1. Introduction
Lithium–sulfur (Li-S) batteries are considered to be one of the most promising battery systems due to their high specific capacity (1675 mAh g
−1), high energy density (2600 wh kg
−1), and low cost [
1,
2,
3,
4,
5,
6]. Despite these benefits, there are still several challenges facing Li-S batteries, such as the dissolution and diffusion of lithium polysulfides (Li
2S
x, 4 ≤ x ≤ 8), the volume variation of S/Li
2S, and the poor electrical conductivity of S materials, which result in a low specific capacity and decayed cycle life [
7,
8,
9,
10,
11]. Generally, the cathode of a Li-S battery mainly includes four components: a current collector, active material, a conductive carbon agent, and a polymer binder [
12]. As an indispensable component, the binder promotes an effective connection among the active substances, conductive agents, and sulfur material, which is of great importance to ensure the rapid transfer of electrons and ions [
13,
14,
15,
16]. Moreover, the integrity of the cathode after the cycling process is closely related to the molecular architecture of the binder. Therefore, the application of a binder is particularly important for Li-S batteries [
17,
18,
19].
Polyvinylidene fluoride (PVDF), a commercial binder, is widely used in research on sulfur cathodes [
20,
21,
22,
23,
24]. PVDF has the advantages of a high bonding strength, high thermal stability, and a wide electrochemical window. However, PVDF binder fails to suppress the shuttle effect of lithium polysulfide dissolved during the charge/discharge process, resulting in rapid capacity decay [
25,
26]. Therefore, it is of great significance to make advances in an advisable binder for Li-S batteries to promote electrochemical performance by confining lithium polysulfide. Water-soluble binders have attracted more and more attention due to their advantages of having a strong lithium polysulfide suppression ability, non-toxicity, and being environmentally friendly.
Sodium carboxymethyl cellulose (CMC) and its derivatives display good flexibility and are conducive to enhancing the strength and structural stability of sulfur cathodes. SBR-CMC binder is not only a high adhesion agent, but also a dispersion medium that favors the uniform distribution between insulating sulfur particles and conductive carbon black, and it also ensures good electrical contact, leading to higher sulfur utilization [
27].
The pollution caused by synthetic materials is the main problem we have to face. Biomass-based binders are renewable and biodegradable. They are conducive to sustainable development. Biomass-based binders are derived from animals, plants, microorganisms, and other living organisms. They are mainly composed of organic polymer materials. In terms of chemical composition, biomass is mainly composed of carbon, hydrogen, and oxygen. Because they are derived from animals, plants, microorganisms, and other living organisms, unmodified biomass-based binders are easily degraded into water, carbon dioxide, and other small molecules by natural microorganisms, and their products can enter the natural cycle again. So, the harm to the environment is weak.
However, biomass materials lack active sites, and the effect of lithium polysulfide adsorption is poor [
28]. Wan et al. studied a natural peach gum (PG) binder in Li-S batteries, and its good bonding properties maintained the electrode integrity during the charge and discharge processes and enhanced ion/electron conduction. In addition, the PG structure is rich in hydroxyl and carboxyl groups, which not only shows strong adhesion performance, but also presents a strong combination effect with polysulfide [
29]. Tung oil contains long fatty acid chains and can also be used as a binder in Li-S batteries. Good tensile properties are beneficial to impede the cathode collapse caused by the expansion/contraction of active material [
30]. Li et al. investigated the electrochemical performance of gum arabic (GA) binder in Li-S batteries. The polysulfide shuttle effect could be inhibited due to the large amount of hydroxyl in the structure; therefore, the cycling life of batteries is prolonged [
31].
In this article, the influence of conventional binders (PVDF, PVP, CMC, PAN, and PEO) on the electrochemical performance of sulfur cathodes is analyzed through extensive tests. Remarkably, a new biomass-based binder is proposed in this work to promote the electrochemical performance of Li-S batteries. γ-polyglutamic acid (γ-PGA) is the main component of natto gum, which shows excellent bonding ability, and the large number of amino groups contained in its molecules can effectively confine the lithium polysulfides through the secondary valence bond. Therefore, it is expected that the electrochemical properties of Li-S batteries can be improved via the application of γ-PGA. During long-term cycling tests, a high initial capacity of 991 mAh g−1 is delivered, indicating the high electrochemical activity of the cathode, and high-capacity retention is maintained at 62.5%, suggesting the effective role of the γ-PGA binder.
