Improvement of the Mechanical Properties and Corrosion Resistance of CSS-42L Steel with a Novel TiAlMoNbW Nitrid Film Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Characterization
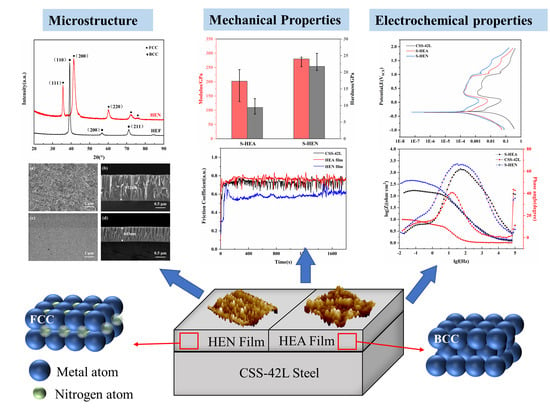
3. Results
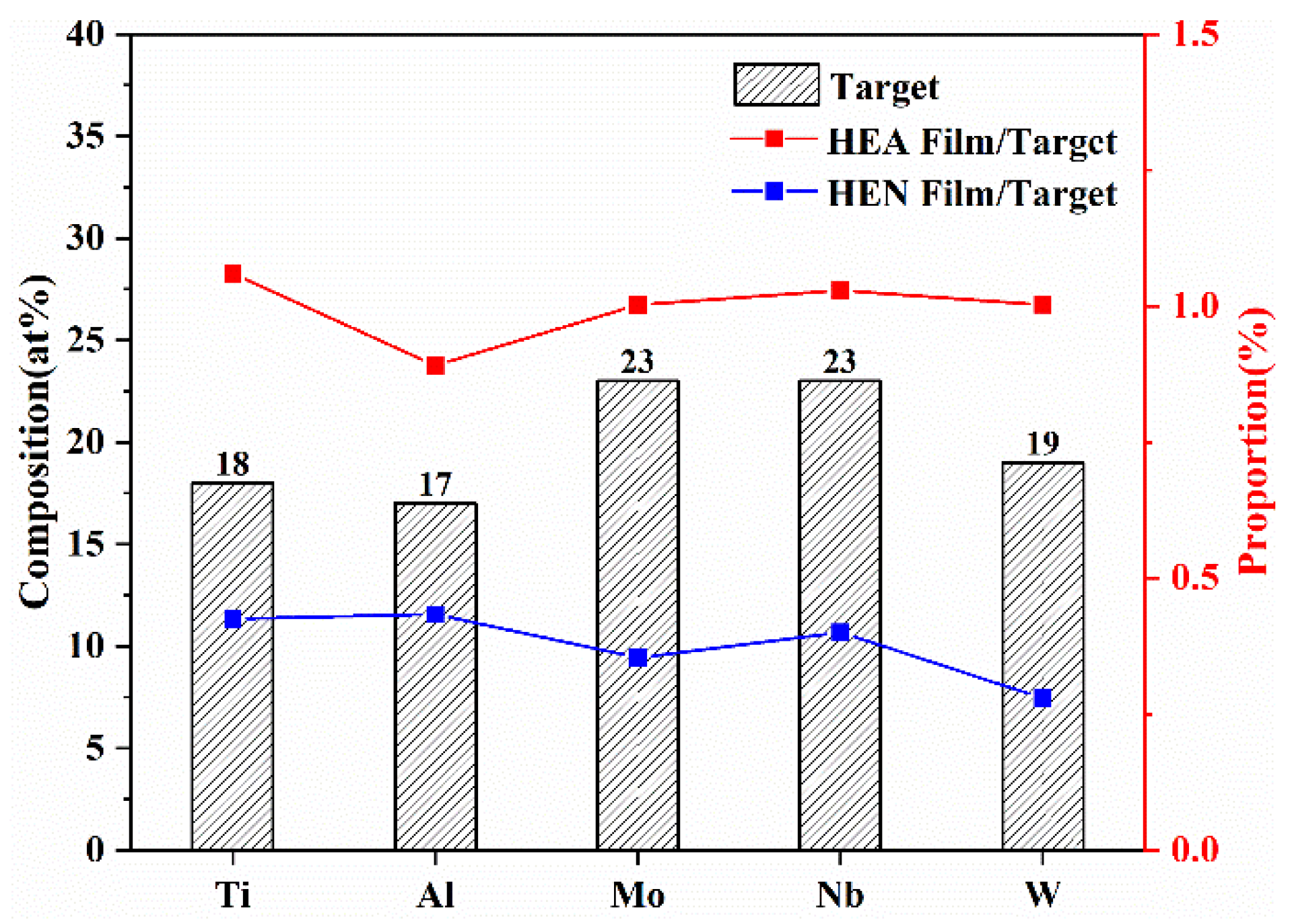
3.1. Chemical Composition and Phase Analysis
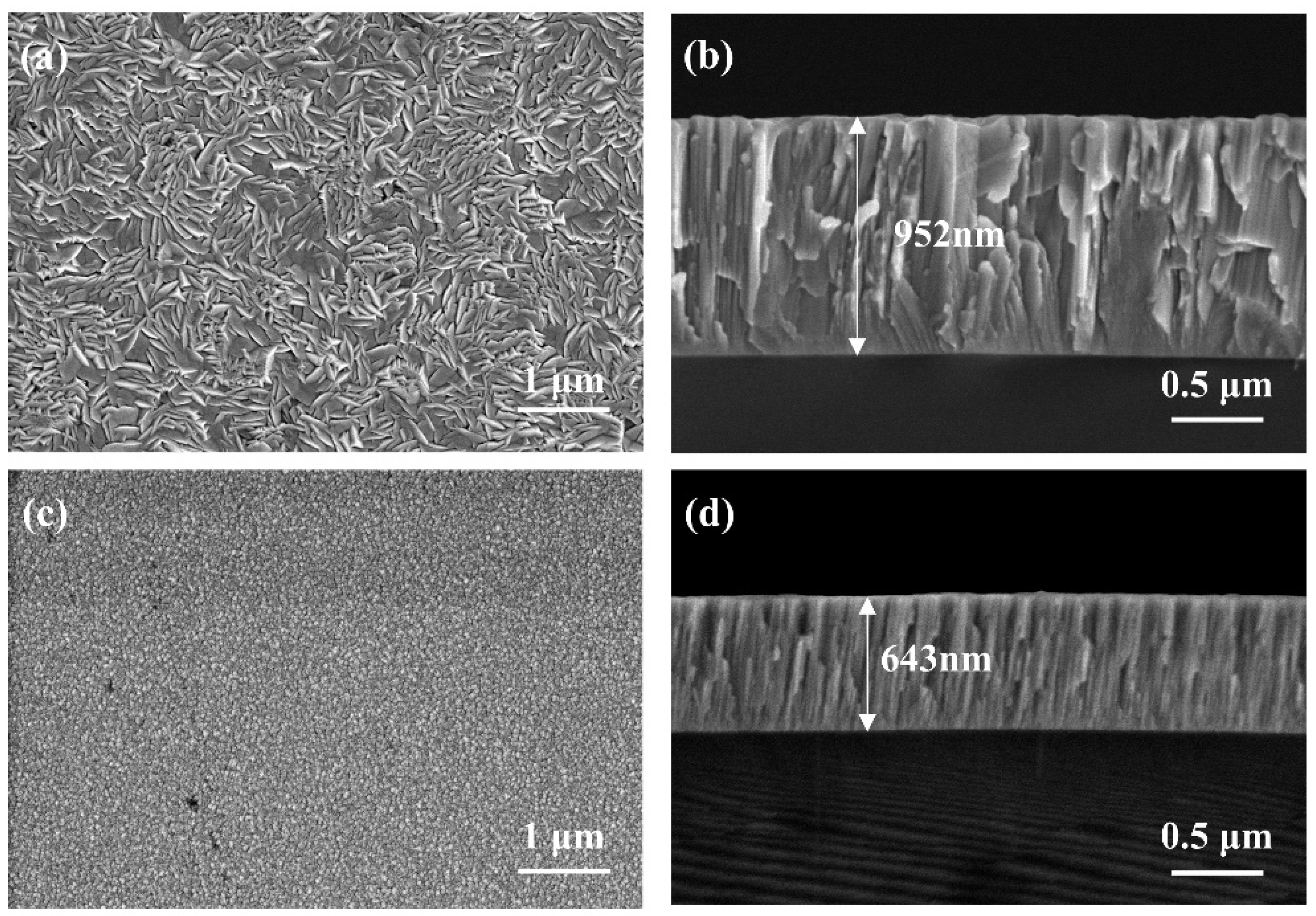
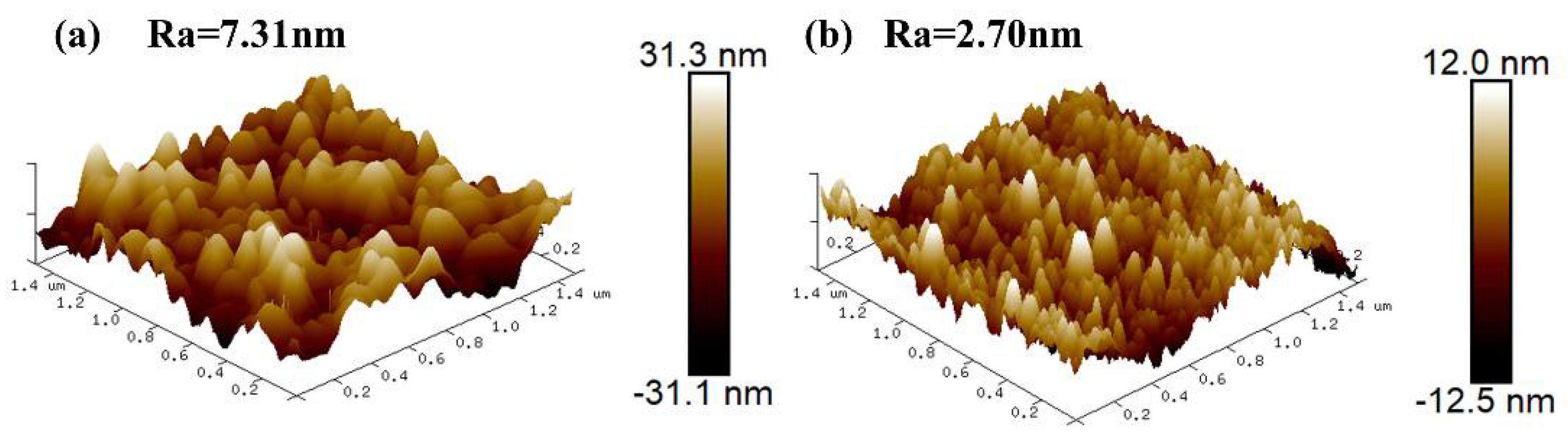
3.2. Microstructure
3.3. Mechanical Properties
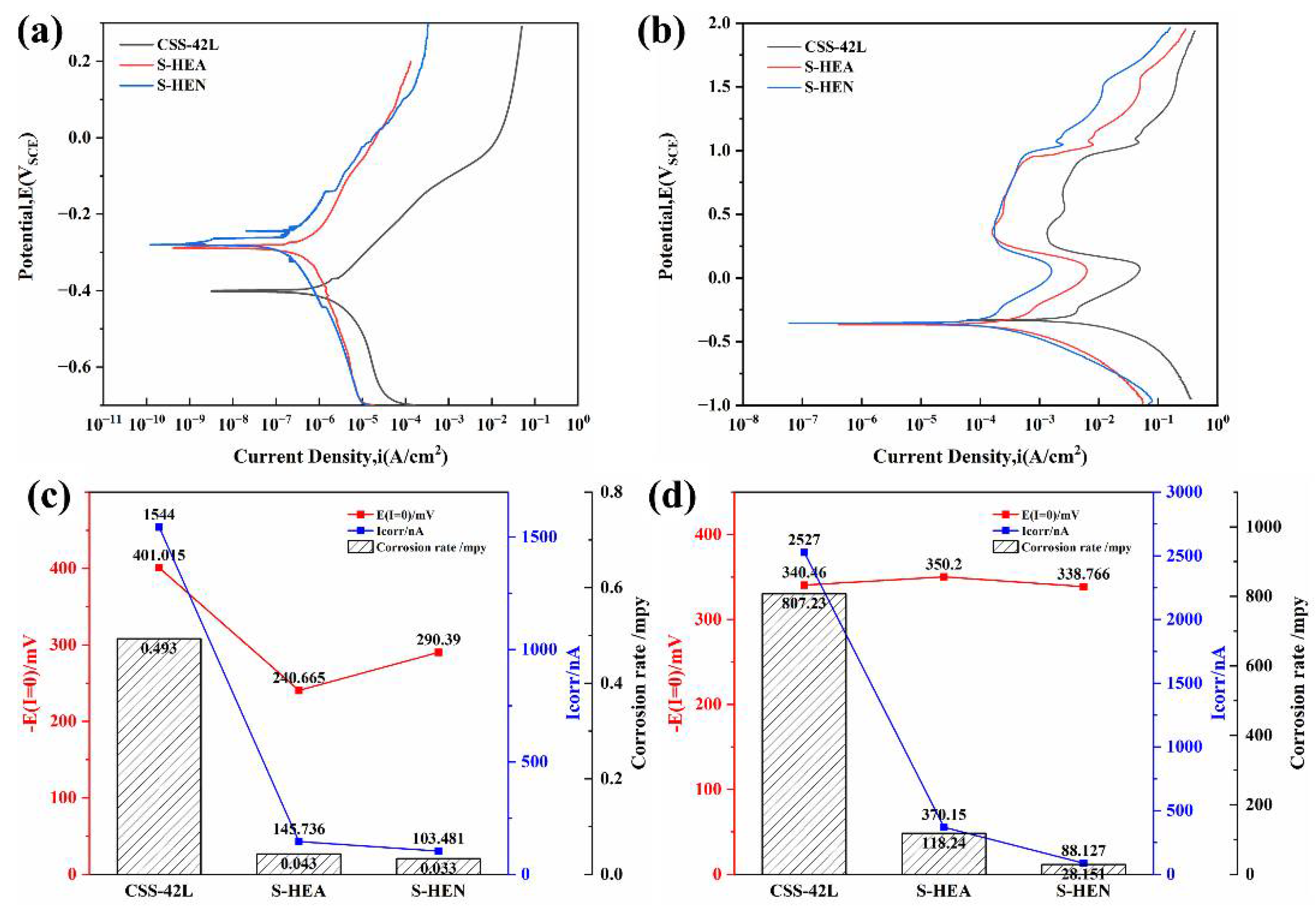
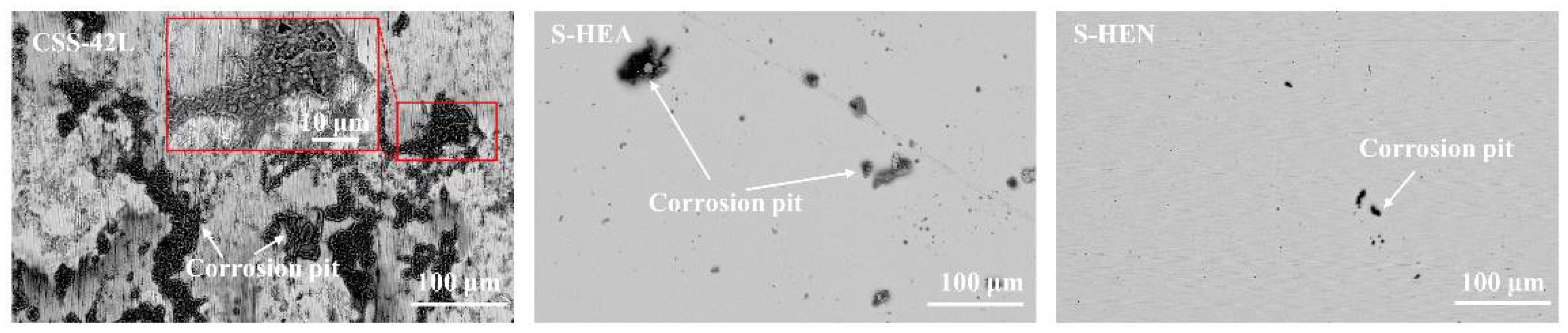
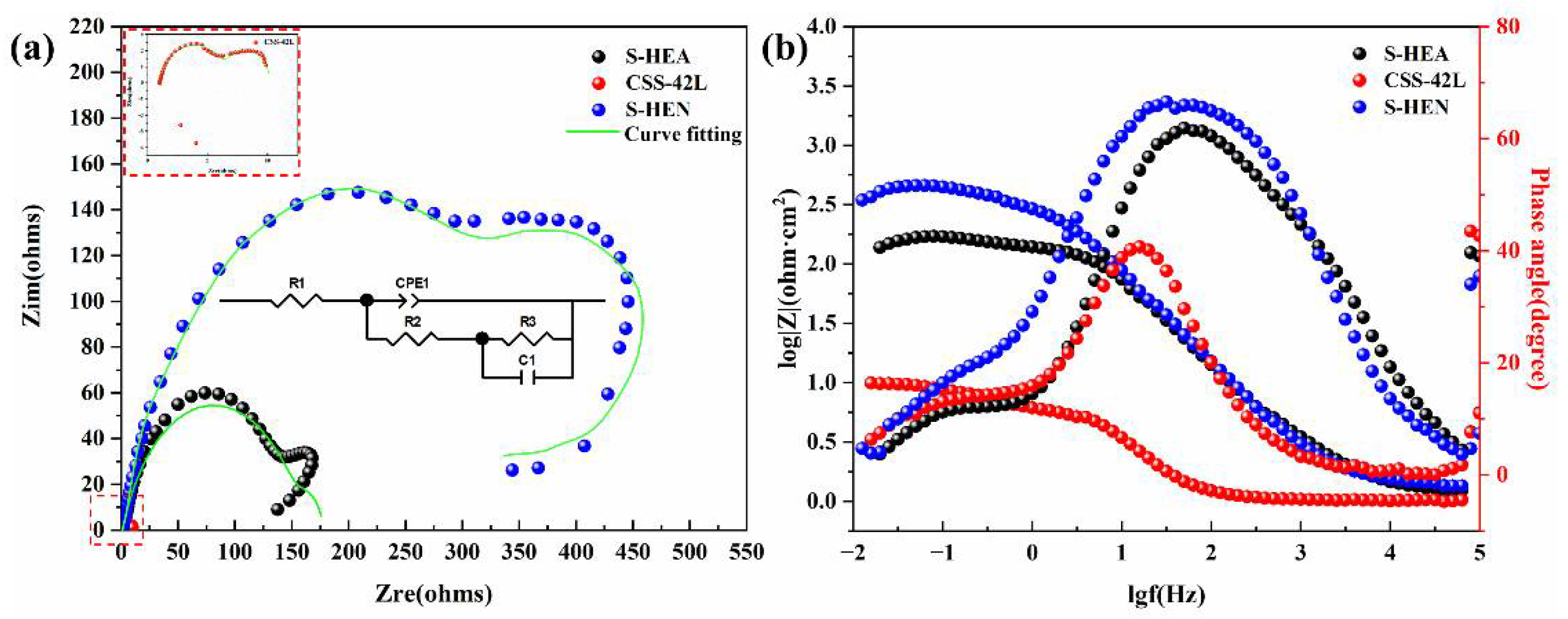
3.4. Electrochemical Corrosion Behavior
4. Discussion
4.1. Formation Mechanism of HEA and HEN Films
4.2. Improvement in Wear and Corrosion Resistance
5. Conclusions
- The films prepared by the TiAlMoNbW target have excellent mechanical properties. Affected by the solid-solution strengthening effect and the saturated nitride phase, the hardness, and modulus of the HEN film are higher than those of the HEA film, increasing from 11.42 GPa and 233.1 GPa to 25.7 GPa and 313.6 GPa, respectively.
- Benefiting from the high toughness nanoparticle-like FCC structure, lower surface roughness, and formed nitride phase with high hardness of the HEN film, the friction coefficient of the HEN film-coated samples is reduced from 0.73 to 0.57, and the wear rate is decreased from 1.848 × 10−5 mm3/(N·mm) to 0.376 × 10−5 mm3/(N·mm).
- In the neutral and acidic environments of this experiment, the HEN films have lower Icorr and an order of magnitude lower corrosion rate than the CSS-42L steel substrate. The corrosion rate is as low as 0.033 mpy and 28.151 mpy, respectively, indicating that the presence of HEN films effectively improves the corrosion resistance of the base steel.