Abstract
Adhesive resins with guided application protocols have been developed with the main goal of reducing the number of clinical steps. Universal Adhesives (UA) can be applied with both Self-Etch (SE) or Etch-and-Rinse (ER) adhesion strategies. This review aims to evaluate the bond strength of UA, applied to dental tissues, by a systematic bibliometric review of in vitro studies. The research question, through the PICO model, aimed to assess the current knowledge of the immediate and long-term bond strength of UA, applied with a direct restorative technique. PubMed and ScienceDirect database searches focused on the bond resistance of UA applied with the ER and SE strategies. Studies assessing shear bond strength and microtensile bond strength, in both enamel and dentin, were included. From 1109 screened articles, 12 fulfilled the inclusion criteria. The bond strength of UA to enamel showed better results with the ER approach, while the adhesion strategy did not significantly affect the bond strength of UA to dentin. Evidence from in vitro studies has tended to suggest that the use of the SE adhesion approach seems to be a better choice to improve the bond strength to the dentin. The selective enamel etching is advisable when applied with the SE adhesion approach to optimize the UA bond strength to the enamel.
Keywords:
dental bonding; adhesives; universal adhesives; dental tissues; in vitro; composite resins 1. Introduction
With the evolution of minimally invasive dentistry, many dental professionals have requested the development of adhesive products with simplified adhesion protocols that enable a reduction in the number of clinical steps during restorative procedures. These products should be suitable for a greater variety of dental restorations that overlook the guarantee of adhesion between the dental substrates and the restorative material applied. The increased demand for these materials has led to Universal Adhesives (AU), which could be applied according to the adhesion strategy, Self-Etch (SE) and Etch-and-Rinse (ER) [1].
The ER adhesion strategy requires the application of orthophosphoric acid and abundant washing with water to remove the hydroxyapatite microcrystalline, the organic particles and the smear layer, thereby demineralizing the enamel and dentin surface layers. Those adhesives systems can involve 2 (ER-2) or 3 (ER-3) clinical steps. In the three-step ER system, the hydrophilic primer and the hydrophobic bonding (fluid resin) are presented in two separate bottles/steps [2].
In the SE adhesion strategy, the previous orthophosphoric acid-conditioning step is eliminated as those adhesive products contain acidic primers in their compositions. Those components enable preparation of the dental substrates by integrating the smear layer with the adhesive interface, thus using it as an adhesive substrate. In this way, the SE adhesion mode simultaneously demineralizes and infiltrates enamel and dentin dental tissues. These adhesive systems may contemplate one (SE-1) or two (SE-2) steps, which varies according to the presentation of the acidic primer and the fluid resin, which may be combined or separated. SE adhesives can be classified as “soft” (pH > 2), “moderate” (pH between 1 and 2) and “aggressive” (pH < 1), according to the increasing demineralization potential effect [3].
Universal adhesives were introduced to the market in 2011 and gained popularity among dental professionals, owing to their unique properties, such as the potential to bond to different kinds of clinical dental substrates and fewer technical steps. UA can bond to dental substrates, ceramic, composites and metal substrates. Therefore, they are also referred to as “multi-mode” adhesives [4].
Universal adhesives can generally be applied as one-step SE or two-step ER when phosphoric acid is used. Additionally, UAs present, in their constitution, specific carboxylate and/or phosphate monomers that ionically bind to the calcium contained in hydroxyapatite. The most common functional monomer is 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP), which creates a strong chemical interaction with hydroxyapatite. 10-MDP induces the surface dissolution of hydroxyapatite with the subsequent formation of MDP–calcium salts [5].
The concentration of 10-MDP varies between different UAs, which influences the bond strength of the adhesive. It has been shown that the higher the monomer concentration, the stronger the bond strength of the adhesive [6]. Some studies have reported the formation of a “nano-layer” between the 10-MDP and the tooth structure. The occurrence of this phenomenon constitutes a key component of the adhesive interface, which may contribute to the longevity of the bond [7].
