Abstract
In this study we present an NIR carbon dioxide gas sensor based on an inner filter process that includes an ionic liquid (IL), 1-ethyl-3-methylimidazolium tetrafluoroborate (EMIMBF4), to improve its stability, dynamic behavior and lifetime, which are usually the main drawbacks with these sensors. The presence of CO2 causes a displacement of a simple boron-dipyrromethene-type fluorophore, azaBODIPY, as the pH indicator towards its acid form. This increases the emission intensity of Cr(III)-doped gadolinium aluminium borate (GAB) as the luminophore. The characterization of the prepared sensor was carried out and a discussion of the results is presented. The response and recovery times improved considerably, 23 and 49 s, respectively, with respect to the sensor without IL, at 60 and 120 s, respectively,. Additionally, the measurement range is extended when using IL, able in this case to measure in the complete range up to 100% CO2; without IL the measurement range is limited to 60% CO2. The detection limit ranges from 0.57% CO2 without IL to 0.26% CO2 when IL is added. The useful lifetime of the sensing membrane was 20 days for membranes with IL and only 6 days for membranes without IL, with the sensor always kept in the dark and without the need to maintain a special atmosphere.
1. Introduction
So-called green solvent ionic liquids are salts that remain as a liquid at temperatures below 100 °C, specifically composed of a combination of organic cations and organic/inorganic anions. They present a wide liquid range together with good characteristic properties: high thermal and chemical stability, low volatility, significant electrical conductivity, high polarity, and great to dissolve an extensive range of compounds [1]. This makes them suitable for a wide variety of applications in different areas: catalytic organic transformations [2], such as lubricants; heat transfer fluids in biocatalysis [3]; biomedical and pharmaceutical applications [3]; separation and extraction processes [4]; as well as biotechnology [5] and nanoscience [6], as ideal electrolytes for use in a great number of electrochemical devices [7] such as batteries, capacitors, fuel cells, and solar cells [8]; biomolecule sensing [9,10]; explosives [11]; the determination of gas compounds [11,12]; and the development of sensors [13,14,15].
One of the most highly studied gases is carbon dioxide, because of the increasing levels found in the atmosphere owing to the increase in industrial activity. Carbon dioxide occurs in the combustion of fossil fuels and is considered one of the gases that contributes to the global warming of the planet. This makes the accurate, continuous monitoring of CO2 levels a matter of necessity [16]. It is also important in medicine [17], clinical and biological research [18], industrial and biotechnological processes [19,20], and in intelligent packaging [21]. In this respect, the growth of sensors with raised sensitivity and selectivity capable of detecting and quantifying CO2 gas reversibly and economically is highly useful.
Ionic liquids have more CO2 holding capacity than polymeric materials, and CO2 can be reversibly dissolved in imidazolium-based ionic liquids [22]. Ionic liquid-based CO2 optical sensors [23] and electrochemical sensors [24] have been developed. CO2 is reversibly soluble in imidazolium-based ionic liquids [25], and this gas is more soluble than ethane, methane, oxygen, nitrogen, argon, hydrogen and carbon monoxide in 1-ethyl-3-methylimidazolium tetrafluoroborate (EMIMBF4) [26].
EMIMBF4 has been used in CO2 gas sensors in different ways, for example, with filled photonic crystal fibres to detect the presence of CO2 by optical transmission [27] with an ion pair formed of 1-hydroxy-3,6,8-pyrenetrisulfonate (HPTS) in an ethyl cellulose matrix to provide longer storage time, enhanced repeatability and dynamic range. However, the sensor has relatively lower sensitivity, between 0 and 5% CO2, so it is unsuitable for monitoring CO2 in physiologically relevant [28], and the high response and recovery time, in the order of minutes [29]. Recently our research group found an improvement that involves including ionic liquids in sensing membranes for the determination of gaseous carbon dioxide in fluorescent optical sensors [30].
Two types of sensors are commercially available for the determination and continuous monitoring of CO2. The first is based on metal oxide semiconductors, which are relatively inexpensive compared with other sensing technologies, offering fast and robust responses, although most of these sensors have a working range from 2000 to 10,000 ppm. Responses to low CO2 concentrations are required in many applications, such as environmental operations, disease control and diagnosis and smart packaging [31]. The second employs non-dispersive infrared sensors (NDIR) [32]. The main limitation with this type of sensor is that the miniaturization possibilities are limited when a measurement using very small volumes is needed.