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ragen, M.A.; Anthony, D.L.; Spitzer, R.F. A comparison of the mechanical and physical properties of contemporary and new alloys for aerospace bearing applications. Bear Steel Technol. 2002, 1419, 362–374. [Google Scholar]
- Qiu, Z.F.; Wang, F.F.; Li, Q.S.; Zheng, L.J.; Zhang, F.X.; Zhang, H. Corrosion and mechanical properties for Cr-coated CSS-42L bearing steel after Ti and C ions co-implantation. Appl. Surf. Sci. 2020, 509, 145293. [Google Scholar] [CrossRef]
- Yang, C.Y.; Xu, J.H.; Fu, Y.C.; Ding, W.F.; Song, H.H. Investigation on the Grindab145293.ility of CSS-42L Stainless Steel. Trans. Nanjing Univ. Aeronaut. Astronaut. 2016, 33, 706–713. [Google Scholar]
- Darisuren, S.; Park, J.H.; Pyun, Y.S.; Amanov, A. A Study on the Improvement of the Fatigue Life of Bearings by Ultrasonic Nanocrystal Surface Modification Technology. Metals 2019, 9, 1114. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Tsai, D.C.; Chang, Z.C.; Kuo, B.H.; Lin, T.N.; Shiao, M.H.; Shieu, F.S. Interfacial reactions and characterization of (TiVCrZrHf)N thin films during thermal treatment. Surf. Coat. Technol. 2014, 240, 160–166. [Google Scholar] [CrossRef]
- Feng, X.G.; Zhang, K.F.; Zheng, Y.G.; Zhou, H.; Wan, Z.H. Chemical state, structure and mechanical properties of multi-element (CrTaNbMoV)Nx films by reactive magnetron sputtering. Mater. Chem. Phys. 2020, 239, 121991. [Google Scholar] [CrossRef]
- Braic, M.; Balaceanu, M.; Vladescu, A.; Zoita, C.N.; Braic, V. Deposition and characterization of multi-principal-element (CuSiTiYZr)C coatings. Appl. Surf. Sci. 2013, 284, 671–678. [Google Scholar] [CrossRef]