3. Results and Discussion
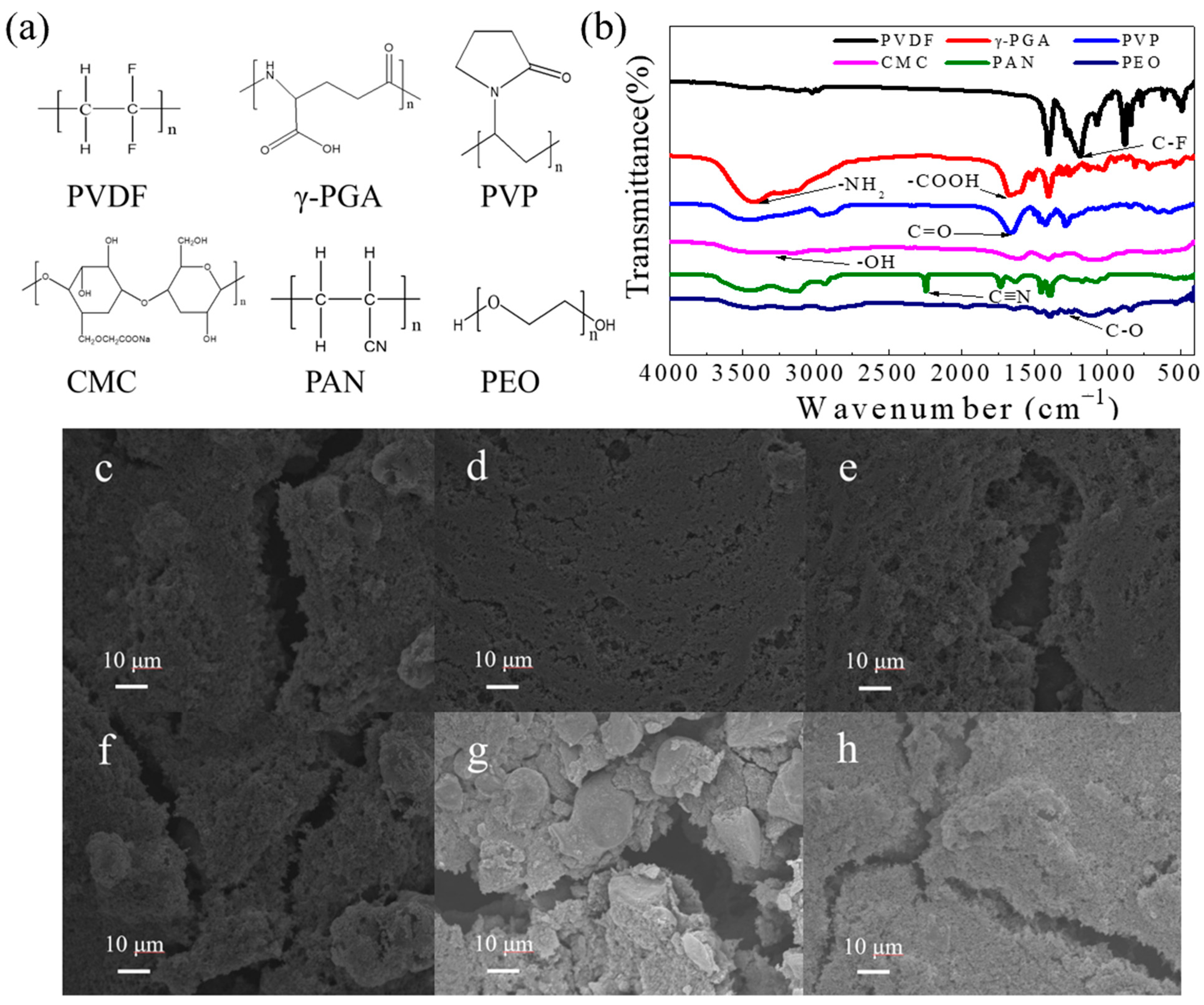
Figure 1a,b show the structural formula and FTIR spectra of the PVDF, γ-PGA, PVP, CMC, PAN, and PEO binders. PVDF consists of fluorine–carbon bonds (the characteristic absorption peak of C-F is located at 1187 cm
−1) and presents a high dielectric constant, good chemical stability, and excellent mechanical properties. PVP is a non-ionic polymer compound and can be dissolved in water. Its high viscosity makes PVP a good binder in lithium batteries, and the characteristic absorption peaks of C=O and C-N can be observed at 1657 cm
−1 and 1289 cm
−1. CMC is a carboxymethylated derivative of cellulose that is usually prepared via the reaction of natural cellulose with caustic soda and monochloroacetic acid. CMC is conducive to improving the viscosity of the slurry and to prevent slurry precipitation during the cathode preparation process. However, the viscosity of CMC is easy to decrease with temperature and is easy to lose after absorbing moisture. In the FTIR spectra, the hydroxyl group in CMC shows an obvious absorption peak at 3420 cm
−1. PAN is obtained by the free radical polymerization of the monomer acrylonitrile (the characteristic absorption peak of C≡N is located at 2244 cm
−1), which has been used as an electrolyte matrix and binder in lithium batteries for a long time. PEO is a linear polymer with repeating units of –[C-C-O]–groups (absorption peaks can be observed at 1120 cm
−1) that is conducive to the transportation of Li ions and is treated as a promising polymer electrolyte candidate in Li batteries. Moreover, PEO shows high viscosity after being dissolved in water, and when used as a Li-S binder, the ionic conductivity and the mechanical properties of cathodes can be improved. Γ-PGA is a biomass-based polymer that comprises amino (the characteristic absorption peaks is at 3422 cm
