The Influence of CBD Parameters on the Energy Gap of ZnS Narcissus-Like Nanostructured Thin Films
Abstract
1. Introduction
2. Materials and Methods
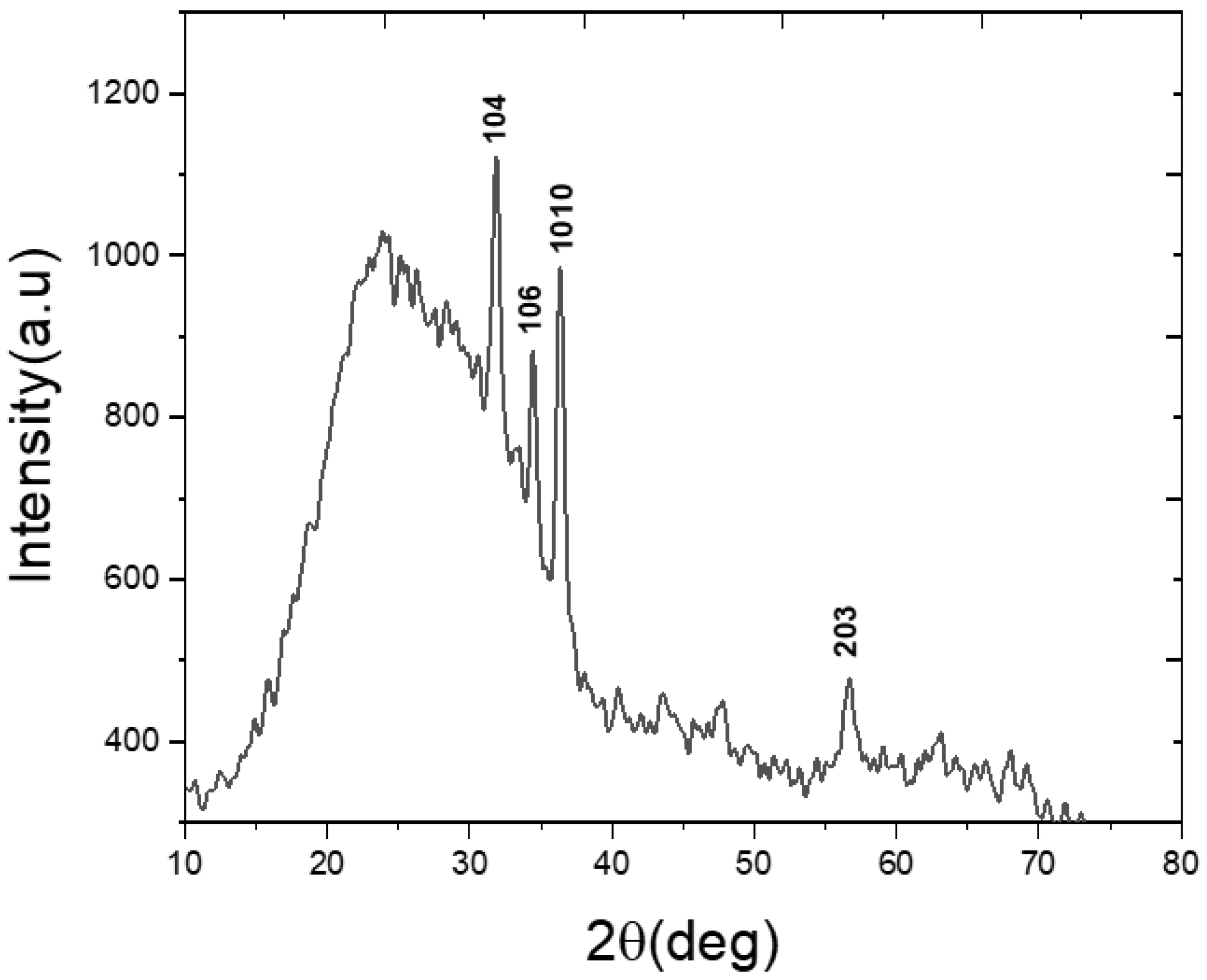
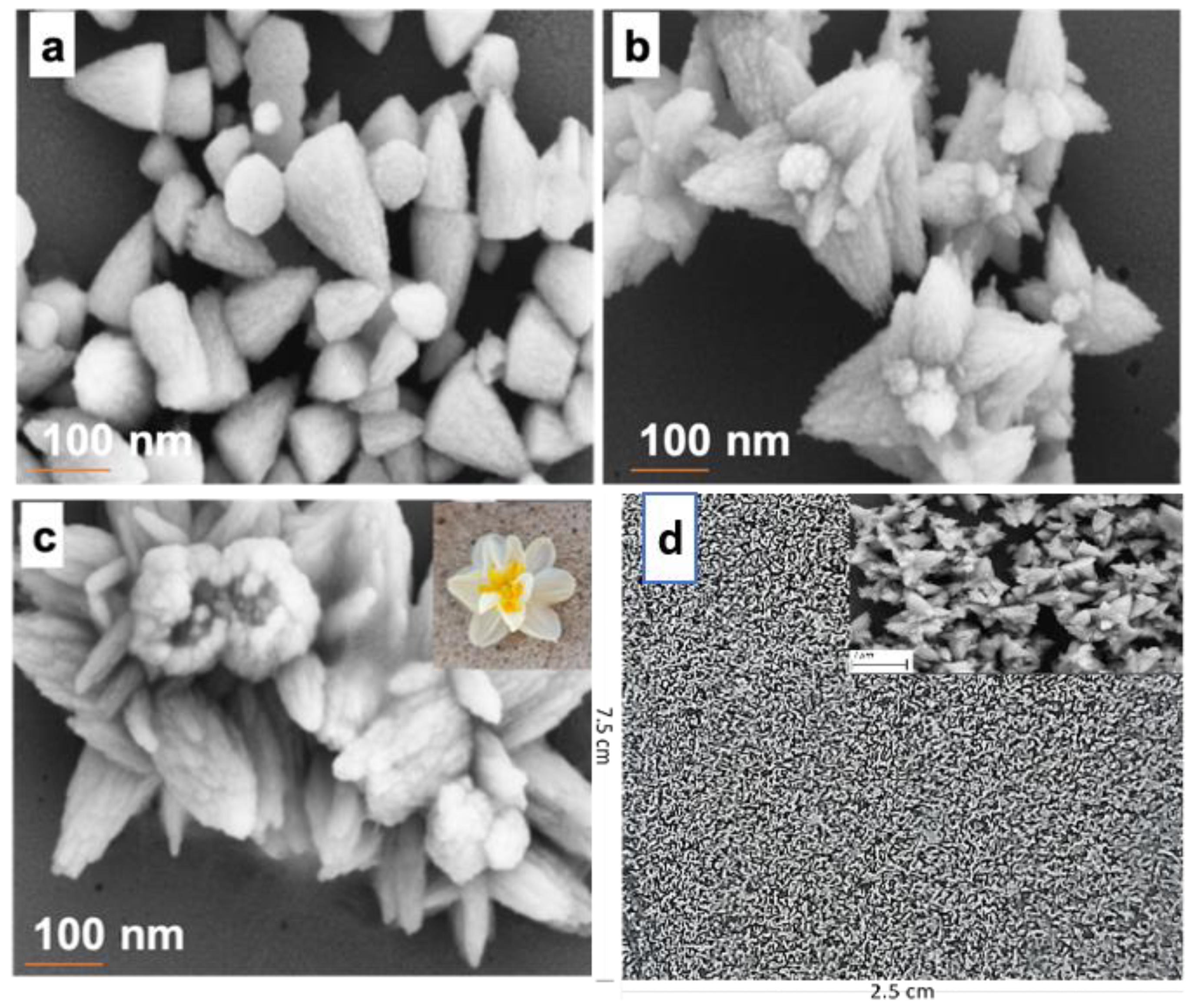
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choudapur, V.; Bennal, A.; Raju, A. Influence of pH on optoelectronic properties of zinc sulphide thin films prepared using hydrothermal and spin coating method. Mater. Res. Express 2018, 5, 045201. [Google Scholar] [CrossRef]
- Goudarzi, A.; Namghi, A.D.; Ha, C.-S. Fabrication and characterization of nano-structured ZnS thin films as the buffer layers in solar cells. RSC Adv. 2014, 4, 59764–59771. [Google Scholar] [CrossRef]
- Long, F.; Wang, W.-M.; Cui, Z.-k.; Fan, L.-Z.; Zou, Z.-g.; Jia, T.-k. An improved method for chemical bath deposition of ZnS thin films. Chem. Phys. Lett. 2008, 462, 84–87. [Google Scholar] [CrossRef]
- Li, Y. Photoluminescent Zinc Sulfide Optical Ceramics. Master’s Thesis, New York State College of Ceramics at Alfred University, Alfred, NY, USA, 2015. [Google Scholar]
- La Porta, F.A.; Andrés, J.; Li, M.S.; Sambrano, J.R.; Varela, J.A.; Longo, E. Zinc blende versus wurtzite ZnS nanoparticles: Control of the phase and optical properties by tetrabutylammonium hydroxide. Phys. Chem. Chem. Phys. 2014, 16, 20127–20137. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, W.J.; Jie, J.S.; Meng, X.M.; Zapien, J.A.; Lee, S.T. Homoepitaxial growth and lasing properties of ZnS nanowire and nanoribbon arrays. Adv. Mater. 2006, 18, 1527–1532. [Google Scholar] [CrossRef]
