The Phase Evolution and Photocatalytic Properties of a Ti-TiO2 Bilayer Thin Film Prepared Using Thermal Oxidation
Abstract
:1. Introduction
2. Materials and Methods
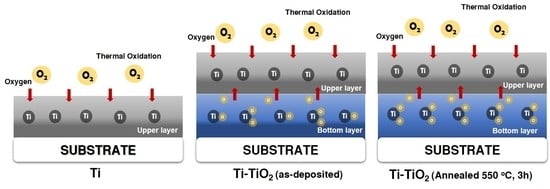
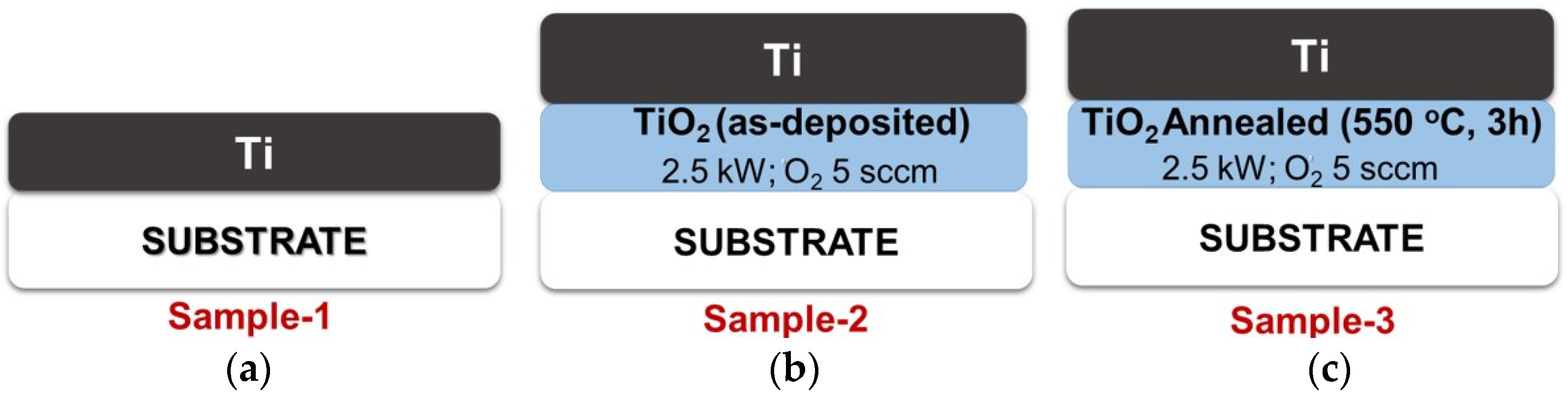
2.1. Preparation of TiO2 Thin Films as a Bottom Layer
2.2. Ti-TiO2 Bilayer Thin-Film Preparation
2.3. Sample Characterization
2.4. Photocatalytic Activity
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Graham, M.E.; Li, G.; Gray, K.A. Fabricating highly active mixed phase TiO2 photocatalysts by reactive DC magnetron sputter deposition. Thin Solid Film 2006, 515, 1176–1181. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, X.; Zhao, Q. Photocatalytic activity of nanometer TiO2 thin films prepared by the Sol–Gel Method. Mater. Chem. Phys. 2001, 69, 25–29. [Google Scholar] [CrossRef]
- Francioso, L.; Presicce, D.S.; Siciliano, P.; Ficarella, A. Combustion conditions discrimination properties of Pt-doped TiO2 thin film oxygen sensor. Sens. Actuators B Chem. 2007, 123, 516–521. [Google Scholar] [CrossRef]
- Weihao, L.; Shengnan, C.; Jianxun, W.; Shaohui, J.; Chenlu, S.; Yong, L.; Gaorong, H. Study on structural, optical and hydrophilic properties of FTO/TiO2 tandem thin film prepared by aerosol-assisted chemical vapor deposition method. Surf. Coat. Technol. 2019, 358, 715–720. [Google Scholar] [CrossRef]
- Greczynski, G.; Petrov, I.; Greene, J.E.; Hultman, L. Paradigm shift in thin-film growth by magnetron sputtering: From gas-ion to metal-ion irradiation of the growing film. J. Vac. Sci. Technol. A Vac. Surf. Film 2019, 37, 60801. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.; Shrivastava, M. Photocatalytic removal of hazardous dye cyanosine from industrial waste using titanium dioxide. J. Hazard. Mater. 2008, 152, 216–220. [Google Scholar] [CrossRef]