Bond strength is a key element in assessing the effectiveness of an adhesive. The two tests currently available to evaluate the adhesive strength between the two substrates are the (1) “shear bond strength test”, i.e., the load that a material is capable of withstanding in a direction parallel to the face of the material, which is the maximum shear stress in the adhesive before failure under torsional loading (using a universal testing machine of the Instron type) and the (2) “microtensile bond strength”, i.e., the division of resin-bonded teeth into plates with a thickness between 0.5 and 1.0 mm, which are then trimmed in such a way that the tensile force will be concentrated at the bonded interface during the test [8,9].
The materials studied in this systematic research are polymeric materials used in daily practice in restorative dentistry. The need to expand the current knowledge of dental research in this field is crucial to achieve a greater understanding of the in vitro performance of diverse UAs that are available on the market.
The main purpose of this systematic review is to analyze the immediate and long-term bond strength of universal adhesives, which are applied with direct restorative techniques to the tooth substrate, both enamel and dentin tissues. The type of surface conditioning recommended for each substrate will also be discussed.
2. Materials and Methods
This systematic review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analysis) guidelines [10].
The research question, based on the PICO model, aimed to assess the in vitro outputs and current knowledge of the immediate and long-term bond strength of UA to both enamel and dentin dental tissues when applied by ER and SE adhesive strategies.
This review has been submitted to PROSPERO (The International Prospective Register of Systematic Reviews) and is registered under the code CRD42022339745.
2.1. Inclusion and Exclusion Criteria
Only in vitro studies involving permanent teeth and assessing shear bond strength or microtensile bond strength on enamel and dentin were included. In vitro outputs of UA bond strength, when applied by both ER and SE direct restorative strategies, were included and analyzed.
Only articles written in the English language and published between 2007 and 2022 were considered in this review. Published research other than those considered in the inclusion criteria, such as those that assessed another UA property, written in a language other than English and whose in vitro sample size was less than 20 teeth, were excluded.
The inclusion and exclusion criteria were established by a consensus reached by three examiners (F.T., L.P.S. and B.L.) after discussion and consideration of the research question and the purposes of this study, while aiming for an ample range of results to be provided by the search.
2.2. Search Strategy
2.2.1. Sources of Information
An electronic search was made in PubMed and ScienceDirect electronic databases. The structured search strategy of the articles and data extraction were conducted by two calibrated examiners (F.T. and L.P.S.) to identify all the in vitro studies on UA applied by both the ER and SE adhesion strategies.
2.2.2. Search Terms
The search strategy included Mesh (Medical Subjects Headings) terms: “Dental Bonding”, “Dental Adhesives”, “Composite Resins”, “Dental Materials” and 8 uncontrolled descriptors: “Universal Adhesives”, “Multi-mode Adhesives“, “Bond Strength”, “Shear Bond Strength”, “Microtensile Bond Strength”, “Bonding Performance”, “Resin-based Composite” and “Dental Resins”. Boolean operators (“OR” and “AND”) were used to join search terms that were relevant to the search question (Table 1). The last search was performed in March 2022.

Table 1.
Search strategy used in each electronic database.
2.2.3. Study Selection
Articles identified using the search terms were exported to EndNote desktop 20.3 software to check for duplicates. A first screening of record titles and abstracts was carried out by three independent examiners (J.D., L.T. and P.M.-M.) according to the inclusion and exclusion criteria. The remaining studies were assessed for eligibility and qualitative synthesis by full-text screening.
2.2.4. Study Data
Bibliometric analysis was performed by recording the following variables: Authors and year of publication. The methodology of analysis included the aims, materials and methods and outputs of the included studies, i.e., the results (expressed in MPa) of two variables on the bond test, namely the mean value of the microtensile bond strength (µTBS) and mean value of the shear bond strength (SBS), with their respective Standard Deviation (SD).
For the synthesis of outcomes, studies were categorized in terms of the significant results found regarding the UAs used.
2.3. Quality Assessment
The quality of the studies was assessed using the modified CONSORT checklist of items for reporting pre-clinical studies on dental materials/devices [11].
3. Results
3.1. Study Selection and Flow Diagram
A total of 1109 preliminary references were assessed (Figure 1). After excluding duplicates, the remaining articles were screened and 1069 were excluded after reading the title and/or abstract.
