Development of Indium Titanium Zinc Oxide Thin Films Used as Sensing Layer in Gas Sensor Applications
Abstract
:1. Introduction
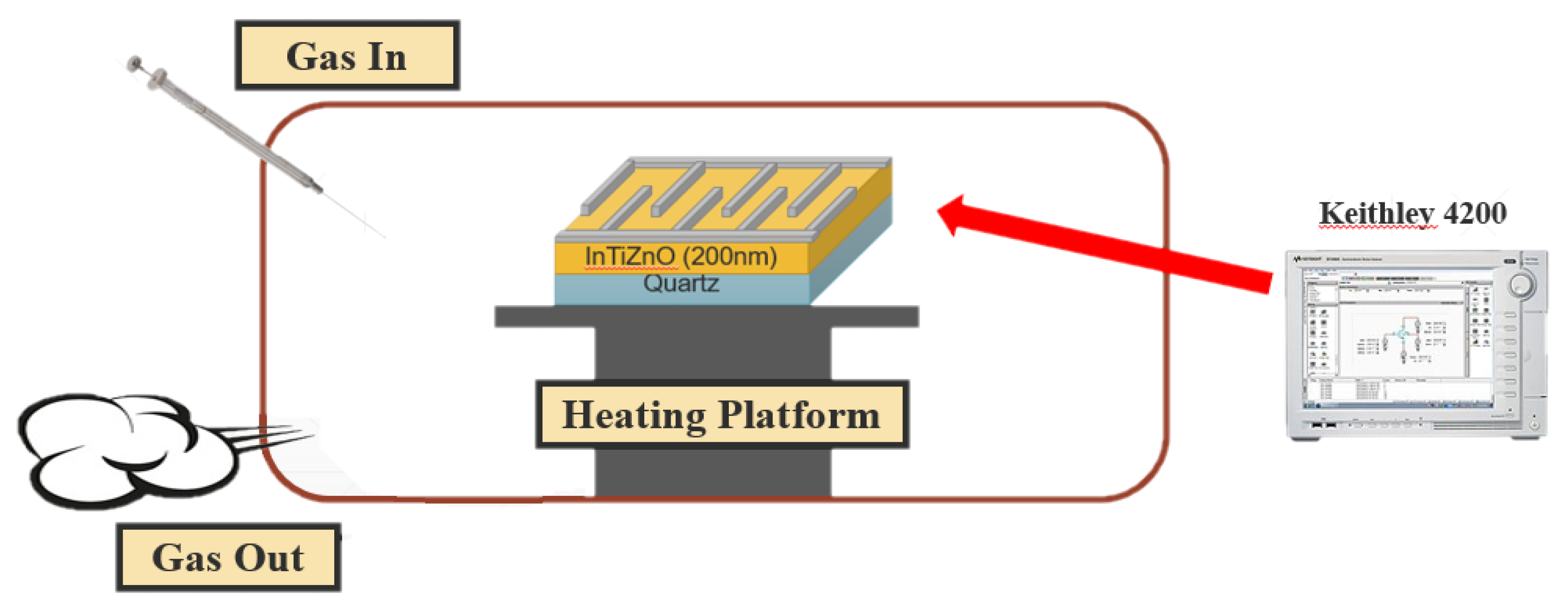
2. Materials and Methods
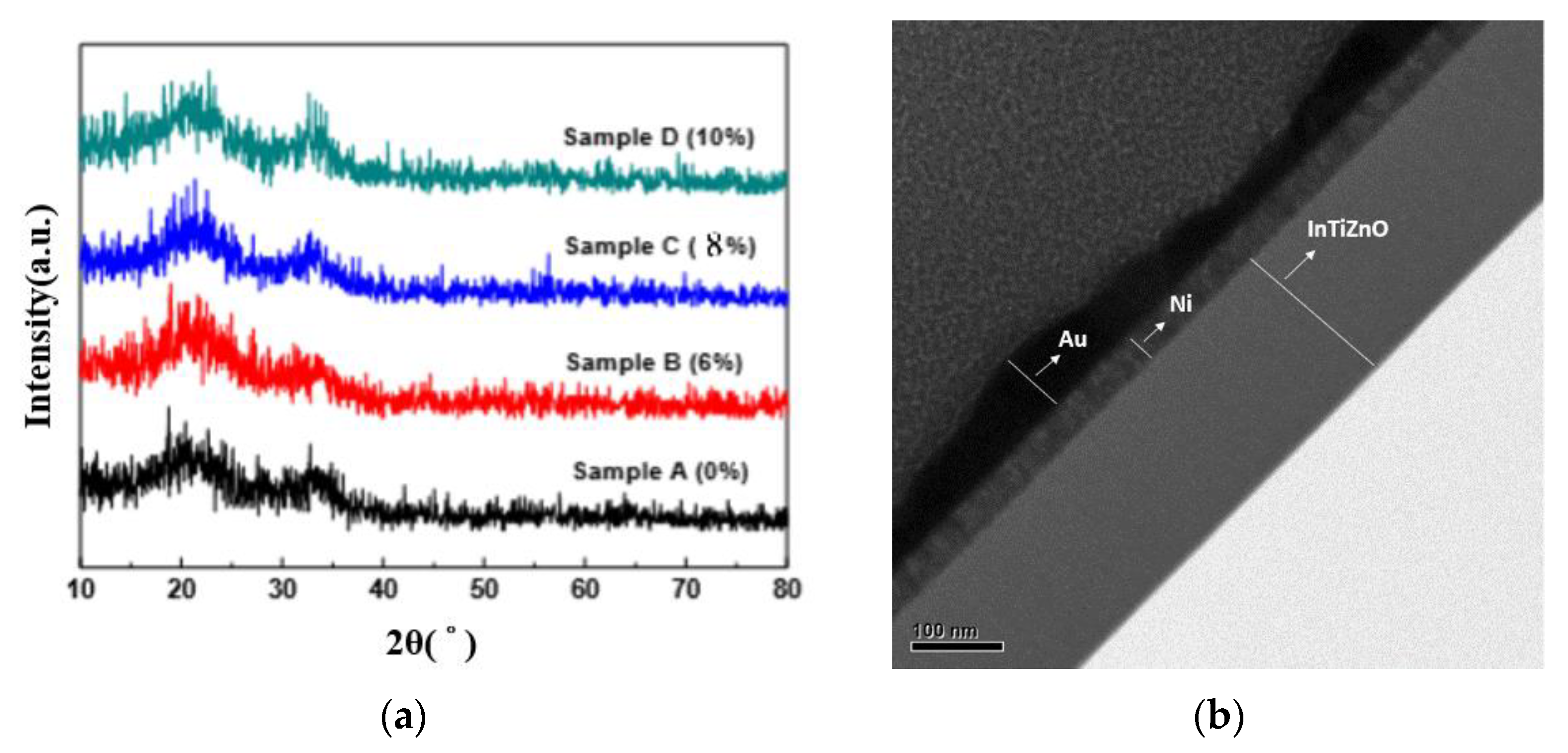
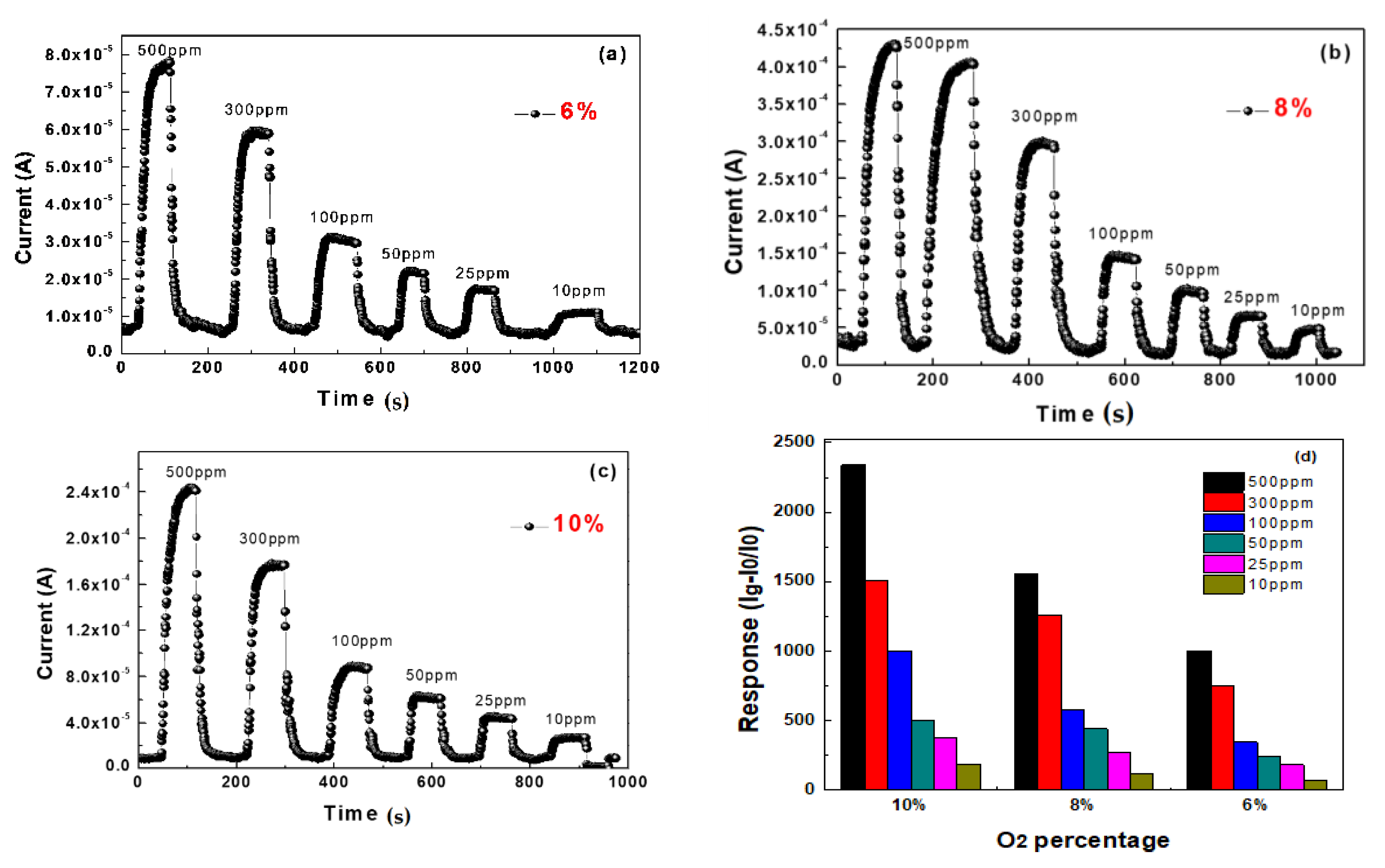
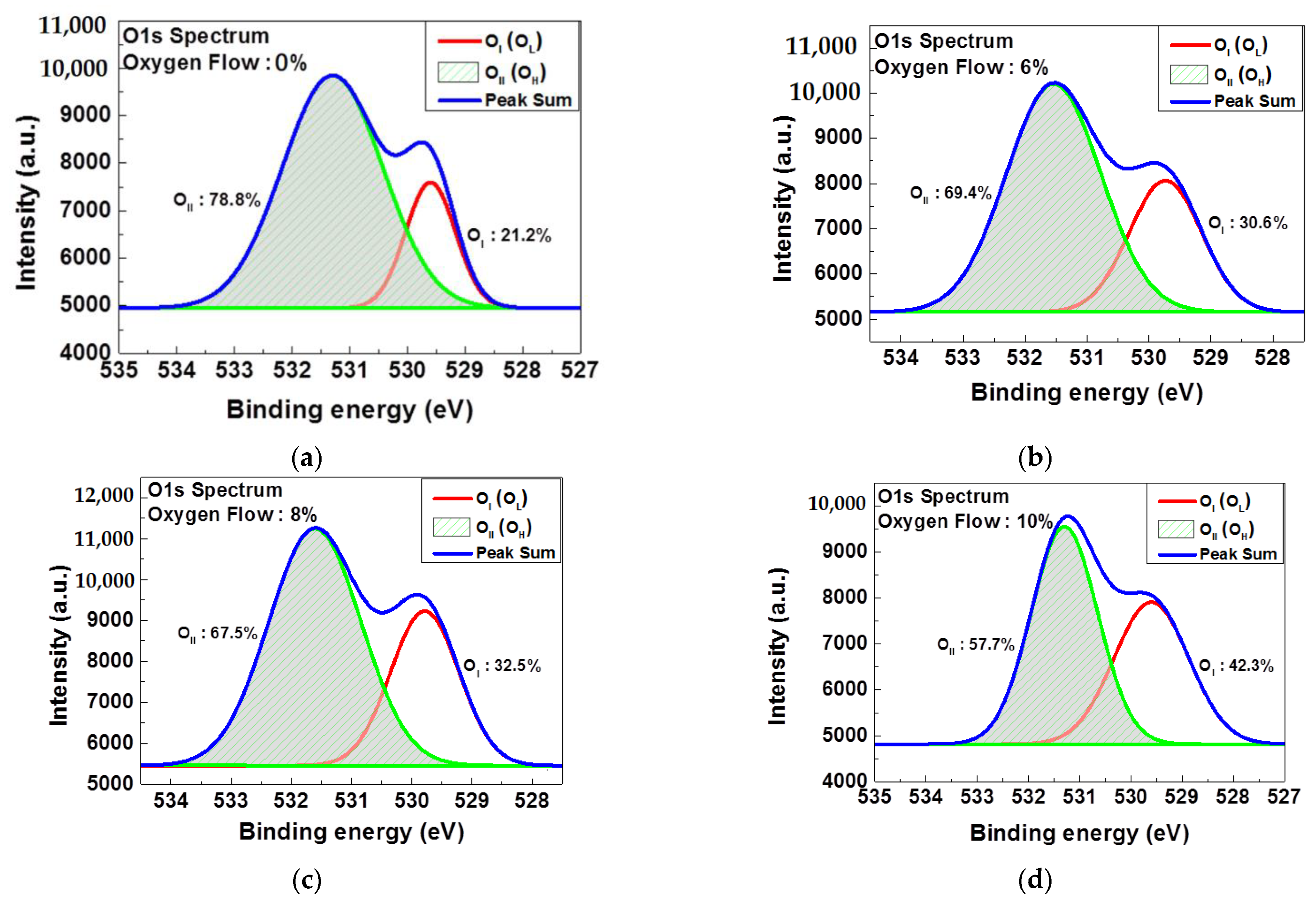
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B Adv. Funct. Solid State Mater. 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Korotcenkov, G. The role of morphology and crystallographic structure of metal oxides in response of conductometric-type gas sensors. Mater. Sci. Eng. R Rep. 2008, 61, 1–39. [Google Scholar] [CrossRef]
- Rieu, M.; Camara, M.; Tournier, G.; Viricelle, J.P.; Pijolat, C.; de Rooij, N.F.; Briand, D. Fully inkjet printed SnO2 gas sensor on plastic substrate. Sens. Actuator B Chem. 2016, 236, 1091–1097. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuator A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Urasinska-Wojcik, B.; Vincent, T.A.; Chowdhury, M.F.; Gardner, J.W. Ultrasensitive WO3 gas sensors for NO2 detection in air and low oxygen environment. Sens. Actuator B Chem. 2017, 239, 1051–1059. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Wang, H.; Xiong, M.; Yang, T.; Zakharova, G.S. Highly sensitive and selective ammonia gas sensors based on PbS quantum dots/TiO2 nanotube arrays at room temperature. Sens. Actuator B Chem. 2016, 236, 529–536. [Google Scholar] [CrossRef]
- Afshar, M.; Preiß, E.M.; Sauerwald, T.; Rodner, M.; Feili, D.; Straub, M.; Seidel, H. Indium-tin-oxide single-nanowire gas sensor fabricated via laser writing and subsequent etching. Sens. Actuator B Chem. 2015, 215, 525–535. [Google Scholar] [CrossRef]
- Behera, B.; Chandra, S. An innovative gas sensor incorporating ZnO-CuO nanoflakes in planar MEMS technology. Sens. Actuator B Chem. 2016, 229, 414–424. [Google Scholar] [CrossRef]
- Lee, K.I.; Kim, E.K.; Kim, H.D.; Kang, H.I.; Song, J.T. Low temperature Al doped ZnO films on a flexible substrate by DC sputtering. Phys. Status Solidi C 2008, 5, 3344–3347. [Google Scholar] [CrossRef]
- Minami, T. Transparent conducting oxide semiconductors for transparent electrodes. Semicond. Sci. Technol. 2005, 20, S35–S44. [Google Scholar] [CrossRef]
- Nomura, K.; Kamiya, T.; Ohta, H.; Hirano, M.; Hosono, H. Defect passivation and homogenization of amorphous oxide thin-film transistor by wet O2 annealing. Appl. Phys. Lett. 2008, 93, 192107. [Google Scholar] [CrossRef]
