Evaluation of Corrosion Protection Performance of New Polymer Composite Coatings on Carbon Steel in Acid Medium by Electrodeposition Methods
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
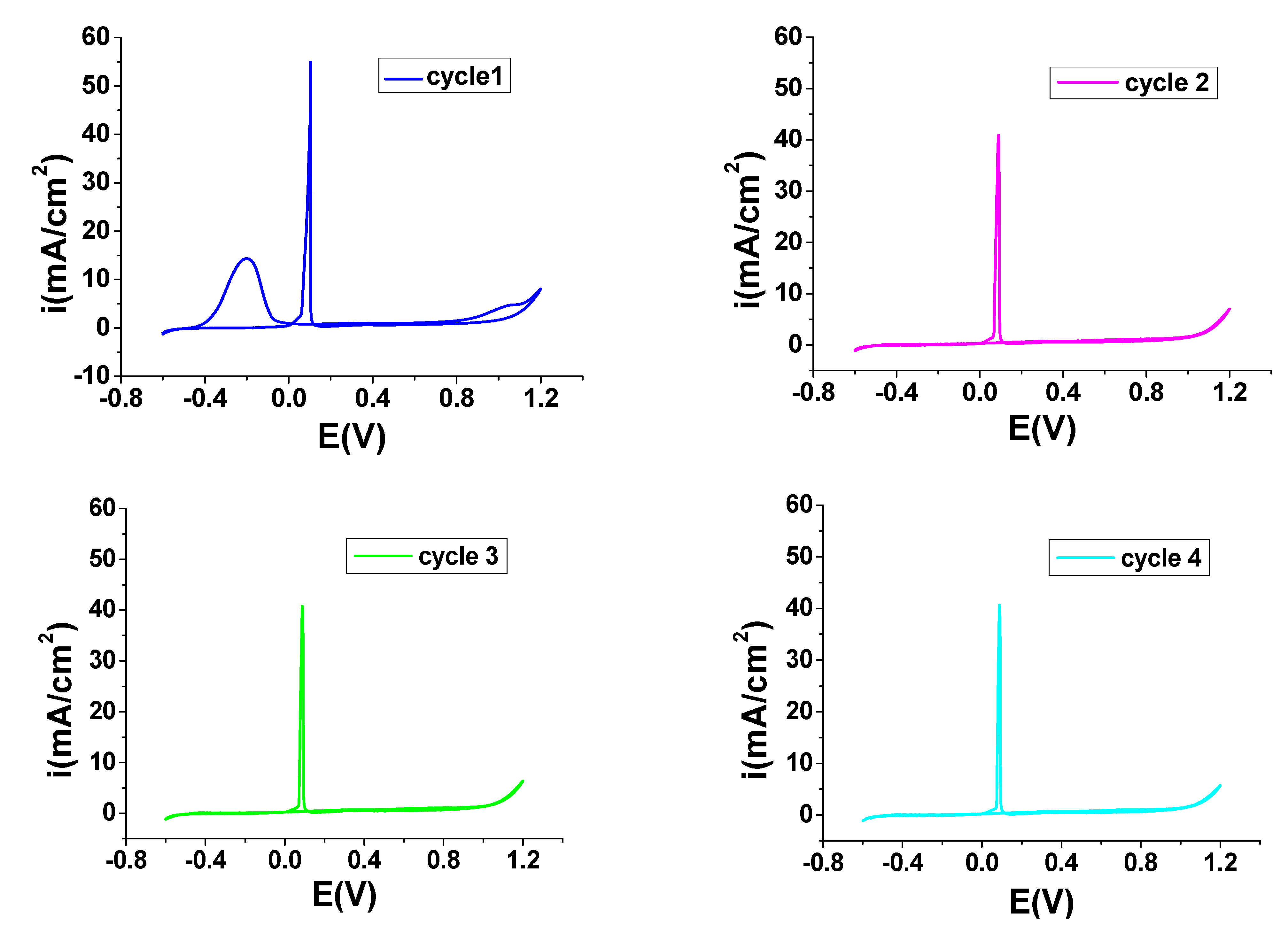
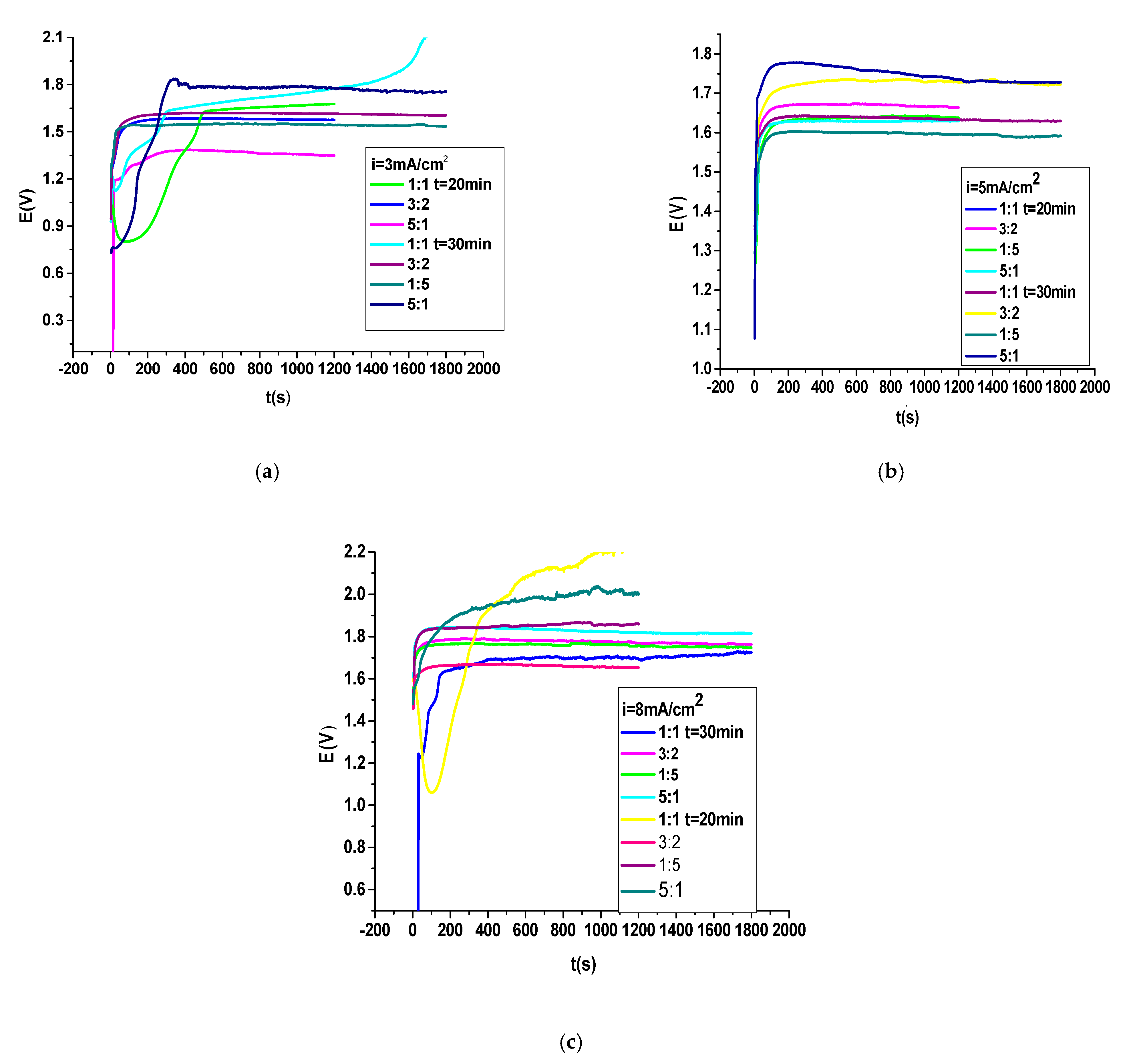
3.1. Electrodeposition PNMPY-TRX100/PNNDEA Coating on OL 37 Electrode
3.2. Electrocharacterization of PNMPY-TRX100/PNNDEA Coating
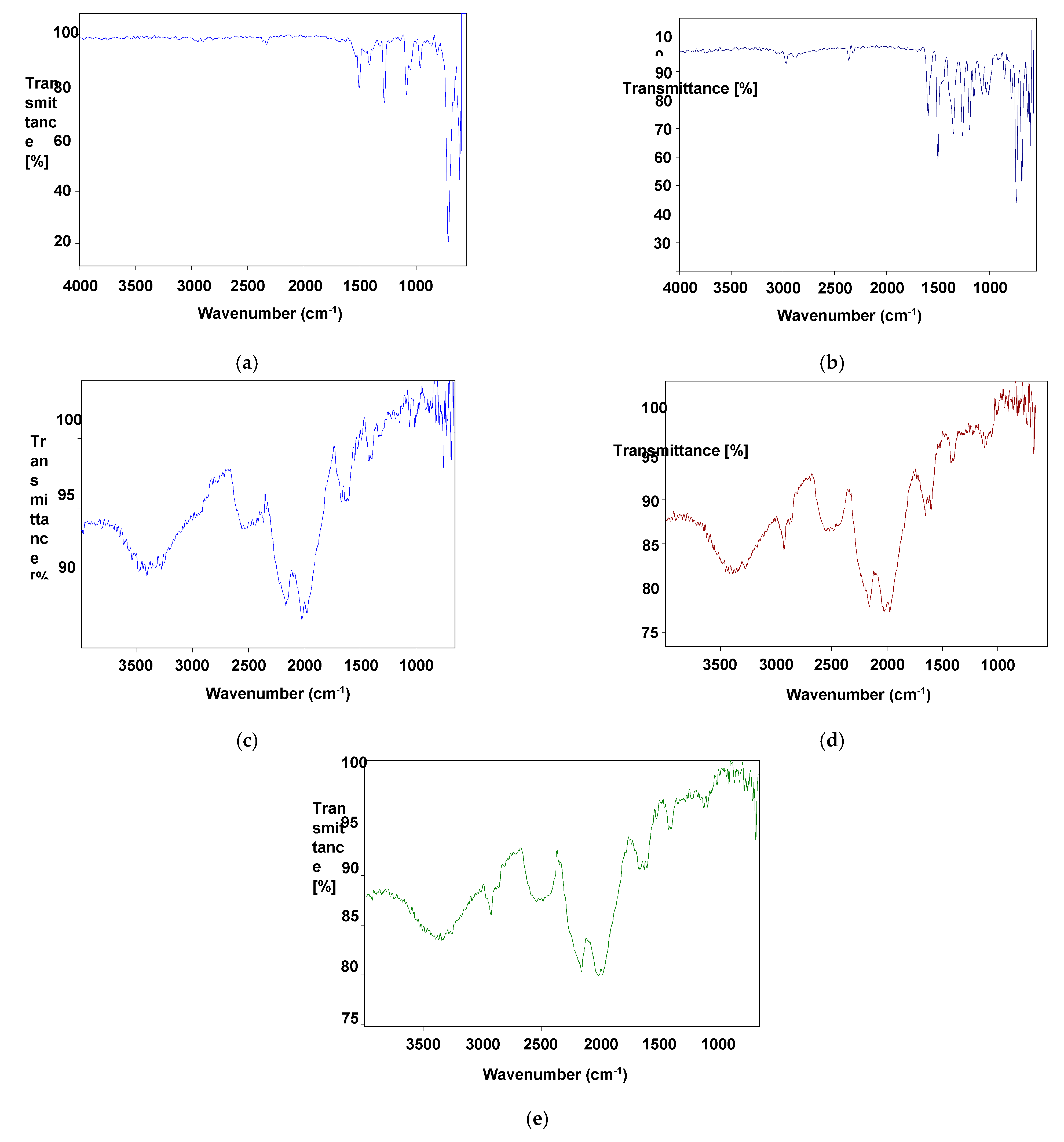
3.3. FT-IR Studies
3.4. Electrochemical Studies
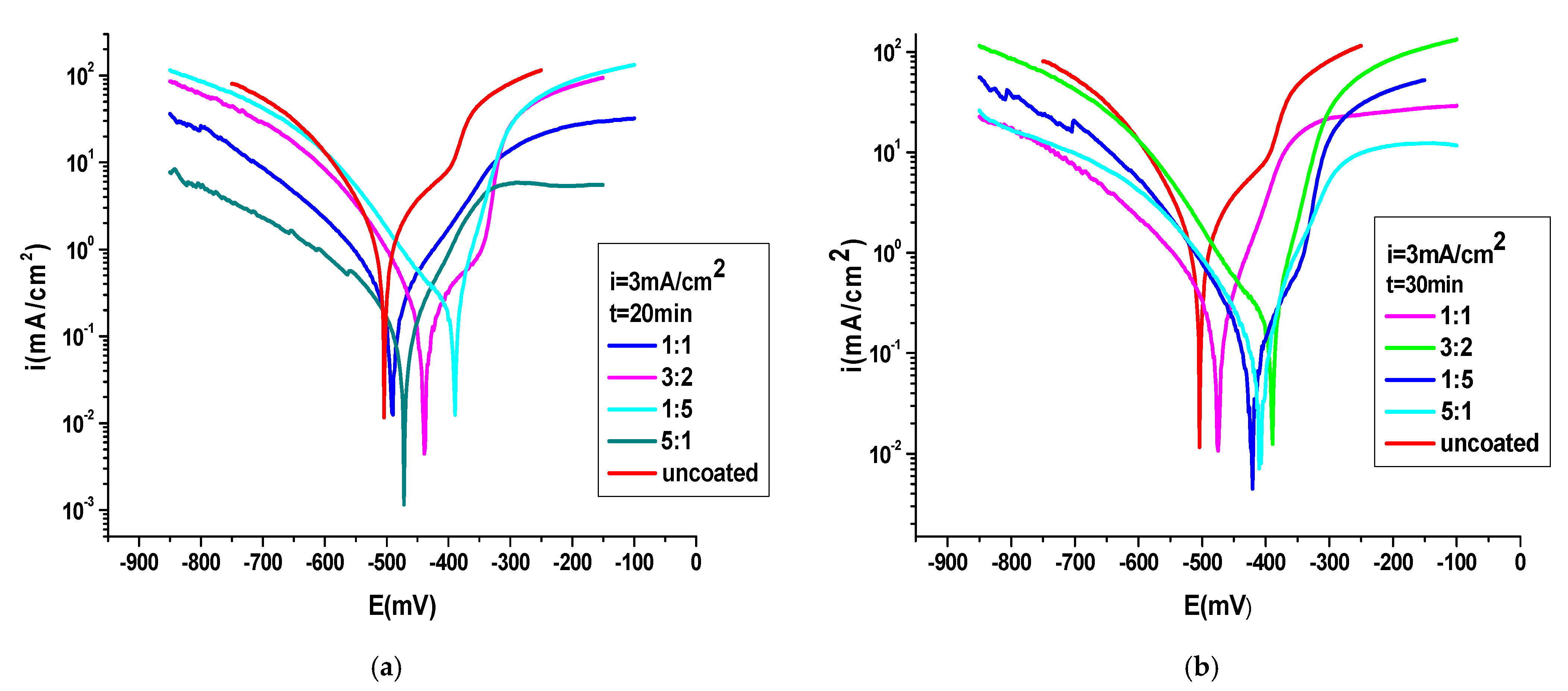
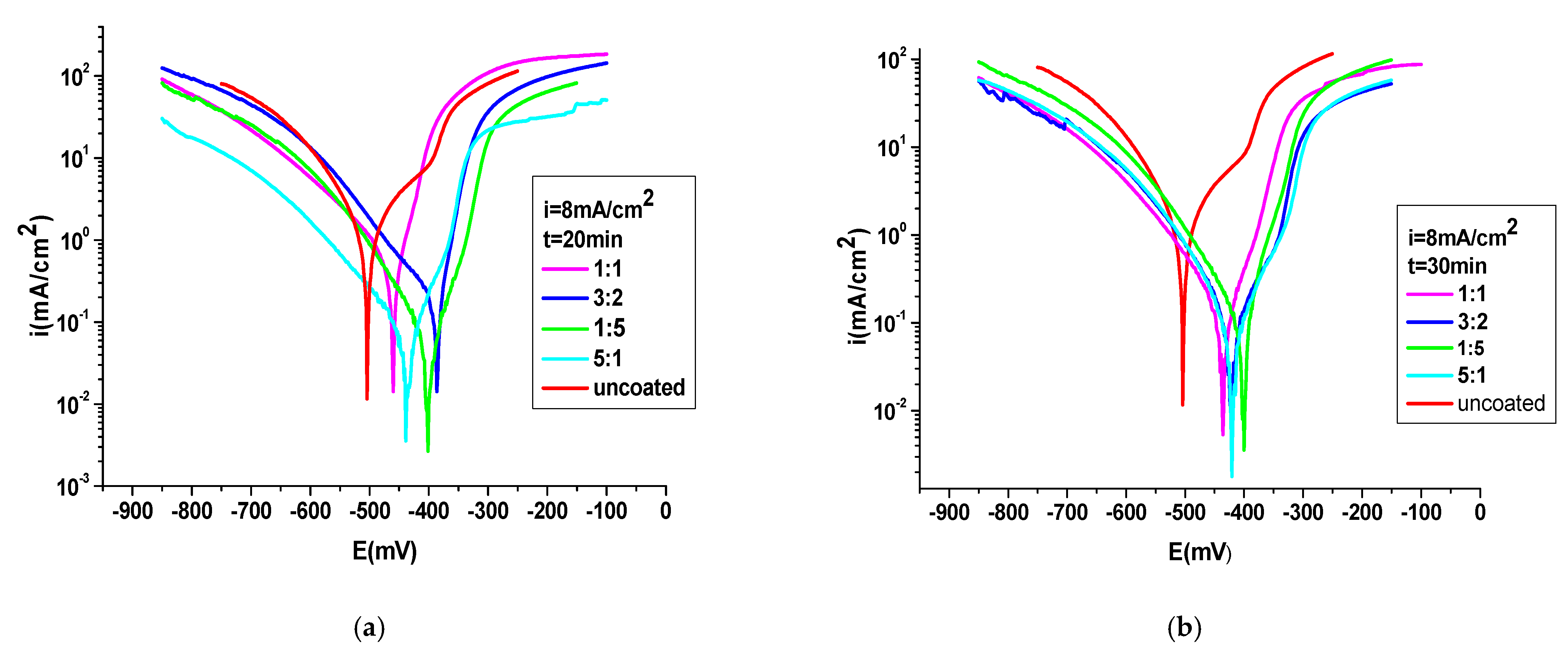
3.4.1. Potentiodynamic Polarization Method
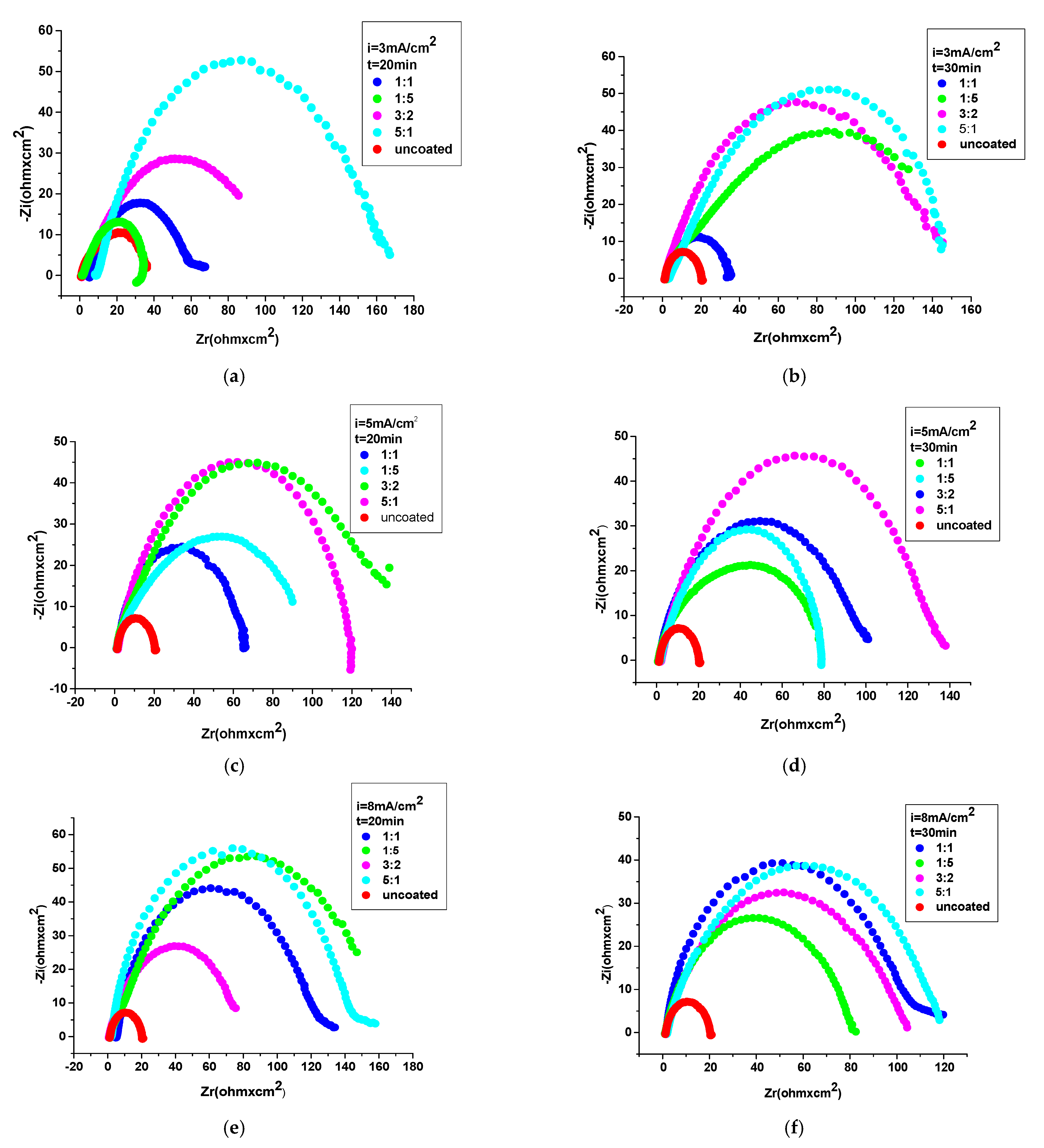
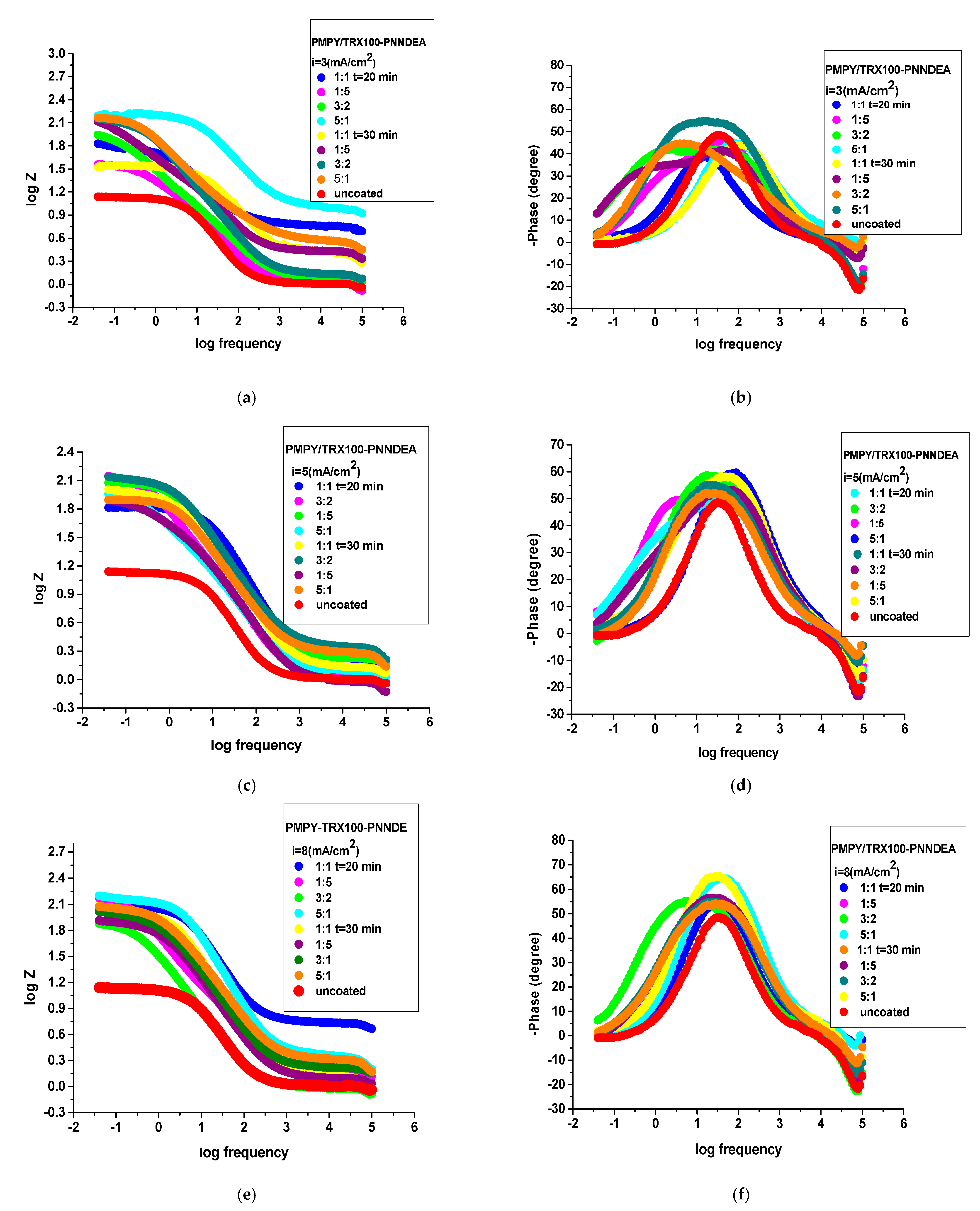
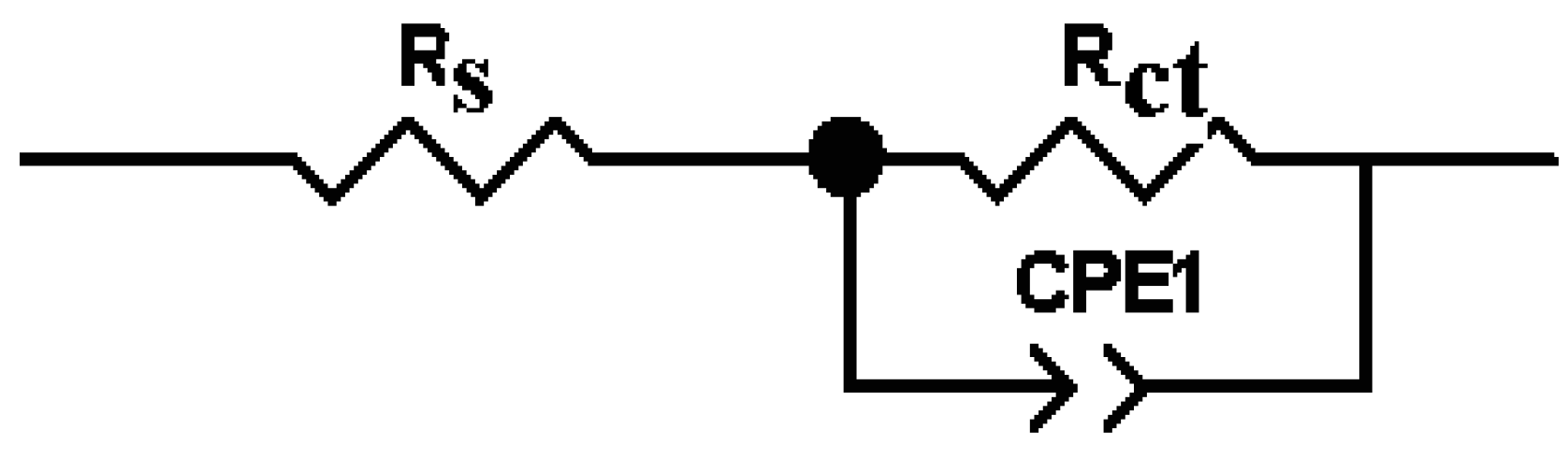
3.4.2. Electrochemical Impedance Spectroscopy (EIS)
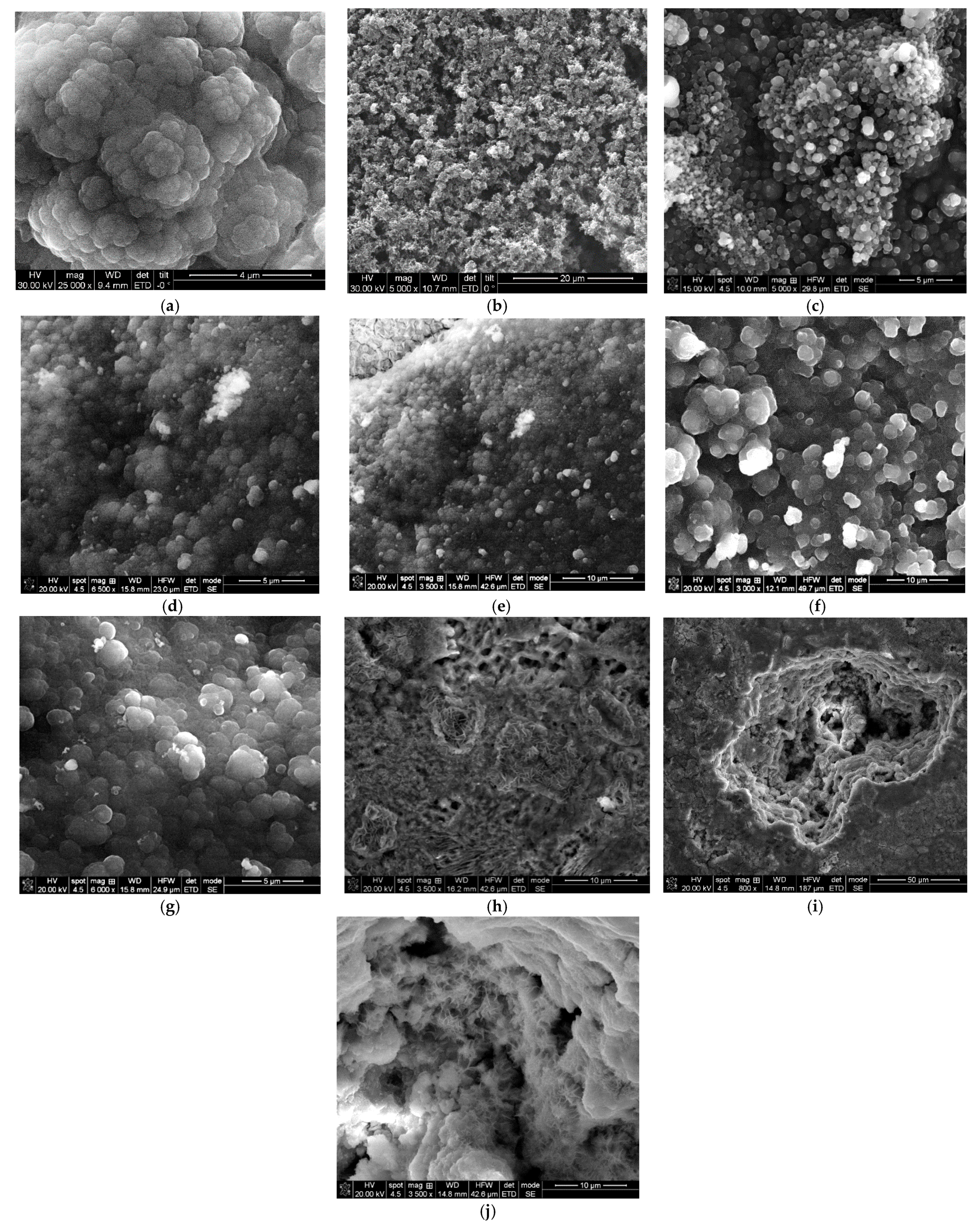
3.5. SEM Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhai, Y.; Pan, K.; Zhang, E. Anti-Corrosive Coating of Carbon-Steel Assisted by Polymer-Camphor sulfonic Acid Embedded within Graphene. Coatings 2020, 10, 879. [Google Scholar] [CrossRef]
- Kumar Gupta, D.; Neupane, S.; Singh, S.; Karki, N.; Yadav, A.P. The effect of electrolytes on the coating of polyaniline on mild steel by electrochemical methods and its corrosion behavior. Prog. Org. Coat. 2021, 152, 106127. [Google Scholar] [CrossRef]
- Gallegos-Melgar, A.; Serna, S.A.; Lázaro, I.; Gutiérrez-Castañeda, J.; Mercado-Lemus VArcos-Gutierrez, H.H.; Hernández-Hernández, M.; Porcayo-Calderón Jan Mayen, J.; Del Angel Monroy, M. Potentiodynamic Polarization Performance of a Novel Composite Coating System of Al2O3/Chitosan-Sodium Alginate, Applied on an Aluminum AA6063 Alloy for Protection in a Chloride Ions Environment. Coatings 2020, 10, 45. [Google Scholar] [CrossRef] [Green Version]
- Tavandashti, N.P.; Almas, S.M.; Esmaeilzadeh, E. Corrosion protection performance of epoxy coating containing alumina/PANI nanoparticles doped with cerium nitrate inhibitor on Al-2024 substrates. Prog. Org. Coat. 2021, 152, 106133. [Google Scholar] [CrossRef]
- Li, J.; He, Y.; Sun, Y.; Zhang, X.; Shi, W.; Ge, D. Synthesis of Polypyrrole/V2O5 Composite Film on the Surface of Magnesium Using a Mild Vapor Phase Polymerization (VPP) Method for Corrosion Resistance. Coatings 2020, 10, 402. [Google Scholar] [CrossRef] [Green Version]
