Abstract
Development of green flame retardants has become a core part of the attention of material scientists and technologists in a paradigm shift from general purpose to specific sustainable products. This work is the first report on the use of coffee biowastes as sustainable flame retardants for epoxy, as a typical highly flammable polymer. We used spent coffee grounds (SCG) as well as SCG chemically modified with phosphorus (P-SCG) to develop a sustainable highly efficient flame retardant. A considerable reduction in the peak of heat release rate (pHRR) by 40% was observed in the pyrolysis combustion flow calorimeter analysis (PCFC), which proved the merit of the used coffee biowastes for being used as sustainable flame retardants for polymers. This work would open new opportunities to investigate the impact of other sorts of coffee wastes rather than SCG from different sectors of the coffee industry on polymers of different family.
1. Introduction
Polymer materials scientists together with environmental engineers today are at a critical juncture to find practical solutions to the huge amount of plastic wastes. In this regard, exploring and formulating green materials, developing green and clean technologies, and unifying low-cost recycling strategies have become the subjects of heated debates. In line with such attempts, several guidelines and legislations have been proposed and launched by the policy makers and managers for the treatment of biowastes in a value-added pathway rather than making biowastes landfilled or burnt [1,2,3]. The polymer industry these days makes good use of biowastes to develop sustainable polymer composites. Nevertheless, biowastes in their raw form may lead to poor properties mainly arising from inadequate interfacial adhesion. Therefore, it seems necessary to modify biowastes to attain high-performance polymer/biowastes composites [4,5].
Flame retardancy is a signature of performance of a polymer. Different sorts of flame retardants have been examined in developing flame-retardant polymer systems. In recent years, there has been a worldwide shift in the use of flame retardant additives from conventional additives to bio-based flame retardants. Several sorts of bio-based flame retardants have been used in polymer matrices, Figure 1 [6]. The majority of these bio-based molecules being used as a flame retardant, or as a part of a flame retardant system, are extracted from the raw biomaterials, which itself necessitates applying several steps of pre- and/or post-treatment. Moreover, in some cases like lignin, the botanical origin of molecule as well as the method of extraction may to a large extent govern the final properties of product [7]. Thus, modification of bio-based flame retardants seems crucial.
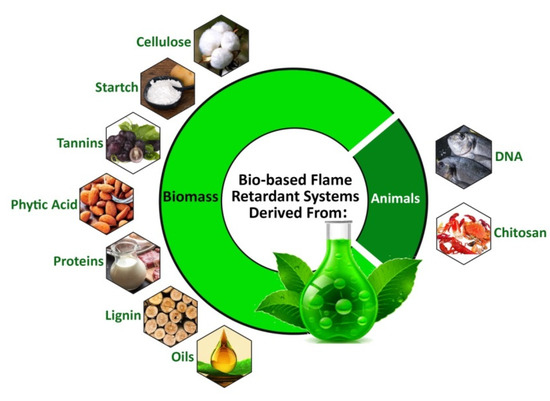
Figure 1.
Schematic representation of bio-based flame retardant systems derived from animals (DNA and chitosan) and biomass (cellulose, starch, tannins, phytic acid, proteins, lignin, and oils) [6]. Reproduced/Adapted with permission from [6]. Copyright (2021, Elsevier).
From the sustainability point of view, the use of biowaste materials in flame retardant systems can be considered as a promising solution to plastic disposal and biowaste landfill, mainly due to the availability and low cost of biowastes. So far, several biowastes have been incorporated as flame retardants into several polymers, including “oyster shell powder” in polypropylene [8], polyurethane [9], polyethylene [10], and acrylonitrile-butadiene-styrene copolymer (ABS) [11], “eggshell” in ethylene-vinyl acetate copolymer (EVA) [12] and for intumescent coating system [13], and “rice husk” in epoxy [14]. Sustainable flame retardants are at the core of attention in view of providing environmental safety, cost-effectiveness, and health safety considerations (Figure 2). Nevertheless, finding industrial biowastes in large quantities that guarantee all aspects of sustainability remains as a debate.
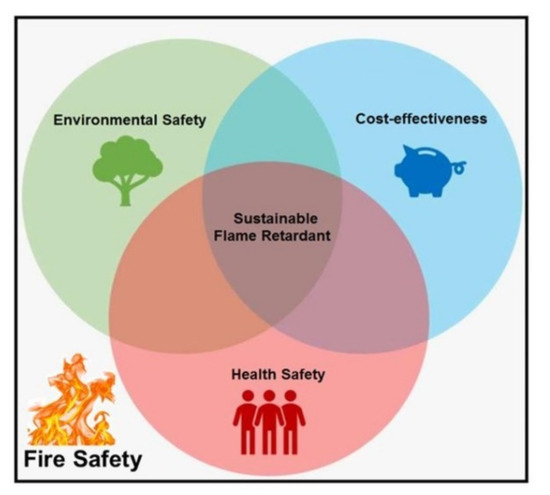
Figure 2.
