Study of the Passivation Film on S32750 Super-Duplex Stainless Steel Exposed in a Simulated Marine Atmosphere
Abstract
:1. Introduction
2. Experimental
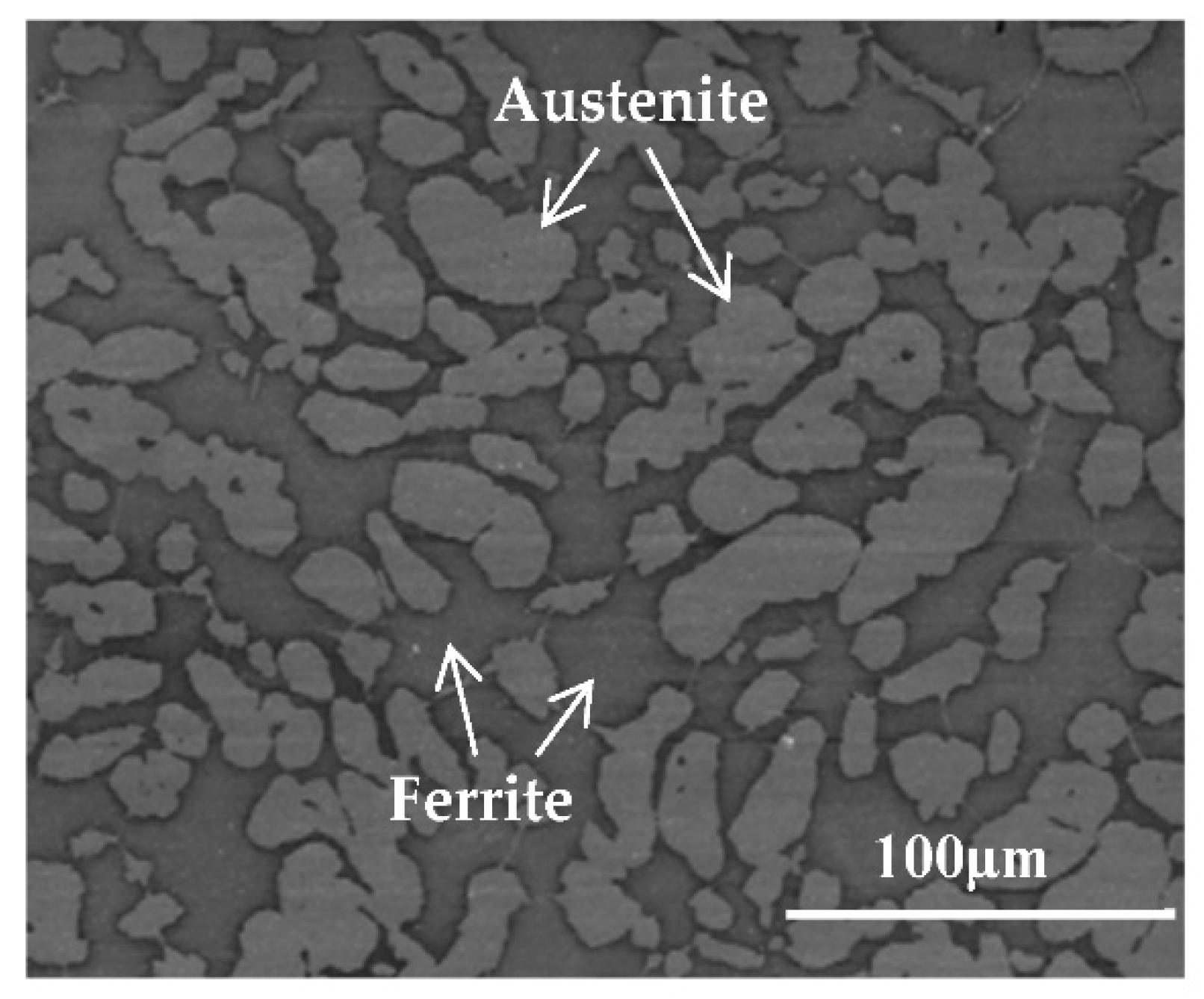
2.1. Materials
2.2. Exposure Conditions
2.3. Electrochemical Measurements
2.4. Analysis and Characterization Methods
3. Results and Discussion
3.1. Electrochemical Measurements after NSS Test
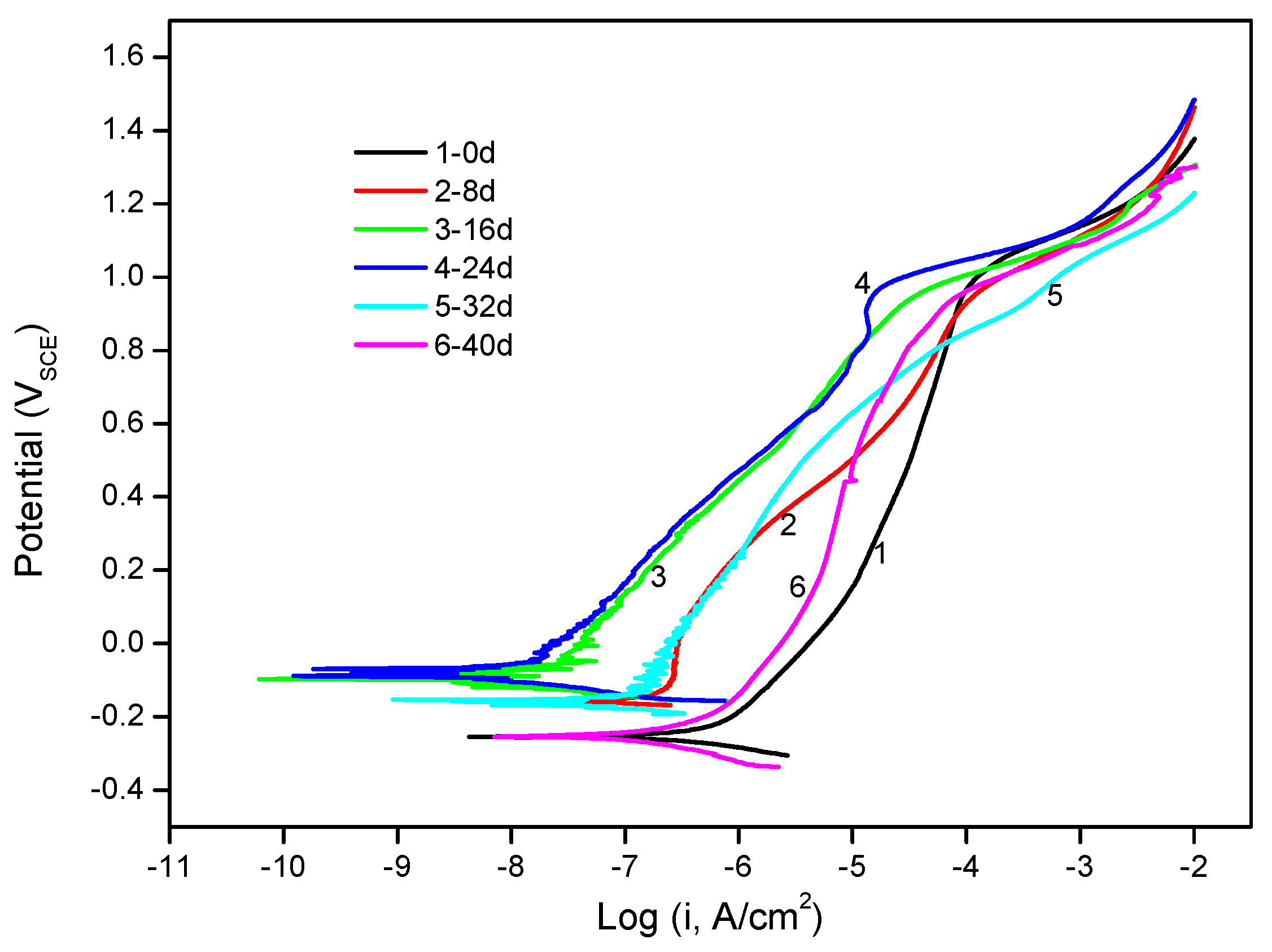
3.1.1. Potentiodynamic Polarization Curves
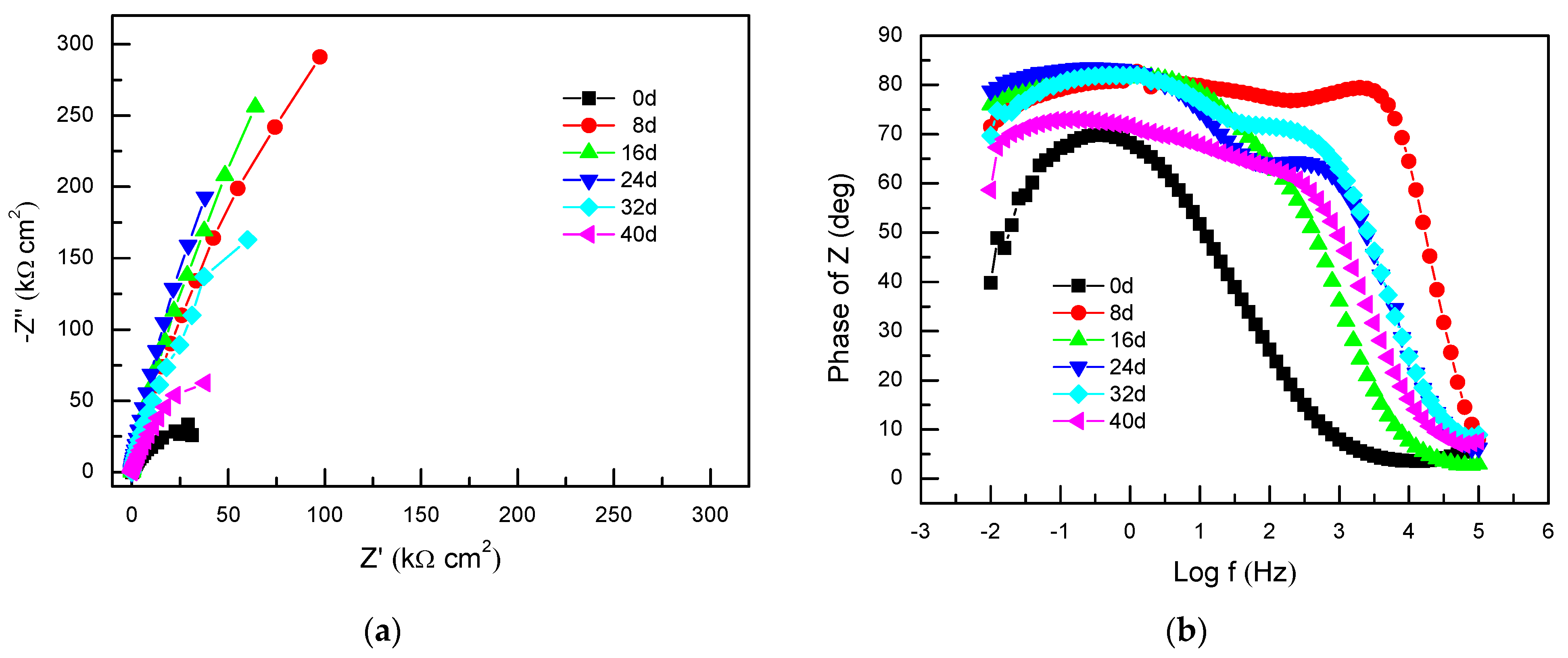
3.1.2. Electrochemical Impedance Spectroscopy
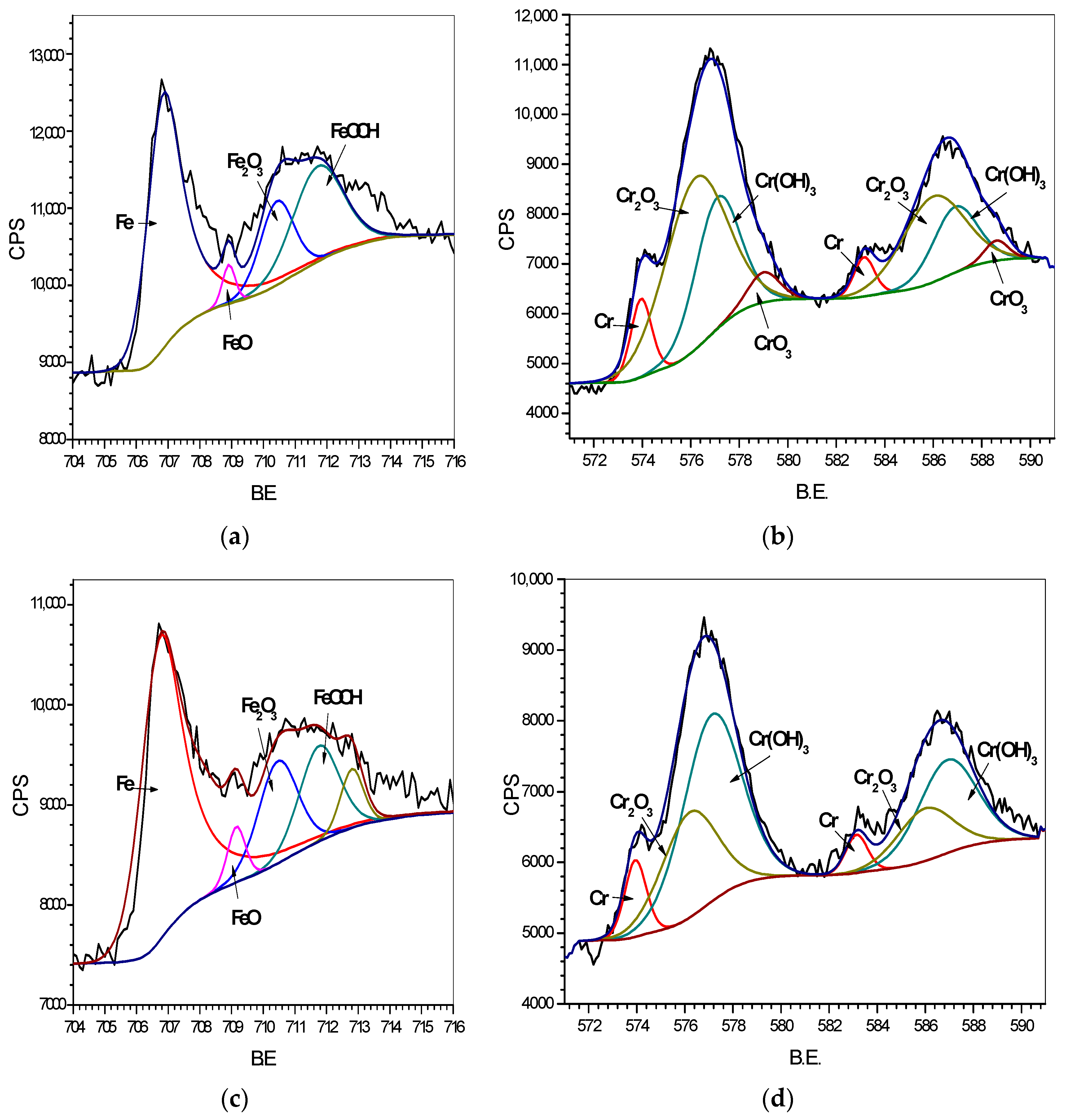
3.2. XPS Analysis
3.2.1. Surface Analysis of Oxide Films
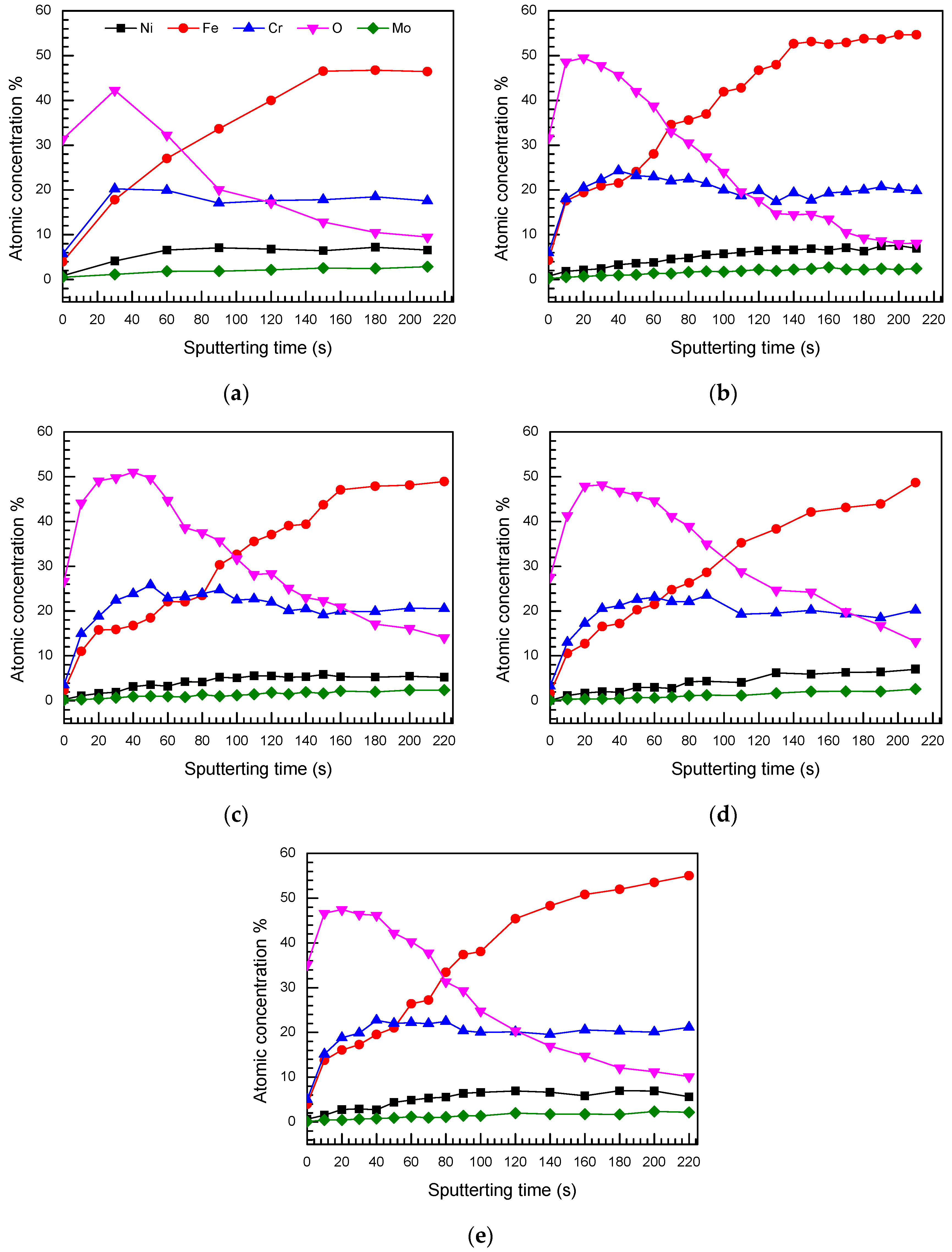
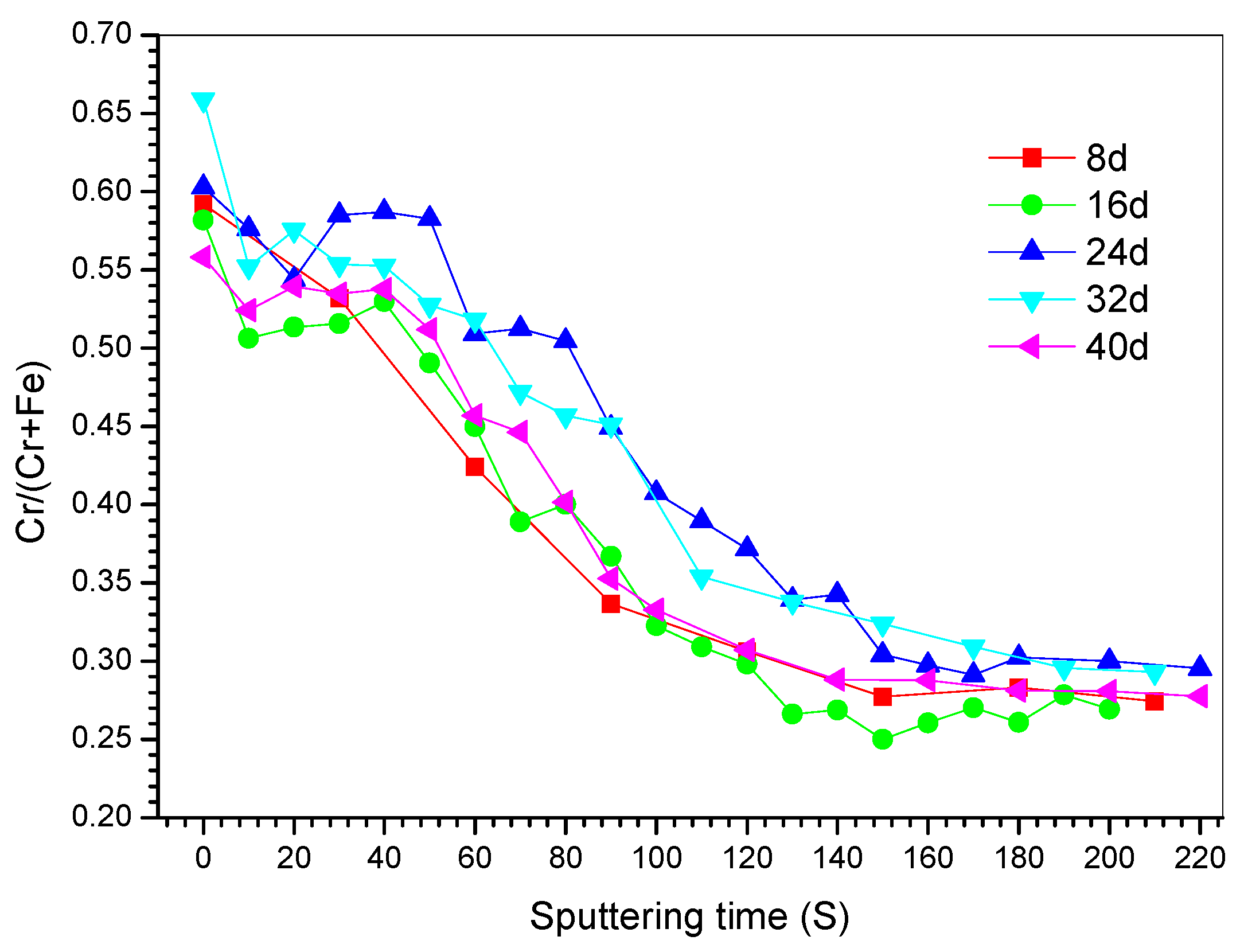
3.2.2. XPS Depth Profiles
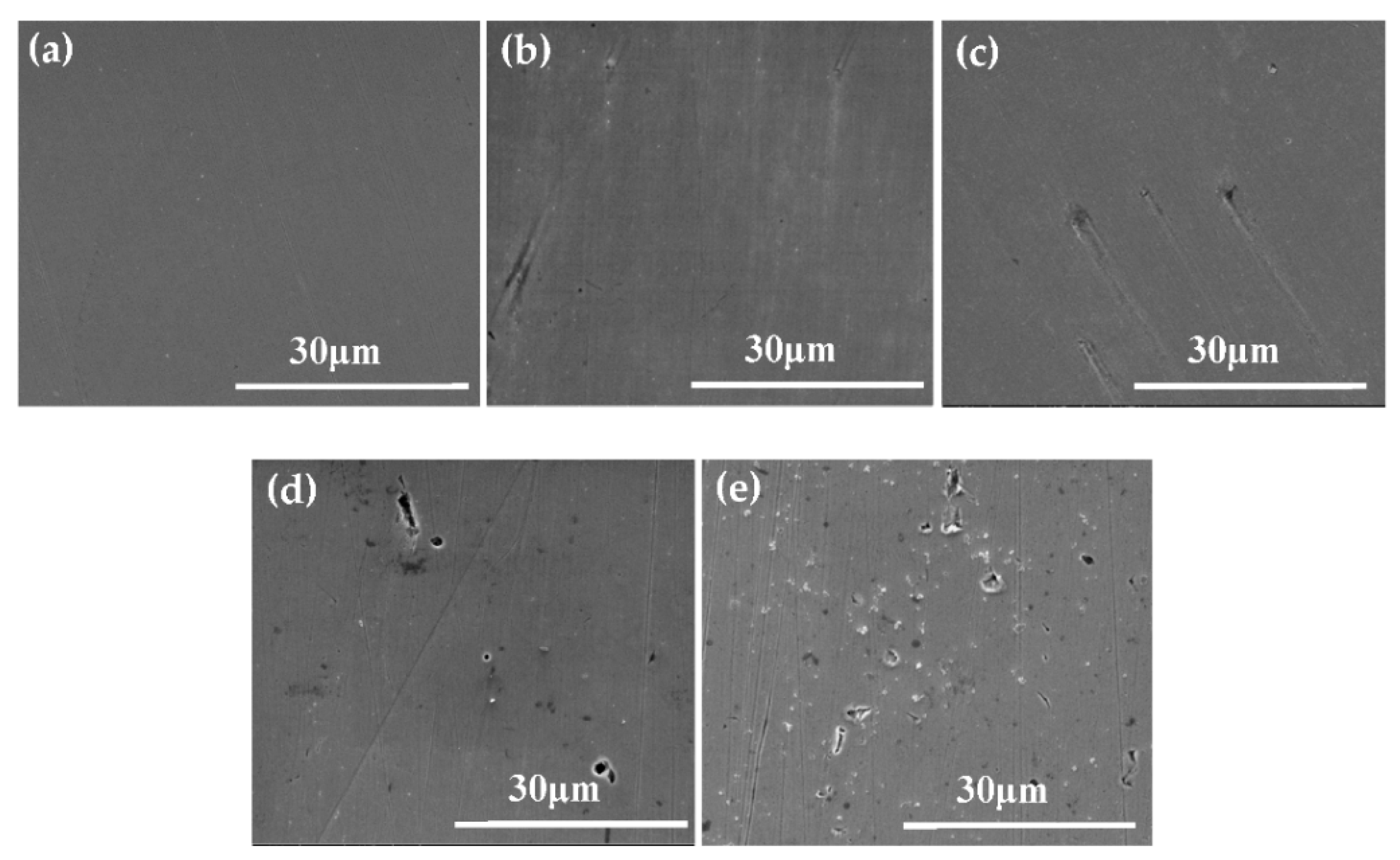
3.3. Surface Morphologies
4. Conclusions
- (1)
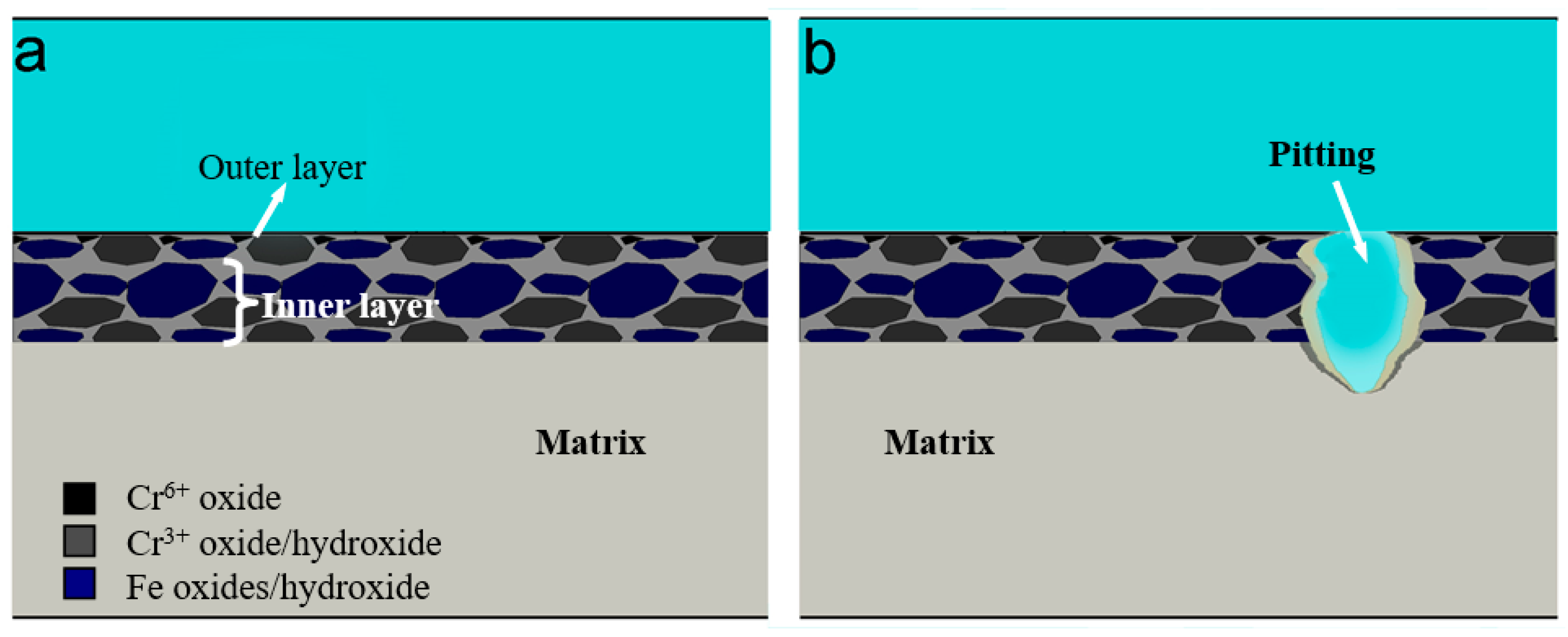
- Two processes (passivation and local corrosion) occurred on the metal surface when S32750 SDSS was exposed in the simulated marine atmospheric environment. The specimen exposed for 24 d exhibited the best corrosion resistance. The thickness of the passivation film slightly changed, but became dense when the exposure time was prolonged. Pittings occurred when the exposure time was more than 24 d.
- (2)