- Fouda, A.S.; Heakal, F.E.; Radwan, M.S. Role of some thiadiazole derivatives as inhibitors of the corrosion of. C-steel in 1MH2SO4. J. Appl. Electrochem. 2009, 39, 391–402. [Google Scholar] [CrossRef]
- Asami, K.; Hashimoto, K. Importance of initial surface film in the degradation of stainless steels by atmospheric exposure. Corros. Sci. 2003, 45, 2263–2283. [Google Scholar] [CrossRef]
- Aliyu, I.K.; Kumar, M.; Abdul Samad Mohammed, A. Wear and corrosion resistance performance of UHMWPE/GNPs nanocomposite coatings on AA2028 Al alloys. Prog. Org. Coat. 2021, 151, 106072. [Google Scholar] [CrossRef]
- Cheng, L.; Wu, H.; Li, J.; Zhao, H.; Wang, L. Polydopamine modified ultrathin hydroxyapatite nanosheets for anti-corrosion reinforcement in polymeric coatings. Corros. Sci. 2021, 178, 10906. [Google Scholar] [CrossRef]
- Boshkova, N.; Kamburova, K.; Koprinarov, N.; Konstantinova, M.; Boshkov, N.; Radeva, T. Obtaining and Corrosion Performance of Composite Zinc Coatings with Incorporated Carbon Spheres. Coatings 2020, 10, 665. [Google Scholar] [CrossRef]
- Liu, A.; Tian, H.; Li, S.; Xiaodan, J.; Yang, H.; Sun, Y.; Wang, L.; Li, W. Bioinspired layered hybrid coatings with greatly enhanced barrier effect and active corrosion protection performance. Prog. Org. Coat. 2021, 152, 106131. [Google Scholar] [CrossRef]
- Ji, S.; Gui, H.; Guan, G.; Zhou, M.; Guo, Q.; Tan, M.Y. Molecular design and copolymerization to enhance the anti-corrosion performance of waterborne acrylic coatings. Prog. Org. Coat. 2021, 153, 106140. [Google Scholar] [CrossRef]
- Branzoi, F.; Branzoi, V. Enzymatic electrode obtained by immobilizing of urease into a nanocomposite film based on conducting polymers and different additives. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 549–556. [Google Scholar] [CrossRef]
- Yağan, A.; Pekmez, N.Ö.; Yıldız, A. Poly(N-methylaniline) coatings on stainless steel by electropolymerization. Corros. Sci. 2007, 49, 2905–2919. [Google Scholar] [CrossRef]
- Branzoi, F.; Branzoi, V.; Pahom, Z. Monolayer and bilayer conducting polymer coatings for corrosion protection of copper in 5 M H2SO4 solutions. Rev. Roum. Chim. 2013, 58, 49–58. [Google Scholar]