Schematic representation of some important parameters centered to development of sustainable flame retardants.
The byproducts of the coffee industry as well as the spent coffee grounds are huge in amount [15,16]. Some research works already reported the use of spent coffee grounds to produce biodiesel [17,18], biosorbent [19], biogas [20], biocomposites [21], and biofoams [22,23]. Coffee biowastes have also been used in water treatment [24], and gas storage [25] (Figure 3). Recycled coffee grounds contain proteins, minerals, carbohydrates, and especially lignin, cellulose, and hemicellulose [26,27,28]. Therefore, such biowastes could potentially be useful in flame retardancy because they possess lignin, cellulose, and hemicellulose, which have been found to be promising green flame retardants for different polymers [29]. Their mode of action in flame retardancy is generally in the condensed phase where they generate and increase the char residue. The char formed thereof acts as a physical barrier against heat and mass transfer during the combustion. It is demonstrated that the combination of these materials with phosphorus-based flame retardants improve the quantity and quality of the formed char [29,30].

Figure 3.
Applications of spent coffee grounds including agriculture, food industry deodorization, water treatment, construction materials, and bioenergy.
This work aims to unravel, for the first time, the potential of the spent coffee grounds as a flame retardant for polymer materials. In a comprehensive review, we already discussed flame retardancy of epoxy and classified epoxy composites in terms of Flame Retardancy Index (FRI) [31]. Thus, epoxy resin was selected herein as a host polymer due to its versatility in a wide range of application from structural composites to organic coatings and also because of high flammability of epoxy as a model polymer. Two types of the biowastes including spent coffee grounds and phosphorus-modified spent coffee grounds were used varying the loading level. Several composites containing the aforementioned coffee grounds were prepared and evaluated for thermal degradation and micro-calorimetry flame retardancy behaviors.
2. Materials and Methods
Diglycidyl ether of bisphenol a epoxy resin (EPON™ 828) with epoxide equivalent weight of 450–550 g/eq. was purchased from Hexion (Shanghai, China). The amine-based curing agent of Epikure™ F205 with hydrogen equivalent weight of 105 g/eq. and viscosity of 500–700 mPa.s was provided by Hexion (China). Dimethyl phosphite (98%) was manufactured by Sigma-Aldrich (Saint Louis, MO, USA) and used as received. The coffee waste was obtained from the spent coffee grounds collected from a coffee machine. The brand of coffee beans was Lavazza, Arabica 40%/Robusta 60%.
For the preparation of phosphite-treated coffee biowaste (P-coffee), 15 g of coffee waste was added to 150 mL dimethyl phosphite under stirring at 30 °C for 24 h. The resulting phosphite-treated coffee biowaste was collected utilizing centrifugation (2000× g, 5 min) and dried at room temperature for seven days.
Epoxy containing coffee biowaste and phosphite-treated coffee biowaste (P-coffee) were prepared varying the amount of biowaste as 5, 15, and 30 wt.% (Table 1). To prepare composites, the fixed amounts of coffee biowaste ground and epoxy resin were mixed and sonicated two times for 5 min. Then, F205 curing agent was added to the resulting mixtures at 2:1 resin to curing agent stoichiometric ratio and thoroughly mixed for 2 min. The resulting composite was casted on a glass substrate and then put in an oven for 3 h at 70 °C for curing. Then, the post-curing was performed for two weeks at ambient temperature. For peeling off films from glass substrate, they were immersed in water for 24 h.

Table 1.
Formulations and sample codes of epoxy/coffee biowaste composites (Ep.: epoxy, Coffee: untreated coffee waste, P-coffee: treated coffee waste with dimethyl phosphite).
Microstructure and chemical constituents were analyzed using a scanning electron microscope (SEM) Zeiss Gemini SEM 500 (Jena, Germany) with accelerating voltage of 6 keV, coupled with an energy-dispersive X-ray spectroscopy (EDAX) Element SDD Silicon Drift Detector X-Ray Spectrometer 30 mm2.
Thermal decomposition of samples was investigated using a Setaram Labsys Evo thermogravimetric analyzer (France) in the temperature range of 25–900 °C, at a heating rate of 10 °C·min−1 under nitrogen atmosphere with gas flow rate fixed at 100 cm3·min−1.
Fourier-transform infrared (FTIR) spectroscopy was also conducted on pure coffee and treated coffee wastes in form of powder using a Bruker FTIR Alpha with a resolution of 4 cm−1 and 64 scans.