- Fang, X.; Bando, Y.; Liao, M.; Zhai, T.; Gautam, U.K.; Li, L.; Koide, Y.; Golberg, D. An efficient way to assemble ZnS nanobelts as ultraviolet-light sensors with enhanced photocurrent and stability. Adv. Funct. Mater. 2010, 20, 500–508. [Google Scholar] [CrossRef]
- Li, H.; Shih, W.Y.; Shih, W.-H. Non-heavy-metal ZnS quantum dots with bright blue photoluminescence by a one-step aqueous synthesis. Nanotechnology 2007, 18, 205604. [Google Scholar] [CrossRef]
- Farhangfar, S.; Yang, R.; Pelletier, M.; Nielsch, K. Atomic layer deposition of ZnS nanotubes. Nanotechnology 2009, 20, 325602. [Google Scholar] [CrossRef]
- Zhu, Y.-C.; Bando, Y.; Xue, D.-F.; Golberg, D. Nanocable-aligned ZnS tetrapod nanocrystals. J. Am. Chem. Soc. 2003, 125, 16196–16197. [Google Scholar] [CrossRef]
- Gilbert, B.; Frazer, B.; Zhang, H.; Huang, F.; Banfield, J.; Haskel, D.; Lang, J.; Srajer, G.; De Stasio, G. X-ray absorption spectroscopy of the cubic and hexagonal polytypes of zinc sulfide. Phys. Rev. B 2002, 66, 245205. [Google Scholar] [CrossRef]
- Shin, S.W.; Kang, S.R.; Gurav, K.; Yun, J.H.; Moon, J.-H.; Lee, J.Y.; Kim, J.H. A study on the improved growth rate and morphology of chemically deposited ZnS thin film buffer layer for thin film solar cells in acidic medium. Sol. Energy 2011, 85, 2903–2911. [Google Scholar] [CrossRef]
- Haque, F.; Khan, N.; Rahman, K.; Islam, M.; Alam, M.; Sopian, K.; Amin, N. Prospects of zinc sulphide as an alternative buffer layer for CZTS solar cells from numerical analysis. In Proceedings of the 8th International Conference on Electrical and Computer Engineering, Dhaka, Bangladesh, 20–22 December 2014; pp. 504–507. [Google Scholar]
- Shen, F.; Que, W.; Yin, X.; Huang, Y.; Jia, Q. A facile method to synthesize high-quality ZnS (Se) quantum dots for photoluminescence. J. Alloys Compd. 2011, 509, 9105–9110. [Google Scholar] [CrossRef]