- Liu, A.; Liu, G.X.; Shan, F.K.; Zhu, H.H.; Shin, B.C.; Lee, W.J.; Cho, C.R. High-performance InTiZnO thin-film transistors deposited by magnetron sputtering. Chin. Phys. Lett. 2013, 30, 127301. [Google Scholar] [CrossRef]
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Kim, T.W. Recent advances in energy-saving chemiresistive gas sensors: A review. Nano Energy 2021, 79, 105369. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Chang, L.F.; Hsueh, T.J. Light-activated humidity and gas sensing by ZnO nanowires grown on LED at room temperature. Sens. Actuator B Chem. 2017, 249, 265–277. [Google Scholar] [CrossRef]
- Dhahri, R.; Hijiri, M.; Mir, L.E.; Banavita, A.; Iannazzo, D.; Latino, M.; Donato, N.; Neri, G. Gas sensing properties of Al-doped ZnO for UV-activated CO detection. J. Phys. D Appl. Phys. 2016, 49, 135502. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, Y.; Xie, C.S.; Wu, J.; Zheng, D.W.; Liao, Y.C. A comparative study on UV light activated porous TiO2 and ZnO film sensors for gas sensing at room temperature. Ceram. Int. 2012, 38, 503–509. [Google Scholar] [CrossRef]
- Gui, Y.; Li, S.; Xu, J.; Li, C. Study on TiO2-doped ZnO thick film gas sensors enhanced by UV light at room temperature. Microelectron. J. 2008, 39, 1120–1125. [Google Scholar] [CrossRef]
- Gong, J.; Li, T.; Zhai, X.; Hu, Z.; Deng, Y. UV-light-activated ZnO fibers for organic gas sensing at room temperature. J. Phys. Chem. C 2010, 114, 1293–1298. [Google Scholar] [CrossRef]
- Hsueh, T.J.; Chang, S.J.H.; Hsu, C.L.; Lin, Y.R.; Chen, I.C. Highly sensitive ZnO nanowire ethanol sensor with Pd adsorption. Appl. Phys. Lett. 2007, 91, 053111. [Google Scholar] [CrossRef] [Green Version]
- Hsueh, T.J.; Hsu, C.L.; Chang, S.J.; Chen, I.C. Laterally grown ZnO nanowire ethanol gas sensors. Sens. Actuator B Chem. 2007, 126, 473–477. [Google Scholar] [CrossRef]