Pyrolysis combustion flow calorimeter analysis (PCFC) was performed to evaluate the flammability behavior of samples according to ASTM D7309 [32]. This test was conducted on a Fire Testing Technology (FTT) Company machine, East Grinstead, UK. Small quantity of samples between 2 and 4 mg was pyrolyzed at a heating rate of 1 °C/s. The gases obtained were conducted to another chamber and combustion took place in presence of oxygen (20%) at 900 °C. The Huggett’s relation [33] (1 kg of consumed oxygen corresponds to 13.1 MJ of released energy) was used to calculate the flammability parameters including the peak of heat release rate (pHRR), temperature at pHRR (TpHRR), and the total heat release (THR). A non-conventional horizontal burning test was performed on samples with dimensions of 30 × 100 × 4 mm3. A Bunsen burner flame was placed on the bottom of sample for 3 s and after the apparition of flame it moved up to continue burning to the end of test. The digital videos were recorded, and selected images were also extracted.
3. Results and Discussions
3.1. Analysis of Phosphorus Modification of Coffee
Pure coffee biowastes (hereafter referred to as Coffee) and treated-coffee (P-coffee) were imaged on SEM. Their chemical composition was also investigated using EDX. Figure 4 shows micrographs obtained from Coffee and P-coffee, and Table 2 summarizes their chemical compositions. The size of particles was between 10 µm and 100 µm. Some more SEM micrographs are available in the Appendix A.
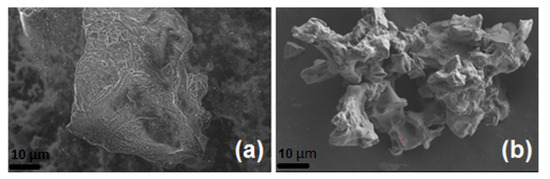
Figure 4.
SEM micrographs of unmodified coffee biowaste (Coffee) (a) and coffee biowaste modified with phosphorus (P-coffee) (b).

Table 2.
Chemical composition of unmodified coffee biowaste (Coffee) and coffee biowaste modified with phosphorus (P-coffee) obtained in EDX (the mean of the obtained values at three different points). The elemental analysis proves the presence of phosphorus in P-coffee. The percentage of phosphorus was estimated to be 20 wt.% for P-coffee. Moreover, carbon and oxygen are increased after modification of Coffee.
Fourier-transform infrared (FTIR) spectroscopy was also conducted on the treated and untreated coffee. Figure 5 displays the FTIR spectra of coffee and P-coffee. The spent coffee grounds generally contain several components such as cellulose, lignin, fatty acids, and hemicellulose [18]. The broad band between 3100 cm−1 and 3500 cm−1 was attributed to the stretching of −OH groups related to the inter- and intra-molecular hydrogen bonding of some polymeric compounds, such as cellulose and lignin. Two bands at 2921 cm−1 and 2851cm−1 can be assigned to symmetric or asymmetric C-H stretching vibration of aliphatic acids, respectively. The band at 1742 cm−1 was associated to the vibration of C=O groups in aliphatic esters. For the P-coffee sample, the absorption peak of P=O was situated in the range of 1350–1250 cm−1. Moreover, two bands at 994 cm−1 and 925 cm−1 corresponds to P-O-C stretching. Although it is not possible to confirm chemical reaction between coffee biowastes and phosphite, the intensity of peaks in the case of P-coffee are indicative of appropriate interaction between components.
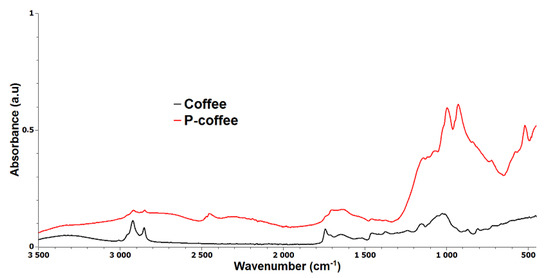
Figure 5.
FTIR spectra of untreated (Coffee) and phosphorus treated coffee (P-coffee) samples.
3.2. Analysis of Thermal Degradation
TGA thermograms of Coffee and P-coffee biowastes are compared in Figure 6. Increase in the residual mass is generally a signature of flame retardancy improvement, which reflects the contribution of condensed phase action in controlling the flammability. On one hand, the formation of more char residue is indicative of the decrease in the amount of gases in the vapor phase. Although the content of phosphorus ingredient was 20%, the difference in the TGA thermograms is almost close to 30%, a further evidence for strong interaction between phosphorus and coffee wastes leading to formation of more char residue. On the other hand, the presence of char during the combustion process on the surface of the polymer reduces the release rate of gases to the vapor phase and also protects the underlying polymer from the flame [6].