−1) and carboxyl groups (the characteristic absorption peaks are at 1403 cm
−1). This is the first time that γ-PGA has been used as a binder, and it is expected to develop the electrochemical performance of Li-S batteries due its abundant polar groups with its lithium polysulfide-capturing ability.
The surface morphologies of electrodes with different binders were also tested using SEM (
Figure 1c–h). Higher-stability slurries can maintain the good dispersion morphology inherited from the wet state. There was a large number of cracks on the surface of the cathode prepared with PVDF (
Figure 1c), which is not conducive to the transfer of Li ions and may result in the rapid capacity decay of the battery. As for the cathode prepared with γ-PGA, a dense and uniform surface can be observed, and the sulfur particles and conductive carbon are unevenly distributed (
Figure 1d), which may be attributed to the good flexibility and adhesion of γ-PGA and is expected to perform well during long-term cycling. Due to the hydrophobic surface, a water-based slurry is more likely to find particle aggregation between conductive carbon additives. Therefore, it is necessary to make the slurry coating more uniform via more sufficient stirring.
Figure 1e–h show the cathodes prepared with PVP, CMC, PAN, and PEO binders. Compared to the cathodes prepared with γ-PGA binder, the surfaces of these cathodes display different degrees of fracture, indicating that these binders are not conducive to the uniform distribution of active substances and conductive carbon, which will exert an adverse impact on the transmission of electrons and ions in the subsequent cycle and lead to a short cycle life. A rough surface is favorable for electrolyte wetting. This is because more sulfur is dissolved in the electrolyte during cycling. However, the binding agent has a weak capacity to fix sulfur, which leads to capacity attenuation.