Optical CO2 sensors are a promising alternative for portable devices, as they have a comparably small sensing component. Additionally, the optoelectronic reader can be separate, increasing the possible applications. One of the problems with optical sensors for CO2 is the response and recovery time, which are usually high. In this article, this problem is addressed through the introduction of an ionic liquid in the optical membrane that works in the near infrared region [33], reducing these times. The measurement in the NIR region has a number of advantages, including low light scattering, a drastic reduction in autofluorescence, the availability of excitation sources and low-cost photodetectors [34]. Moreover, including an ionic liquid improves the useful life of the sensor and the response and recovery time. In this way, sensors are obtained that increased the sensitivity of CO2 monitoring, using lower amounts of reagents. Subsequently, they can be included in devices adapted for applications in the environment, clinics, and in smart packaging.
2. Experimental Section
2.1. Reagents and Materials
Hydroxypropyl methylcellulose (HPMC, Methocel E-5, LV USP/EP premium grade) (Dow Chemical Iberia S.L., Tarragona, Spain), 9004-65-3, was used as the membrane polymer. 1-Ethyl-3-methylimidazolium tetrafluoroborate (EMIMBF4), 143314-16-3, was used as IL; tetramethylammonium hydroxide pentahydrate (TMAOH), 10424-65-4, as the phase transfer agent; and Tween 20, 9005-64-5, as the surfactant, all sourced from Sigma. Microcrystalline Cr(III)-doped gadolinium aluminium borate (GAB) powder was synthesized according to [35] using the solution combustion technique, and azaBODIPY was synthesized according to [36,37] and characterized according to [33]. Sheets of Mylar-type polyester from Goodfellow (Cambridge, UK) were used as the support for the membranes.
The standard mixtures to characterize the sensing membranes were prepared using N2 as the diluting gas, controlling the flow rates of the high purity CO2 and N2 gases that enter the mixing chamber with computer-controlled mass flow controllers (Air Liquide España S.A., Madrid, Spain). The system worked at a total pressure of 760 Torr and a flow rate of 500 cm3·min−1.
2.2. Sensing Membrane Preparation
The sensing membrane for CO2 contains 0.5 mg azaBODIPY dissolved in 0.5 mL of ethanol, 1 mL of HPMC 1% in water, 5 µL of Tween 20, 5 µL of a TMAOH solution containing 14 mg TMAOH in 1 mL 1:1 ethanol:water, 6 µl IL, and 1.5 mg GAB.
The CO2 sensor consists of only one membrane prepared by casting 25 µL of the solution indicated above on a Mylar support using a spin-coating technique and then, adding 1.5 mg of GAB, leaving the microcrystals of the salt retained on the surface of the sensing zone. The membrane was then left to dry in the darkness in a box for 6 h at room temperature (20 °C) and humidity (55% relative humidity (RH)). The prepared membranes have a calculated thickness approximately of 2 µm.
2.3. Instrumentation
Cary Eclipse Varian Inc. (Palo Alto, CA, USA) luminescence spectrometer steady-state luminescence measurements were used as the analytical parameter to study the CO2 sensing membranes. A homemade cell holder composed of two metallic triangular prisms was used to make the measurement [38] that supports the membrane, measuring the membrane by reflection at a 60° angle in the cell. This is because sensing membranes that include solid GAB show low transparency. The fluorescence of the membranes was measured with excitation and emission slit widths of 2.5 and 5 nm, λex = 620 nm and λem = 731 nm, respectively. All measurements were made in triplicate to check for experimental errors.
In order to prepare the gas standard mixtures of CO2 gas to characterize the membranes were used with computer-controlled mass flow controllers (Air Liquide España S.A.), operating at a total pressure of 760 Torr and a gas flow rate between 100 and 500 cm3·min−1, controlling the flow rates of the high purity CO2 and N2 (as the diluting gas) gases that enter the mixing chamber. A standard of 5% CO2 in nitrogen was used to prepare the gas mixtures with a CO2 concentration lower than 0.4%, with the lowest CO2 concentration tested being 0.02%.