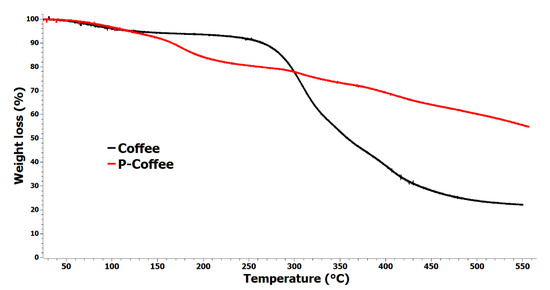
Figure 6.
TGA thermograms of unmodified coffee biowaste (Coffee) and coffee biowaste modified with phosphorus (P-coffee) under nitrogen at 10 °C·min−1.
The graph confirmed successful modification of coffee biowastes with phosphorus, on the bedrock of lower thermal stability of P-coffee between 150 °C and 300 °C compared with Coffee. Surprisingly, a significant improvement in thermal stability was observed for P-coffee above 300 °C. At 350 °C, P-coffee lost only 27% of its initial masse, while for Coffee it was ca. 48%. More prominently, degradation of P-coffee is single-stage, indicative of strong interaction or formation of a complex between coffee biowastes and phosphite. The final residues of Coffee and P-coffee coffee were 22 and 55%, respectively. Therefore, the difference of remaining residue for Coffee and P-coffee at the end of the test is more than a theoretical addition of dimethyl phosphite on coffee waste. This difference showed that some chemical interactions occurred during the decomposition, mainly consisting of the formation of double bonds (C=C), cyclization, aromatization, fusion of aromatics, and graphitization leading to formation of char residue.
TGA thermograms of epoxy and epoxy composites containing Coffee and P-coffee biowastes are compared in Figure 7a,b, and some important parameters extracted from the figures are listed in Table 3. Overall, lower thermal stability of composites at the early stage of TGA can be explained by P-coffee and the release of some chemicals. In fact, the presence of P-coffee stabilized and increased the char yield.
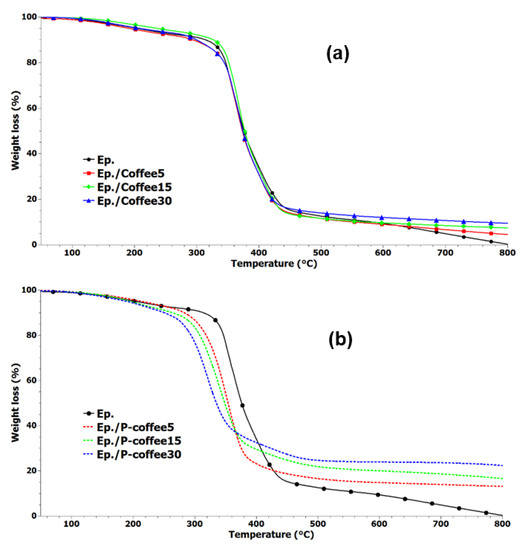
Figure 7.
TGA thermograms of epoxy and epoxy composites containing various amounts of unmodified coffee biowaste (Coffee); (a) those of coffee biowaste modified with phosphorus (P-coffee) (b).

Table 3.
Thermal decomposition characteristics of neat epoxy and epoxy composites extracted from TGA diagrams.
For samples containing Coffee (Figure 7a), the thermal decomposition mechanism of epoxy remained almost unaffected, while above 500 °C, composite samples show improved thermal stability. For samples containing P-coffee (Figure 7b), however, decomposition started at a lower temperature with respect to the neat epoxy; the more the P-coffee amount the higher the rate of thermal decomposition. At higher temperatures (above 450 °C), composites obviously have higher thermal stability compared to epoxy and systems containing Coffee, seen in Figure 7a. In Table 3, T5 and T10 are temperatures at which 5% and 10% weight loss takes place, respectively. T10 vividly increased from 314 °C for neat epoxy to 326 °C for Ep./Coffee15 sample. Overall, composites were not thermally stable and roughly degraded up to 800 °C. The residue at 800 °C were 4.5, 7.5, and 9.5% for epoxy composites containing 5, 15, and 30 wt.% Coffee, respectively. For instance, the value of T10 for Ep./P-coffee30 sample was 250 °C, while it was 314 °C for the neat epoxy. It can be concluded that stabilization of char was observed after 428 °C and all composites showed improved thermal stability compared with the neat epoxy. The remaining residue at 800 °C was 13, 16.5, and 22.5% for epoxy composites containing 5, 15, and 30 wt.% P-coffee, respectively. This suggests the effectiveness of phosphorus modification of coffee biowastes. Derivative thermogravimetric (DTG) curves of epoxy and epoxy composites containing Coffee and P-coffee are also shown in Figure 8a,b. It demonstrates that the main degradation steps of all samples containing unmodified coffee biowaste (Figure 8a) occurs between 280 °C and 470 °C. The presence of modified coffee waste at 15 wt.% and 30 wt.% of loading led to decrease of Tmax (temperature, at which sample lost maximum of its weight). Tmax of pure epoxy and Ep./P-coffee5 was 353 °C, while it was 341 °C and 318 °C for Ep./P-coffee15 and Ep./P-coffee30, respectively.