- The passivation film of S32750 SDSS was composed of two chromium-enriched layers. The outer layer had a very thin film at the metal/atmosphere interface of the specimen surface and had higher chromium content than the inner layer, which appeared depleted.
- (3)
- The outer and inner layers had similar Fe components, and Fe3+ oxide/hydroxide was the primary oxide in the film. However, the layers had different Cr components; the outer layer contained CrO3, whereas the inner layer had Cr2O3/Cr(OH)3 as the primary oxide.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, M.; He, J.S.; Wang, M.M.; Li, J.S.; Xing, S.L.; Gui, K.X.; Wang, G.; Liu, Q. Effects of deep cold rolling on the evolution of microstructure, microtexture, and mechanical properties of 2507 duplex stainless steel. Mat. Sci. Eng. A-Struct. 2022, 845, 143224. [Google Scholar] [CrossRef]
- Ma, C.Y.; Zhou, L.; Zhang, R.X.; Li, D.G.; Shu, F.Y.; Song, X.G.; Zhao, Y.Q. Enhancement in mechanical properties and corrosion resistance of 2507 duplex stainless steel via friction stir processing. J. Mater. Res. Technol. 2020, 9, 8296–8305. [Google Scholar] [CrossRef]
- Huang, F.F.; Liu, E.J.; Qin, Y.; Wang, Q.R.; Jin, Y.; Wen, L.; Chang, H. A Study of Multi-Pass Laser-Cladding 2205 Duplex StainlessSteel Coating: Microstructure, Electrochemical Corrosion Behavior, and Wear-Resistance Properties. Coatings 2022, 12, 229. [Google Scholar] [CrossRef]