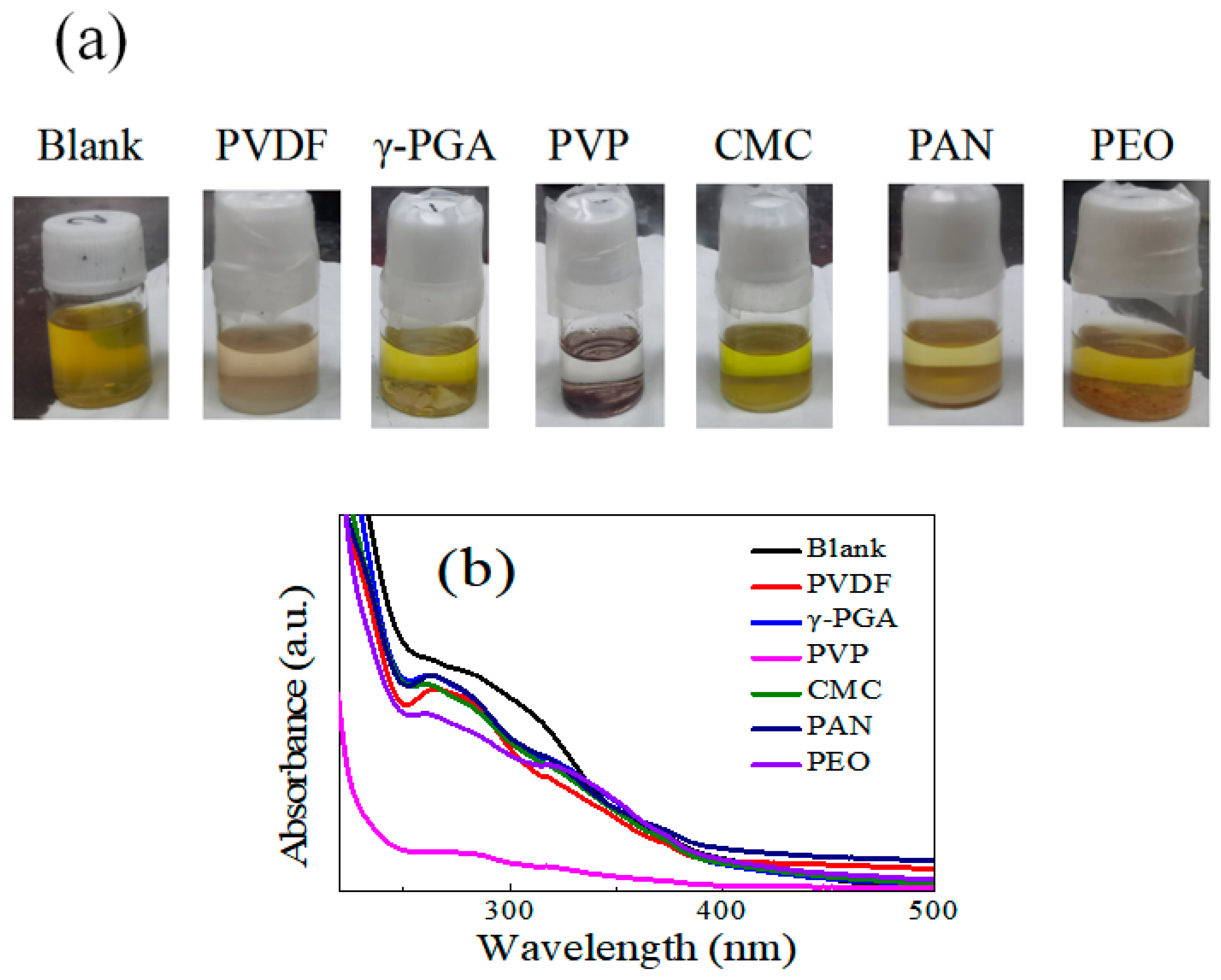
The interaction between binders and LiPSs is verified by the color variation in the Li
2S
6 solution upon mixing with the PVDF, γ-PGA, PVP, CMC, PAN, and PEO binders. In order to explain the adsorption of the binder on lithium polysulfide, Li
2S
6 solution was selected as the adsorption experiment according to the scheme of adsorption experiments in the literature, and the problem was briefly analyzed to reflect this problem. This indicates that polyglutamic acid binder has a certain adsorption capacity. Digital images are displayed in
Figure 2a. It can be seen that the color of the Li
2S
6 solution becomes lighter after adding PVDF binder. As for γ-PGA, the amino and carboxyl groups present a strong adsorption effect on Li
2S
6, but it is easy for it to agglomerate in ethylene glycol dimethyl ether, and most functional groups cannot exert an adsorption role; therefore, the adsorption capacity is relatively low. CMC shows similar color variation due to the same reason. As for PVP, the solution becomes colorless owing to the strong adsorption ability of the N atoms and carbonyl. No noticeable color variation is observed for the solution with added PAN or PEO.
To further demonstrate the adsorption ability of different binders, UV-vis spectroscopy was conducted by adding 0.1 g polymers to 5 mL 2 mmol L
−1 Li
2S
6 into a 1,3-dioxolane (DOL)/1,2-dimethoxyethane (DME) solution (1:1
v/
v) to track variation in the polysulfide concentration over 4 h. The spectra of the Li
2S
6 solution display a broad absorbance peak at around 250–280 nm, which belongs to the S
62− anion (
Figure 2b). The intensity of the Li
2S
6/PVDF solution decreases slightly during the test period, suggesting weak adsorption ability to Li
2S
6. The intensities of the solutions exposed to γ-PGA and CMC show minimal decrease. Compared to the solutions with PVDF and γ-PGA, the typical S
62− anion peak cannot be detected in the solution with PVP binder, indicating the strong adsorption of PVP on LiPSs and consistent with the results of the digital images.
