Improved Corrosion Protection of Acrylic Waterborne Coating by Doping with Microencapsulated Corrosion Inhibitors
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
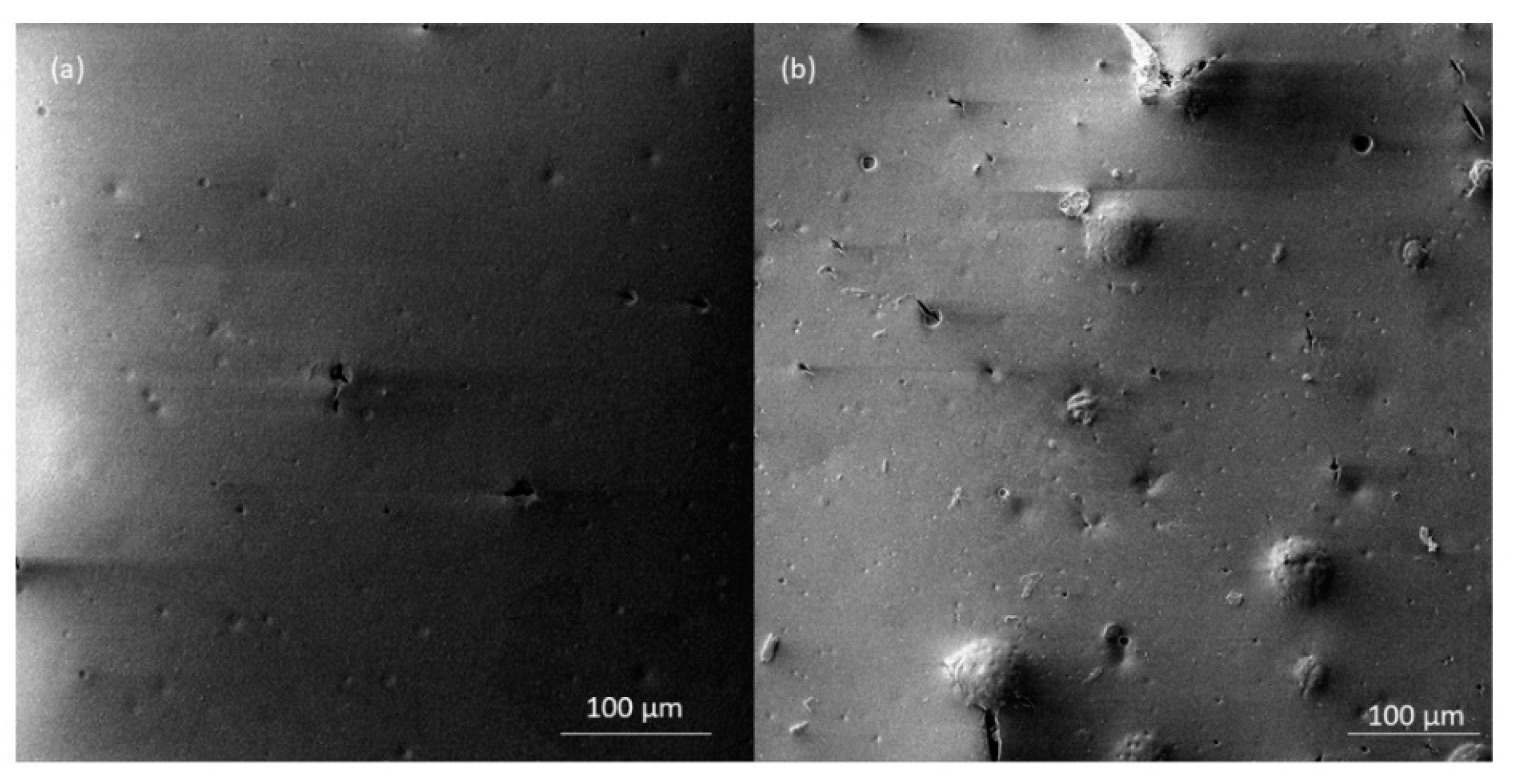
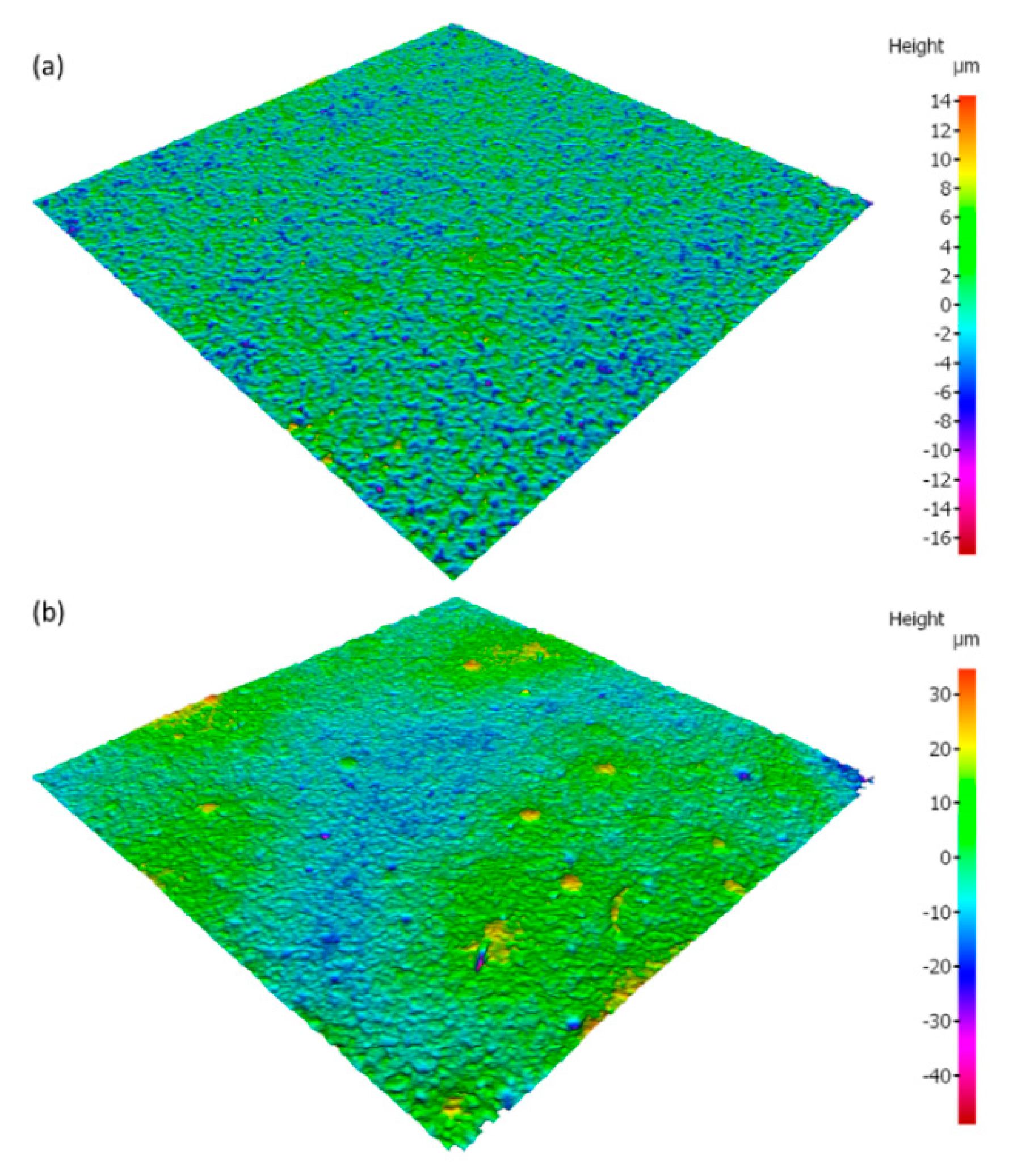