- Huang, Y.S.; Chen, L.; Lui, H.W.; Cai, M.H.; Yeh, J.W. Microstructure, hardness, resistivity and thermal stability of sputtered oxide films of AlCoCrCu0.5NiFe high-entropy alloy. Mat. Sci. Eng. A-Struct. 2007, 457, 77–83. [Google Scholar] [CrossRef]
- Ren, B.; Yan, S.Q.; Zhao, R.F.; Liu, Z.X. Structure and properties of (AlCrMoNiTi)Nx and (AlCrMoZrTi)N1-x films by reactive RF sputtering. Surf. Coat. Technol. 2013, 235, 764–772. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, G.J.; Dai, P.Q. Evolution of the microstructure and properties of laser-clad FeCrNiCoBx high-entropy alloy coatings. Mater. Sci. Technol. 2016, 32, 1666–1672. [Google Scholar] [CrossRef]
- Hsueh, H.T.; Shen, W.J.; Tsai, M.H.; Yeh, J.W. Effect of nitrogen content and substrate bias on mechanical and corrosion properties of high-entropy films (AlCrSiTiZr) (100−x) Nx. Surf. Coat. Technol. 2012, 206, 4106–4112. [Google Scholar] [CrossRef]
- Zheng, S.J.; Cai, Z.B.; Pu, J.B.; Zeng, C.; Chen, S.Y.; Chen, R.; Wang, L.P. A feasible method for the fabrication of VAlTiCrSi amorphous high entropy alloy film with outstanding anti-corrosion property. Appl. Surf. Sci. 2019, 483, 870–874. [Google Scholar] [CrossRef]
- Dou, D.; Li, X.C.; Zheng, Z.Y.; Li, J.C. Coatings of FeAlCoCuNiV high entropy alloy. Surf. Eng. 2016, 32, 766–770. [Google Scholar] [CrossRef]
- Ji, X.L.; Duan, H.; Zhang, H.; Ma, J.J. Slurry Erosion Resistance of Laser Clad NiCoCrFeAl3 High-Entropy Alloy Coatings. Tribol. Trans. 2015, 58, 1119–1123. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, W.F.; He, Y.Z.; Li, M.X.; Guo, S. Formation of core–shell structure in high entropy alloy coating by laser cladding. Appl. Surf. Sci. 2016, 363, 543–547. [Google Scholar] [CrossRef]