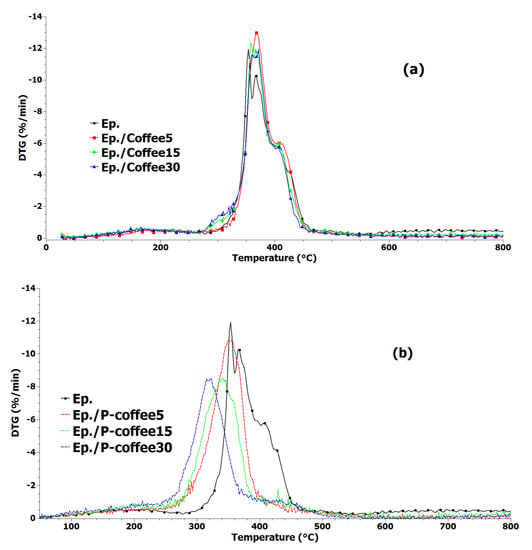
Figure 8.
DTG curves of epoxy and epoxy composites containing unmodified coffee biowaste (Coffee) (a) and coffee biowaste modified with phosphorus (P-coffee) (b).
3.3. Analysis of Flammability
Figure 9 compares the heat release rate (HRR) curves of samples as a function of temperature. The main characteristics of PCFC including peak of heat release rate (pHRR), total heat release (THR), and temperature at pHRR (TpHRR) are extracted from curves and summarized in Table 4.
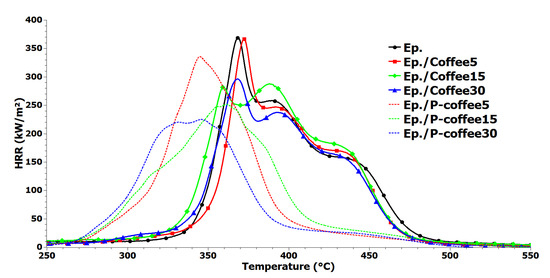
Figure 9.
Heat release rate (HRR) curves for the neat epoxy and epoxy composites containing both Coffee and P-coffee obtained in PCFC test.

Table 4.
Summary results obtained in PCFC tests for the neat epoxy and epoxy containing Coffee and P-coffee.
From Figure 9, two different flammability behaviors are observed for composite samples containing Coffee or P-coffee. For all composites containing Coffee, the curve of HRR initiated around 325 °C, which was more similar to that of neat epoxy. Then, a sharp increase in HRR was observed, before reaching the pHRR. A completely different behavior was observed for samples containing P-coffee, where HRR curves followed a moderate ascending trend in the vicinity of 275 °C. A moderate slope compared with those of the composites containing Coffee was also seen, which ended in pHRR rise and then fall around 420 °C. Such early-stage variation in HRR curves can be explained on account of phosphorous chemical used in treatment of coffee biowaste.
The values of pHRR were almost similar for neat epoxy and epoxy/Coffee5 (370 W·g−1 and 365 W·g−1, respectively). At higher loadings of 15 and 30 wt.%, however, Coffee more substantially affected pHRR compared to P-coffee (with a reduction of 24% and 20% in pHRR compared with that of neat epoxy). The temperature at which pHRR occurred also remained in 359–372 °C for neat epoxy and epoxy composites containing Coffee. Interestingly, reduction in pHRR was more salient for the samples containing P-coffee, which demonstrated the efficiency of chemical treatment of coffee biowastes in improvement of flammability of polymers. For samples containing 30 wt.% of P-coffee, pHRR was significantly increased by 40%, while for 5 wt.% and 15 wt.% loadings the values of reduction in pHRR were 10% and 32%, respectively. Moreover, temperature at pHRR was significantly decreased for samples containing P-coffee. Since PCFC is not a real flame test, a horizontal flame test was performed to evaluate the flame behavior of samples. Figure 9 displays the extracted photos from recorded videos during the horizontal flame test. All videos are presented as Supporting Information section for experts. After the ignition, a big flame instantaneously appeared in the case of neat epoxy sample leading to a complete and rapidly burning of this sample. At flameout around 30 s, no remaining residue was observed for neat epoxy (Figure 10a). In the presence of Coffee, the inflammation rate was decreased, leading to an increase in time to inflammation up to 90 s. A small quantity of remaining residue was obtained at the end of tests, which is essentially ascribed to the presence of coffee biowaste. Surprisingly, the flame behavior of epoxy was significantly changed in the presence of P-coffee. The rate of burning decreased even for Ep./P-coffee5 sample. An self-extinguishable behavior was observed for samples containing 15 wt.% and 30 wt.% P-coffee. For Ep./P-coffee15, the self-extinguishability was appeared after 40 s of ignition. The self-extinguishability character was more salient for the Ep./P-coffee30 sample even at the beginning of the test. Therefore, the ignition was repeated to initiate the inflammation, but the self-extinguishability character came back after 40 s. Thus, these samples were not consumed due to the aforementioned behavior and a compact char formed, on the zone of inflammation (Figure 10f,g). These flame tests clearly demonstrated the efficiency of treatment of coffee biowaste with phosphorus in flame retardancy of epoxy resins.