- Rui, M.; Zhu, A. The synthesis and corrosion protection mechanisms of PANI/CNT nanocomposite doped with organic phosphoric acid. Prog. Org. Coat. 2021, 153, 106134. [Google Scholar] [CrossRef]
- González, M.B.; Saidman, S.B. Electrodeposition of polypyrrole on 316L stainless steel for corrosion prevention. Corros. Sci. 2011, 53, 276–282. [Google Scholar] [CrossRef]
- Herrasti, P.; Patil, P. Corrosion protective poly (o-ethoxyaniline) coatings on copper. Electrochim. Acta 2007, 53, 927–933. [Google Scholar]
- Branzoi, F.; Branzoi, V. Investigation of Protective Effect of Polymeric Film Coatings on Carbon Steel in Aggressive Solutions. Int. J. Electrochem. Sci. 2016, 11, 6564–6579. [Google Scholar] [CrossRef]
- Duran, B.; Turhan, M.C.; Bereket, G.; Sarac, A.S. Electropolymerization, characterization and corrosion performance of poly(N-ethylaniline) on copper. Electrochim. Acta 2009, 55, 104–112. [Google Scholar] [CrossRef]
- Yagan, A.; Pekmez, O.N.; Yildiz, A. Poly(N-ethylaniline) coatings on 304 stainless steel for corrosion protection in aqueous HCl and Na Clsolutions. Electrochim. Acta 2008, 53, 2474–2482. [Google Scholar] [CrossRef]
- Shi, S.; Zhao, Y.; Zhang, Z.; Yu, L. Corrosion protection of a novel SiO2@PANI coating for Q235 carbon steel. Prog. Org. Coat. 2019, 132, 227–234. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Iroh, J.O. Characterization of polyaniline–polypyrrole composite coatings on low carbon steel: A XPS and infrared spectroscopy study. Appl. Surf. Sci. 2003, 218, 58–69. [Google Scholar] [CrossRef]
- Abdollah, O.; Hussein, R.; Razieh, M. Electrochemical synthesis of polypyrrole/polyhedral oligomeric silsesquioxane nanocomposite on copper for corrosion protection. Prog. Org. Coat. 2016, 90, 331–338. [Google Scholar]
- Al Juhaiman, L.A. Polyvinyl pyrrolidone as a Corrosion Inhibitor for Carbon Steel in HCl. Int. J. Electrochem. Sci. 2016, 11, 1621–1631. [Google Scholar]
- Yagan, A.; Pekmez Ozcicek, N.; Yildiz, A. Electrodeposition of poly(N-methyl aniline) on mildsteel: Synthesis, Characterization and corrosion protection. J. Electroanal. Chem. 2005, 578, 231–238. [Google Scholar] [CrossRef]