- Rajaram, G.; Jain, A. Characteristics of alumina coating on SAE 52100 bearing steel with Ni-Cr bond coat. Int. Mater. Eng. Innov. 2018, 9, 20–33. [Google Scholar] [CrossRef]
- Yao, C.Z.; Zhang, P.; Liu, M.; Li, G.R.; Ye, J.Q.; Liu, P.; Tong, Y.X. Electrochemical preparation and magnetic study of Bi–Fe–Co–Ni–Mn high entropy alloy. Electrochim. Acta 2008, 53, 8359–8365. [Google Scholar] [CrossRef]
- Liu, D.; Cheng, J.B.; Ling, H. Electrochemical behaviours of (NiCoFeCrCu)95B5 high entropy alloy coatings. Mater. Sci. Technol. 2015, 31, 1159–1164. [Google Scholar] [CrossRef]
- Li, W.; Liu, P.; Liaw, P.K. Microstructures and properties of high-entropy alloy films and coatings: A review. Mater. Res. Lett. 2018, 6, 199–229. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.J.; Qi, Y.F.; Zhang, L.X.; Tang, Y.H.; Qi, C.; Shen, Q.; Ma, Y.T.; Wang, B. The effect of alloy elements on corrosion and oxidative resistance of W-based alloy films. Surf. Coat. Technol. 2022, 434, 128165. [Google Scholar] [CrossRef]
- Braeckman, B.R.; Boydens, F.; Hidalgo, H.; Dutheil, P.; Jullien, M.; Thomann, A.L.; Depla, D. High entropy alloy thin films deposited by magnetron sputtering of powder targets. Thin Solid Films 2015, 580, 71–76. [Google Scholar] [CrossRef]
- Yan, X.H.; Li, J.S.; Zhang, W.R.; Zhang, Y. A brief review of high-entropy films. Mater. Chem. Phys. 2018, 210, 12–19. [Google Scholar] [CrossRef]
- Liu, K.Q.; Ostadhassan, M.; Xu, X.M. A comparison study of the unloading behavior in shale samples in nanoindentation experiments using different models. Pet. Sci. Eng. 2020, 186, 106715. [Google Scholar] [CrossRef]