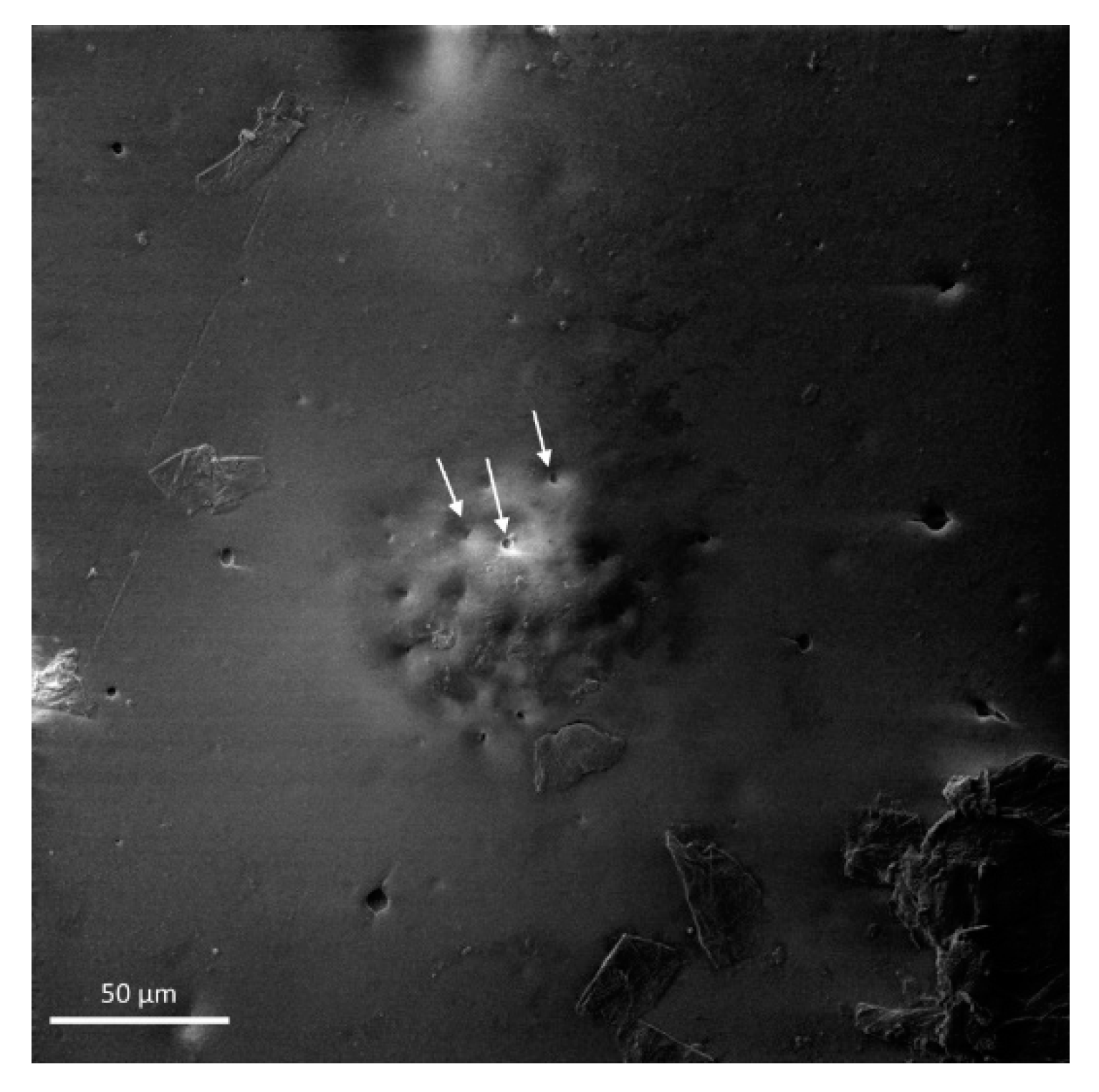
- The addition of microcapsules to the coating increased the coating roughness, however the appearance of cracks did not change significantly. In a thin coating of 50 μm thickness, the larger microcapsules can be seen on the coating surface.