Temperature and humidity have an appreciable effect on the sensitivity of CO2 sensors based on its acid-base character [39].
In order to establish different humidity conditions (from 10% to 100% RH), a controlled evaporation and mixing system (CEM) was used, consisting of a CEM three-way mixing valve and evaporator for control of the liquid source, a mass flow meter for liquid (MiniCoriflow) with a range from 0.4 to 20 g·h−1 of liquid (water in this case), and a mass flow for measurement and control of the carrier gas flow (N2 gas). The liquid is mixed with the carrier gas flow, and both are heated with a temperature controlled heat-exchanger, resulting in the total complete evaporation of the liquid (100 °C was selected for water).
3. Results and Discussion
3.1. CO2 Sensing Scheme
All the reagents are dissolved in an aqueous solution of HPMC as the polymeric matrix. The luminophore, GAB, is excited in the red (λexc. 620 nm in the HPMC membrane), showing a broad emission in the NIR (λems. 731 nm in the HPMC membrane). Its luminescence decay time is about 85 µs and it shows high chemical and photochemical inertness. The pH indicator is an azaBODIPY with a pKa value suitable for gaseous CO2 determination. Also included is a hydrophobic quaternary ammonium hydroxide, in this case TMAOH, which acts as an internal buffering system to turn the pH indicator into the deprotonated form, stabilized as a hydrated ion pair:
where is the ion pair formed between the tetramethylammonium cation and the deprotonated form of azaBODIPY, and K is the equilibrium constant. In the presence of CO2 gas, the protonated form of the azaBODIPY is formed, reducing the attenuation of the GAB luminescence, with an increase in the luminescent signal. The overlapping between the GAB spectrum (donor) and the azaBODIPY spectrum (acceptor) emission was reduced by the increase in the CO2 concentration, increasing the fluorescent signal.
The working mechanism is based on an inner filter effect [33] similar to the sensor without IL because there was no change in the decay time within the full range of CO2 concentrations. Consequently, the presence of IL in the sensing membrane fundamentally contributes to improving the sensitivity of the sensor and improving its characteristics [30].
3.2. Membrane Composition
The CO2 sensing ability of these membranes is based on the luminescence quenching of a luminophore by one of the forms of an azaBODIPY-type non-fluorescent pH indicator present in a membrane that also contains TMAOH acting as an internal buffer. The change in the position of the acid-base equilibrium by the CO2 and the concomitant change in colour result in a change in luminescence emission due to the overlap of the absorption spectrum of the basic form of the pH indicator and the emission of the luminescent GAB. CO2 diffuses through the HPMC, dissolves and reacts with TMAOH, and gradually displaces the equilibrium of the indicator towards its acid form with a concomitant increase in the phosphorescent emission of GAB by an inner filter process [33].
Of the known ionic liquids, EMIMBF4 was selected for its good solubility in aqueous media and good characteristics for CO2 sensing [30]. Thanks to the presence of both species in the solution, the amount of TMAOH needed to deprotonate azaBODIPY and change it to its basic form is considerably small. It has also been reported that in the presence of the ionic liquid anion ([BF4]−), the anion that would decompose into HF and , which would catalyse the formation of hydrogen carbonate, stimulating the hydration of CO2 and its posterior protolysis [28].
The amount of pH indicator was set at 0.5 mg. The initial sensing membrane without the inclusion of EMIMBF4 was prepared with a 2% HPMC polymer, the minimum concentration to produce a membrane with the required mechanical properties and a good response. However, it was observed that, after adding the ionic liquid, the optical and mechanical characteristics of the membrane worsened. Therefore, the percentage of HPMC had to be reduced considerably. Different membranes were prepared with percentages of HPMC in the range of 1.0 to 2.0% w/w. In each case, the maximum response was reached when the percentage of HPMC was 1.0% w/w and this value was selected as optimal for successive experiments (Figure 1a).