- Yu, L.H.; Luo, H.; Bian, J.G.; Ju, H.B.; Xu, J.H. Research on Microstructure, Mechanical and Tribological Properties of Cr-Ti-B-N Films. Coatings 2017, 7, 137. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Cui, H.Z.; Wang, M.L.; Zhao, Y.Q.; Sun, K. Effect of Al content on microstructure and corrosion resistance of AlxCoCrFeNi high entropy alloys. Trans. Mater. Heat Treat. 2018, 39, 29–36. [Google Scholar]
- Cheng, K.H.; Lai, C.H.; Lin, S.J.; Yeh, J.W. Structural and mechanical properties of multi-element (AlCrMoTaTiZr)N-x coatings by reactive magnetron sputtering. Thin Solid Films 2011, 519, 3185–3190. [Google Scholar] [CrossRef]
- Wang, J.J.; Kuang, S.F.; Yu, X.; Wang, L.Q.; Huang, W.J. Tribo-mechanical properties of CrNbTiMoZr high-entropy alloy film synthesized by direct current magnetron sputtering. Surf. Coat. Technol. 2020, 403, 126374. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys:Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.K.; Yeh, J.W. Effects of nitrogen content on structure and mechanical properties of multi-element (AlCrNbSiTiV)N coating. Surf. Coat. Technol. 2009, 203, 1891–1896. [Google Scholar] [CrossRef]
- Cui, P.P.; Li, W.; Liu, P.; Zhang, K.; Ma, F.C.; Chen, X.H.; Feng, R.; Liaw, P.K. Effects of nitrogen content on microstructures and mechanical properties of (AlCrTiZrHf)N high-entropy alloy nitride films. J. Alloys Compd. 2020, 834, 155063. [Google Scholar] [CrossRef]
- Tsai, D.C.; Huang, Y.L.; Lin, S.R.; Jung, D.R.; Chang, S.Y.; Shieu, F.S. Diffusion barrier performance of TiVCr alloy film in Cu metallization. Appl. Surf. Sci. 2010, 257, 4923–4927. [Google Scholar] [CrossRef]
- Feng, X.B.; Zhang, J.Y.; Wang, Y.Q.; Hou, Z.Q.; Wu, K.; Liu, G.; Sun, J. Size effects on the mechanical properties of nanocrystalline NbMoTaW refractory high entropy alloy thin films. Int. J. Plast. 2017, 95, 264–277. [Google Scholar] [CrossRef]