- Yao, K.S.; Wang, D.Y.; Chang, C.Y.; Weng, K.W.; Yang, L.Y.; Lee, S.J.; Cheng, T.C.; Hwang, C.C. Photocatalytic disinfection of phytopathogenic bacteria by dye-sensitized TiO2 thin film activated by visible light. Surf. Coat. Technol. 2007, 202, 1329–1332. [Google Scholar] [CrossRef]
- Ferrec, A.; Keraudy, J.; Jacq, S.; Schuster, F.; Jouan, P.-Y.; Djouadi, M.A. Correlation between mass-spectrometer measurements and thin film characteristics using DcMS and HiPIMS discharges. Surf. Coat. Technol. 2014, 250, 52–56. [Google Scholar] [CrossRef]
- Del Giudice, L.; Adjam, S.; La Grange, D.; Banakh, O.; Karimi, A.; Sanjinés, R. NbTiN thin films deposited by hybrid HiPIMS/DC magnetron co-sputtering. Surf. Coat. Technol. 2016, 295, 99–106. [Google Scholar] [CrossRef]
- Greczynski, G.; Mráz, S.; Hans, M.; Primetzhofer, D.; Lu, J.; Hultman, L.; Schneider, J.M. Unprecedented Al supersaturation in single-phase rock salt structure VAlN films by Al+ subplantation. J. Appl. Phys. 2017, 121, 171907. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, T.; Komiya, H.; Teranishi, Y.; Morikawa, K.; Nagasaka, H.; Yang, M. Pressure dependence of (Ti, Al) N film growth on inner walls of small holes in high-power impulse magnetron sputtering. Thin Solid Film 2017, 624, 189–196. [Google Scholar] [CrossRef]
- Zauner, L.; Ertelthaler, P.; Wojcik, T.; Bolvardi, H.; Kolozsvári, S.; Mayrhofer, P.H.; Riedl, H. Reactive HiPIMS Deposition of Ti-Al-N: Influence of the deposition parameters on the cubic to hexagonal phase transition. Surf. Coat. Technol. 2020, 382, 125007. [Google Scholar] [CrossRef]
- Lou, B.-S.; Yang, Y.-C.; Qiu, Y.-X.; Diyatmika, W.; Lee, J.-W. Hybrid high power impulse and radio frequency magnetron sputtering system for TiCrSiN thin film depositions: Plasma characteristics and film properties. Surf. Coat. Technol. 2018, 350, 762–772. [Google Scholar] [CrossRef]