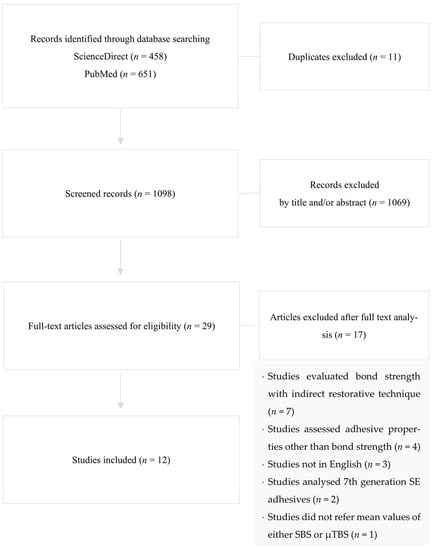
Figure 1.
Systematic review search flowchart, according to PRISMA, illustrating the study inclusion.
The resulting 29 articles were examined at a full-text level, and out of these, 17 articles were excluded due to their evaluation of bond strength when indirect restorative techniques were applied (n = 7), assessment of adhesive properties other than bond strength (n = 4), not written in the English language (n = 3), appraisal of seventh generation SE adhesives (n = 2) and not referring to the exact mean values of either SBS or µTBS (n = 1).
3.2. Study Characteristics
Study Type
To evaluate the results of the bond tests and the mean values of SBS and of µTBS with regards to UAs, a total of 12 in vitro studies were considered. All the reviewed studies are summarized in Table 2.

Table 2.
Summary of the reviewed in vitro studies [12,13,14,15,16,17,18,19,20,21,22,23].
3.3. Quality Assessment
All the in vitro studies analysed with the modified CONSORT checklist (Table 3) presented a structured abstract (item 1) and an introduction that provided scientific background on UA (Item 2a) and clear objectives and hypotheses (Item 2b). The description of methodology and the included variables was sufficiently clear to allow for replication in all the studies (Items 3 and 4), but the majority of them did not present a detailed report on the calculation of the sample size, random allocation sequence or the inclusion of a control group (Items 5–9). All the studies indicated that the statistical method used (Item 10) presented the significance level as p values, but did not all register the confidence intervals (Item 11). The discussions included a brief synopsis of the key findings, comparisons with relevant findings from other published studies and limitations of the studies (Item 12). The sources of funding (if any) were indicated in the majority of studies (Item 13) and indications of access to full trial protocols were obviated in all studies (Item 14).

Table 3.
Results of the assessment of in vitro studies [12,13,14,15,16,17,18,19,20,21,22,23] by the use of the modified CONSORT checklist [11]. Cells marked with an asterisk “*” represent study fulfilment for the given quality assessment parameter. Cells left blank represent non-fulfilment.
3.4. Study Results
3.4.1. Mean Microtensile Bond Strength (µTBS) Analysis
Nine studies analyzed µTBS values. Six papers only examined µTBS in dentin dental tissue (Marchesi et al., 2014; Jang et al., 2016; Cardoso et al., 2019; Ahmed et al., 2020; Burrer et al., 2020; and Giacomini et al., 2020). One paper solely studied µTBS on enamel (De Goes, Shinohara and Freitas, 2014). Two papers evaluated µTBS in both dentin and enamel tissues (Frattes et al., 2017 and Cruz et al., 2019).
In four studies, both immediate and long-term bond strength analysis was performed after six-months (Cardoso et al., 2019; Ahmed et al., 2020; and Burrer et al., 2020) and after six-months and one-year (Marchesi et al., 2014).
The studies by Frattes et al. (2017) and Cardoso et al. (2019) were not performed in human teeth, but in bovine teeth
Frattes et al. (2017) performed a study without a control group, given that the authors compared bond strength values between randomized groups with and without eroded surfaces.
The main outcomes, as well as the means and standard deviations values obtained in each study, are presented in Table 2.