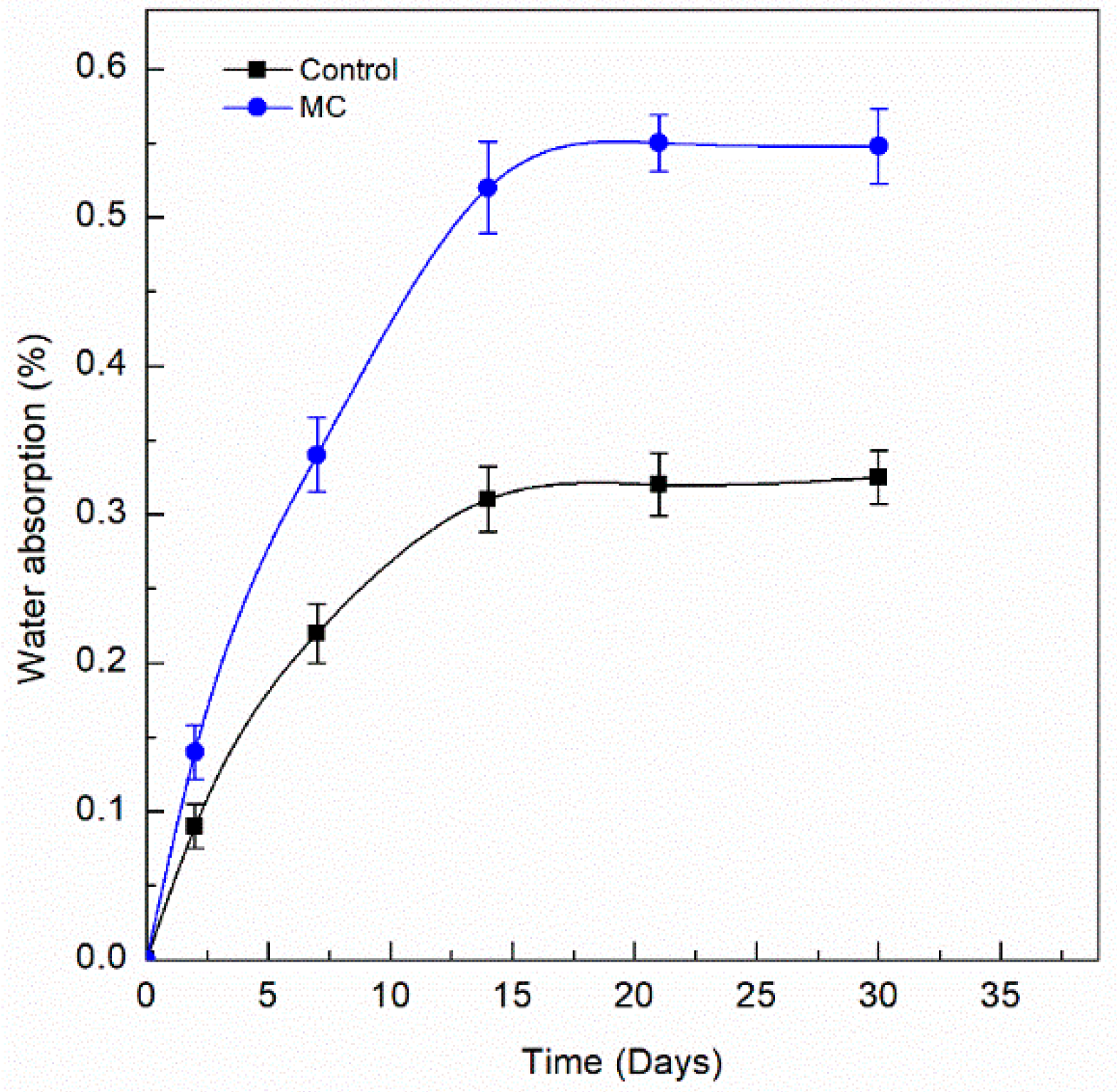
- The water absorption of the MC doped coating showed increased values compared to the control coating of nearly double. This is accounted for by the increased number of pores present in the coating after exposure to test media, due to the colophony microcapsule release mechanism.