- Cao, F.J.; Huang, G.Q.; Hou, W.T.; Ni, R.Y.; Sun, T.; Hu, J.P.; Shen, Y.F.; Gerlich, A.P. Simultaneously enhanced strength-ductility synergy and corrosion resistance in submerged friction stir welded super duplex stainless steel joint via creating ultrafine microstructure. J. Mater. Process. Technol. 2022, 307, 117660. [Google Scholar] [CrossRef]
- Tan, H.; Jiang, Y.M.; Deng, B.; Sun, T.; Xu, J.; Li, J. Effect of annealing temperature onthe pitting corrosion resistance of super duplex stainless UNS S32750. Mater. Charact. 2009, 60, 1049. [Google Scholar] [CrossRef]
- Zhong, C.; Tang, X.; Cheng, Y.F. Corrosion of steel under the defected coatingstudied by localized electrochemical impedance spectroscopy. Electrochim. Acta 2008, 53, 4740–4747. [Google Scholar] [CrossRef]
- Gerald, O.J.; Li, W.G.; Li, Z.; Zhao, Y.T.; Li, C.L. Corrosion behaviour of 2205 duplex stainless steel in marine conditions containing Erythrobacter pelagi bacteria. Mater. Chem. Phys. 2020, 239, 122010. [Google Scholar] [CrossRef]
- Zanotto, F.; Grassi, V.; Balbo, A.; Monticelli, C.; Zucchi, F. Stress corrosion cracking of LDX 2101® duplex stainless steel in chloridesolutions in the presence of thiosulphate. Corros. Sci. 2014, 80, 205–212. [Google Scholar] [CrossRef]