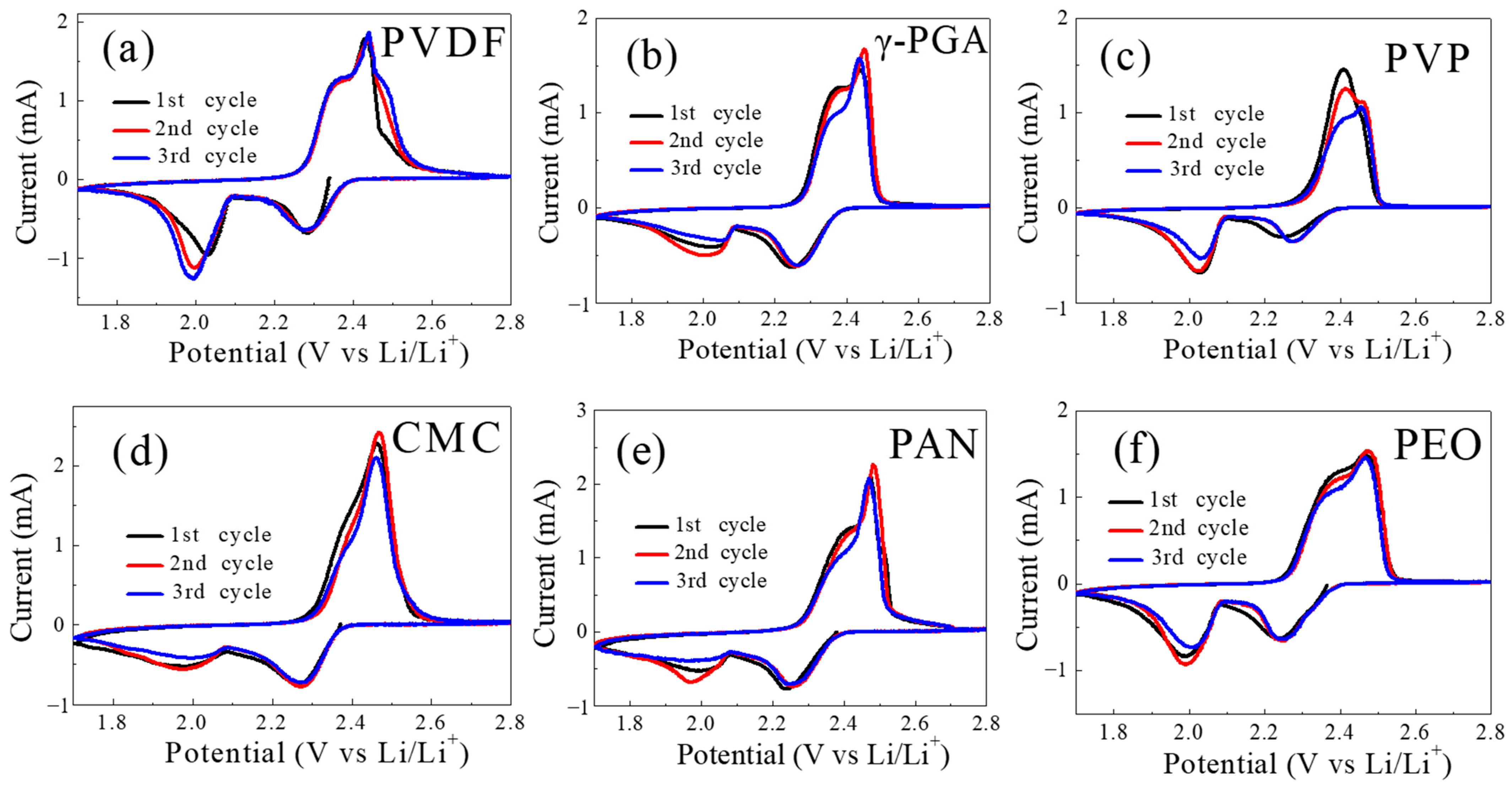
The activity of the different binders on the kinetics of the redox reactions was evaluated using cyclic voltammetry in the working range of 1.7–2.8 V at the scanning speed of 0.1 mV s
−1 for three cycles. All of the cyclic voltammogram (CV) profiles in
Figure 3 show typical anodic peaks (oxidation peak) at around 2.3–2.4 V (corresponding to the conversion of lithium sulfide to solid sulfur) and cathodic peaks (reduction peaks) at about 2.3 V (corresponding to the conversion of solid sulfur to high-order liquid lithium polysulfide) and 2.05 V (corresponding to the conversion of liquid lithium polysulfide to solid Li
2S
2/Li
2S). The peak at 2.05 V is assigned to accessible sulfur utilization at the applied scan rate. During the first cycle, a large amount of lithium polysulfide was dissolved in the electrolyte. In the process of reducing lithium polysulfide to solid Li
2S
2/Li
2S, 2 V has an obvious reduction peak. As the cycle progresses, the reduction of lithium polysulfide into solid Li
2S
2/Li
2S decreases, so the reduction peak weakens [
32]. Remarkably, the peak intensities of the γ-PGA/sulfur cathode are significantly higher than those of the PVDF/sulfur cathode and its other counterparts, indicating the optimized redox reactivity of electrodes endowed with γ-PGA. Furthermore, the polarization between the reduction and oxidation peaks of γ-PGA is smaller than those of other counterparts, suggesting the suppressed parasitic reaction and effective confined lithium polysulfide effect provided by the binder.
In order to test the effects of different binders on the cycling stability of Li-S batteries, the corresponding cycling test was conducted at 0.2 C after an activation process at 0.1 C for the first five cycles. As shown in
Figure 4a, the initial discharge specific capacity of the γ-PGA/sulfur cathode is 991 mAh g
−1. After 100 cycles, a specific capacity of 620 mAh g
−1 with a capacity retention rate of 62.5% remained. The initial specific capacity of the PVDF/sulfur cathode was 845 mAh g
−1 and had a low capacity of 456 mAh g