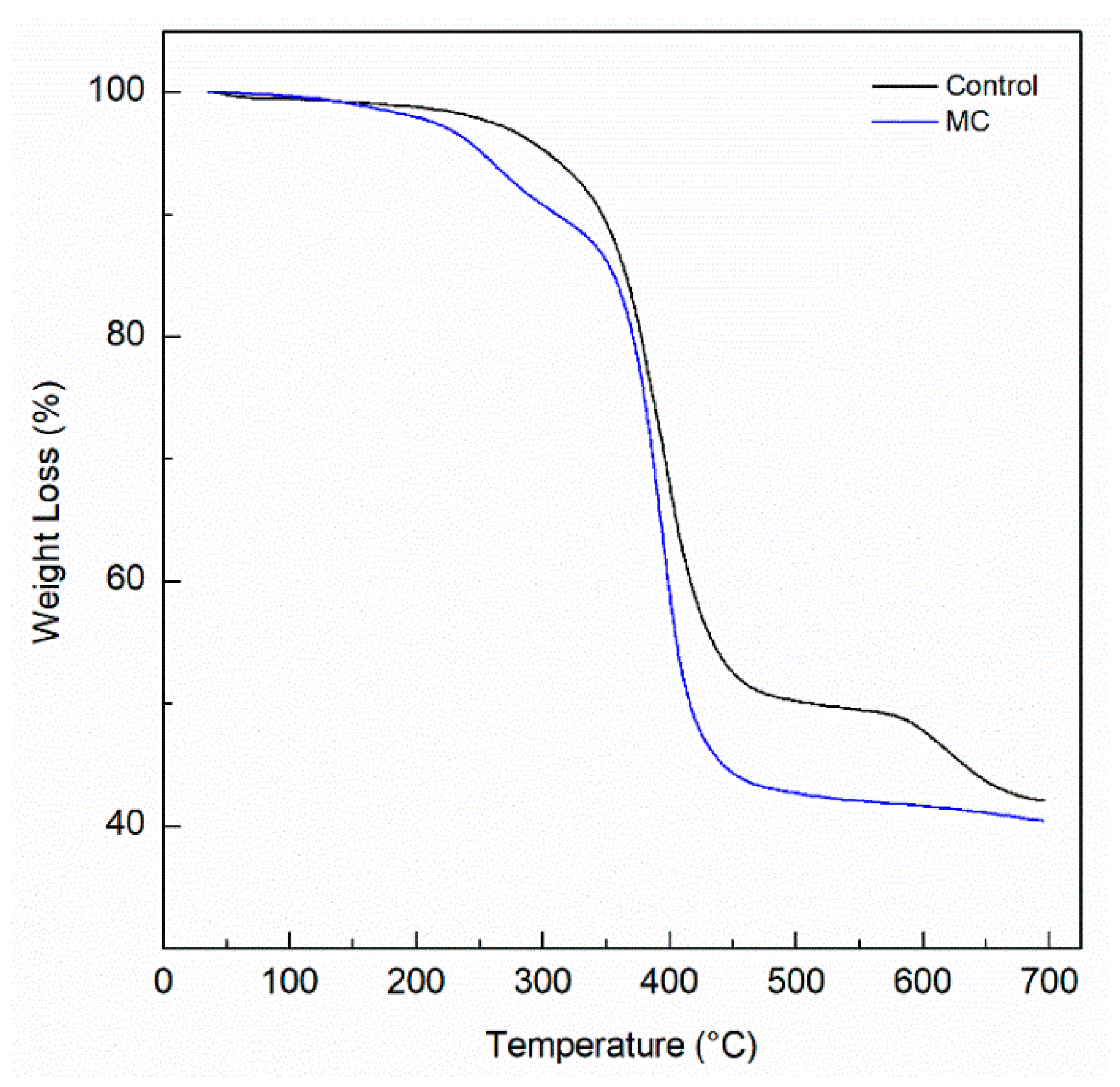
- The thermal stability of the coating did not change significantly, however the degradation of the microcapsules occurs near 200 °C, before the coating begins to degrade around 300 °C.
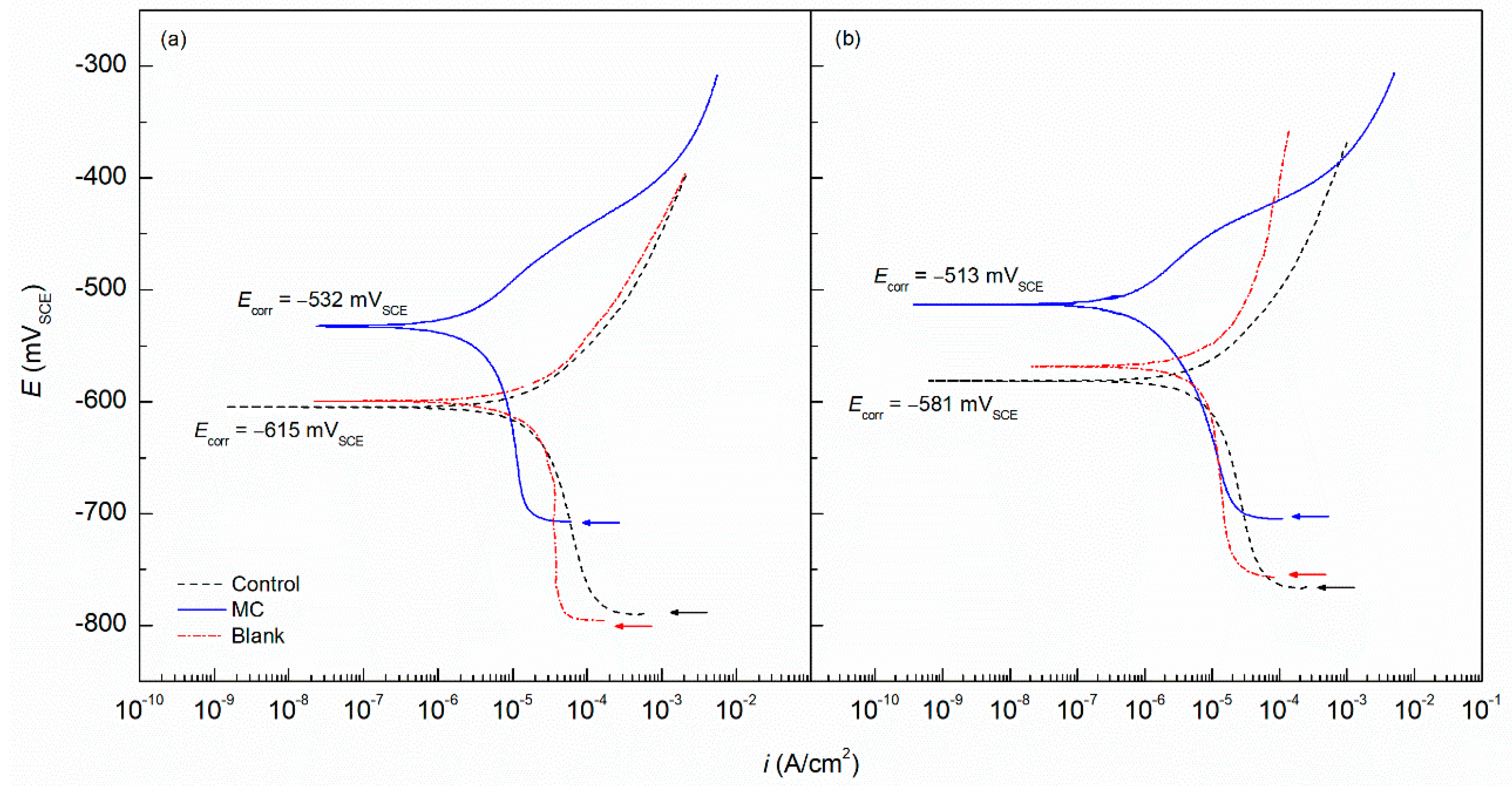
- The MC coating specimens maintained more noble Ecorr values compared to the control in DI and SCPS solutions with −532 and −513 mVSCE, respectively. Additionally, the MC polarization results showed lower icorr values by nearly one order of magnitude for both conditions. Therefore, the MC coating provided more protection from chloride attack on the substrate as well as the deleterious effects of low pH on carbon steel.
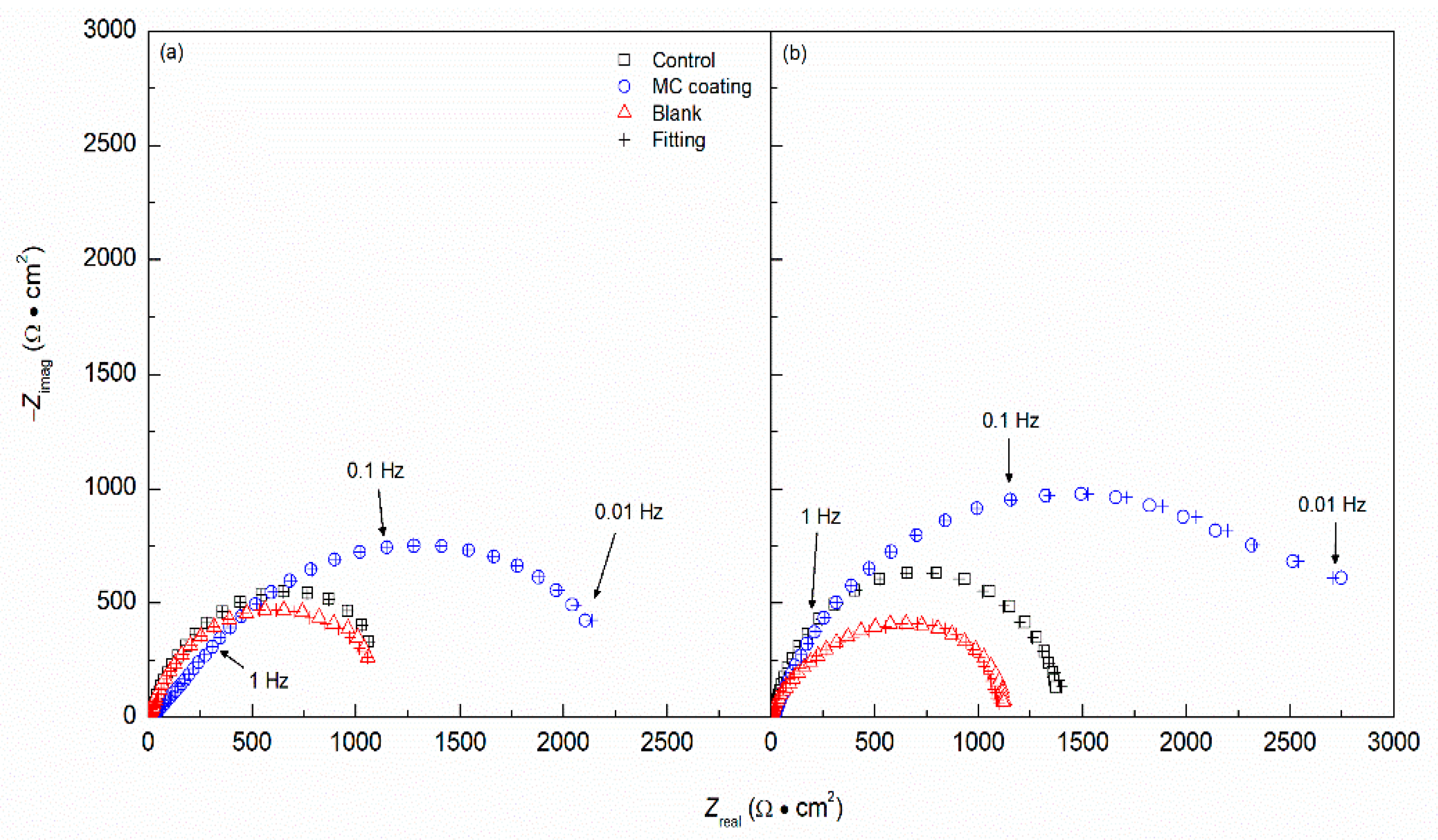
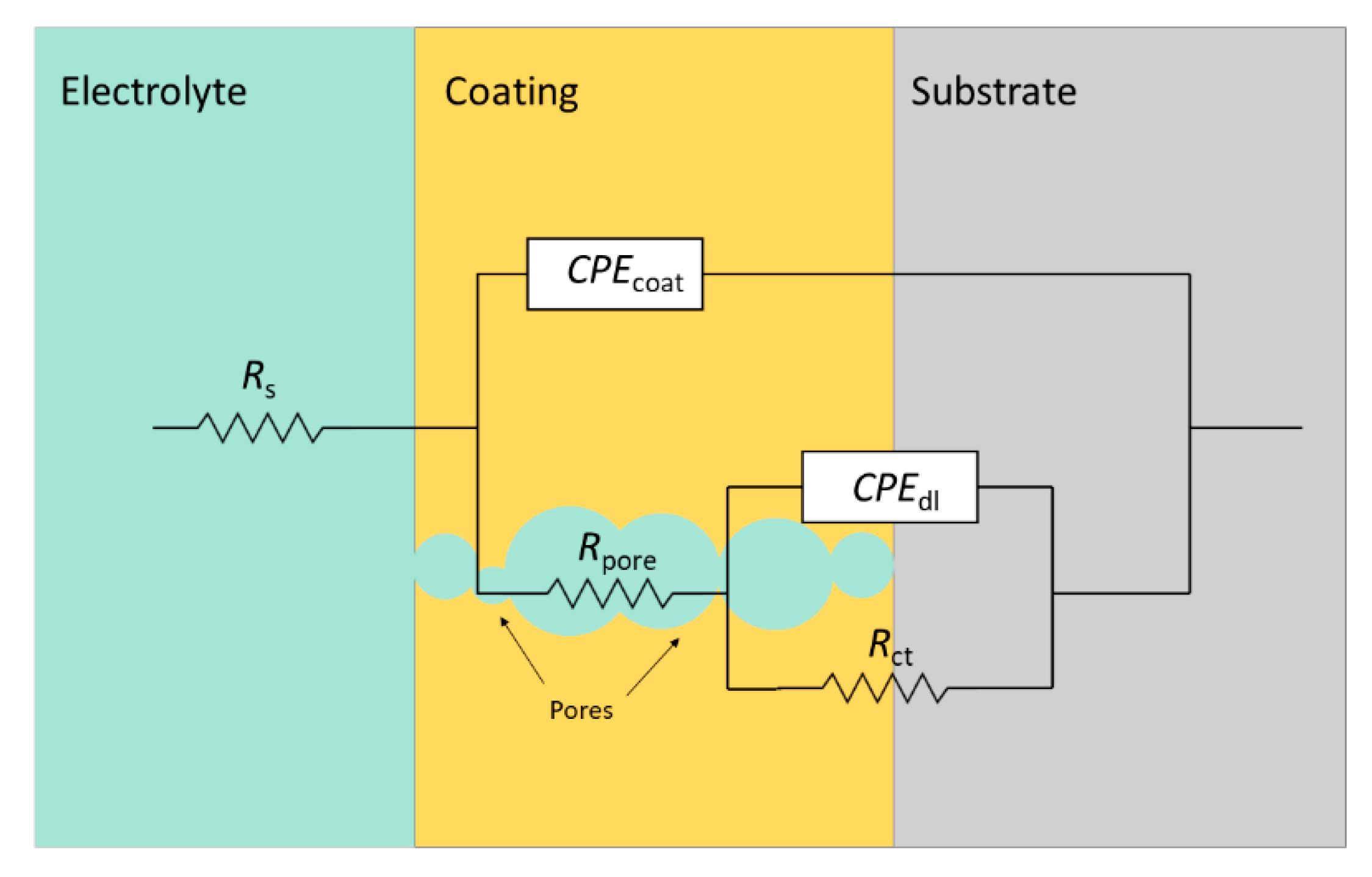
- The EIS analysis corroborated the DC polarization results, showing increased corrosion resistance for the MC coated specimens compared to the control. Moreover, the Rpore and Rct are much higher than the control, indicating the protection of the inhibitors. The Ceff,dl also shows lower values for the MC coating than the control, showing a more protective and less doped double layer.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morris, W.; Vico, A.; Vázquez, M. Chloride induced corrosion of reinforcing steel evaluated by concrete resistivity measurements. Electrochim. Acta 2004, 49, 4447–4453. [Google Scholar] [CrossRef]
- Qu, L.; Wang, Q.; Xu, S.; Wang, N.; Shi, Z. Chloride corrosion resistance of double-layer anticorrosive coating in simulated concrete pore solution. Constr. Build. Mater. 2021, 295, 123682. [Google Scholar] [CrossRef]
- Afshar, A.; Jahandari, S.; Rasekh, H.; Shariati, M.; Afshar, A.; Shokrgozar, A. Corrosion resistance evaluation of rebars with various primers and coatings in concrete modified with different additives. Constr. Build. Mater. 2020, 262, 120034. [Google Scholar] [CrossRef]