- Branzoi, F.; Băran, A.; Ludmila, A.; Alexandrescu, E. The inhibition action of some organic polymers on the corrosion carbon steel in acidic media. Chem. Pap. 2020, 74, 4315–4335. [Google Scholar] [CrossRef]
- Chaudhari, S.; Patil, P.P. Inhibition of steel corrosion by electrosynthesizedpoly(o-anisidine)-dodecylbenzenesulfonate coatings. Electrochim. Act. 2010, 55, 6715–6723. [Google Scholar] [CrossRef]
- Hasanov, R.; Bilgic, S. Monolayer and bilayer conducting polymer coatings for corrosion protection of steel in 1 M H2SO4 solution. Prog. Org. Coat. 2009, 64, 435–445. [Google Scholar] [CrossRef]
- Brânzoi, V.; Branzoi, F.; Pilan, L. Characterization of electrodeposited polymeric and composite modified electrodes on cobalt based alloy. Mater. Chem. Phys. 2009, 118, 197–202. [Google Scholar] [CrossRef]
- Zeybek, B.; Pekmez, N.O.; Kilic, E. Electrochemical synthesis of bilayer coatings of poly(N-methylaniline) and polypyrrole on mild steel and their corrosion protection performances. Electrochim. Acta 2011, 56, 9277–9286. [Google Scholar] [CrossRef]
- Branzoi, F.; Brânzoi, V.; Musina, A. Fabrication and characterisation of conducting composite films based on conducting polymers and functionalised carbon nanotubes. Surf. Interface Anal. 2012, 44, 1076–1080. [Google Scholar] [CrossRef]
- Sazou, D.; Kourouzidou, M.; Pavlidou, E. Potentiodynamic and potentiostatic deposition of polyaniline on stainless steel: Electrochemical and structural studies for a potential application to corrosion control. Electrochim. Acta 2007, 52, 4385–4397. [Google Scholar] [CrossRef]
- Branzoi, F.; Branzoi, V.; Musina, A. Coatings based on conducting polymers and functionalized carbon nanotubes obtained by electropolymerization. Prog. Org. Coat. 2013, 76, 632–638. [Google Scholar] [CrossRef]
- Pekmez, N.O.; Cınkıllı, K.; Zeybek, B. The electrochemical copolymerization of pyrrole and bithiophene on stainless steel in the presence of SDS in aqueous medium and its anticorrosive performance. Prog. Org. Coat. 2014, 77, 1277–1287. [Google Scholar] [CrossRef]
- Menkuer, M.; Ozkazanc, H. Anticorrosive polypyrrole/zirconium-oxide composite film prepared in oxalic acid and dodecylbenzene sulfonic acid mix electrolyte. Prog. Org. Coat. 2020, 147, 105815. [Google Scholar] [CrossRef]


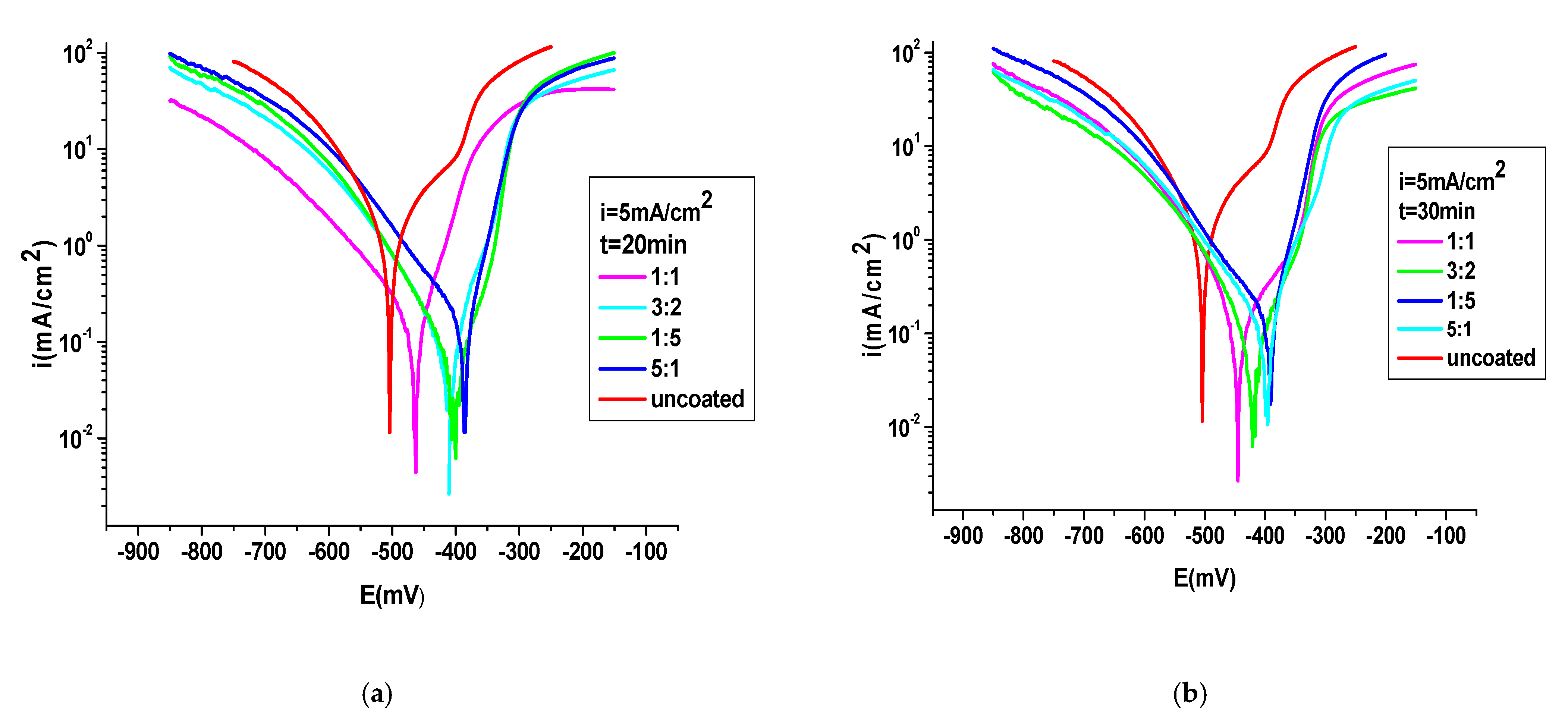