−1 a capacity retention rate of 53.9% maintained after cycling. The relatively poor cycling performance may be related to the inability of PVDF to inhibit the shuttle of lithium polysulfide. As for the cathodes prepared with PVP, CMC, PAN, and PEO binders, the capacities of 406 mAh g
−1, 311 mAh g
−1, 364 mAh g
−1, and 421 mAh g
−1 were maintained after cycling, with capacity retention rates of 52.8%, 40.7%, 51.5%, and 67%, respectively. The γ-PGA/sulfur cathode displays the highest discharge specific capacity and capacity retention due to the presence of amino and carboxyl groups that can capture the lithium polysulfide effectively and maintain the integrity of the electronic and ion circuits. PEO presents the second stable cycling performance due to the existence of polar groups, while CMC shows the worst stability due to the brittle mechanical behavior of the molecular structure. Other binders are not conducive to the dispersion of active material and demonstrate a weak adsorption capacity of lithium polysulfide; therefore, rapid capacity decay is observed after long-term cycling.
The related charge/discharge curves are displayed in
Figure 4b–g. The discharge profiles with different binders show two distinct plateaus at about 2.3–2.4 V (the reduction of elemental sulfur (S
8) to lithium polysulfides (Li
2S
n, 2 < n < 8)) and 2.0–2.1 V (the reduction of high-order polysulfides to lithium sulfide). With the progress of the cycle, the discharge platforms of the electrodes prepared with PVP, PAN, and PEO show a significant decline, indicating the increasing polarization and the severe side reactions happening with the deepening of the discharge, which may be caused by the shuttle of lithium polysulfide and the generation of large amounts of non-conductive Li
2S at the anode interface.
Figure 4c shows the charge/discharge curve of the γ-PGA/sulfur cathode. Minor polarization is displayed and is closely related to the capability of the binder to construct the electron/ion transmission circuit and to carry out lithium polysulfide inhibition.