- Hsueh, T.J.; Chang, S.J.; Hsu, C.L.; Lin, Y.R.; Chen, I.C. ZnO nanotube ethanol gas sensors. J. Electrochem. Soc. 2008, 155, K152–K155. [Google Scholar] [CrossRef]
- Giessibl, F.J. Principle of NC-AFM. In Noncontact Atomic Force Microscopy; Springer: Berlin/Heidelberg, Germany, 2002; pp. 11–46. [Google Scholar]
- Wang, C.X.; Yin, L.W.; Zhang, L.Y.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [Green Version]

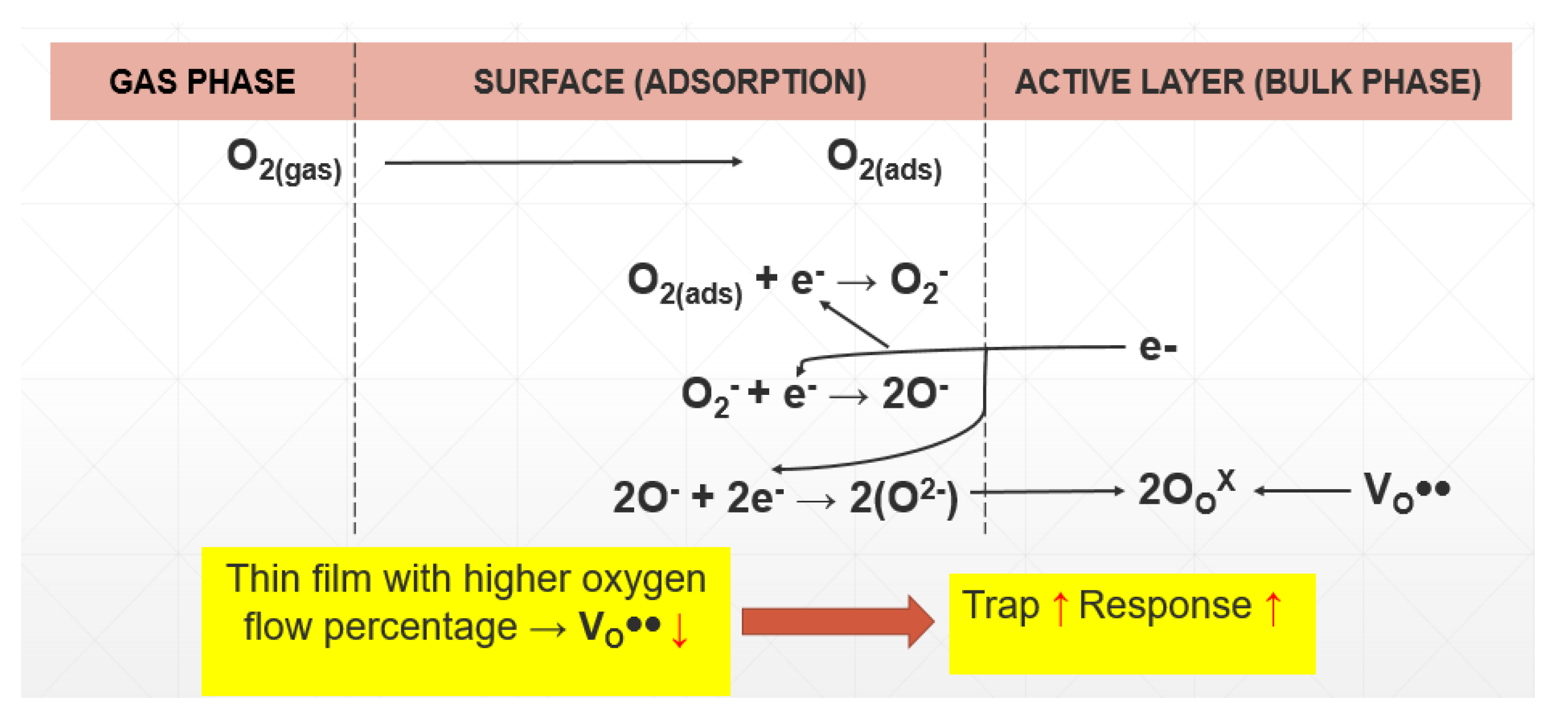

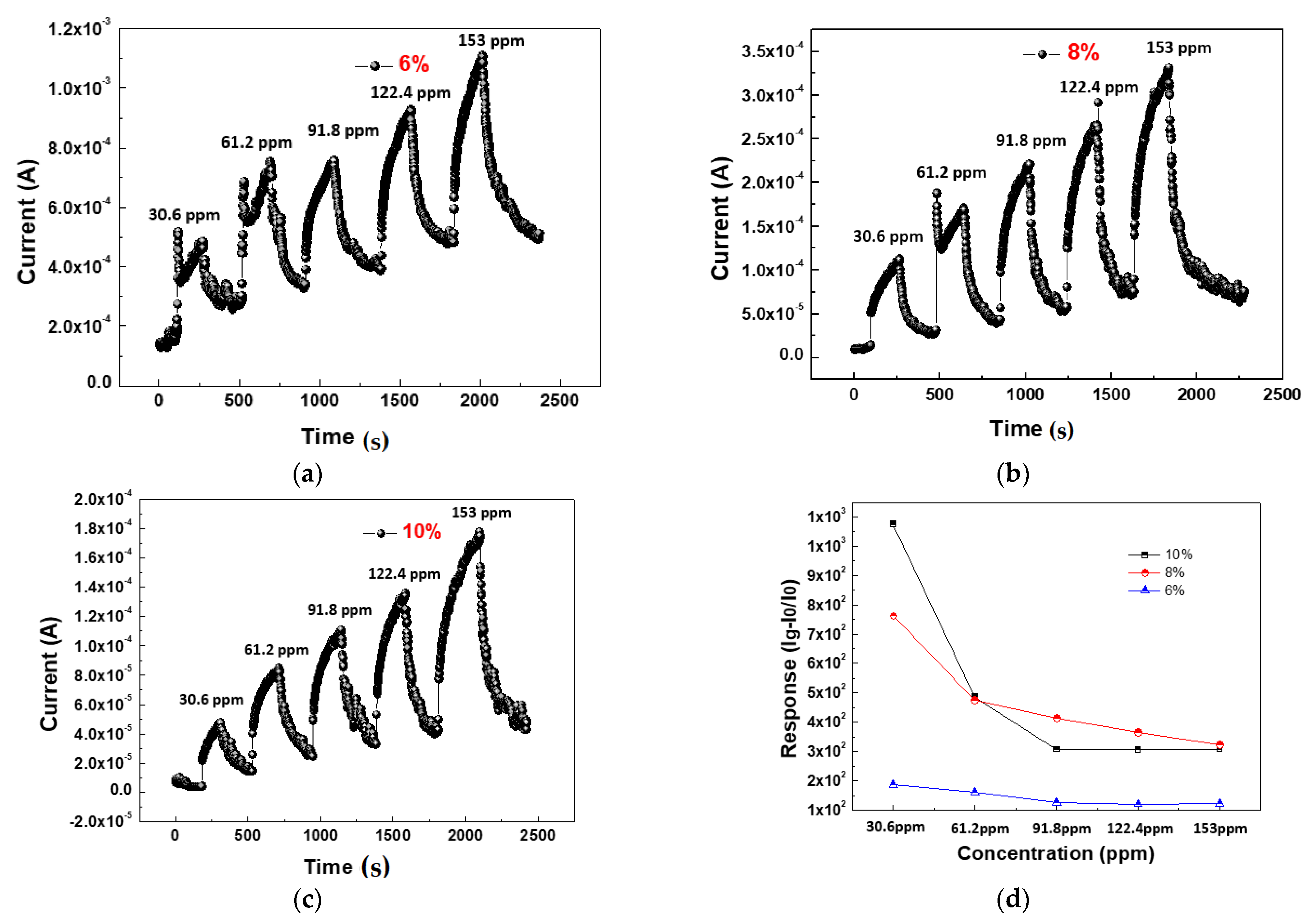

| SO2 /Oxygen Flow | 30.6 ppm | 61.2 ppm | 91.8 ppm | 122.4 ppm | 153 ppm |
|---|---|---|---|---|---|
| 10% | 1075 | 486 | 307 | 306 | 307 |
| 8% | 762 | 475 | 415 | 365 | 323 |
| 6% | 186 | 160 | 125 | 120 | 122 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.-P.; Yang, R.-H.; Lin, C.-H. Development of Indium Titanium Zinc Oxide Thin Films Used as Sensing Layer in Gas Sensor Applications. Coatings 2021, 11, 807. https://doi.org/10.3390/coatings11070807
Chang S-P, Yang R-H, Lin C-H. Development of Indium Titanium Zinc Oxide Thin Films Used as Sensing Layer in Gas Sensor Applications. Coatings. 2021; 11(7):807. https://doi.org/10.3390/coatings11070807
Chicago/Turabian StyleChang, Sheng-Po, Ren-Hao Yang, and Chih-Hung Lin. 2021. "Development of Indium Titanium Zinc Oxide Thin Films Used as Sensing Layer in Gas Sensor Applications" Coatings 11, no. 7: 807. https://doi.org/10.3390/coatings11070807
APA StyleChang, S.-P., Yang, R.-H., & Lin, C.-H. (2021). Development of Indium Titanium Zinc Oxide Thin Films Used as Sensing Layer in Gas Sensor Applications. Coatings, 11(7), 807. https://doi.org/10.3390/coatings11070807