| The System PNMPY-TRX100/PNNDEA/OL 37 | Ecorr (mV) | icorr (mA/cm2) | Rp (Ωcm) | Rmpy | Pmm/year | Kg (g/m2h) | ba (mV/ Decade) | −bc (mV/ Decade) | E (%) | %P |
|---|---|---|---|---|---|---|---|---|---|---|
| OL 37 + 0.5 M H2SO4 | −504 | 0.892 | 16 | 387 | 9.81 | 8.79 | 98 | 93 | - | |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 1:1 molar ratio, t = 20 min | −442 | 0.171 | 78 | 74 | 1.88 | 1.68 | 85 | 80 | 81 | 0.04 |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 3:2 molar ratio | −443 | 0.144 | 106 | 62.4 | 1.58 | 1.41 | 99 | 73 | 84 | 0.03 |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 5:1 molar ratio | −452 | 0.083 | 160 | 35.53 | 0.90 | 0.81 | 68 | 94 | 91 | 0.024 |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 1:5 molar ratio | −393 | 0.170 | 66 | 73.66 | 1.86 | 1.43 | 45 | 113 | 81 | 0.017 |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 1:1 molar ratio t = 30 min | −470 | 0.157 | 72 | 68.03 | 1.72 | 1.54 | 58 | 86 | 82 | 0.085 |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 3:2 molar ratio | −400 | 0.092 | 144 | 40.3 | 1.02 | 0.91 | 47 | 99 | 90 | 0.006 |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 5:1 molar ratio | −409 | 0.086 | 159 | 37.52 | 0.95 | 0.85 | 54 | 84 | 90.5 | 0.007 |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 1:5 molar ratio | −422 | 0.098 | 162 | 42.46 | 1.07 | 0.96 | 80 | 85 | 89 | 0.01 |
| The System PNMPY-TRX100/PNNDEA/OL 37 | Ecorr (mV) | icorr (mA/cm2) | Rp (Ωcm) | Rmpy | Pmm/year | Kg (g/m2h) | ba (mV/ Decade) | −bc (mV/ Decade) | E (%) | %P |
|---|---|---|---|---|---|---|---|---|---|---|
| OL 37 + 0.5 M H2SO4 | −504 | 0.892 | 16 | 387 | 9.81 | 8.79 | 98 | 93 | ||
| PNMPY-TRX100/PNNDEA 5 mA/cm2 1:1 molar ratio, t = 20 min | −458 | 0.101 | 118 | 43.76 | 1.11 | 0.99 | 48 | 89 | 89 | 0.036 |
| PNMPY-TRX100/PNNDEA 5 mA/cm2 3:2 molar ratio | −409 | 0.068 | 192 | 29.46 | 0.74 | 0.66 | 51 | 79 | 92 | 0.007 |
| PNMPY-TRX100/PNNDEA 5 mA/cm2 5:1 molar ratio | −405 | 0.057 | 228 | 24.7 | 0.62 | 0.56 | 50 | 83 | 94 | 0.008 |
| PNMPY-TRX100/PNNDEA 5 mA/cm2 1:5 molar ratio | −386 | 0.091 | 102 | 39.43 | 1.01 | 0.89 | 40 | 82 | 90 | 0.006 |
| PNMPY-TRX100/PNNDEA 5 mA/cm2 1:1 molar ratio t = 30 min | −446 | 0.107 | 125 | 49.93 | 1.26 | 1.13 | 102 | 70 | 88 | 0.024 |