- Lu, T.W.; Feng, C.S.; Wang, Z.; Liao, K.W.; Liu, Z.Y.; Xie, Y.Z.; Hu, J.G.; Liao, W.B. Microstructures and mechanical properties of CoCrFeNiAl0.3 high-entropy alloy thin films by pulsed laser deposition. Appl. Surf. Sci. 2019, 494, 72–79. [Google Scholar] [CrossRef]
- Fritze, S.; Koller, C.M.; Fieandt, L.v.; Malinovskis, P.; Johansson, K.; Lewin, E.; Mayrhofer, P.H.; Jansson, U. Influence of Deposition Temperature on the Phase Evolution of HfNbTiVZr High-Entropy Thin Films. Materials 2019, 12, 587. [Google Scholar] [CrossRef] [Green Version]
- Li, X.C.; Zheng, Z.Y.; Dou, D.; Li, J.C. Microstructure and Properties of Coating of FeAlCuCrCoMn High Entropy Alloy Deposited by Direct Current Magnetron Sputtering. Mater. Res. 2016, 19, 802–806. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.F.; Wang, X.D.; Cao, Q.P.; Zhao, G.H.; Li, J.X.; Zhang, D.X.; Zhu, J.J.; Jiang, J.Z. Microstructure characterization of AlxCo1Cr1Cu1Fe1Ni1( x=0 and 2.5) high-entropy alloy films. J. Alloys Compd. 2014, 609, 137–142. [Google Scholar] [CrossRef]
- Ren, B.; Shen, Z.; Liu, Z. Structure and mechanical properties of multi-element (AlCrMnMoNiZr)Nx coatings by reactive magnetron sputtering. J. Alloys Compd. 2013, 560, 171–176. [Google Scholar] [CrossRef]
- Feng, X.G.; Tang, G.Z.; Sun, M.R.; Ma, X.X.; Wang, L.Q.; Yukimura, K. Structure and properties of multi-targets magnetron sputtered ZrNbTaTiW multi-elements alloy thin films. Surf. Coat. Technol. 2013, 228, S424–S427. [Google Scholar] [CrossRef]
- Liang, S.C.; Tsai, D.C.; Chang, Z.C.; Sung, H.S.; Lin, Y.C.; Yeh, Y.J.; Deng, M.J.; Shieu, F.S. Structural and mechanical properties of multi-element (TiVCrZrHf)N coatings by reactive magnetron sputtering. Appl. Surf. Sci. 2011, 258, 399–403. [Google Scholar] [CrossRef]