- Murai, H.; Abe, T.; Matsuda, J.; Sato, H.; Chiba, S.; Kashiwaba, Y. Improvement in the light emission characteristics of CdS: Cu/CdS diodes. Appl. Surf. Sci. 2005, 244, 351–354. [Google Scholar] [CrossRef]
- Chen, Z.-G.; Zou, J.; Liu, G.; Lu, H.F.; Li, F.; Lu, G.Q.; Cheng, H.M. Silicon-induced oriented ZnS nanobelts for hydrogen sensitivity. Nanotechnology 2008, 19, 055710. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, Z.; Huang, H.; Liu, Z.; Chen, D.; Shen, G. Gas sensors, thermistor and photodetector based on ZnS nanowires. J. Mater. Chem. 2012, 22, 6845–6850. [Google Scholar] [CrossRef]
- Rajesh; Das, B.K.; Srinives, S.; Mulchandani, A. ZnS nanocrystals decorated single-walled carbon nanotube based chemiresistive label-free DNA sensor. Appl. Phys. Lett. 2011, 98, 013701. [Google Scholar] [CrossRef]
- Elidrissi, B.; Addou, M.; Regragui, M.; Bougrine, A.; Kachouane, A.; Bernede, J. Structure, composition and optical properties of ZnS thin films prepared by spray pyrolysis. Mater. Chem. Phys. 2001, 68, 175–179. [Google Scholar] [CrossRef]
- Bacha, K.B.; Timoumi, A.; Bitri, N.; Bouzouita, H. Structural, morphological and optical properties of sprayed ZnS thin films on various substrate natures. Optik 2015, 126, 3020–3024. [Google Scholar] [CrossRef]
- Bosco, J.; Tajdar, F.; Atwater, H. Molecular beam epitaxy of n-type ZnS: A wide band gap emitter for heterojunction PV devices. In Proceedings of the 2012 38th IEEE Photovoltaic Specialists Conference, Austin, TX, USA, 3–8 June 2012; pp. 002513–002517. [Google Scholar]
- Nicolau, Y. Solution deposition of thin solid compound films by a successive ionic-layer adsorption and reaction process. Appl. Surf. Sci. 1985, 22, 1061–1074. [Google Scholar] [CrossRef]