3.4.2. Mean Shear Bond Strength (SBS) Analysis
Three studies analyzed SBS values: two papers solely examined SBS in enamel tissue (Beltrami et al. (2016) and Kharouf et al. (2020)) and one paper evaluated SBS in both dentin and enamel tissues (Jacker-Guhr, Sander and Luehrs (2019)).
In the study performed by Jacker-Guhr, Sander and Luerhs (2019), the samples were aged by thermocycling (10.000 cycles of 5°/55 °C, dwell time 30 s, transfer time 10 s). Beltrami et al. (2016) and Kharouf et al. (2020) solely analyzed immediate SBS bond strength.
The studies by Beltrami et al. (2016) and Jacker-Guhr, Sander and Luehrs (2019) were not performed in human teeth, but in bovine teeth.
The main outcomes, as well as the means and standard deviations values obtained by each study, are presented in Table 2.
4. Discussion
This updated systematic review was carried out with respect to the bond strength of the UAs to different types of tooth substrates (enamel and dentin), depending on the application strategy, namely the SE or ER adhesion modes. It can be stated that there is an association between the nature of dental substrate and the mode of application used, however it is impossible to state which UA had better results, due to the fact that each study used different experimental methodologies, namely the type of test performed (the SBS or μTBS test).
The SBS and µTBS tests are used to evaluate the adhesion between different materials and substrates, thus contributing greatly to the advancement of adhesive systems. These micro-tests have the advantages of being able to identify, with more precision, a greater percentage of cohesive failures with low coefficients of variation and the possibility of assessing different areas of the same specimen, while enabling the calculation of the mean and standard deviation [24].
Similar to the µTBS test, the SBS technique involves the testing of small areas and admits preparation of multiple specimens from the same tooth. However, the splitting and trimming steps, which may generate early microcracking, are avoided [25].
Hence, micro-tests are considered to be more reliable, which is more closely reflective of the interfacial bond strength, as it offers more uniform stress distribution. While clinical trials yield the most reliable evidence, in vitro tests provide immediate information regarding the bonding effectiveness of new adhesive materials [26].
According to the studies by De Goes, Shinohara and Freitas (2014), Beltrami et al. (2016) and Kharouf et al. (2021), which solely evaluated the bond strength on enamel, the authors agree that a prior acid conditioning of the surface significantly improves adhesion [13,14,23]. In enamel, acid etching creates microporosities that are readily penetrated, even by common hydrophobic bonding agents, by capillary attraction. The polymerization of monomers and the mechanical retention of small resin connections with the enamel surface provides the best bond to this tooth substrate [2].
It is important to state that, in the studies by De Goes, Shinohara and Freitas (2014), Beltrami et al. (2016) and Kharouf et al. (2021), the adhesives selected for the control groups are considered to be gold standards and thus always showed better or equal results when compared to the UAs tested. Hence, none of the authors found differences in statistical significance on the UAs tested. Nevertheless, it is of relevance to the work by Kharouf et al. (2021), in which a higher bond strength value for the UA was found. These could be related to the friction technique of the adhesive during application.
In contrast, the results found for dentin are conflicting. Giacomini et al. (2020) showed better immediate and long-term μTBS results with the SE adhesion strategy [22], however the studies by Ahmed et al. (2020), Marchesi et al. (2014) and Cardoso et al. (2019) showed better immediate bond strengths for the ER testing group [12,19,20]. Nonetheless, for this same group, after a follow-up period of six months, the SE group showed more reliable results with higher bond strength values. The authors suggested that these results can be explained by hydrolytic degradation, due to a greater aggressiveness of the acid, with a consequent dissolution of the smear layer. Those findings and the consequent reasoning are also supported by several other studies [1,27,28].
The hybrid layer created by the adhesive, applied with the ER adhesion mode, can suffer degradation in a period of time ranging from six-months to three–five years because of the loss of collagen fiber cross bands and an increase in water absorption, thus resulting in a decrease in bond strength between the surfaces [29].
Jacker-Guhr, Sander and Luehrs (2019) agree with the results of the studies listed above, however the authors did not perform a long-term assessment in their study [18]. Jang et al. (2016), Frattes et al. (2017) and Cruz et al. (2019) stated that UAs showed promising bond strength values, regardless of the type of adhesion strategy used [15,16,17].