- Zakeri, M.; Moayed, M.H. Investigation on the effect of nitrate ion on the critical pittingtemperature of 2205 duplex stainless steel along a mechanistic approachusing pencil electrode. Corros. Sci. 2014, 85, 222–231. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, Q.; Yuan, Y.F.; Guo, S.Y. Effect of microstructure and passive film on corrosion resistance of 2507 super duplex stainless steel prepared by different cooling methods in simulated marine environment. Int. J. Min. Met. Mater. 2020, 27, 1100–1114. [Google Scholar] [CrossRef]
- Zeng, H.T.; Yang, Y.; Zeng, M.H.; Li, M.C. Effect of dissolved oxygen on electrochemical corrosion behavior of 2205 duplex stainless steel in hot concentrated seawater. J. Mater. Sci. Technol. 2021, 66, 177–185. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, Q.; Yuan, Y.F.; Guo, S.Y.; Huang, Y.Z. Study on the correlation between passive film and AC corrosion behavior of 2507 super duplex stainless steel in simulated marine environment. J. Electroanal. Chem. 2020, 864, 114072. [Google Scholar] [CrossRef]
- Abdo, H.S.; Seikh, A.H.; Samad, U.A.; Fouly, A.; Mohammed, J.A. Electrochemical Corrosion Behavior of Laser Welded 2205 Duplex Stainless-Steel in Artificial Seawater Environment under Different Acidity and Alkalinity Conditions. Crystals 2021, 11, 1025. [Google Scholar] [CrossRef]