- Yano, S.; Schroeder, R.; Ullrich, B.; Sakai, H. Absorption and photocurrent properties of thin ZnS films formed by pulsed-laser deposition on quartz. Thin Solid Films. 2003, 423, 273–276. [Google Scholar] [CrossRef]
- Izi, M.; Heidari, G.; Khoie, S.M.; Najafi, J. Comparison of ZnS thin films fabricated by electrodeposition and spray pyrolysis methods. Surf. Eng. Appl. Electrochem. 2017, 53, 245–249. [Google Scholar] [CrossRef]
- Tsai, P.-C.; Pai, I.; Shieh, H.-P.D. Two-stage chemical bath deposition for well-covered and stoichiometric ZnS thin films. In Proceedings of the 2012 38th IEEE Photovoltaic Specialists Conference, Austin, TX, USA, 3–8 June 2012; pp. 001992–001994. [Google Scholar]
- Naseema, K.; Ribin, K.; Navya, N.; Prasannan, P. Thermal conversion of CBD grown ZnS thin films to ZnO. Z. Nat. A 2021, 76, 65–73. [Google Scholar]
- Liu, Q.; Mao, G. Comparison of CdS and ZnS thin films prepared by chemical bath deposition. Surf. Rev. Lett. 2009, 16, 469–474. [Google Scholar] [CrossRef]
- Liu, J.; Wei, A.; Zhao, Y. Effect of different complexing agents on the properties of chemical-bath-deposited ZnS thin films. J. Alloys Compd. 2014, 588, 228–234. [Google Scholar] [CrossRef]
- Zein, R.; Alghoraibi, I. Influence of bath temperature and deposition time on topographical and optical properties of nanoparticles ZnS thin films synthesized by a chemical bath deposition method. J. Nanomater. 2019, 2019, 7541863. [Google Scholar] [CrossRef]