- Fan, Q.; Liang, Y.; Wu, Z.; Liu, Y.; Wang, T. Microstructure and properties of CrAlSiN coatings deposited by HiPIMS and direct-current magnetron sputtering. Coatings 2019, 9, 512. [Google Scholar] [CrossRef] [Green Version]
- Greczynski, G.; Lu, J.; Jensen, J.; Bolz, S.; Kölker, W.; Schiffers, C.; Lemmer, O.; Greene, J.E.; Hultman, L. A Review of metal-ion-flux-controlled growth of metastable TiAlN by HIPIMS/DCMS co-sputtering. Surf. Coat. Technol. 2014, 257, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Greczynski, G.; Lu, J.; Jensen, J.; Petrov, I.; Greene, J.E.; Bolz, S.; Kölker, W.; Schiffers, C.; Lemmer, O.; Hultman, L. Strain-free, single-phase metastable Ti0.38Al0.62N alloys with high hardness: Metal-ion energy vs. momentum effects during film growth by hybrid high-power pulsed/Dc magnetron cosputtering. Thin Solid Film 2014, 556, 87–98. [Google Scholar] [CrossRef]
- Sartale, S.D.; Ansari, A.A.; Rezvani, S.J. Influence of Ti film thickness and oxidation temperature on TiO2 thin film formation via thermal oxidation of sputtered Ti film. Mater. Sci. Semicond. Process. 2013, 16, 2005–2012. [Google Scholar] [CrossRef]
- Zhou, B.; Jiang, X.; Liu, Z.; Shen, R.; Rogachev, A.V. Preparation and characterization of TiO2 thin film by thermal oxidation of sputtered Ti film. Mater. Sci. Semicond. Process. 2013, 16, 513–519. [Google Scholar] [CrossRef]
- Munguti, L.; Dejene, F. Influence of annealing temperature on structural, optical and photocatalytic properties of ZnO–TiO2 composites for application in dye removal in water. Nano-Struct. Nano-Objects 2020, 24, 100594. [Google Scholar] [CrossRef]
- Astinchap, B.; Moradian, R.; Gholami, K. Effect of sputtering power on optical properties of prepared TiO2 thin films by thermal oxidation of sputtered Ti layers. Mater. Sci. Semicond. Process. 2017, 63, 169–175. [Google Scholar] [CrossRef]