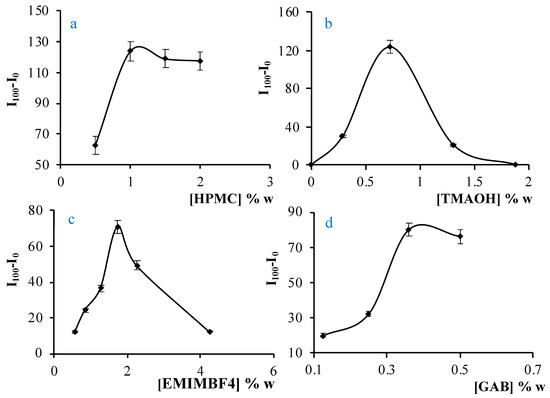
Figure 1.
Optimization of reagent concentration for sensing membrane containing IL: (a) HPMC; (b) TMAOH; (c) EMIMBF4; (d) GAB.
The amount of the TMAOH transfer agent phase was optimized preparing different membranes with TMAOH contents from 0 to 1.8% w/w. With small amounts of TMAOH, the difference between I100-I0 is very small because there is not enough TMAOH for all the azaBODIPY to be in its basic form, preventing the membrane from responding to CO2. Similarly, when the TMAOH concentration is too high (1.8% w/w), the pH is too basic and the sensitivity is low. The maximum signal was obtained with 0.75% TMAOH (Figure 1b).
To optimize the amount of EMIMBF4 in the sensing membrane, eight different sensors were prepared with amounts ranging from 0.5% to 4.3% w/w. Figure 1c shows that the best result was obtained for 1.5% w/w. The inclusion of surfactants in the membrane increases the response of the sensor and the preparation of the membranes is improved by spin coating. The surfactant selected was Tween 20, because of its effectiveness with this sensing membrane over the other surfactants tested [30,40]. To study the influence of Tween 20, different membranes were prepared containing between 0 and 1.15% w/w. In all cases, the maximum signal was obtained for 0.38% w/w; this amount was thus selected.
The optimization of the amount of GAB was made taking into account the I100-I0 parameter and the response and recovery times, as the goal was to obtain the maximum signal in the shortest possible time. Table 1 shows the results of the optimization of GAB.

Table 1.
Optimization of the amount of GAB versus three variables: I100-I0 parameter, response time and recovery time.
For 0.12 and 0.25% w/w of GAB, the values of I100-I0 are very similar, but the response and recovery times improve for 0.12% of GAB. For 0.5% w/w of GAB, the response time is very fast, but the parameter I100-I0 is too small to be considered adequate to work with. The amount of 0.35% w/w of GAB was selected as the optimum value in the end (Figure 1d).
It is well known that temperature and humidity have a considerable effect on the sensitivity of luminescent sensors [41], particularly at high values of both parameters. The dependence of the relative humidity of the sensing membrane at room temperature (22 ± 1 °C) was studied, obtaining the calibration function at relative humidities between 10% and 100% and observing a reduction in sensitivity with as the % RH increases (Figure 2). The experiments assays were carried out at a constant room temperature, 22 ± 1 °C, in all cases, although there is a known decrease in sensitivity as the temperature increases [42]. This fact occurs owing to the opposite dependence of CO2 solubility in the pH indicator membrane on the temperature [43].
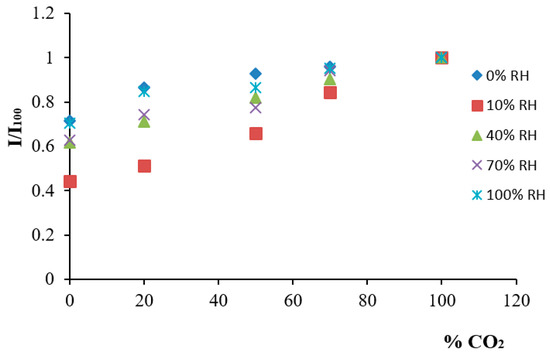
Figure 2.
Humidity dependence of the sensing membrane:  : 0% RH;
: 0% RH;  : 10% RH;
: 10% RH;  : 40% RH;
: 40% RH;  : 70% RH;
: 70% RH;  : 100% RH.
: 100% RH.