| PNMPY-TRX100/PNNDEA 5 mA/cm2 3:2 molar ratio | −403 | 0.094 | 123 | 41 | 1.03 | 0.92 | 52 | 99 | 89 | 0.009 |
| PNMPY-TRX100/PNNDEA 5 mA/cm2 5:1 molar ratio | −421 | 0.055 | 199 | 23.83 | 0.60 | 0.54 | 63 | 65 | 94 | 0.008 |
| PNMPY-TRX100/PNNDEA 5 mA/cm2 1:5 molar ratio | −400 | 0.131 | 96 | 56.7 | 1.44 | 1.28 | 54 | 119 | 85 | 0.011 |
| The System PNMPY-TRX100/PNNDEA/OL 37 | Ecorr (mV) | icorr (mA/cm2) | Rp (Ωcm) | Rmpy | Pmm/year | Kg (g/m2h) | ba (mV/ Decade) | −bc (mV/ Decade) | E (%) | %P |
|---|---|---|---|---|---|---|---|---|---|---|
| OL 37 + 0.5 M H2SO4 | −504 | 0.892 | 16 | 387 | 9.81 | 8.79 | 98 | 93 | - | |
| PNMPY-TRX100/PNNDEA 8 mA/cm2 1:1 molar ratio, t = 20 min | −430 | 0.074 | 116 | 32.06 | 0.81 | 0.72 | 50 | 69 | 92 | 0.018 |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 3:2 molar ratio | −422 | 0.098 | 160 | 42.46 | 1.07 | 0.96 | 81 | 84 | 89 | 0.017 |
| PNMPY-TRX100/PNNDEA 8 mA/cm2 5:1 molar ratio | −423 | 0.057 | 176 | 24.7 | 0.62 | 0.56 | 63 | 65 | 94 | 0.009 |
| PNMPY-TRX100/PNNDEA 8 mA/cm2 1:5 molar ratio | −401 | 0.092 | 143 | 39.43 | 1.01 | 0.89 | 114 | 86 | 90 | 0.007 |
| PNMPY-TRX100/PNNDEA 8 mA/cm2 1:1 molar ratio t = 30 min | −416 | 0.071 | 179 | 30.6 | 0.78 | 0.39 | 70 | 79 | 92 | 0.008 |
| PNMPY-TRX100/PNNDEA 8 mA/cm2 3:2 molar ratio | −412 | 0.102 | 107 | 44.2 | 1.12 | 1.04 | 72 | 41 | 89 | 0.0094 |
| PNMPY-TRX100/PNNDEA 8 mA/cm2 5:1 molar ratio | −405 | 0.052 | 221 | 22.53 | 0.56 | 0.51 | 57 | 92 | 94 | 0.0059 |
| PNMPY-TRX100/PNNDEA 8 mA/cm2 1:5 molar ratio | −404 | 0.067 | 218 | 29.03 | 0.73 | 0.63 | 59 | 88 | 92.5 | 0.0058 |
| The System PNMPY-TRX100/PNNDEA/OL 37 | Immersion Time (h) | Ecorr (mV) | icorr (mA/cm2) | Rp (Ωcm) | Rmpy | Pmm/year | Kg (g/m2h) | ba (mV/ Decade) | −bc (mV/ Decade) | E (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| OL 37 + 0.5 M H2SO4 | −504 | 0.892 | 16 | 387 | 9.81 | 8.79 | 98 | 93 | ||