- Hone, F.G.; Abza, T. Short review of factors affecting chemical bath deposition method for metal chalcogenide thin films. Int. J. Thin Films Sci. Technol. 2019, 8, 43–52. [Google Scholar]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, M.M.; Hasan, N.H.; Mohammed, H.I.; Mohamed, G.H.; Al-Hamdani, K.A.; Abdulameer, A.F. Investigation of optical properties of the PbS/CdS thin films by thermal Evaporation. J. Electron. Devices 2012, 12, 761–766. [Google Scholar]
- Mokili, B.; Froment, M.; Lincot, D. Chemical deposition of zinc hydroxosulfide thin films from zinc (II)-ammonia-thiourea solutions. Le J. Phys. IV 1995, 5, C3-261–C3-266. [Google Scholar] [CrossRef][Green Version]
- García-Valenzuela, J. Simple thiourea hydrolysis or intermediate complex mechanism? Taking up the formation of metal sulfides from metal–thiourea alkaline solutions. Comments Inorg. Chem. 2017, 37, 99–115. [Google Scholar] [CrossRef]
- Bera, K.; Saha, S.; Jana, P.C. Temperature Dependent Synthesis of Zinc Sulfide Nanocrystal. Orient. J. Chem. 2018, 34, 1665. [Google Scholar]
- Wei, A.; Liu, J.; Zhuang, M.; Zhao, Y. Preparation and characterization of ZnS thin films prepared by chemical bath deposition. Mater. Sci. Semicond. Process. 2013, 16, 1478–1484. [Google Scholar] [CrossRef]
- Bolanle, Y.; Babatunde, R.; Adegboyo, O. Effects of deposition time of ZnS thin film on optical and morphological properties of ZnS deposited by chemical bath deposition method for photovoltaic application. Phys. Mem. J. Theor. Appl. Phys. 2019, 1, 38–45. [Google Scholar]
- Iwashita, T.; Ando, S. Preparation and characterization of ZnS thin films by the chemical bath deposition method. Thin Solid Films 2012, 520, 7076–7082. [Google Scholar] [CrossRef]