- Ress, J.; Martin, U.; Bosch, J.; Bastidas, D.M. Protection of carbon steel rebars by epoxy coating with smart environmentally friendly microcapsules. Coatings 2021, 11, 113. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nguyen, T.A. Protection of steel rebar in salt-contaminated cement mortar using epoxy nanocomposite coatings. Int. J. Electrochem. 2018, 2018, 8386426. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Huang, X.; Bi, J. Corrosion protection of Q235 steel using epoxy coatings loaded with calcium carbonate microparticles modified by sodium lignosulfonate in simulated concrete pore solutions. Materials 2021, 14, 1982. [Google Scholar] [CrossRef]
- Erdoğdu, Ş.; Bremner, T.W.; Kondratova, I.L. Accelerated testing of plain and epoxy-coated reinforcement in simulated seawater and chloride solutions. Cem. Concr. Res. 2001, 31, 861–867. [Google Scholar] [CrossRef]
- Hettiarachchi, N.M.; De Silva, R.T.; Prasanga Gayanath Mantilaka, M.M.M.G.; Pasbakhsh, P.; De Silva, K.M.N.; Amaratunga, G.A.J. Synthesis of calcium carbonate microcapsules as self-healing containers. RSC Adv. 2019, 9, 23666–23677. [Google Scholar] [CrossRef]
- Dogan-Guner, E.M.; Brownell, S.; Schueneman, G.T.; Shofner, M.L.; Meredith, J.C. Enabling zero added-coalescent waterborne acrylic coatings with cellulose nanocrystals. Prog. Org. Coat. 2021, 150, 105969. [Google Scholar] [CrossRef]
- Jiao, C.; Sun, L.; Shao, Q.; Song, J.; Hu, Q.; Naik, N.; Guo, Z. Advances in waterborne acrylic resins: Synthesis principle, modification strategies, and their applications. ACS Omega 2021, 6, 2443–2449. [Google Scholar] [CrossRef]
- González, E.; Stuhr, R.; Vega, J.M.; García-Lecina, E.; Grande, H.-J.; Leiza, J.R.; Paulis, M. Assessing the effect of CeO2 nanoparticles as corrosion inhibitor in hybrid biobased waterborne acrylic direct to metal coating binders. Polymers 2021, 13, 848. [Google Scholar] [CrossRef]
- Ecco, L.G.; Fedel, M.; Deflorian, F.; Becker, J.; Iversen, B.B.; Mamakhel, A. Waterborne acrylic paint system based on nanoceria for corrosion protection of steel. Prog. Org. Coat. 2016, 96, 19–25. [Google Scholar] [CrossRef]
- Chimenti, S.; Vega, J.M.; García-Lecina, E.; Grande, H.-J.; Paulis, M.; Leiza, J.R. In-situ phosphatization and enhanced corrosion properties of films made of phosphate functionalized nanoparticles. React. Funct. Polym. 2019, 143, 104334. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, J.; Liu, W.; Guo, F.; Pei, J.; Zhu, C.; Zhang, W. Preparation and characterization of self-healing microcapsules embedding waterborne epoxy resin and curing agent for asphalt materials. Constr. Build. Mater. 2018, 183, 384–394. [Google Scholar] [CrossRef]