The Burrer et al. (2020) study only evaluated the ER adhesion mode, but used different adhesive application times and durations of acid pre-etching [21]. The authors found no significant differences in bond strength values (µTBS exclusively in dentin) between the tested groups, in which several times were used for the etching application. However, the lowest bond strength value was found for the group with the shortest acid pre-etching time (10 s). Earlier studies established a correlation between extended pre-etching time and the consequent depth of the tissue demineralization, thus ensuring a poorly infiltrated hybrid layer [30,31,32,33], increased surface roughness [32] and a reduced bond strength value [33].
Hence, despite the reliability of the adhesion to enamel, dentin bonding has been found to be less predictable. The main obstacle is the heterogeneity of dentin, with hydroxyapatite crystals interposed within collagen fibers [34]. Furthermore, dentin is intimately connected to the pulp tissue by means of numerous fluid-filled tubules that run through the dentin from the pulp to the dentin–enamel junction. Once under constant external pressure, this fluid makes the exposed dentin surface moist and therefore intrinsically hydrophilic, thus being the greatest challenge for adhesion on this dental substrate [35].
The different results found for dentin can be explained by the different compositions of the UAs analyzed—those with the functional monomer 10-MDP in their composition showed better results in terms of bond strength and stability over time due to the strong hydrophobicity of the hybrid layer formed (10-MDP and hydroxyapatite crystals). This layer protects against the hydrolytic degradation process, as demonstrated in the outputs of the study by Jin et al. (2022). The authors concluded that 10-MDP forms a stable collagen-phosphate complex with the collagen present in dentin, generating MDP-calcium salts that are deposited on the dentin collagen frame, which protects it from hydrolytic degradation. Both free 10-MDP and MDP-calcium salts inhibit metallopeptidases and proteases [36].
Another factor that may also interfere and influence the bond strength values is the adhesive’s pH value. The use of the ER adhesion strategy can improve the bond strength results of UAs with mild (pH ≈ 2; granting an interaction depth of 1 µm) and ultra-mild (pH > 2.5; allowing a true nano interaction zone, contrary to the conventional thicker hybrid layer) pH values [37,38,39]. Mild and ultra-mild adhesives demineralize dentin superficially, hence the smear plug is not completely removed from the dentine tubule. As a result, a shallow hybrid layer is formed [40].
Generally, phosphoric acid is used in gel form, with a concentration between 30% and 40% (pH ranging from 0.1 to 0.4) [41]. Selective enamel etching is a pre-requisite for ultra-mild UAs applied in a self-etch strategy, since they have proven to be unable to etch enamel at the same depth of phosphoric acid [40].
However, it must be considered that the etch application may negatively interfere with the long-term adhesive-dentin bond, causing the degradation of collagen fibers inside the demineralized dentin [41,42]. This factor was not considered in the studies included in this systematic review.
Moreover, further longitudinal studies regarding UA clinical performance are also required to support, or not, those in vitro findings. Recently, the two-year follow up results of a randomized clinical trial, which evaluated the performance of UA, showed differences in the clinical performance (cumulative decrease in clinical performance with the SE adhesion strategy), functional success (decreased marginal adaptation with UAs applied by both the SE and ER modes) and retention rates (overall retention rate: 94.8%; the annual failure rate showed higher values for the control group (12.5%) than for the UAs tested) of the UAs applied [43].
Another study by Cruz et al. revealed better results for clinical outputs when the UA was applied by the SE mode rather than by the ER adhesion mode [44].
5. Conclusions
The bond strength of UA to enamel showed better results with the ER adhesion mode, while the adhesion strategy did not significantly affect the bond strength of UA to dentin. Evidence from in vitro studies also suggested that the use of SE adhesion approach seems to be a better choice to improve the bond strength of UA to the dentin tissues.