 : 0% RH;
: 0% RH;  : 10% RH;
: 10% RH;  : 40% RH;
: 40% RH;  : 70% RH;
: 70% RH;  : 100% RH.
: 100% RH.
In order to study the effect of different concentrations gases, such as O2 and H2, and the minor constituents, such as SO2, HCl, and CH4, were exposed to the sensing membrane It was concluded that only gases with acid-base properties, like HCl and SO2, produce a strong interference, as expected owing to the sensing mechanism, while other gaseous species such as CH4 and O2 did not produce any change in the signal when present.
3.3. Analytical Characterization of the Sensing Membranes
The hyperbolic response of both colorimetric and luminescent CO2 sensors between the measured signal and CO2 concentration is well known [44,45,46]. Different approaches have been used to linearize the experimental data, such as the Stern-Volmer equation [47] and the equation proposed by Nakamura and Amao [48]. In this case, the calibration function (Figure 3) was obtained using Equation (1), because of the good fit for linearizing the experimental data. Each measurement was obtained as a mean value of three replicates covering the whole range of concentrations working at room temperature (22 ± 1 °C):
where the values of the coefficients are as follows: a = 0.72 ± 0.05; b = 0.12 ± 0.05; c = 0.17 ± 0.05 and r2 = 0.9961. The signal increases exponentially until it reaches 100% CO2. For that reason, the detection limit, LOD, was calculated from the raw exponential experimental data by using the first three points (Figure 3 insert), which can be adjusted to a straight line, I/I100 = 0.0051[% CO2] + 0.856; R2 = 0.989 [49], using (LOD = t0N − 3s0), where t0N is the blank or average value in the absence of CO2 and s0 is the critical level or standard deviation of the blank, which was calculated from six replicate measurements. The limit of quantification (LOQ) of the instrumental procedure was obtained from the linear Stern-Volmer calibration function by using LOQ = t0N + 10s0. The LOD found with this approach was 0.26% CO2 and the LOQ was 1.64% CO2.
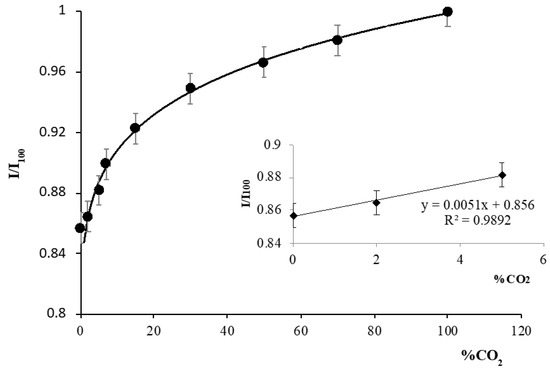
Figure 3.
Sensor response to CO2 concentration. Insert: linearization of the first three experimental raw data for LOD and LQD calculation.
The precision was evaluated at two CO2 concentrations (0.6% and 16.7%), performing 15 measurements each and obtaining relative standard deviations. Comparing the results to those obtained using other sensors described by us [30] that include ionic liquids in their composition, we found that the relative standard deviations in both cases are lower than 5%. For the proposal of determining the reversibility of the sensor, alternative atmospheres of pure CO2 and pure nitrogen with a response time between 10% and 90% of the maximum signal and a recovery time from 90% to 10% were exposed on the sensing membrane. In Figure 4, one can observe no hysteresis during the measurements as well as the fact that the signal change was fully reversible.

Figure 4.
Response and recovery time of CO2 sensing membrane while increasing and decreasing CO2 concentrations (from 10% to 90% and vice-versa).
The stability of the membrane was studied by measuring the I100-I0 value for 10 months from a set of membranes using a Shewhart control chart. We defined the lifetime of the sensor (T1) as that in which the sensor output signal remains within the control line on the Shewhart chart for one constant concentration of CO2 gas concentration. The lifetime of the sensing membrane (T2) is the time during which the membrane responds to CO2, even though it is necessary to recalibrate it. Figure 5 shows the control chart for the sensing membrane containing IL. The sensor signal remains within the established control limits up to 20 days, after which the sensor responds up to 300 days, as it is possible to recalibrate the signal within this period. After this period, the sensor must be replaced because no response is obtained.