Figure 10.
Snapshots taken from videos showing the evolution of flame propagation as a function of time in a horizontal flame test configuration; (a) Ep., (b) Ep./Coffee5, (c) Ep./Coffee15, (d) Ep./Coffee30, (e) Ep./P-coffee5, (f) Ep./P-coffee15, (g) Ep./P-coffee30.
3.4. Possible Flame Retardancy Mechanism
The sustainable coffee biowastes used as flame retardant for epoxy as a typical highly flammable polymer can be explained mechanistically. Possible mechanisms of action of P-coffee in epoxy are both actions in the gas and condensed phases. The simultaneous presence of a carbon source, coffee biowaste, and phosphorus in epoxy facilitated and accelerated the formation of a carbonaceous residue playing the role of a barrier against heat and mass diffusion of gases to the gas phase. In the meanwhile, the released phosphorus chemicals captured free radicals in the gas phase and avoided the propagation of flame (Figure 11).
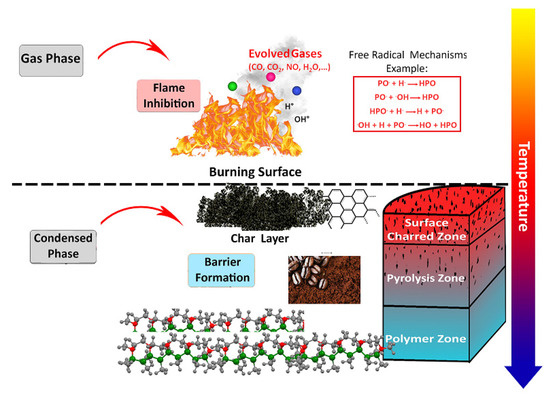
Figure 11.
Possible mechanism of flame retardancy of sustainable coffee biowastes in epoxy.
4. Conclusions and Future Perspectives
In this work, spent ground coffee was treated with dimethyl phosphite using a simple method and then incorporated into epoxy resin as flame retardant at 5, 15, and 30 wt.% of loading. The analysis of modified coffee biowaste showed that it contained a high amount of phosphorus (20 wt.%). The remaining residue at the end of TGA test for epoxy containing 30 wt.% modified coffee (P-coffee) was about 22.5% against 0% for the blank epoxy and 9.5% for the epoxy containing 30 wt.% unmodified coffee biowaste (Coffee). The flammability analysis in PCFC demonstrated a significant reduction in the pHRR (40%) for epoxy containing 30 wt.% P-coffee. Moreover, the burning tests revealed an self-extinguishable character for this sample. From these results, it is clear that coffee biowaste has a huge potential to be used as flame retardant. A recent survey highlighted the importance of visionary management of food industry biowastes such as agricultural, food industry, and spent coffee ground biowastes [34]. The need for improving the rate of recycling and composting as well as sustainable strategies for future developments have also been discussed. Nevertheless, there is a global need to enrich the cultural standpoints in a paradigm shift towards minimizing household biowastes. The idea of circular economy is grounded on prevention of waste production as well as management of the natural resources and investment on green and clean productions rather than recycling or reuse of waste materials. There have been some attempts in recent years in the quest for implementing circular economy in polymer industry. For example, life cycle assessment (LCA) is applied as a criterion to assess environmental impact of recycling polyethylene terephthalate (PET) wastes in relation to additives used in processing and also economical features [35]. LCA is a measure of the possibility of management of wastes having eyes at the life cycle of plastics. The use of sustainable biowastes makes it possible to significantly shorten LCA and circular economy interval. The outcome of this short communication unveiled the potential of coffee biowaste as a flame retardant for polymers. Considering the huge amount of coffee industry byproducts as well as collection of coffee biowastes all around the world, future horizon seems promising. The point is that using coffee biowastes not merely allows for taking big steps in line with circular economy requirements, but auspiciously gives rise to developing high-performance polymer materials, herein highly flame-retardant polymer composites.