Figure 5a compares the rate performance of Li-S batteries prepared with different binders. The specific discharge capacities of the PVDF/sulfur cathode at the 0.1 C, 0.2 C, 0.5 C, and 1 C rates are 690 mAh g
−1, 619 mAh g
−1, 551 mAh g
−1, and 527 mAh g
−1. The specific discharge capacity of the PEO/sulfur cathode at 0.1 C and 0.2 C are 787 mAh g
−1 and 641 mAh g
−1, which are higher than those obtained for the PVDF counterpart. The specific discharge capacities at 0.5 C (551 mAh g
−1) and 1 C (527 mAh g
−1) are lower. The specific discharge capacities of the PVP/sulfur cathode are 637 mAh g
−1, 564 mAh g
−1, 460 mAh g
−1, and 364 mAh g
−1. The specific discharge capacities of the CMC/sulfur cathode are 659 mAh g
−1, 289 mAh g
−1, 133 mAh g
−1, and 97 mAh g
−1. The specific discharge capacities of the PAN/sulfur cathode at the 0.1 C, 0.2 C, 0.5 C, and 1 C rates are 671 mAh g
−1, 482 mAh g
−1, 223 mAh g
−1, and 195 mAh g
−1. The specific discharge capacities of the γ-PGA/sulfur cathode are 765 mAh g
−1, 680 mAh g
−1, 604 mAh g
−1, and 552 mAh g
−1. The Li-S batteries prepared with the γ-PGA binder shows excellent electrochemical performance at each current density, indicating that γ-PGA binder can better fix more sulfur on the cathode to participate in the recycling process of Li-S batteries.
The related charge/discharge curves are provided in
Figure 5b–g. It can be seen that the discharge platform decreases and the polarization increases with the C-rate for every sample. The discharge platform of the γ-PGA/sulfur cathode decreases slowly as the current density increases (2.10 V, 2.09 V, 2.07 V, and 2.05 V at 0.1 C, 0.2 C, 0.5 C, and 1 C). This shows that the γ-PGA/sulfur cathode possess small polarization and fast reaction kinetics, which may be ascribed to its good polysulfide adsorption capacity and to the maintenance of cathode integrity.
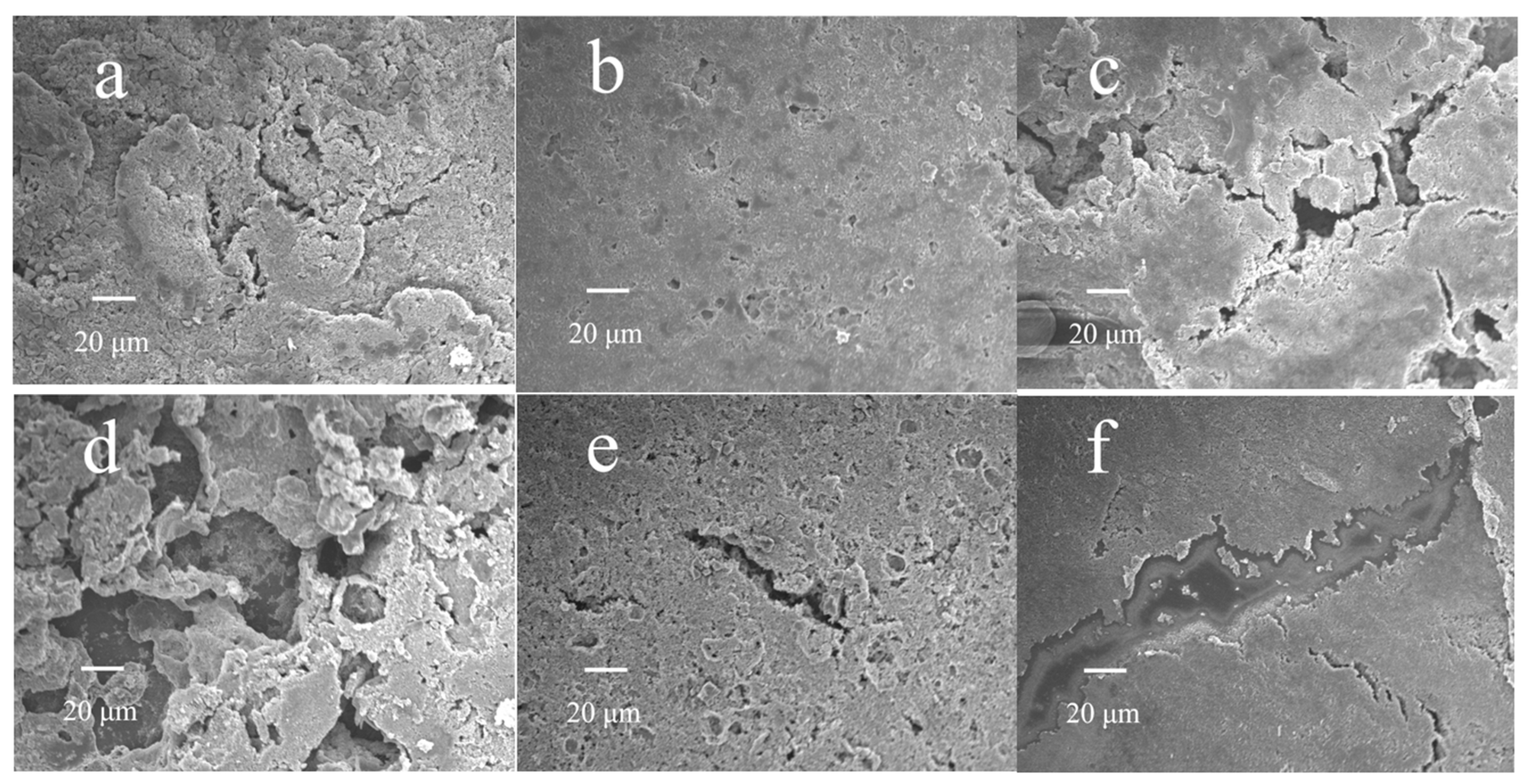
To further explore the effects of binders exerted on the electrochemical performance of Li-S batteries, the surface morphology of the cycled cathode is characterized using SEM.
Figure 6a shows SEM images of the PVDF/sulfur cathode. It can be seen that many cracks are generated and that the circuits of electron and Li
+-ion transmission are damaged, which may be ascribed to the poor mechanical behavior of the binder and lead to poor cycling performance.
Figure 6b shows the SEM of the γ-PGA/sulfur cathode. The surface is relatively complete and homogeneous; thus, the electron and ion transmission paths maintained during cycling, and excellent electrochemical performance can be achieved.