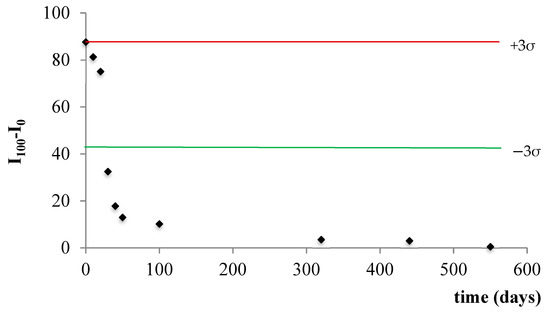
Figure 5.
Shewhart control chart for sensing membrane lifetime.
Table 2 shows the comparison of the analytical characteristics of the sensing membrane proposed here versus the sensing membrane without IL [33]. We observed an improvement in the LOD for the membranes containing IL (from 0.57% to 0.26%). The precision measured at two CO2 levels (0.6% and 16.7% CO2) was better when using IL. The biggest difference in performance characteristics was related to response time. The membrane with IL reduced the response time t90 to 38% (from 60 s to 23 s) and recovery time t10 to 34% (from 120 s to 49 s).

Table 2.
Comparison of the analytical characteristics of sensing membranes for CO2 determination with and without IL.
The lifetime (T1) of the sensor with IL is higher than the reference T1 value (15 days), noting once again that including IL increases the lifetime. Table 3 summarizes the principal analytical characteristics of the CO2 gas sensor with IL in its composition found in the literature. The fluorescent pH indicator 1-hydroxypyrene3,6,8-trisulfonate (HPTS) (τ0~5 ns) has been widely used with this type of sensor to analyse carbon dioxide gas. Additionally, it was the first to include an ionic liquid in diverse polymeric matrices such as ethyl cellulose, poly(methyl methacrylate), prepared on an inert Mylar support or using an electrospinning technique. Other indicators such as α-naphtholphthalein and azaBODIPY have also been used in different matrices, as in this case, and the response time and recovery time are considerably improved compared with the rest of the sensors developed to date. Additionally, the precision, as RSD (%), is significantly lower for the proposed sensor than for those developed previously, although it does not have the lowest LOD of all the sensors.

Table 3.
Performance comparison of CO2 sensing membranes based on IL.
The inclusion of ionic liquids, together with an azaBODIPY forming sensing membrane, in this study, considerably improves the detection limit and response time of the sensor, with its response in the near-infrared (NIR) region. It has several advantages, including low light scattering, dramatically reduced autofluorescence and the availability of low-cost photodetectors and excitation sources.
4. Conclusions
In this study, we report the development of a CO2 sensing membrane that operates with an inner filter process and in which a very stable inorganic phosphor and an ionic liquid were included for extremely fast CO2 capture. This considerably improves both the sensitivity of the sensor and its response and recovery time to determine and monitor CO2 in the infrared region.
A significant improvement on an NIR optical sensor based on azaBODIPY is obtained for the determination of gaseous CO2 using the measurement of the fluorescence intensity of the GAB nanoparticles included in the HPMC membrane with TMAOH as a phase transfer agent and EMIMBF4 as IL by shifting the acid-base equilibrium of the included aza-BODIPY. The advantages of working in the NIR region combined with those of including an ionic liquid, that is, improvements in sensitivity (LOD 0.26%), precision (RSD less than 0.5%) and response and recovery times (t90 23 s and t10 49 s) and useful life (20 days), make this sensor especially attractive for application in biological systems.
The sensor can be included in specific instrumentation for application in the environment, diagnostics and disease tracking and smart packaging, all goals for future research.
