12101499
APA StyleFathy, N., Abdel Halim, K. S., Hafez, K. M., Ramadan, M., Ayadi, B., Alghamdi, A. S., & Ibrahim, K. M. (2022). Influence of Pre-Tinning Process on Coating Morphology and Interface Structure of Low Carbon Steel Dipped in Molten 6061 Al Alloy. Coatings, 12(10), 1499. https://doi.org/10.3390/coatings12101499