Selective enamel etching is advisable when the SE adhesion approach is applied to optimize the UA bond strength to the enamel tissue. Selective etching corresponds to a technique in which solely the enamel margin surfaces are etched with 30–40% phosphoric acid, prior to the adhesive application, to ensure a stronger and adequate bond to the enamel surface.
Dental professionals should select the most suitable adhesion strategy for each type of dental tissues as this step is essential for a better prognosis of the overall direct restorative technique. More systematic or meta-analysis reviews should be performed to determine the relationship of the in vitro bond strength results and the main clinical performance outputs of UA, and of adhesion approaches to both enamel and dentin tissues.
Author Contributions
Conceptualization and data extraction F.T. and L.P.d.S.; methodology, F.T. and L.P.d.S.; validation in vitro studies, J.D., L.T. and P.M.-M.; resources, F.T. and L.P.d.S.; data curation, F.T., L.P.d.S., B.F.L., J.D. and L.T.; writing—original draft preparation, F.T., L.P.d.S. and B.F.L.; writing—review and editing, F.T., L.P.d.S., B.F.L. and P.M.-M.; visualization, L.T. and P.M.-M.; supervision, L.P.d.S., B.F.L. and P.M.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nagarkar, S.; Theis-Mahon, N.; Perdigão, J. Universal dental adhesives: Current status, laboratory testing, and clinical performance. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 107, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Perdigão, J.; Araujo, E.; Ramos, R.Q.; Gomes, G.; Pizzolotto, L. Adhesive dentistry: Current concepts and clinical considerations. J. Esthet. Restor. Dent. 2020, 33, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; De Munck, J.; Van Landuyt, K.L. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef]
- Perdigão, J.; Swift, J.E. Contemporary Issues-Universal Adhesives. J. Esthet. Restor. Dent. 2015, 27, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, E.; Cardoso, M.; Ferreira, M.M.; Marto, C.M.; Paula, A.; Coelho, A.S. 10-MDP Based Dental Adhesives: Adhesive Interface Characterization and Adhesive Stability—A Systematic Review. Materials 2019, 12, 790. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Nagaoka, N.; Okihara, T.; Kuroboshi, M.; Hayakawa, S.; Maruo, Y.; Nishigawa, G.; De Munck, J.; Yoshida, Y.; Van Meerbeek, B. Functional monomer impurity affects adhesive performance. Dent. Mater. 2015, 31, 1493–1501. [Google Scholar] [CrossRef]
- Yoshihara, K.; Yoshida, Y.; Hayakawa, S.; Nagaoka, N.; Irie, M.; Ogawa, T.; Van Landuyt, K.L.; Osaka, A.; Suzuki, K.; Minagi, S.; et al. Nanolayering of phosphoric acid ester monomer on enamel and dentin. Acta Biomater. 2011, 7, 3187–3195. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Carvalho, R.M.; Sano, H.; Nakajima, M.; Yoshiyama, M.; Shono, Y.; A Fernandes, C.; Tay, F. The microtensile bond test: A review. J. Adhes. Dent. 1999, 1, 299–309. [Google Scholar]
- De Munck, J.; Van Landuyt, K.L.; Peumans, M.; Poitevin, A.; Lambrechts, P.; Braem, M.; Van Meerbeek, B. A critical review of the durability of adhesion to tooth tissue: Methods and results. J. Dent. Res. 2005, 84, 118–132. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed]
- Faggion, C.M. Guidelines for Reporting Pre-clinical In Vitro Studies on Dental Materials. J. Évid. Based Dent. Pract. 2012, 12, 182–189. [Google Scholar] [CrossRef] [PubMed]
- De Goes, F.; Shinohara, M.S.; Freitas, M.S. Performance of a New One-Step Multi-mode Adhesive on Etched vs. Non-etched Enamel on Bond Strength and Interfacial Morphology. J. Adhes. Dent. 2014, 16, 243–250. [Google Scholar] [PubMed]