- Rothschild, A.; Edelman, F.; Komem, Y.; Cosandey, F. Sensing behavior of TiO2 thin films exposed to air at low temperatures. Sens. Actuators B Chem. 2000, 67, 282–289. [Google Scholar] [CrossRef]
- Vaquila, I.; Passeggi, M.C.G.; Ferrón, J. Oxidation process in titanium thin films. Phys. Rev. B 1997, 55, 13925. [Google Scholar] [CrossRef]
- Martin, M.; Mader, W.; Fromm, E. Oxidation of iron, aluminium and titanium films in the temperature range 50–200 °C. Thin Solid Film 1994, 250, 61–66. [Google Scholar] [CrossRef]
- Aniołek, K. The influence of thermal oxidation parameters on the growth of oxide layers on titanium. Vacuum 2017, 144, 94–100. [Google Scholar] [CrossRef]
- Ting, C.-C.; Chen, S.-Y.; Liu, D.-M. Structural evolution and optical properties of TiO2 thin films prepared by thermal oxidation of sputtered Ti films. J. Appl. Phys. 2000, 88, 4628–4633. [Google Scholar] [CrossRef]
- Sarvadii, S.Y.; Gatin, A.K.; Kharitonov, V.A.; Dokhlikova, N.V.; Ozerin, S.A.; Grishin, M.V.; Shub, B.R. Oxidation of thin titanium films: Determination of the chemical composition of the oxide and the oxygen diffusion factor. Crystals 2020, 10, 117. [Google Scholar] [CrossRef] [Green Version]
- Tayade, R.J.; Natarajan, T.S.; Bajaj, H.C. Photocatalytic degradation of methylene blue dye using ultraviolet light emitting diodes. Ind. Eng. Chem. Res. 2009, 48, 10262–10267. [Google Scholar] [CrossRef]
- Hasan, M.M.; Haseeb, A.; Saidur, R.; Masjuki, H.H.; Hamdi, M. Influence of substrate and annealing temperatures on optical properties of RF-sputtered TiO2 thin films. Opt. Mater. 2010, 32, 690–695. [Google Scholar] [CrossRef]
- Chung, Y.-L.; Gan, D.-S.; Ou, K.-L.; Chiou, S.-Y. Formation of anatase and TiO from Ti thin film after anodic treatment and thermal annealing. J. Electrochem. Soc. 2011, 158, C319. [Google Scholar] [CrossRef]