- Ke, H.; Duo, S.; Liu, T.; Sun, Q.; Ruan, C.; Fei, X.; Tan, J.; Zhan, S. Effect of temperature on structural and optical properties of ZnS thin films by chemical bath deposition without stirring the reaction bath. Mater. Sci. Semicond. Process. 2014, 18, 28–35. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Cho, S.; Kim, S.; Park, I.Y.; Choi, Y.D. Role of growth parameters on structural and optical properties of ZnS nanocluster thin films grown by solution growth technique. Mater. Chem. Phys. 2003, 77, 254–260. [Google Scholar] [CrossRef]
- Oliva, A.I.; Gonzalez-Chan, I.; Rejón, V.; Rojas, J.; Patiño, R.; Aguilar, D. Chemical bath method for ZnS thin films preparation. In Proceedings of the 2010 7th International Conference on Electrical Engineering Computing Science and Automatic Control, Tuxtla Gutierrez, Mexico, 8–10 September 2010; pp. 500–503. [Google Scholar]
- Arun, S.; Kameswara, R.V.; Shrivastava, A.R.; Sanjay, U.; Deepika, J. Controlled Synthesis of Water Soluble Micrometer Sized Highly Luminescent Zinc Sulfide Flowers Using Green Chemistry Approach and Their Characterization. ChemXpress 2016, 9, 108. [Google Scholar]
- Trindade, T.; de Jesus, J.D.P.; O’Brien, P. Preparation of zinc oxide and zinc sulfide powders by controlled precipitation from aqueous solution. J. Mater. Chem. 1994, 4, 1611–1617. [Google Scholar] [CrossRef]
- Nasr, T.B.; Kamoun, N.; Guasch, C. Physical properties of ZnS thin films prepared by chemical bath deposition. Appl. Surf. Sci. 2008, 254, 5039–5043. [Google Scholar] [CrossRef]
- AL-Diabat, A.M.; Ahmed, N.M.; Hashim, M.; Chahrour, K.M.; Bououdina, M. Effect of deposition temperature on structural and optical properties of chemically sprayed ZnS thin films. Procedia Chem. 2016, 19, 485–491. [Google Scholar] [CrossRef]