Figure 6c shows the SEM of the PVP/sulfur cathode. It can be clearly seen that serious cracks appear on the surface, which are related to the molecular structure of PVP, and the existence of a five-membered ring leads to poor mechanical strength, resulting in failure to maintain the complete cathode structure after cycling.
Figure 6d shows the SEM of the CMC/sulfur cathode, in which it is difficult to disperse active materials and conductive agents due to the high viscosity. Therefore, serious structural damage happens during cycling.
Figure 6e,f show the SEM of cathode prepared by PAN and PEO and obvious cracks can be observed. The maintenance of cathode integrity is consistent with the electrochemical performance results, and these results imply that a Li-S battery made of γ-PGA binder can make the electrode surface have better morphology, thereby making the Li-S battery cycle more stable.
The electrochemical performance of Li-S batteries is not only related to the integrity of the cathode, but is also affected by the stability of the lithium anode because the lithium polysulfide produced in the working process is easy to diffuse to the anode side and can be reduced by lithium. A large number of electronic inert by-products such as Li
2S/Li
2S
2 are generated and gathered, which further aggravates the electrochemical performance of Li-S batteries.
Figure 7a shows the surface morphology of the lithium anodes disassembled with the PVDF/sulfur cathode. It can be seen that a large number of by-products were deposited on the surface of lithium anode, which may have resulted from the decomposition of the solvent, the uneven products resulting from lithium deposition, and the reduction of Li
2S/Li
2S
2. As a sharp contrast, the surface of the lithium anode disassembled with the γ-PGA/sulfur cathode is relatively flat and dense (
Figure 7b), which is closely related to the shuttle-effect inhibition of γ-PGA. In
Figure 7c–f, the surface of the lithium anodes disassembled with PVP/sulfur, CMC/sulfur, PAN/sulfur, and PEO/sulfur cathodes present uneven and loose morphologies, which may be caused by an incomplete electrochemical reaction, the reduction of lithium polysulfide, and the uneven deposition of lithium. The above results show that the γ-PGA binder is conducive to promoting the electrochemical reaction, reducing the damage of lithium polysulfide to the anode, and inhibiting the occurrence of side reactions on both electrodes.