- Jin, S.; Liu, C.M.; Lin, X.; Li, M.J.; Cao, H.B.; Wang, H.Z. Effect of inorganic anions on the corrosion behavior of UNS S32750 duplex stainless steel in chloride solution. Mater. Corros. 2015, 66, 1077–1083. [Google Scholar] [CrossRef]
- Han, Y.D.; Zhang, Y.K.; Jing, H.Y.; Gao, Z.Q.; Xu, L.Y.; Zhang, Z.Q.; Zhao, L. Microstructure and Corrosion Studies on Different Zones of Super Duplex Stainless Steel UNS S32750, Weldment. Front. Mater. 2020, 7, 251. [Google Scholar] [CrossRef]
- Brooks, A.R.; Clayton, C.R.; Doss, K.; Lu, Y.C. On the role of Cr in the passivity of stainless steel. J. Electrochem. Soc. 1986, 133, 2459. [Google Scholar] [CrossRef]
- Kocijan, A.; Donik, Č.; Jenko, M. Electrochemical and XPS studies of the passivefilm formed on stainless steels in borate bufferand chloride solutions. Corros. Sci. 2007, 49, 2083–2098. [Google Scholar] [CrossRef]
- Cui, Z.Y.; Chen, S.S.; Dou, Y.P.; Han, S.K.; Wang, L.W.; Man, C.; Wang, X.; Chen, S.G.; Cheng, Y.F.; Li, X.G. Passivation behavior and surface chemistry of 2507 super duplex stainlesssteel in artificial seawater: Influence of dissolved oxygen and pH. Corros. Sci. 2019, 150, 218–234. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zhao, H.; Zhang, H.Z.; Hu, J.; Jin, J.R. Microstructure evolution and pitting corrosion behavior of UNSS32750 super duplex stainless steel welds after short-time heattreatment. Corros. Sci. 2017, 121, 22–31. [Google Scholar] [CrossRef]