- Nasr, T.B.; Kamoun, N.; Kanzari, M.; Bennaceur, R. Effect of pH on the properties of ZnS thin films grown by chemical bath deposition. Thin Solid Films 2006, 500, 4–8. [Google Scholar] [CrossRef]
- Liu, W.-L.; Yang, C.-S.; Hsieh, S.-H.; Chen, W.-J.; Fern, C.-L. Effect of deposition variables on properties of CBD ZnS thin films prepared in chemical bath of ZnSO4/SC (NH2)2/Na3C3H5O7/NH4OH. Appl. Surf. Sci. 2013, 264, 213–218. [Google Scholar] [CrossRef]
- Gumus, C.; Erkena, O.; Gunes, M.; Ozaslan, D. Effect of the deposition time on optical and electrical properties of semiconductor ZnS thin films prepared by chemical bath deposition. Indian J. Pure Appl. Phys. IJPAP 2017, 55, 471–477. [Google Scholar]
- Haddad, H.; Chelouche, A.; Talantikite, D.; Merzouk, H.; Boudjouan, F.; Djouadi, D. Effects of deposition time in chemically deposited ZnS films in acidic solution. Thin Solid Films 2015, 589, 451–456. [Google Scholar] [CrossRef]
- Borah, J.; Sarma, K. Optical and optoelectronic properties of ZnS nanostructured thin film. Acta Phys. Pol. Ser. A Gen. Phys. 2008, 114, 713. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Yoshida, T.; Lincot, D.; Minoura, H. Mechanistic study of chemical deposition of ZnS thin films from aqueous solutions containing zinc acetate and thioacetamide by comparison with homogeneous precipitation. J. Phys. Chem. B 2003, 107, 387–397. [Google Scholar] [CrossRef]
- Gao, X.; Li, X.; Yu, W. Morphology and optical properties of amorphous ZnS films deposited by ultrasonic-assisted successive ionic layer adsorption and reaction method. Thin Solid Films 2004, 468, 43–47. [Google Scholar] [CrossRef]
- Padmavathy, V.; Sankar, S.; Ponnuswamy, V. Influence of thiourea on the synthesis and charac-terization of chemically deposited nano structured zinc sulphide thin films. J. Mater. Sci. Mater. Electron. 2018, 29, 7739–7749. [Google Scholar] [CrossRef]