- Beltrami, R.; Chiesa, M.; Scribante, A.; Allegretti, J.; Poggio, C. Comparison of Shear Bond Strength of Universal Adhesives on Etched and Nonetched Enamel. J. Appl. Biomater. Funct. Mater. 2016, 14, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Kharouf, N.; Mancino, D.; Haikel, Y.; Rapp, G.; Zghal, J.; Arntz, Y.; Reitzer, F. Does Etching of the Enamel with the Rubbing Technique Promote the Bond Strength of a Universal Adhesive System? J. Contemp. Dent. Pract. 2020, 21, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, M.C.; Scaffa, P.M.C.; Gonçalves, R.S.; Zabeu, G.S.; Vidal, C.D.M.P.; Carrilho, M.R.D.O.; Honório, H.M.; Wang, L. Profile of a 10-MDP-based universal adhesive system associated with chlorhexidine: Dentin bond strength and in situ zymography performance. J. Mech. Behav. Biomed. Mater. 2020, 110, 103925. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.H.; Yoshihara, K.; Mercelis, B.; Van Landuyt, K.; Peumans, M.; Van Meerbeek, B. Quick bonding using a universal adhesive. Clin. Oral Investig. 2019, 24, 2837–2851. [Google Scholar] [CrossRef]
- Marchesi, G.; Frassetto, A.; Mazzoni, A.; Apolonio, F.; Diolosà, M.; Cadenaro, M.; Di Lenarda, R.; Pashley, D.H.; Tay, F.; Breschi, L. Adhesive performance of a multi-mode adhesive system: 1-Year in vitro study. J. Dent. 2013, 42, 603–612. [Google Scholar] [CrossRef]
- Cardoso, G.C.; Nakashini, L.; Isolan, C.P.; Jardim, P.S.; Moraes, R.R. Bond stability of universal adhesives applied to dentin using etch-and-rinse or self-etch strategies. Braz. Dent. J. 2019, 30, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Sarikaya, R.; Ye, Q.; Misra, A.; Tamerler, C.; Spencer, P. Multifunctional monomer acts as co-initiator and crosslinker to provide autonomous strengthening with enhanced hydrolytic stability in dental adhesives. Dent. Mater. 2020, 36, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Scotti, N.; Giovanni, C.; Massimo, G.; Breschi, L. New adhesives and bonding techniques. Why and when? Int. J. Esthet. Dent. 2017, 12, 524–535. [Google Scholar] [PubMed]
- Da Rosa, W.L.; Piva, E.; da Silva, A.F. Bond strength of Universal Adhesives: A systematic review and meta-analysis. J. Dent. 2015, 43, 765–776. [Google Scholar] [CrossRef]
- Jacker-Guhr, S.; Sander, J.; Luehrs, A.-K. How “Universal” is Adhesion? Shear Bond Strength of Multi-mode Adhesives to Enamel and Dentin. J. Adhes. Dent. 2019, 21, 87–95. [Google Scholar]
- Jang, J.-H.; Lee, M.G.; Woo, S.U.; Lee, C.O.; Yi, J.-K.; Kim, D.-S. Comparative study of the dentin bond strength of a new universal adhesive. Dent. Mater. J. 2016, 35, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Frattes, F.C.; Augusto, M.G.; Torres, C.R.G.; Pucci, C.R.; Borges, A. Bond Strength to Eroded Enamel and Dentin Using a Universal Adhesive System. J. Adhes. Dent. 2017, 19, 121–127. [Google Scholar] [PubMed]
- Cruz, J.; Sousa, B.; Coito, C.; Lopes, M.; Vargas, M.; Cavalheiro, A. Microtensile bond strength to dentin and enamel of self-etch vs. etch-and-rinse modes of universal adhesives. Am. J. Dent. 2019, 32, 174–182. [Google Scholar] [PubMed]
- Burrer, P.; Dang, H.; Par, M.; Attin, T.; Tauböck, T.T. Effect of Over-Etching and Prolonged Application Time of a Universal Adhesive on Dentin Bond Strength. Polymers 2020, 12, 2902. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Chowdhury, A.F.M.A.; Saikaew, P.; Matsumoto, M.; Hoshika, S.; Yamauti, M. The microtensile bond strength test: Its historical background and application to bond testing. Jpn. Dent. Sci. Rev. 2019, 56, 24–31. [Google Scholar] [CrossRef]