- Bao, Y.; Yan, Y.; Chen, Y.; Ma, J.; Zhang, W.; Liu, C. Facile fabrication of BTA@ZnO microcapsules and their corrosion protective application in waterborne polyacrylate coatings. Prog. Org. Coat. 2019, 136, 105233. [Google Scholar] [CrossRef]
- Njoku, C.N.; Bai, W.; Arukalam, I.O.; Yang, L.; Hou, B.; Njoku, D.I.; Li, Y. Epoxy-based smart coating with self-repairing polyurea-formaldehyde microcapsules for anticorrosion protection of aluminum alloy AA2024. J. Coat. Technol. Res. 2020, 17, 797–813. [Google Scholar] [CrossRef]
- Ress, J.; Martin, U.; Bosch, J.; Bastidas, D.M. pH-triggered release of NaNO2 corrosion inhibitors from novel colophony microcapsules in simulated concrete pore solution. ACS Appl. Mater. Interfaces 2020, 12, 46686–46700. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramanian, M.; Haran, B.S.; Popova, S.; Popov, B.N.; Petrou, M.F.; White, R.E. Inhibiting action of calcium nitrite on carbon steel rebars. J. Mater. Civ. Eng. 2001, 13, 10–17. [Google Scholar] [CrossRef]
- Garcés, P.; Saura, P.; Zornoza, E.; Andrade, C. Influence of pH on the nitrite corrosion inhibition of reinforcing steel in simulated concrete pore solution. Corros. Sci. 2011, 53, 3991–4000. [Google Scholar] [CrossRef]
- Söylev, T.A.; Richardson, M.G. Corrosion inhibitors for steel in concrete: State-of-the-art report. Constr. Build. Mater. 2008, 22, 609–622. [Google Scholar] [CrossRef]
- Cánovas, M.F.; Selv, N.H.; Kawiche, G.M. Influence on the physical-mechanical properties of portland-cement mortar, have admixtures of colophony and tannin. Mater. Construct. 1989, 216, 15–22. [Google Scholar] [CrossRef][Green Version]
- Tu, F.; Lee, D. Controlling the stability and size of double-emulsion-templated poly(lactic-co-glycolic) acid microcapsules. Langmuir 2012, 28, 9944–9952. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Serra, C.A.; Vandamme, T.F.; Yu, W.; Anton, N. Double emulsions prepared by two–step emulsification: History, state-of-the-art and perspective. J. Control. Release 2019, 295, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Song, B.; He, P.; Wang, Z.; Wang, J. Electrochemical impedance spectroscopy (EIS) study on the degradation of acrylic polyurethane coatings. RSC Adv. 2017, 7, 13742–13748. [Google Scholar] [CrossRef]