- Lin, C.H.; Duh, J.G.; Yeh, J.W. Multi-component nitride coatings derived from Ti-Al-Cr-Si-V target in RF magnetron sputter. Surf. Coat. Technol. 2007, 201, 6304–6308. [Google Scholar] [CrossRef]
- Lai, C.H.; Lin, S.J.; Yeh, J.W.; Chang, S.Y. Preparation and characterization of AlCrTaTiZr multielement nitride coatings. Surf. Coat. Technol. 2006, 201, 3275–3280. [Google Scholar] [CrossRef]
- Khan, N.A.; Akhavan, B.; Zhou, H.; Chang, L.; Wang, Y.; Sun, L.; Bilek, M.M.; Liu, Z. High entropy alloy thin films of AlCoCrCu0.5FeNi with controlled microstructure. Appl. Surf. Sci. 2019, 495, 143560. [Google Scholar] [CrossRef]
- Shen, W.J.; Tsai, M.H.; Chang, Y.S.; Yeh, J.W. Effects of substrate bias on the structure and mechanical properties of (Al1.5CrNb0.5Si0.5Ti)Nx coatings. Thin Solid Films 2012, 520, 6183–6188. [Google Scholar] [CrossRef]
- Chang, Z.C.; Liang, S.C.; Han, S.; Chen, Y.K.; Shieu, F.S. Characteristics of TiVCrAlZr multi-element nitride films prepared by reactive sputtering. Nucl. Instrum. Methods Phys. Res. Sect. B 2010, 268, 2504–2509. [Google Scholar] [CrossRef]
- Wu, H.; Ian, B.; Liu, Y.; Wu, X.L. Dry sliding tribological behavior of Zr-based bulk metallic glass. Trans. Nonferrous Met. Soc. China 2012, 22, 585–589. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. On the significance of the H/E ratio in wear control: A nanocomposite coating approach to optimised tribological behaviour. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]






| C | Cr | Co | Mo | Ni | V | Nb |
|---|---|---|---|---|---|---|
| 0.10–0.25 | 13.0–19.0 | 5.0–14.0 | 3.0–5.0 | 1.75–5.25 | 0.25–1.25 | 0.01–0.1 |
| Diameters | Pressure/MPa | Temperature/°C | Time/h |
|---|---|---|---|
| 4 inches | 50 | 1600 | 2 |