Author Contributions
Conceptualization, H.V., M.R.S.; methodology, H.V., M.R.S. and S.R.; validation, all authors; formal analysis, H.V., M.J. and T.P.; investigation, H.V., M.J. and T.P.; data curation, H.V., M.J.; writing—original draft preparation, H.V., M.J.; writing—review and editing, all authors; visualization, H.V., M.R.S.; supervision, H.V. and M.R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
H.V. would like to thank IUT de Moselle Est-“Plastinnov” for the PCFC tests.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. SEM Photos Provided from Coffee and P-Coffee Powders (First Line: Coffee, Second Line: P-Coffee)

Table A1.
SEM photos provided from Coffee and P-coffee powders.
Table A1.
SEM photos provided from Coffee and P-coffee powders.
| Coffee |  |  |
| P-coffee |  |  |
References
- Lohri, C.R.; Diener, S.; Zabaleta, I.; Mertenat, A.; Zurbrügg, C. Treatment technologies for urban solid biowaste to create value products: A review with focus on low- and mid-dle-income settings. Rev. Environ. Sci. Bio/Technol. 2017, 16, 81–130. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.; Sims, E.; Bertham, O.; Symington, H.; Bell, N.; Pfaltzgraff, L.; O’Brien, M. Towards a Circular Economy: Waste Management in the EU.; Study: Europ. Union. 2017. Available online: https://www.europarl.europa.eu/RegData/etudes/STUD/2017/581913/EPRS_STU%282017%29581913_EN.pdf (accessed on 25 August 2021).
- Brusselaers, J.; Van Der Linden, A. Bio-waste in Europe—turning challenges into opportunities. EEA Rep. 2020, 4, 56. [Google Scholar]
- Thomas, S.; Mishra, R.K.; Asiri, A.M. Sustainable Polymer Composites and Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Shamsi, R.; Sadeghi, G.M.M.; Vahabi, H.; Seyfi, J.; Sheibani, R.; Zarintaj, P.; Laoutid, F.; Saeb, M.R. Hopes beyond PET recycling: Environmentally clean and engineeringly applicable. J. Polym. Environ. 2019, 27, 2490–2508. [Google Scholar] [CrossRef]
- Vahabi, H.; Laoutid, F.; Mehrpouya, M.; Saeb, M.R.; Dubois, P. Flame retardant polymer materials: An update and the future for 3D printing developments. Mater. Sci. Eng. R Rep. 2021, 144, 100604. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R Rep. 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Shah, A.U.R.; Prabhakar, M.N.; Saleem, M.; Song, J. IDevelopment of biowaste encapsulated polypropylene composites: Thermal, optical, dielectric, flame retardant, me-chanical, and morphological properties. Polym. Compos. 2017, 38, 236–243. [Google Scholar] [CrossRef]
- Liu, C.-H.; Lee, H.-T.; Tsou, C.-H.; Wang, C.-C.; Gu, J.-H.; Suen, M.-C. Preparation and characterization of biodegradable polyurethane composites containing oyster shell powder. Polym. Bull. 2019, 77, 3325–3347. [Google Scholar] [CrossRef]
- Chong, M.H.; Chun, B.C.; Chung, Y.-C.; Cho, B.G. Fire-retardant plastic material from oyster-shell powder and recycled polyethylene. J. Appl. Polym. Sci. 2005, 99, 1583–1589. [Google Scholar] [CrossRef]
- Moustafa, H.; Youssef, A.M.; Duquesne, S.; Darwish, N. ACharacterization of bio-filler derived from seashell wastes and its effect on the mechanical, thermal, and flame re-tardant properties of ABS composites. Polym. Compos. 2017, 38, 2788–2797. [Google Scholar] [CrossRef]
- Oualha, M.A.; Omri, N.; Oualha, R.; Nouioui, M.A.; Abderrabba, M.; Amdouni, N.; Laoutid, F. Development of metal hydroxide nanoparticles from eggshell waste and seawater and their application as flame retardants for ethylene-vinyl acetate copolymer (EVA). Int. J. Biol. Macromol. 2019, 128, 994–1001. [Google Scholar] [CrossRef]
- Yew, M.C.; Sulong, N.H.R.; Amalina, M.; Johan, M.R. Eggshells: A novel bio-filler for intumescent flame-retardant coatings. Prog. Org. Coatings 2015, 81, 116–124. [Google Scholar] [CrossRef]
- Krishnadevi, K.; Selvaraj, V. Biowaste material reinforced cyanate ester based epoxy composites for flame retardant applications. High Perform. Polym. 2016, 28, 881–894. [Google Scholar] [CrossRef]
- Pulgarin, C.; Schwitzguebel, J.P.; Tabacchi, R. Utilization of wastes from coffee production. Biofutur 1991, 102, 43–50. [Google Scholar]
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Rasul, M.; Hassan, N.; Mofijur, M. Waste coffee oil: A promising source for biodiesel production. Energy Procedia 2019, 160, 677–682. [Google Scholar] [CrossRef]