- Ren, F.; Zheng, Y.-F.; Liu, X.-M.; Yue, X.-Y.; Ma, L.; Li, W.-G.; Lai, F.; Liu, J.-L.; Guan, W.-L. An investigation of the oxidation mechanism of abietic acid using two-dimensional infrared correlation spectroscopy. J. Mol. Struct. 2015, 1084, 236–243. [Google Scholar] [CrossRef]
- Gao, F.; Du, A.; Ma, R.; Lv, C.; Yang, H.; Fan, Y.; Zhao, X.; Wu, J.; Cao, X. Improved corrosion resistance of acrylic coatings prepared with modified MoS2 nanosheets. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124318. [Google Scholar] [CrossRef]
- Andrade, C. Evaluation of the degree of carbonation of concretes in three environments. Constr. Build. Mater. 2020, 230, 116804. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J. Concrete: Microstructure, Properties, and Materials, 4th ed.; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Brug, G.J.; Van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interf. Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Lewis, O.D.; Critchlow, G.W.; Wilcox, G.D.; de Zeeuw, A.; Sander, J. A study of the corrosion resistance of a waterborne acrylic coating modified with nano-sized titanium dioxide. Prog. Org. Coat. 2012, 73, 88–94. [Google Scholar] [CrossRef]

| C | Mn | P | S | Si | Cu | Ni | Cr | Mo | V | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.28 | 1.08 | 0.019 | 0.043 | 0.20 | 0.37 | 0.16 | 0.16 | 0.050 | 0.0379 | Bal. |
| TiO | Propylene Glycol | Alumina | Hydrated Aluminum Magnesium Silicate | Lithium Hydroxide Monohydrate | Acrylic Polymer Emulsion |
|---|---|---|---|---|---|
| 20.0 | 10.0 | 2.0 | 1.0 | <0.1 | Bal. |
| pH | Sample | Ecorr (mVSCE) | icorr (A/cm2) | βa (mV/dec) | βc (mV/dec) |
|---|---|---|---|---|---|
| 6.8 | MC | −532 (±21) | 3.24 × 10−6 (±1.24 × 10−6) | 68 (±11) | 143 (±21) |
| Control | −615 (±15) | 4.21 × 10−5 (±1.94 × 10−6) | 63 (±9) | 365 (±35) | |
| Blank | −599 (±13) | 6.89 × 10−5 (±2.67 × 10−5) | 65 (±14) | 221 (±17) | |
| 12.6 | MC | −513 (±10) | 1.20 × 10−6 (±2.29 × 10−6) | 95 (±10) | 119 (±13) |