- Sekhar, M.C.; Kondaiah, P.; Jagadeesh Chandra, S.V.; Mohan Rao, G.; Uthanna, S. Effect of substrate bias voltage on the structure, electric and dielectric properties of TiO2 thin films by DC magnetron sputtering. Appl. Surf. Sci. 2011, 258, 1789–1796. [Google Scholar] [CrossRef]
- Sekhar, M.C.; Kondaiah, P.; Radha Krishna, B.; Uthanna, S. Effect of oxygen partial pressure on the electrical and optical Properties of DC magnetron sputtered amorphous TiO2 films. J. Spectrosc. 2013, 1, 3–10. [Google Scholar] [CrossRef]
- Horprathum, M.; Eiamchai, P.; Chindaudom, P.; Pokaipisit, A.; Limsuwan, P. Oxygen partial pressure dependence of the properties of TiO2 thin films deposited by DC reactive magnetron sputtering. Procedia Eng. 2012, 32, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Güzelçimen, F.; Tanören, B.; Çetinkaya, Ç.; Kaya, M.D.; Efkere, H.İ.; Özen, Y.; Bingöl, D.; Sirkeci, M.; Kınacı, B.; Ünlü, M.B.; et al. The Effect of thickness on surface structure of Rf sputtered TiO2 thin films by XPS, SEM/EDS, AFM and SAM. Vacuum 2020, 182, 109766. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.-N.; Kumar, R. Formation of Oxygen Vacancies and Ti(3+) State in TiO2 Thin Film and Enhanced Optical Properties by Air Plasma Treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-Ray Photoelectron spectroscopy: Towards reliable binding energy referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- Fakhouri, H.; Arefi-Khonsari, F.; Jaiswal, A.K.; Pulpytel, J. Enhanced visible light photoactivity and charge separation in TiO2/TiN bilayer thin films. Appl. Catal. A Gen. 2015, 492, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Gouttebaron, R.; Cornelissen, D.; Snyders, R.; Dauchot, J.P.; Wautelet, M.; Hecq, M. XPS study of TiOx thin films prepared by Dc magnetron sputtering in Ar–O2 gas mixtures. Surf. Interface Anal. Int. J. Devoted Dev. Appl. Tech. Anal. Surf. Interfaces Thin Film 2000, 30, 527–530. [Google Scholar] [CrossRef]