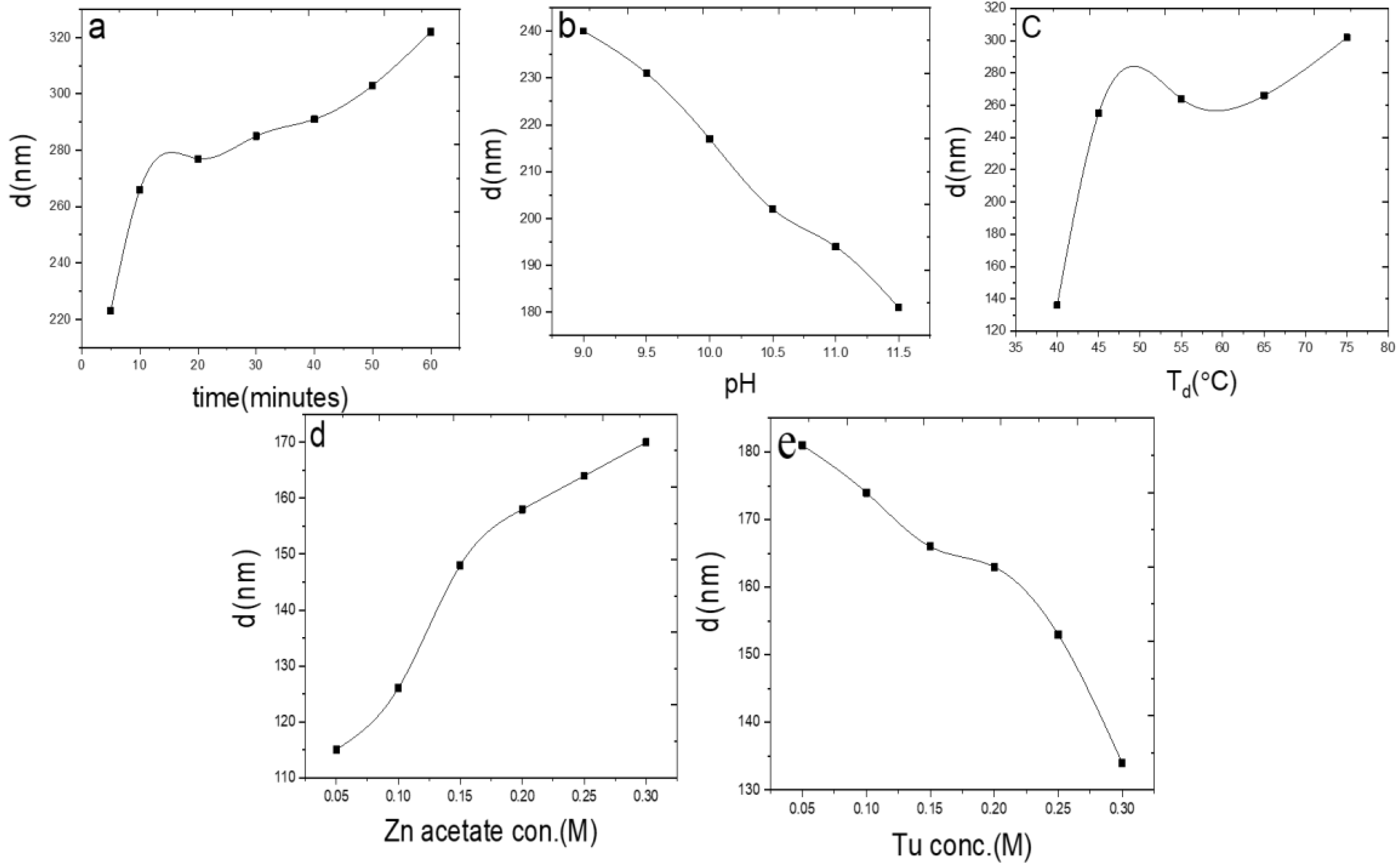
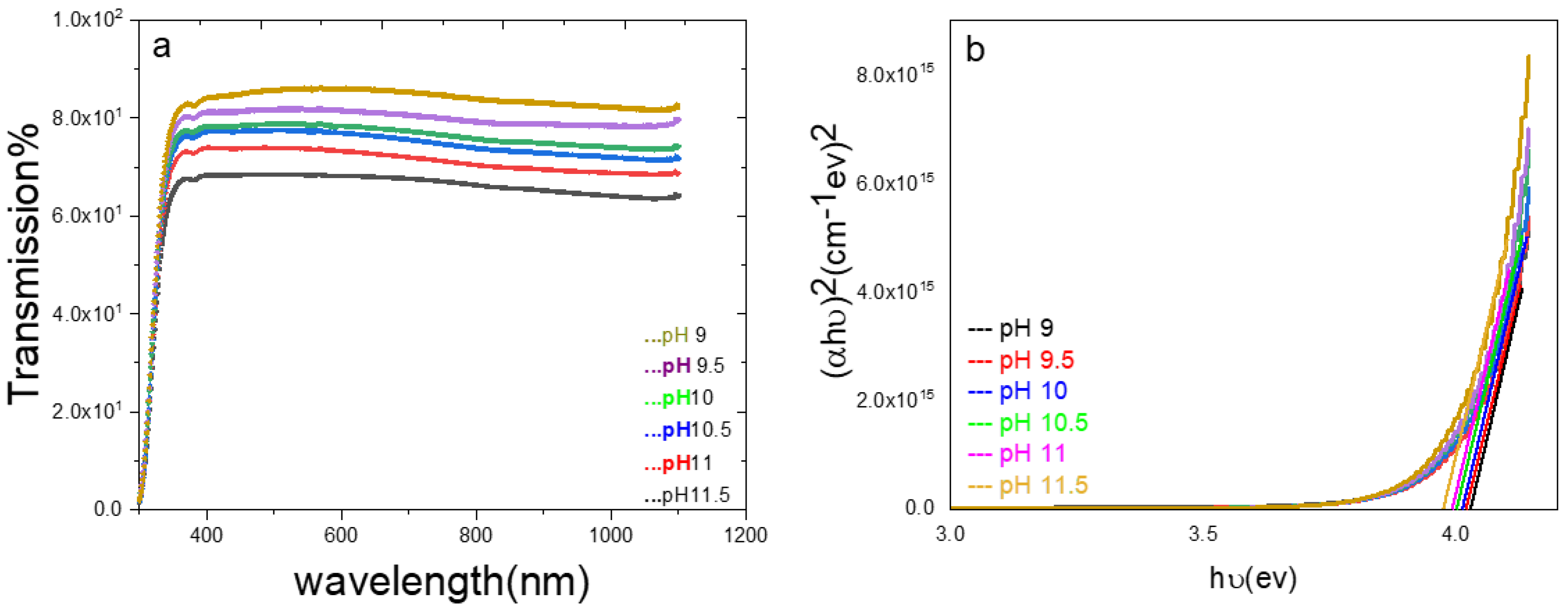
| Time(minutes) Eg (eV) | 5 | 10 | 20 | 30 | 40 | 50 | 60 |
| 4 | 3.995 | 3.99 | 3.98 | 3.97 | 3.94 | 3.92 | |
| pH Eg (eV) | 9 | 9.5 | 10 | 10.5 | 11 | 11.5 | - |
| 4.04 | 4.02 | 4.01 | 4 | 3.99 | 3.97 | - | |
| Temperature (°C) Eg (eV) | 40 | 45 | 55 | 65 | 75 | - | - |
| 4.06 | 4.04 | 4.02 | 4.01 | 3.99 | - | - | |
| Zn (CH3COO)2 (M) Eg (eV) | 0.05 | 0.1 | 0.15 | 0.20 | 0.25 | 0.3 | - |
| 4.03 | 4.016 | 3.997 | 3.984 | 3.978 | 3.958 | ||
| SC(NH2)2 (M) Eg (eV) | 0.05 | 0.1 | 0.15 | 0.20 | 0.25 | 0.3 | - |
| 4.03 | 4.02 | 4.01 | 4 | 3.995 | 3.990 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, M.H.; Mohammed, R.Y.; Ibrahem, M.A. The Influence of CBD Parameters on the Energy Gap of ZnS Narcissus-Like Nanostructured Thin Films. Coatings 2021, 11, 1131. https://doi.org/10.3390/coatings11091131
Khalil MH, Mohammed RY, Ibrahem MA. The Influence of CBD Parameters on the Energy Gap of ZnS Narcissus-Like Nanostructured Thin Films. Coatings. 2021; 11(9):1131. https://doi.org/10.3390/coatings11091131
Chicago/Turabian StyleKhalil, Mohammed Hussein, Raghad Y. Mohammed, and Mohammed Aziz Ibrahem. 2021. "The Influence of CBD Parameters on the Energy Gap of ZnS Narcissus-Like Nanostructured Thin Films" Coatings 11, no. 9: 1131. https://doi.org/10.3390/coatings11091131
APA StyleKhalil, M. H., Mohammed, R. Y., & Ibrahem, M. A. (2021). The Influence of CBD Parameters on the Energy Gap of ZnS Narcissus-Like Nanostructured Thin Films. Coatings, 11(9), 1131. https://doi.org/10.3390/coatings11091131