| Film | Working Gas | Deposition Pressure /Pa | Deposition Power/W | Substrate Temperature/K | Deposition Time/s |
|---|---|---|---|---|---|
| HEA | Ar | 0.8 | 200 | 450 | 2325 |
| HEN | 50%Ar + 50%N2 |
| Sample | R1 | CPE1-T | CPE1-P | R2 | R3 | C1 |
|---|---|---|---|---|---|---|
| CSS-42L | 1.02 | 0.00829 | 0.86799 | 5.749 | 3.431 | 0.3377 |
| S-HEA | 1.266 | 0.00266 | 0.77124 | 34.15 | 11.34 | 0.1322 |
| S-HEN | 1.227 | 0.00047 | 0.76342 | 159.5 | 17.03 | 0.0944 |
| Parameters | ΔSmix (J/K·mol) | ΔHmix (J/K·mol) | Δ (%) | VEC |
|---|---|---|---|---|
| Value | 13.38 | −6.5 | 2.77 | 4.8 |
| Sample | H/E | H3/E2 |
|---|---|---|
| S-HEA | 0.049 | 0.027 |
| S-HEN | 0.083 | 0.173 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, L.; Wang, F.; Chen, H.; Gao, M.; Zhang, H. Improvement of the Mechanical Properties and Corrosion Resistance of CSS-42L Steel with a Novel TiAlMoNbW Nitrid Film Deposition. Coatings 2022, 12, 1048. https://doi.org/10.3390/coatings12081048
Yuan L, Wang F, Chen H, Gao M, Zhang H. Improvement of the Mechanical Properties and Corrosion Resistance of CSS-42L Steel with a Novel TiAlMoNbW Nitrid Film Deposition. Coatings. 2022; 12(8):1048. https://doi.org/10.3390/coatings12081048
Chicago/Turabian StyleYuan, Lin, Fangfang Wang, Haojie Chen, Ming Gao, and Hu Zhang. 2022. "Improvement of the Mechanical Properties and Corrosion Resistance of CSS-42L Steel with a Novel TiAlMoNbW Nitrid Film Deposition" Coatings 12, no. 8: 1048. https://doi.org/10.3390/coatings12081048
APA StyleYuan, L., Wang, F., Chen, H., Gao, M., & Zhang, H. (2022). Improvement of the Mechanical Properties and Corrosion Resistance of CSS-42L Steel with a Novel TiAlMoNbW Nitrid Film Deposition. Coatings, 12(8), 1048. https://doi.org/10.3390/coatings12081048