- Sério, S.; Jorge, M.E.M.; Maneira, M.J.P.; Nunes, Y. Influence of O2 partial pressure on the growth of nanostructured anatase phase TiO2 thin films prepared by DC reactive magnetron sputtering. Mater. Chem. Phys. 2011, 126, 73–81. [Google Scholar] [CrossRef]
- Butt, M.A.; Fomchenkov, S.A. Thermal effect on the optical and morphological properties of TiO2 thin films obtained by Annealing a Ti metal layer. J. Korean Phys. Soc. 2017, 70, 169–172. [Google Scholar] [CrossRef]
- Mechiakh, R.; Meriche, F.; Kremer, R.; Bensaha, R.; Boudine, B.; Boudrioua, A. TiO2 thin films prepared by Sol–Gel method for waveguiding applications: Correlation between the structural and optical properties. Opt. Mater. 2007, 30, 645–651. [Google Scholar] [CrossRef]
- Peerakiatkhajohn, P.; Butburee, T.; Sul, J.-H.; Thaweesak, S.; Yun, J.-H. Efficient and rapid photocatalytic degradation of methyl orange dye using Al/ZnO nanoparticles. Nanomaterials 2021, 11, 1059. [Google Scholar] [CrossRef]
- Wahab, A.K.; Ould-Chikh, S.; Meyer, K.; Idriss, H. On the “Possible” synergism of the different phases of TiO2 in photo-catalysis for hydrogen production. J. Catal. 2017, 352, 657–671. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, Q.; Xia, Y.; Zhan, S.; Li, Y. Understanding the charge separation and transfer in mesoporous carbonate-doped phase-junction TiO2 nanotubes for photocatalytic hydrogen production. Appl. Catal. B Environ. 2018, 225, 433–444. [Google Scholar] [CrossRef]
- Kalarivalappil, V.; Vijayan, B.K.; Kumar, V. Engineering nanocrystalline titania thin films for high photocatalytic activity. Mater. Today Proc. 2019, 9, 621–626. [Google Scholar] [CrossRef]
- Lu, Y.; Matsuzaka, K.; Hao, L.; Hirakawa, Y.; Yoshida, H.; Pan, F.S. Fabrication and photocatalytic activity of TiO2/Ti composite films by mechanical coating technique and high-temperature oxidation. In Proceedings of the ECCM15—15th European Conference on Composite Materials, Venice, Italy, 24–28 June 2012. [Google Scholar]