- Goracci, C.; Sadek, F.T.; Monticelli, F.; Cardoso, P.E.; Ferrari, M. Influence of substrate, shape, and thickness on microtensile specimens’ structural integrity and their measured bond strengths. Dent. Mater. 2004, 20, 643–654. [Google Scholar] [CrossRef]
- Sirisha, K.; Ravishankar, Y.; Ravikumar, P.; Rambabu, T. Validity of bond strength tests: A critical review-Part II. J. Conserv. Dent. 2014, 17, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hann, A.; Gordan, V.V.; Mjor, I. The effect of variation in etching times in dentin bonding. Gen. Dent. 2004, 52, 28–33. [Google Scholar]
- Wang, Y.; Spencer, P. Effect of acid etching time and technique on interfacial characteristics of the adhesive-dentin bond using differenctial staining. Eur. J. Oral Sci. 2004, 112, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S.; Ahmed, N. The effects of acid etching time on surface mechanical properties of dental hard tisses. Dent. Mater. J. 2015, 34, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-J.; Zhang, L.; Tang, L.-H.; Chen, J.-H. Nanoleakage and microtensile bond strength at the adhesive-dentin interface after different etching times. Am. J. Dent. 2010, 23, 335–340. [Google Scholar] [PubMed]
- Oldak-Moradian, J.; George, A. Biomineralization of enamel and dentin mediated by matrix proteins. J. Dent. Res. 2021, 100, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Cadenaro, M.; Maravic, T.; Comba, A.; Mazzoni, A.; Fanfoni, L.; Hilton, T.; Ferracane, J.; Breschi, L. The role of polymerization in adhesive dentistry. Dent. Mater. 2019, 35, e1–e22. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Han, F.; Wang, Q.; Yuan, X.; Zhou, Q.; Xie, H.; Niu, L.; Chen, C. The roles of 10-methacryloyloxydecyl dihydrogen phosphate and its calcium salt in preserving the adhesive–dentin hybrid layer. Dent. Mater. 2022, 38, 1194–1205. [Google Scholar] [CrossRef]
- Cuevas-Suárez, C.; Rosa, W.L.D.O.D.; Lund, R.G.; da Silva, A.F.; Piva, E. Bonding Performance of Universal Adhesives: An Updated Systematic Review and Meta-Analysis. J. Adhes. Dent. 2019, 21, 7–26. [Google Scholar] [PubMed]
- Sezinando, A. Looking for the ideal Adhesive–A Review. Rev. Port. De Estomatol. Med. Dentária E Cir. Maxilofac. 2014, 55, 194–206. [Google Scholar] [CrossRef]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Di Stomatol. 2017, VIII, 1–17. [Google Scholar]
- Perdigão, J.; Sezinando, A.; Monteiro, P.C. Laboratory bonding ability of a multi-purpose dentin adhesive. Am. J. Dent. 2012, 25, 153–158. [Google Scholar] [PubMed]
- Hashimoto, M. A review–Micromorphological evidence of degradation in resin-dentin bonds and potential preventional solutions. J. Biomed. Mater. Res. 2010, 92, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Breschi, L.; Maravic, T.; Cunha, S.R.; Comba, A.; Milena, C.; Tjaderhane, L.; Pashley, D.H.; Tay, F.R.; Mazzoni, A. Dentin bonding systems: From dentin collagen stracture to bond preservation and clinical applications. Dent. Mater. 2018, 34, 78–96. [Google Scholar] [CrossRef] [PubMed]
- Manarte-Monteiro, P.; Domingues, J.; Teixeira, L.; Gavinha, S.; Manso, M.C. Universal Adhesives and Adhesion Modes in Non-Carious Cervical Restorations: 2-Year Randomised Clinical Trial. Polymers 2021, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.; Silva, A.; Eira, R.; Coito, C.; Sousa, B.R.; Lopes, M.; Cavalheiro, A. 24-month clinical performance of a universal adhesive on non-carious cervical lesions: Self-etch and etch-and-rinse techniques. J. Adhes. Dent. 2020, 23, 379–387. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).