- Xin, S.S.; Li, M.C. Electrochemical corrosion characteristics of type 316L stainless steelin hot concentrated seawater. Corros. Sci. 2014, 81, 96–101. [Google Scholar] [CrossRef]
- Klapper, H.S.; Goellner, J.; Burkert, A.; Heyn, A. Environmental factors affecting pitting corrosion of type 304 stainlesssteel investigated by electrochemical noise measurements underpotentiostatic control. Corros. Sci. 2013, 75, 239–247. [Google Scholar] [CrossRef]
- Fredriksson, W.; Petrini, D.; Edström, K.; Björefors, F.; Nyholm, L. Corrosion resistances and passivation of powder metallurgicaland conventionally cast 316L and 2205 stainless steels. Corros. Sci. 2013, 67, 268–280. [Google Scholar] [CrossRef]
- Mesquita, T.J.; Chauveau, E.; Mantel, M.; Bouvier, N.; Koschel, D. Corrosion and metallurgical investigation of two supermartensiticstainless steels for oil and gas environments. Corros. Sci. 2014, 81, 152–161. [Google Scholar] [CrossRef]
- Schultze, J.W.; Lohrengel, M.M. Stability, reactivity and breakdown of passive films. Problems of recent and future research. Electrochum. Acta 2000, 45, 2499–2504. [Google Scholar] [CrossRef]
- Antony, P.J.; Singh Raman, R.K.; Mohanram, R.; Kumar, P.; Raman, R. Influence of thermal aging on sulfate-reducing bacteria (SRB)-influenced corrosion behavior of 2205 duplex stainless steel. Corros. Sci. 2008, 50, 1858–1864. [Google Scholar] [CrossRef]