- Bikondoa, O.; Pang, C.L.; Ithnin, R.; Muryn, C.A.; Onishi, H.; Thornton, G. Direct Visualization of defect-mediated dissociation of water on TiO2. Nat. Mater. 2006, 5, 189–192. [Google Scholar] [CrossRef]
- Minato, T.; Sainoo, Y.; Kim, Y.; Kato, H.S.; Aika, K.; Kawai, M.; Zhao, J.; Petek, H.; Huang, T.; He, W. The electronic structure of oxygen atom vacancy and hydroxyl impurity defects on titanium dioxide (110) surface. J. Chem. Phys. 2009, 130, 124502. [Google Scholar] [CrossRef]
- Shi, H.; Liu, Y.-C.; Zhao, Z.-J.; Miao, M.; Wu, T.; Wang, Q. Reactivity of the defective rutile TiO2 surfaces with two bridging-oxygen vacancies: Water molecule as a probe. J. Phys. Chem. C 2014, 118, 20257–20263. [Google Scholar] [CrossRef]
- Elahifard, M.; Sadrian, M.R.; Mirzanejad, A.; Behjatmanesh-Ardakani, R.; Ahmadvand, S. Dispersion of defects in TiO2 semiconductor: Oxygen vacancies in the bulk and surface of rutile and anatase. Catalysts 2020, 10, 397. [Google Scholar] [CrossRef] [Green Version]
- Mills, A.; Le Hunte, S. An Overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef] [PubMed]
- Stojadinović, S.; Tadić, N.; Radić, N.; Grbić, B.; Vasilić, R. Effect of Tb3+ doping on the photocatalytic activity of TiO2 coatings formed by plasma electrolytic oxidation of titanium. Surf. Coat. Technol. 2018, 337, 279–289. [Google Scholar] [CrossRef]
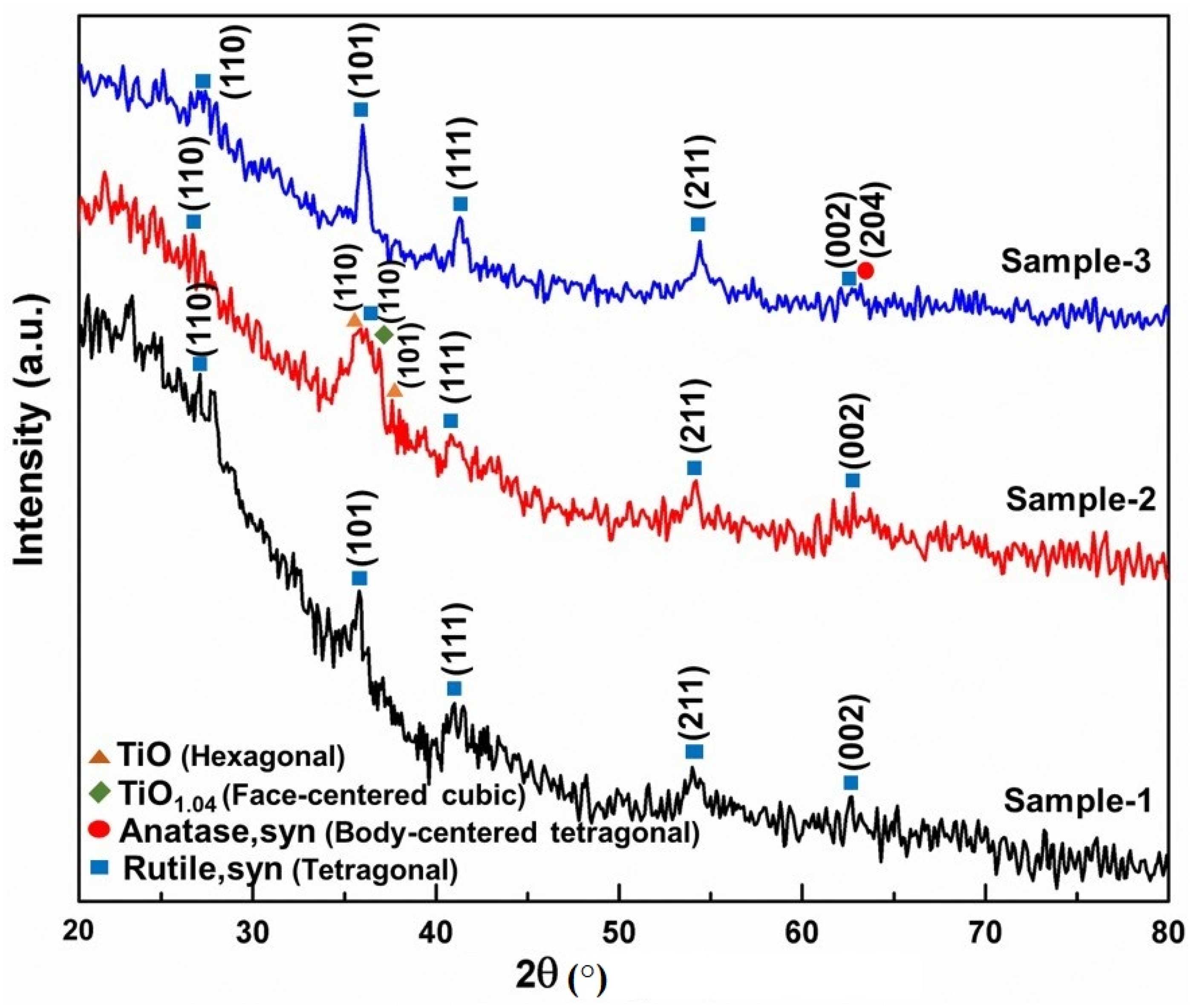
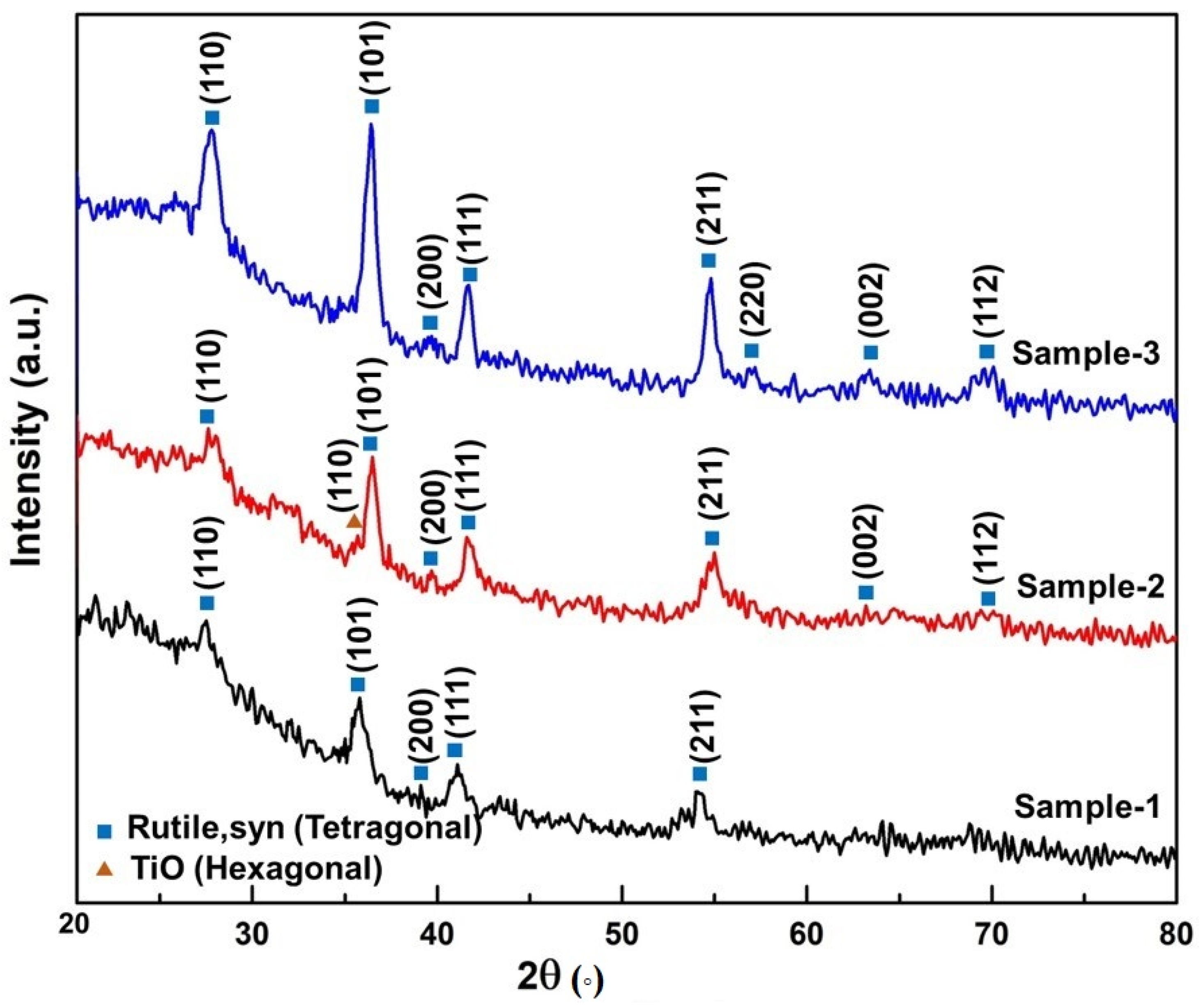
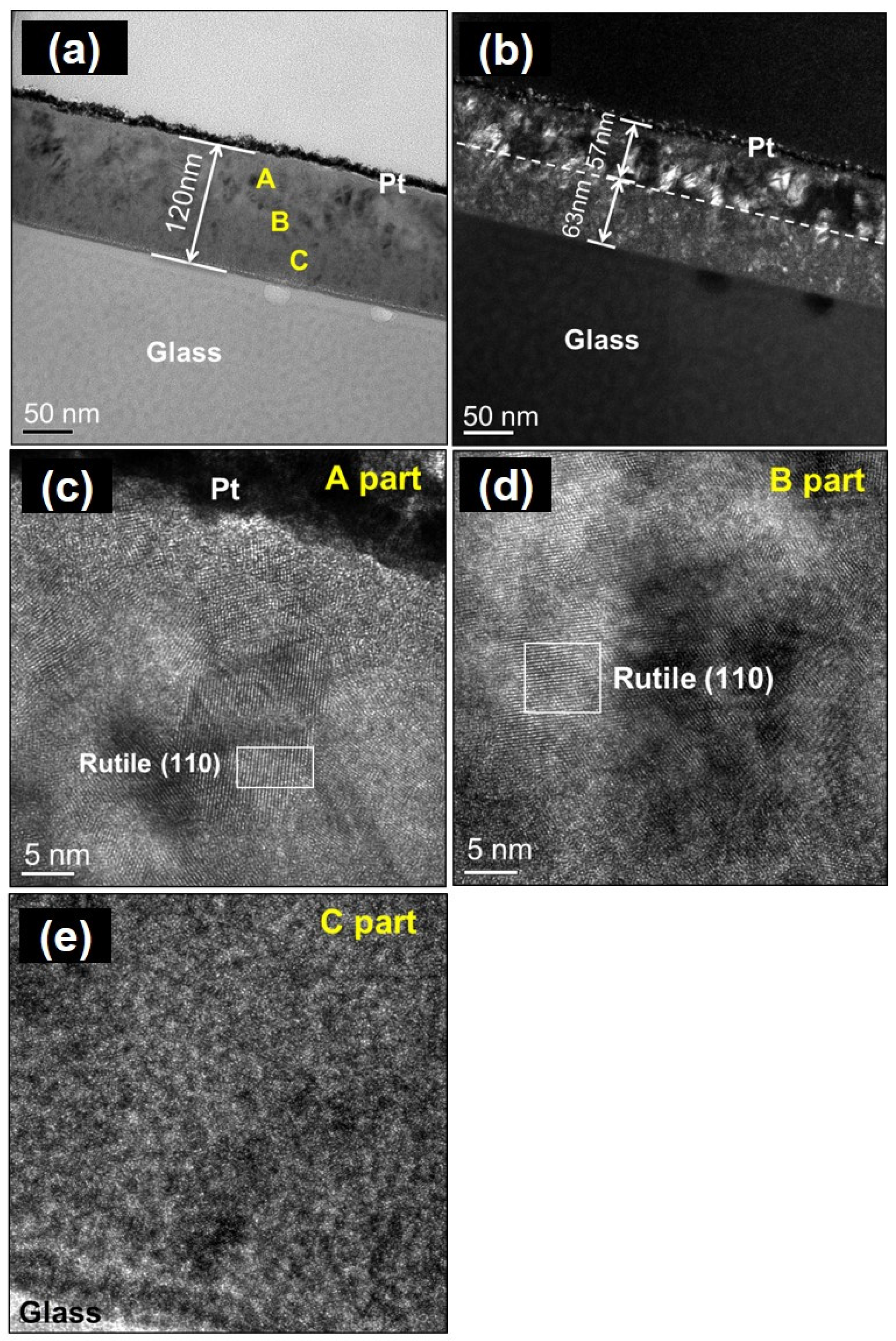
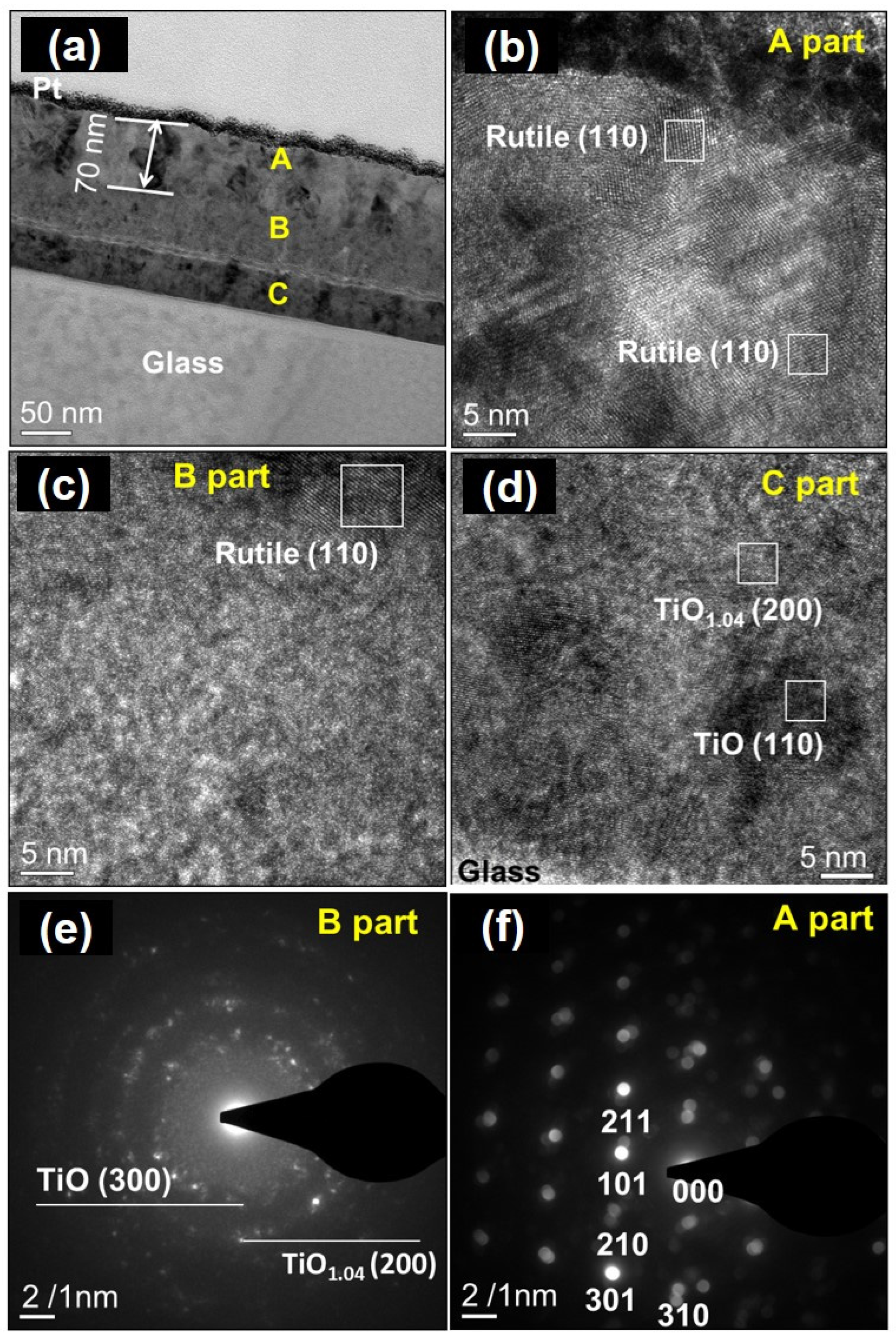
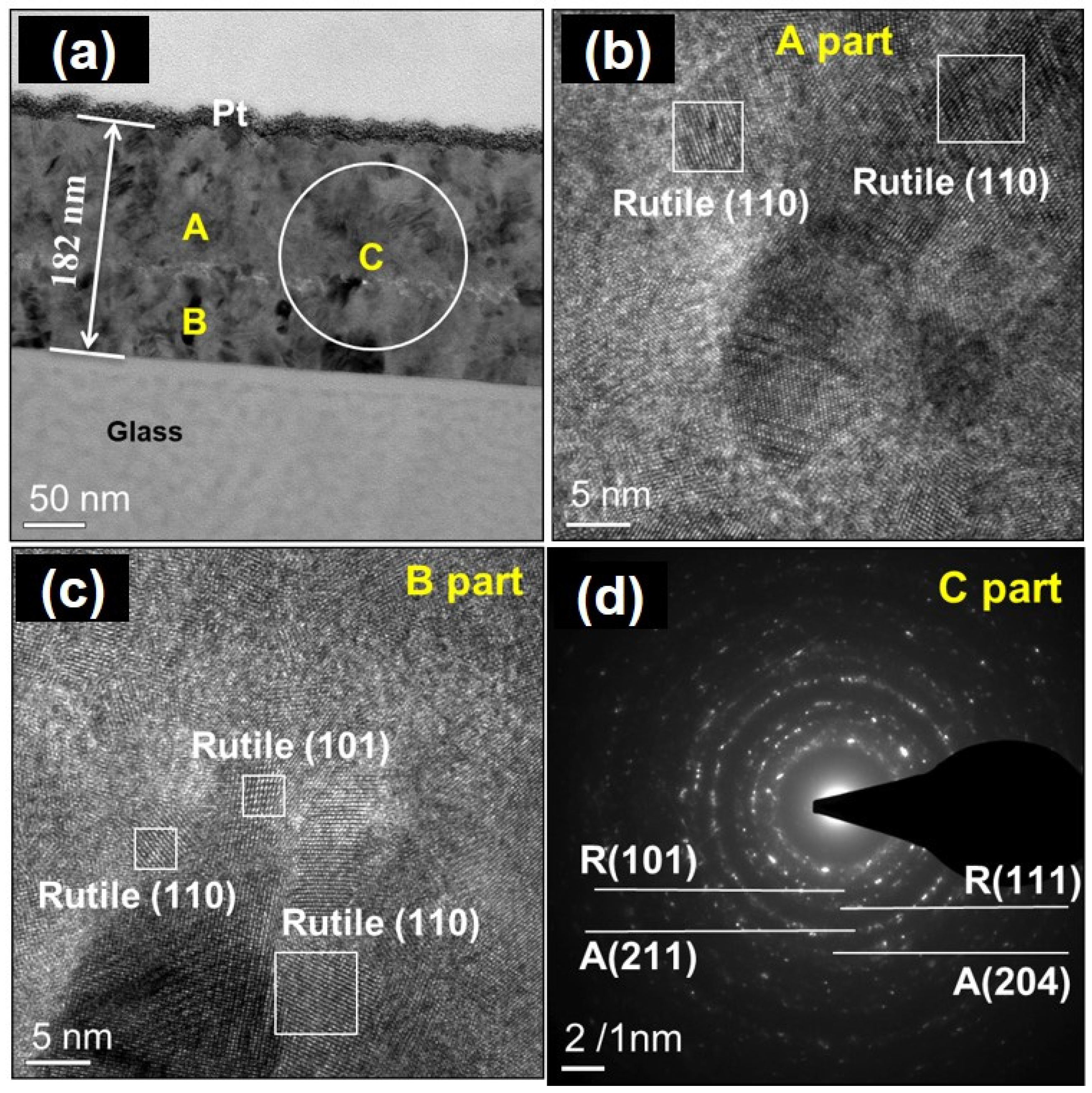
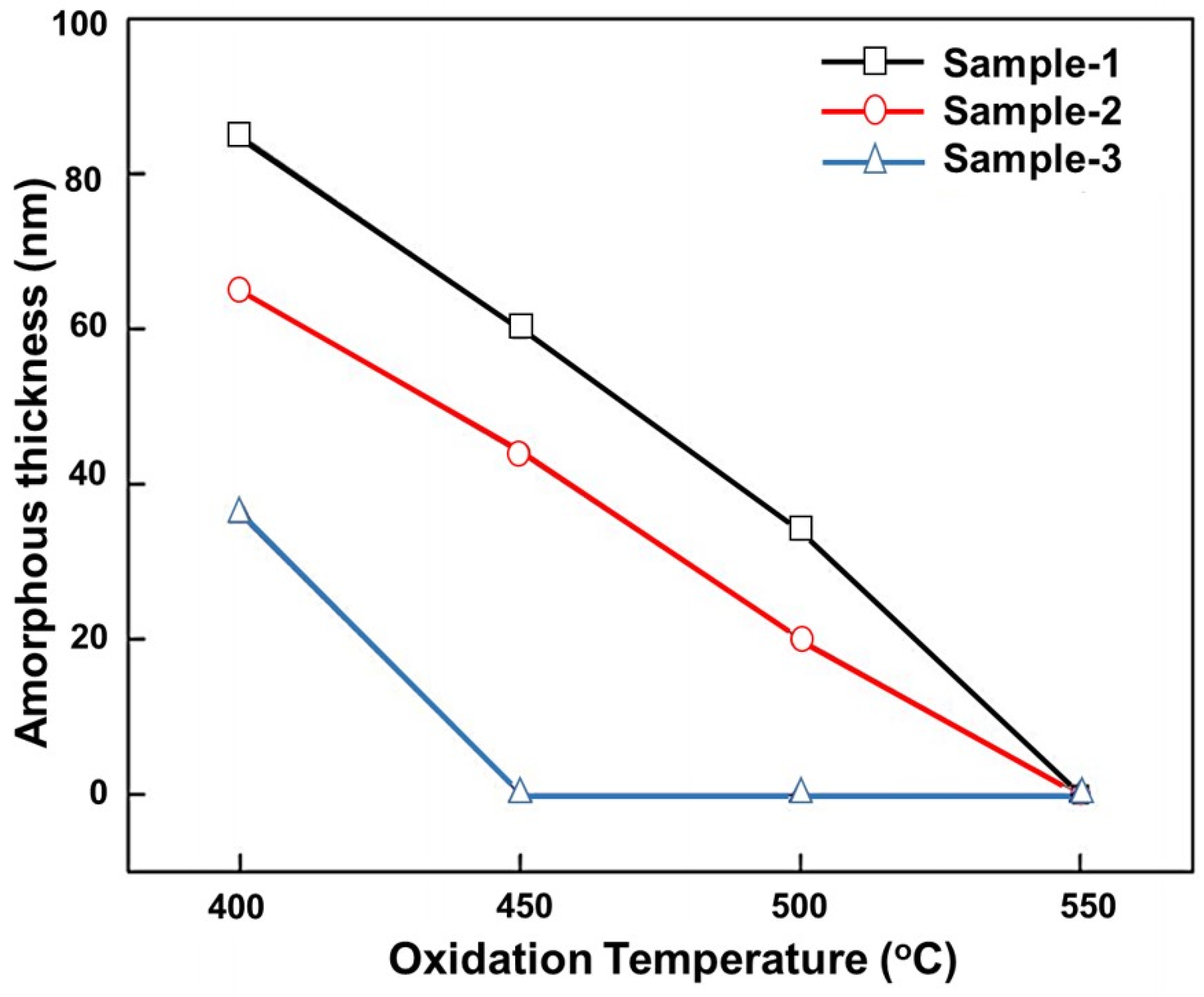
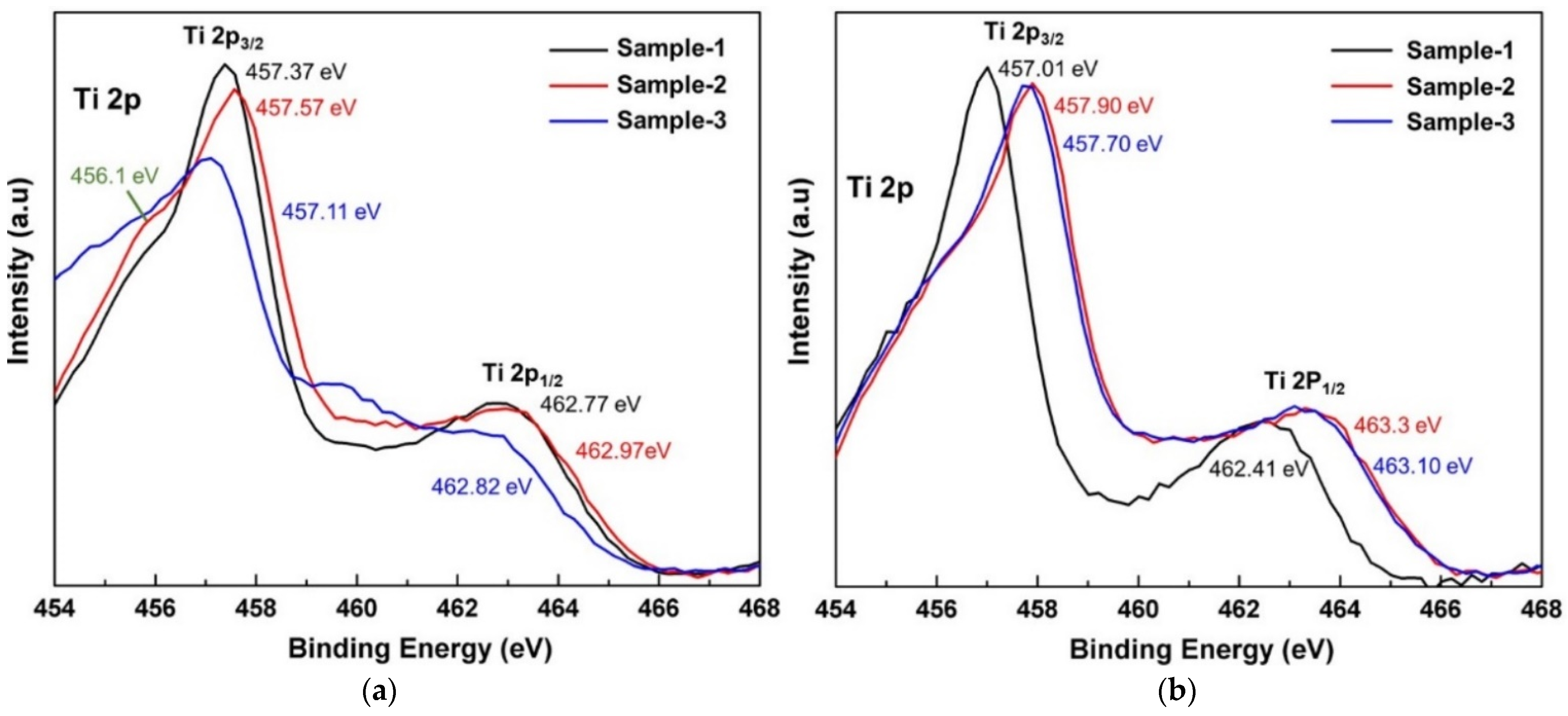
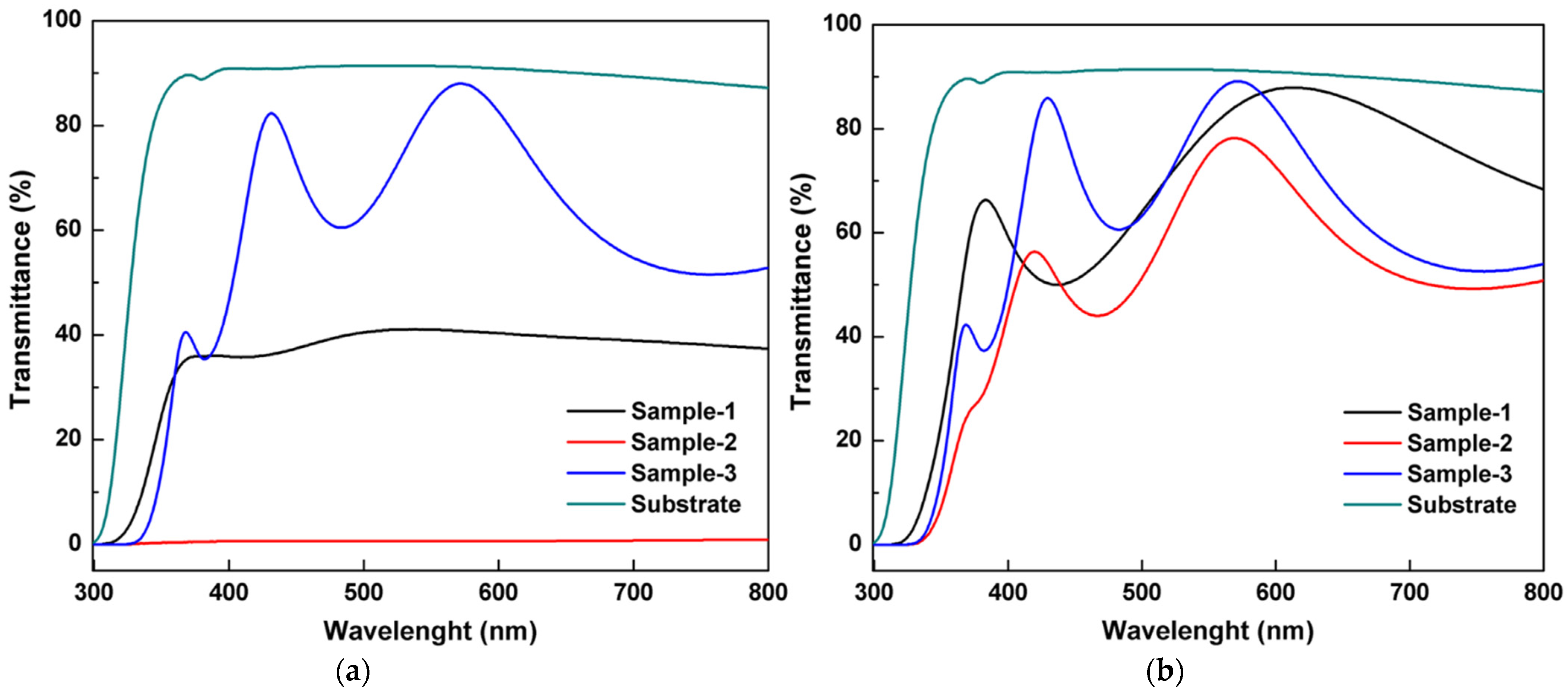
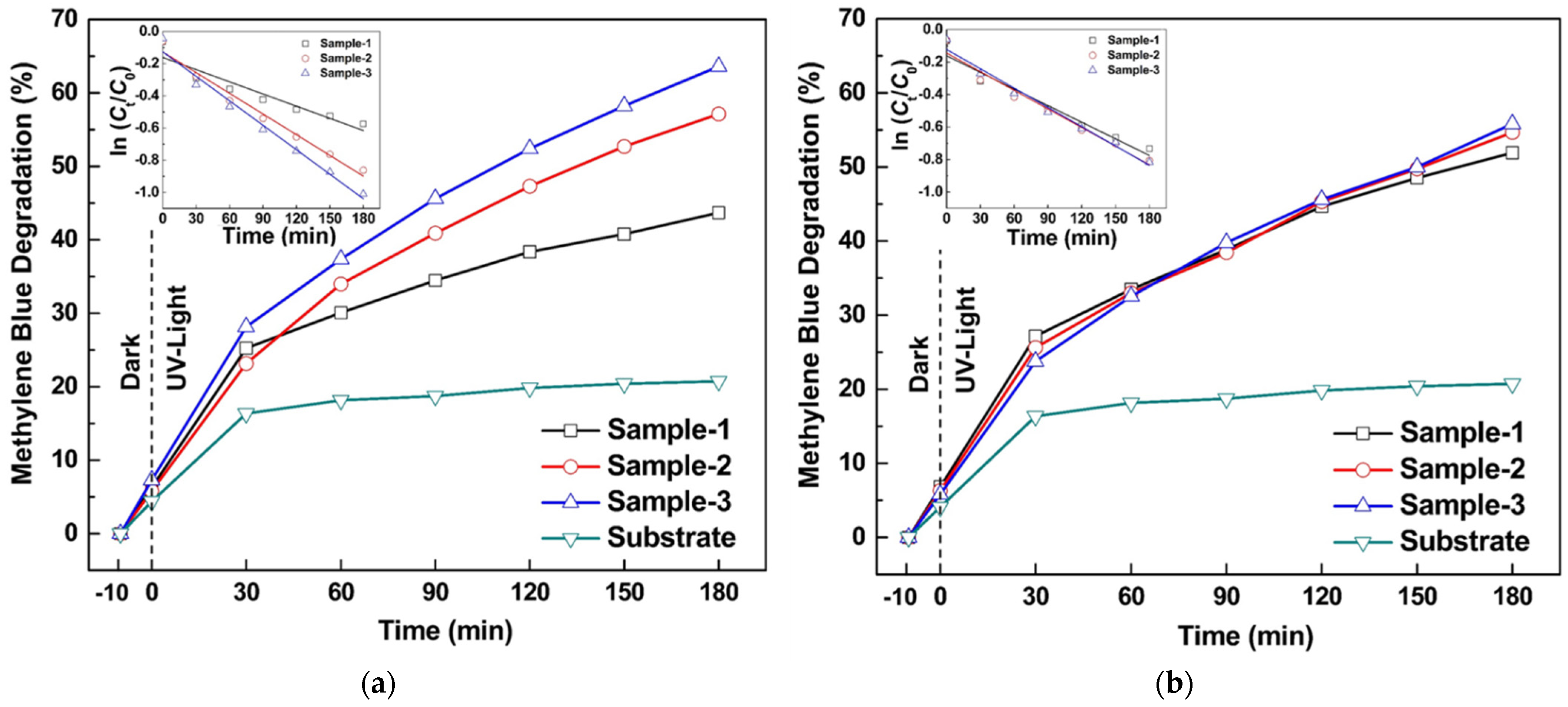
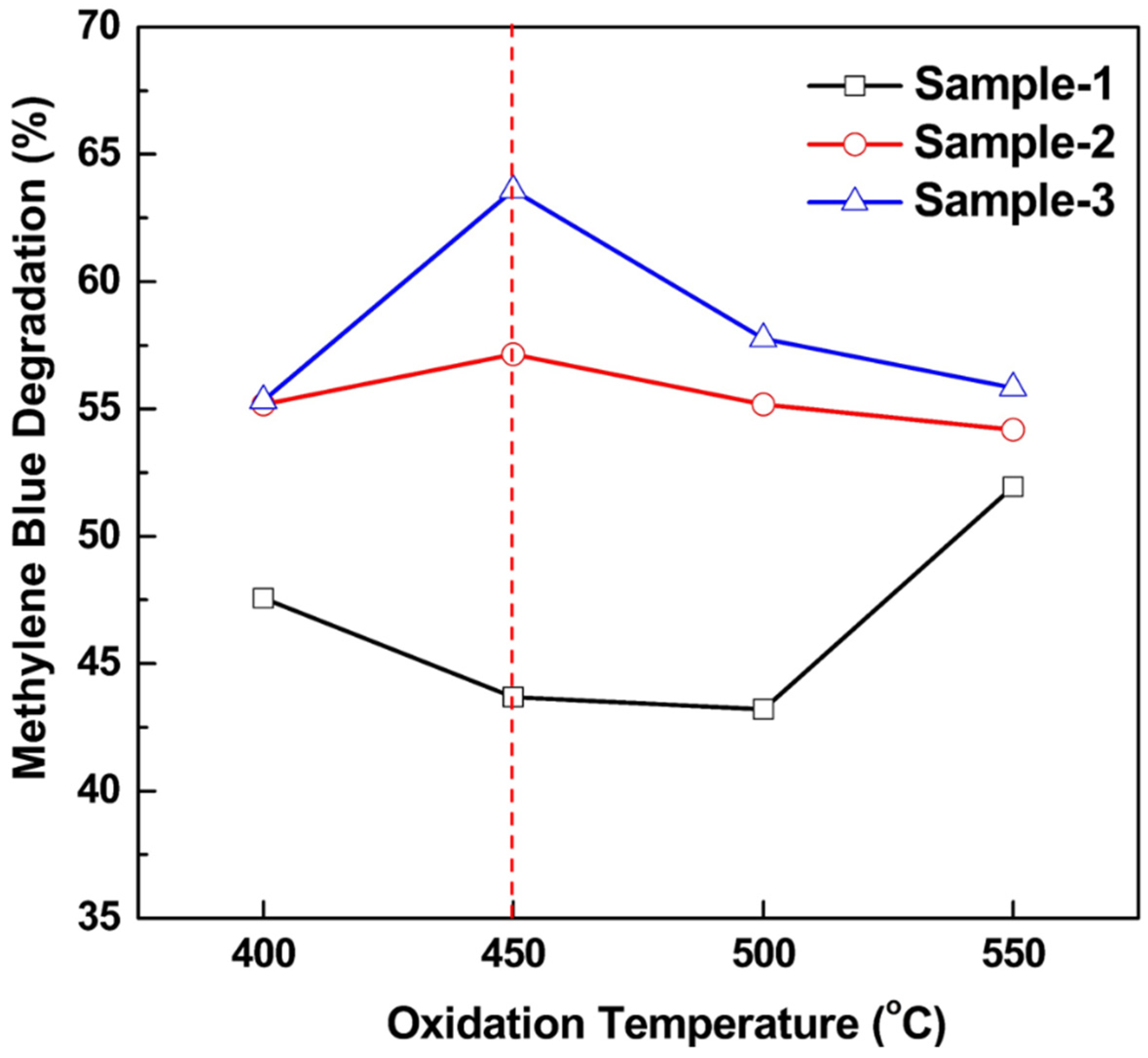
| Oxidation Temperature (°C) | Sample | a (Å) | c (Å) | Unit Cell Volume (Å3) |