| PNMPY-TRX100/PNNDEA 3 mA/cm2 3:2 molar ratio t = 30 min | 0 | −400 | 0.092 | 144 | 39.86 | 1.01 | 0.90 | 47 | 99 | 90 |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 3:2 molar ratio t = 30 min | 24 | −409 | 0.093 | 100 | 40.3 | 1.02 | 0.91 | 41 | 47 | 93 |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 3:2 molar ratio t = 30 min | 48 | −418 | 0.095 | 85 | 41.47 | 1.05 | 0.94 | 59 | 50 | 89 |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 3:2 molar ratio t = 30 min | 72 | −422 | 0.107 | 77 | 43.36 | 1.17 | 0.98 | 96 | 66 | 88 |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 3:2 molar ratio t = 30 min | 96 | −428 | 0.129 | 75 | 55.9 | 1.41 | 1.27 | 101 | 67 | 85.5 |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 3:2 molar ratio t = 30 min | 120 | −426 | 0.131 | 81 | 56.76 | 1.44 | 1.29 | 99 | 71 | 85 |
| PNMPY-TRX100/PNNDEA 5 mA/cm2 5:1 molar ratio, t = 20 min | 0 | −386 | 0.091 | 102 | 39.43 | 1.01 | 0.89 | 44 | 47 | 90 |
| PNMPY-TRX100/PNNDEA 5 mA/cm2 5:1 molar ratio, t = 20 min | 24 | −400 | 0.0827 | 96 | 35.83 | 0.909 | 0.814 | 46 | 82 | 91 |
| PNMPY-TRX100/PNNDEA 5 mA/cm2 5:1 molar ratio, t = 20 min | 96 | −404 | 0.103 | 102 | 44.63 | 1.13 | 1.014 | 74 | 57 | 88.5 |
| PNMPY-TRX100/PNNDEA 5 mA/cm2 5:1 molar ratio, t = 20 min | 120 | −413 | 0.172 | 93 | 74.53 | 1.89 | 1.69 | 99 | 68 | 81 |
| PNMPY-TRX100/PNNDEA 5 mA/cm2 5:1 molar ratio, t = 20 min | 144 | −416 | 0.193 | 85 | 83.63 | 2.12 | 1.90 | 101 | 67 | 78.5 |
| PNMPY-TRX100/PNNDEA 5 mA/cm2 5:1 molar ratio, t = 20 min | 168 | −415 | 0.184 | 87 | 79.73 | 2.02 | 1.81 | 104 | 66 | 79 |
| The System PNMPY-TRX100/PNNDEA/OL 37 | RS (ohm.cm2) | Rct (ohm.cm2) | Cdl (μFcm−2) | E% |
|---|---|---|---|---|
| OL 37 + 0.5 M H2SO4 | 1.032 | 18 | 484 | |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 1:1 molar ratio, t = 20 min | 3.6 | 65 | 220 | 65 |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 3:2 molar ratio | 0.94 | 170 | 84 | 90 |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 5:1 molar ratio | 2.44 | 230 | 62 | 93 |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 1:5 molar ratio | 1.31 | 130 | 109 | 87 |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 1:1 molar ratio t = 30 min | 2.45 | 45 | 317 | 68 |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 3:2 molar ratio | 2.41 | 161 | 89 | 89 |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 5:1 molar ratio | 2.81 | 220 | 65 | 92 |
| PNMPY-TRX100/PNNDEA 3 mA/cm2 1:5 molar ratio | 1.33 | 144 | 99 | 88 |
| PNMPY-TRX100/PNNDEA 5 mA/cm2 1:1 molar ratio, t = 20 min | 1.59 | 66 | 216 | 74 |