| Control | −581 (±18) | 1.15 × 10−5 (±3.04 × 10−6) | 84 (±14) | 275 (±25) | |
| Blank | −568 (±22) | 1.59 × 10−5 (±1.55 × 10−5) | 96 (±17) | 253 (±22) |
| pH | Sample | Rs (Ω·cm2) | Rpore (Ω·cm2) | Ycoat (S·cm−2·Sncoat) | ncoat | Rct (Ω·cm2) | Ydl (S·cm−2·Sndl) | ndl | Ceff,dl (F·cm–2) | χ2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 6.8 | Control | 19 (±5) | 195 (±21) | 1.18 × 10−4 (±4.4 × 10−4) | 0.84 | 1.22 × 103 (±1.1 × 102) | 8.05 × 10−6 (±2.1 × 10−6) | 0.90 | 3.03 × 10−6 | 1.77 × 10−4 |
| MC | 24 (±3) | 280 (±28) | 4.85 × 10−4 (±3.5 × 10−4) | 0.73 | 2.19 × 103 (±1.8 × 102) | 3.47 × 10−6 (±1.6 × 10−6) | 0.91 | 2.26 × 10−6 | 2.51 × 10−4 | |
| Blank | 9 (±2) | – | – | – | 1.01 × 103 (±2.4 × 102) | 6.84 × 10−4 (±2.6 × 10−4) | 0.89 | 359 × 10−6 | 9.23 × 10−4 | |
| 12.6 | Control | 17 (±2) | 286 (±19) | 9.88 × 10−4 (±3.0 × 10−4) | 0.91 | 1.41 × 103 (±0.5 × 102) | 2.20 × 10−6 (±1.5 × 10−6) | 0.90 | 2.24 × 10−6 | 2.93 × 10−4 |
| MC | 16 (±2) | 609 (±25) | 7.90 × 10−4 (±2.7 × 10−4) | 0.76 | 2.61 × 103 (±1.4 × 102) | 4.21 × 10−6 (±1.8 × 10−6) | 0.92 | 1.82 × 10−6 | 7.67 × 10−4 | |
| Blank | 8 (±3) | – | – | – | 1.12 × 103 (±2.3 × 102) | 1.05 × 10−4 (±1.0 × 10−4) | 0.92 | 281 × 10−6 | 3.89 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ress, J.; Martin, U.; Bastidas, D.M. Improved Corrosion Protection of Acrylic Waterborne Coating by Doping with Microencapsulated Corrosion Inhibitors. Coatings 2021, 11, 1134. https://doi.org/10.3390/coatings11091134
Ress J, Martin U, Bastidas DM. Improved Corrosion Protection of Acrylic Waterborne Coating by Doping with Microencapsulated Corrosion Inhibitors. Coatings. 2021; 11(9):1134. https://doi.org/10.3390/coatings11091134
Chicago/Turabian StyleRess, Jacob, Ulises Martin, and David M. Bastidas. 2021. "Improved Corrosion Protection of Acrylic Waterborne Coating by Doping with Microencapsulated Corrosion Inhibitors" Coatings 11, no. 9: 1134. https://doi.org/10.3390/coatings11091134
APA StyleRess, J., Martin, U., & Bastidas, D. M. (2021). Improved Corrosion Protection of Acrylic Waterborne Coating by Doping with Microencapsulated Corrosion Inhibitors. Coatings, 11(9), 1134. https://doi.org/10.3390/coatings11091134