- Hoseinpoor, M.; Momeni, M.; Moayed, M.H.; Davoodi, A. EIS assessment of critical pitting temperature of 2205 duplex stainlesssteel in acidified ferric chloride solution. Corros. Sci. 2014, 80, 197–204. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Jiang, Y.M.; Li, J. In situ investigation of crevice corrosion on UNS S32101 duplex stainlesssteel in sodium chloride solution. Corros. Sci. 2013, 76, 163–169. [Google Scholar] [CrossRef]
- Guo, L.Q.; Lin, M.C.; Qiao, L.J.; Volinsky, A.A. Duplex stainless steel passive film electrical properties studied by in situcurrent sensing atomic force microscopy. Corros. Sci. 2014, 78, 55–62. [Google Scholar] [CrossRef]
- Vignal, V.; Krawiec, H.; Heintz, O.; Mainy, D. Passive properties of lean duplex stainless steels after long-term ageing in airstudied using EBSD, AES, XPS and local electrochemical impedance spectroscopy. Corros. Sci. 2013, 67, 109–117. [Google Scholar] [CrossRef]
- Cheng, X.; Li, X.; Dong, C. Study on the passive film formed on 2205 stainless steelin acetic acid by AAS and XPS. Int. J. Min. Met. Mater. 2009, 16, 170–176. [Google Scholar] [CrossRef]
- ASTM A240/A240M−19 Standard; Specification for Chromium and Chromium-Nickel Stainless Steel Plate, Sheet, and Strip for Pressure Vessels and for General Applications1. ASTM International: West Conshohocken, PA, USA.