|---|---|---|---|---|
| 450 | Sample 1 | 4.5501 | 2.9685 | 61.4581 |
| Sample 2 | 4.6147 | 2.9400 | 62.6086 | |
| Sample 3 | 4.5990 | 2.9649 | 62.7100 | |
| 550 | Sample 1 | 4.5816 | 2.9942 | 62.8510 |
| Sample 2 | 4.5829 | 2.9708 | 62.3964 | |
| Sample 3 | 4.6145 | 2.9734 | 63.3147 |
| Oxidation Temperature (°C) | Element | Atomic Concentration (%) | |||||
|---|---|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | |||||
| a | b | a | b | a | b | ||
| 450 | Ti | 28.34 | 39.10 | 30.47 | 41.22 | 30.70 | 42.60 |
| O | 71.66 | 60.90 | 69.53 | 58.78 | 69.30 | 57.40 | |
| 550 | Ti | 15.46 | 35.56 | 29.91 | 40.01 | 23.46 | 39.96 |
| O | 84.54 | 64.44 | 70.09 | 59.99 | 76.54 | 60.04 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, P.-Y.; Widyastuti, E.; Lin, T.-C.; Chiu, C.-T.; Xu, F.-Y.; Tseng, Y.-T.; Lee, Y.-C. The Phase Evolution and Photocatalytic Properties of a Ti-TiO2 Bilayer Thin Film Prepared Using Thermal Oxidation. Coatings 2021, 11, 808. https://doi.org/10.3390/coatings11070808
Lee P-Y, Widyastuti E, Lin T-C, Chiu C-T, Xu F-Y, Tseng Y-T, Lee Y-C. The Phase Evolution and Photocatalytic Properties of a Ti-TiO2 Bilayer Thin Film Prepared Using Thermal Oxidation. Coatings. 2021; 11(7):808. https://doi.org/10.3390/coatings11070808
Chicago/Turabian StyleLee, Ping-Yuan, Endrika Widyastuti, Tzu-Che Lin, Chen-Tien Chiu, Fu-Yang Xu, Yaw-Teng Tseng, and Ying-Chieh Lee. 2021. "The Phase Evolution and Photocatalytic Properties of a Ti-TiO2 Bilayer Thin Film Prepared Using Thermal Oxidation" Coatings 11, no. 7: 808. https://doi.org/10.3390/coatings11070808
APA StyleLee, P.-Y., Widyastuti, E., Lin, T.-C., Chiu, C.-T., Xu, F.-Y., Tseng, Y.-T., & Lee, Y.-C. (2021). The Phase Evolution and Photocatalytic Properties of a Ti-TiO2 Bilayer Thin Film Prepared Using Thermal Oxidation. Coatings, 11(7), 808. https://doi.org/10.3390/coatings11070808