| PNMPY-TRX100/PNNDEA 5 mA/cm2 3:2 molar ratio | 0.95 | 133 | 107 | 87 |
| PNMPY-TRX100/PNNDEA 5 mA/cm2 5:1 molar ratio | 3.03 | 168 | 85 | 90 |
| PNMPY-TRX100/PNNDEA 5 mA/cm2 1:5 molar ratio | 1.94 | 123 | 115 | 86 |
| PNMPY-TRX100/PNNDEA 5 mA/cm2 1:1 molar ratio t = 30 min | 0.97 | 81 | 176 | 80 |
| PNMPY-TRX100/PNNDEA 5 mA/cm2 3:2 molar ratio | 0.63 | 111 | 128 | 85 |
| PNMPY-TRX100/PNNDEA 5 mA/cm2 5:1 molar ratio | 2.17 | 144 | 99 | 88 |
| PNMPY-TRX100/PNNDEA 5 mA/cm2 1:5 molar ratio | 2.23 | 100 | 142 | 83 |
| PNMPY-TRX100/PNNDEA 8 mA/cm2 1:1 molar ratio t = 20 min | 4.82 | 130 | 109 | 87 |
| PNMPY-TRX100/PNNDEA 8 mA/cm2 3:2 molar ratio | 1.08 | 84 | 170 | 80 |
| PNMPY-TRX100/PNNDEA 8 mA/cm2 5:1 molar ratio | 2.03 | 179 | 80 | 91 |
| PNMPY-TRX100/PNNDEA 8 mA/cm2 1:5 molar ratio | 1.78 | 153 | 93 | 89 |
| PNMPY-TRX100/PNNDEA 8 mA/cm2 1:1 molar ratio t = 30 min | 0.92 | 117 | 121 | 86 |
| PNMPY-TRX100/PNNDEA 8 mA/cm2 3:2 molar ratio | 1.13 | 89 | 160 | 81 |
| PNMPY-TRX100/PNNDEA 8 mA/cm2 5:1 molar ratio | 1.41 | 129 | 110 | 87 |
| PNMPY-TRX100/PNNDEA 8 mA/cm2 1:5 molar ratio | 1.23 | 113 | 126 | 85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branzoi, F.; Băran, A.; Petrescu, S. Evaluation of Corrosion Protection Performance of New Polymer Composite Coatings on Carbon Steel in Acid Medium by Electrodeposition Methods. Coatings 2021, 11, 903. https://doi.org/10.3390/coatings11080903
Branzoi F, Băran A, Petrescu S. Evaluation of Corrosion Protection Performance of New Polymer Composite Coatings on Carbon Steel in Acid Medium by Electrodeposition Methods. Coatings. 2021; 11(8):903. https://doi.org/10.3390/coatings11080903
Chicago/Turabian StyleBranzoi, Florina, Adriana Băran, and Simona Petrescu. 2021. "Evaluation of Corrosion Protection Performance of New Polymer Composite Coatings on Carbon Steel in Acid Medium by Electrodeposition Methods" Coatings 11, no. 8: 903. https://doi.org/10.3390/coatings11080903
APA StyleBranzoi, F., Băran, A., & Petrescu, S. (2021). Evaluation of Corrosion Protection Performance of New Polymer Composite Coatings on Carbon Steel in Acid Medium by Electrodeposition Methods. Coatings, 11(8), 903. https://doi.org/10.3390/coatings11080903