- Brett, C.M.A.; Brett, A.M. Electrochemistry Principles, Methods and Application; Oxford University Press: New York, NY, USA, 1993. [Google Scholar]
- Macdonald, J.R. (Ed.) Impedance Spectroscopy-Emphesizing Solid Materials and Systems; Chapters 1–4; John Wiley& Sons: Hoboken, NJ, USA, 1987. [Google Scholar]
- Wallinder, D.; Pan, J.; Leygraf, C.; Delblanc-Bauer, A. EIS and XPS study of surface modi_cation of205LVM stainless steel after passivation. Corros. Sci. 1999, 41, 275–289. [Google Scholar] [CrossRef]
- Cristofaro, N.D.; Piantini, M.; Zacchetti, N. The influence of temperature on the passivation behavior of a super duplex stainless steel in a boric-borate buffer solution. Corros. Sci. 1997, 39, 2181–2191. [Google Scholar] [CrossRef]
- Doh, S.J.; Je, J.H.; Kim, J.S.; Kim, K.Y.; Kim, H.S.; Lee, Y.D.; Lee, J.M.; Hwu, Y. Influence of Cr and Mo on the passivation of stainless steel430 (18Cr) and 444 (18Cr–2Mo): In situ XANES study. Nucl. Instrum. Meth. B 2003, 199, 211–215. [Google Scholar] [CrossRef]


| Element (wt%) | C | Si | Mn | P | S | Cr | Ni | Mo | Cu | N | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | 0.019 | 0.44 | 0.71 | 0.029 | 0.001 | 25.21 | 6.2 | 3.33 | 0.10 | 0.2512 | Bal. |
| ASTM A240 [31] | ≤0.03 | ≤0.80 | ≤1.2 | ≤0.035 | ≤0.02 | 24–26 | 6–8 | 3–5 | ≤0.5 | 0.24–0.32 | Bal. |
| Exposure Time | Ecorr (V) | Icorr (μA/cm2) | ba (V/dec) |
|---|---|---|---|
| 0 d | −0.25 | 5.49 × 107 | 0.27 |
| 8 d | −0.16 | 1.10 × 107 | 0.11 |
| 16 d | −0.10 | 1.23 × 108 | 0.18 |
| 24 d | −0.09 | 7.69 × 109 | 0.18 |
| 32 d | −0.15 | 5.49 × 108 | 0.16 |
| 40 d | −0.25 | 3.11 × 107 | 0.22 |
| Exposure Time | Rs/ Ω·cm2 | Qpass | Rpass/ Ω·cm2 | Qpit-pass | Rpit-Pass/Ω·cm2 | ||
|---|---|---|---|---|---|---|---|
| Ypass/ Ω−1s−ncm2 | n2 | Ypit-Pass/ Ω−1s−ncm2 | n1 | ||||
| 8 d | 0.60 | 3.64 × 10−5 | 0.89 | 1.80 × 106 | - | - | - |
| 16 d | 11.60 | 4.65 × 10−5 | 0.92 | 2.02 × 106 | 2.87 × 10−4 | 0.76 | 26.86 |
| 24 d | 2.88 | 6.45 × 10−5 | 0.93 | 2.23 × 106 | 1.57 × 10−4 | 0.78 | 58.23 |
| 32 d | 2.15 | 6.81 × 10−5 | 0.91 | 8.09 × 105 | 3.20 × 10−4 | 0.79 | 36.97 |
| 40d | 4.17 | 1.33 × 10−4 | 0.83 | 3.93 × 105 | 5.65 × 10−4 | 0.72 | 60.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Du, W.; Wu, M.; He, J.; Yu, G.; Wang, S.; Song, Z. Study of the Passivation Film on S32750 Super-Duplex Stainless Steel Exposed in a Simulated Marine Atmosphere. Coatings 2022, 12, 1430. https://doi.org/10.3390/coatings12101430
Yang L, Du W, Wu M, He J, Yu G, Wang S, Song Z. Study of the Passivation Film on S32750 Super-Duplex Stainless Steel Exposed in a Simulated Marine Atmosphere. Coatings. 2022; 12(10):1430. https://doi.org/10.3390/coatings12101430
Chicago/Turabian StyleYang, Lijing, Wenwen Du, Minghua Wu, Jin He, Guohong Yu, Shuchang Wang, and Zhenlun Song. 2022. "Study of the Passivation Film on S32750 Super-Duplex Stainless Steel Exposed in a Simulated Marine Atmosphere" Coatings 12, no. 10: 1430. https://doi.org/10.3390/coatings12101430
APA StyleYang, L., Du, W., Wu, M., He, J., Yu, G., Wang, S., & Song, Z. (2022). Study of the Passivation Film on S32750 Super-Duplex Stainless Steel Exposed in a Simulated Marine Atmosphere. Coatings, 12(10), 1430. https://doi.org/10.3390/coatings12101430