- Kondamudi, N.; Mohapatra, S.K.; Misra, M. Spent coffee grounds as a versatile source of green energy. J. Agric. Food Chem. 2008, 56, 11757–11760. [Google Scholar] [CrossRef] [PubMed]
- Mariana, M.; Mahidin, M.; Mulana, F.; Aman, F. Utilization of activated carbon prepared from aceh coffee grounds as bio-sorbent for treatment of fertilizer industrial waste water. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 358, p. 012027. [Google Scholar]
- Kim, J.; Kim, H.; Baek, G.; Lee, C. Anaerobic co-digestion of spent coffee grounds with different waste feedstocks for biogas production. Waste Manag. 2017, 60, 322–328. [Google Scholar] [CrossRef]
- Reis, K.C.; Pereira, L.; Melo, I.C.N.A.; Marconcini, J.M.; Trugilho, P.F.; Tonoli, G.H.D. Particles of coffee wastes as reinforcement in polyhydroxybutyrate (phb) based composites. Mater. Res. 2015, 18, 546–552. [Google Scholar] [CrossRef] [Green Version]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane foams: Past, present, and future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [Green Version]
- Gama, N.; Soares, B.; Freire, C.; Silva, R.; Neto, C.P.; Timmons, A.B.; Ferreira, A. Bio-based polyurethane foams toward applications beyond thermal insulation. Mater. Des. 2015, 76, 77–85. [Google Scholar] [CrossRef]
- Santos, D.C.D.S.; Adebayo, M.; Pereira, S.; Prola, L.; Cataluña, R.; Lima, E.C.; Saucier, C.; Gally, C.R.; Machado, F. New carbon composite adsorbents for the removal of textile dyes from aqueous solutions: Kinetic, equilibrium, and thermodynamic studies. Korean J. Chem. Eng. 2014, 31, 1470–1479. [Google Scholar] [CrossRef]
- Akasaka, H.; Takahata, T.; Toda, I.; Ono, H.; Ohshio, S.; Himeno, S.; Kokubu, T.; Saitoh, H. Hydrogen storage ability of porous carbon material fabricated from coffee bean wastes. Int. J. Hydrog. Energy 2011, 36, 580–585. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef] [Green Version]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.A.; Roberto, I.C.; Teixeira, J.A. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydr. Polym. 2011, 83, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Pujol, D.; Liu, C.; Gominho, J.; Olivella, M.; Fiol, N.; Villaescusa, I.; Pereira, H. The chemical composition of exhausted coffee waste. Ind. Crop. Prod. 2013, 50, 423–429. [Google Scholar] [CrossRef]
- Mandlekar, N.; Cayla, A.; Rault, F.; Giraud, S.; Salaün, F.; Malucelli, G.; Guan, J.-P. An overview on the use of lignin and its derivatives in fire retardant polymer systems. In Lignin-Trends and Applications; Poletto, M., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Vahabi, H.; Shabanian, M.; Aryanasab, F.; Mangin, R.; Laoutid, F.; Saeb, M.R. Inclusion of modified lignocellulose and nano-hydroxyapatite in development of new bio-based adjuvant flame retard-ant for poly (lactic acid). Thermochim. Acta 2018, 666, 51–59. [Google Scholar] [CrossRef]
- Movahedifar, E.; Vahabi, H.; Saeb, M.R.; Thomas, S. Flame retardant epoxy composites on the road of innovation: An analysis with flame retardancy index for future development. Molecules 2019, 24, 3964. [Google Scholar] [CrossRef] [Green Version]
- Sonnier, R.; Vahabi, H.; Ferry, L.; Lopez-Cuesta, J.-M. Pyrolysis-combustion flow calorimetry: A powerful tool to evaluate the flame retardancy of polymers. In Fire and Polymers VI: New Advances in Flame Retardant Chemistry and Science; ACS Symposium Series; Wilkie, C.A., Morgan, A.B., Nelson, G.L., Eds.; American Chemical Society: Washington, DC, USA, 2012. [Google Scholar]
- Huggett, C. Estimation of rate of heat release by means of oxygen consumption measurements. Fire Mater. 1980, 4, 61–65. [Google Scholar] [CrossRef]
- Bigdeloo, M.; Teymourian, T.; Kowsari, E.; Ramakrishna, S.; Ehsani, A. Sustainability and circular economy of food wastes: Waste reduction strategies, higher recycling methods, and improved valorization. Mater. Circ. Econ. 2021, 3, 1–9. [Google Scholar] [CrossRef]
- Sadeghi, B.; Marfavi, Y.; Aliakbari, R.; Kowsari, E.; Ajdari, F.B.; Ramakrishna, S. Recent studies on recycled PET fibers: Production and applications: A review. Mater. Circ. Econ. 2021, 3, 1–18. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).