Author Contributions
Conceptualization, M.D.F.-R.; Methodology, M.D.F.-R., and F.M.-M.; Formal Analysis, M.D.F.-R., and F.M.-M.; Investigation, M.D.F.-R., F.M.-M., and I.M.P.d.V.-S.; Resources, M.D.F.-R., F.M.-M., I.M.P.d.V.-S., and L.F.C.-V.; Data Curation, M.D.F.-R. and I.M.P.d.V.-S.; Writing-Original Draft Preparation, M.D.F.-R. and L.F.C.-V.; Writing-Review and Editing, M.D.F.-R., I.M.P.d.V.-S., and L.F.C.-V.; Visualization, M.D.F.-R.; Supervision, M.D.F.-R. and I.M.P.d.V.-S.; Project Administration, L.F.C.-V.; Funding Acquisition, L.F.C.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was founded by Spanish “Ministerio de Economía y Competitividad” (Projects PID2019-103938RB-I00 and CTQ2017-86125-P) and Junta de Andalucía (Projects B-FQM-243-UGR18 and P18-RT-2961). The projects were partially supported by European Regional Development Funds (ERDF).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors would like to acknowledge Antonio Rodríguez Diéguez and Francisco Santoyo González for their help in the synthesis of gadolinium salt and azaBODIPY, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Greaves, T.L.; Drummond, C.J. Protic Ionic Liquids: Properties and Applications. Chem. Rev. 2008, 108, 206–237. [Google Scholar] [CrossRef]
- Vekariya, R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Gao, W.-W.; Zhang, F.-X.; Zhang, G.-X.; Zhou, C.-H. Key factors affecting the activity and stability of enzymes in ionic liquids and novel applications in biocatalysis. Biochem. Eng. J. 2015, 99, 67–84. [Google Scholar] [CrossRef]
- Delgado-Mellado, N.; Ovejero-Perez, A.; Navarro, P.; Larriba, M.; Ayuso, M.; García, J.; Rodríguez, F. Imidazolium and pyridinium-based ionic liquids for the cyclohexane/cyclohexene separation by liquid-liquid extraction. J. Chem. Thermodyn. 2019, 131, 340–346. [Google Scholar] [CrossRef]
- Claus, J.; Sommer, F.O.; Kragl, U. Ionic liquids in biotechnology and beyond. Solid State Ion. 2018, 314, 119–128. [Google Scholar] [CrossRef]
- Arumugam, V.; Redhi, G.; Gengan, R.M. Chapter 12—The application of ionic liquids in nanotechnology. In Fundamentals of Nanoparticles; Barhoum, A., Hamdy Makhlouf, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 371–400. [Google Scholar] [CrossRef]
- Liu, H.; Yu, H. Ionic liquids for electrochemical energy storage devices applications. J. Mater. Sci. Technol. 2019, 35, 674–686. [Google Scholar] [CrossRef]
- Martins, V.L.; Torresi, R.M. Ionic liquids in electrochemical energy storage. Curr. Opin. Electrochem. 2018, 9, 26–32. [Google Scholar] [CrossRef]
- Rostami, S.; Mehdinia, A.; Jabbari, A.; Kowsari, E.; Niroumand, R.; Booth, T.J. Colorimetric sensing of dopamine using hexagonal silver nanoparticles decorated by task-specific pyridinum based ionic liquid. Sens. Actuators B Chem. 2018, 271, 64–72. [Google Scholar] [CrossRef]
- Rauf, S.; Nawaz, M.A.H.; Muhammad, N.; Raza, R.; Shahid, S.A.; Marty, J.L.; Hayat, A. Protic ionic liquids as a versatile modulator and stabilizer in regulating artificial peroxidase activity of carbon materials for glucose colorimetric sensing. J. Mol. Liq. 2017, 243, 333–340. [Google Scholar] [CrossRef]
- Yu, H.A.; DeTata, D.A.; Lewis, S.W.; Silvester, D.S. Recent developments in the electrochemical detection of explosives: Towards field-deployable devices for forensic science. TrAC Trends Anal. Chem. 2017, 97, 374–384. [Google Scholar] [CrossRef]
- Akhmetshina, A.I.; Gumerova, O.R.; Atlaskin, A.A.; Petukhov, A.N.; Sazanova, T.S.; Yanbikov, N.R.; Nyuchev, A.V.; Razov, E.N.; Vorotyntsev, I.V. Permeability and selectivity of acid gases in supported conventional and novel imidazolium-based ionic liquid membranes. Sep. Purif. Technol. 2017, 176, 92–106. [Google Scholar] [CrossRef]
- Baltes, N.; Beyle, F.; Freiner, S.; Geier, F.; Joos, M.; Pinkwart, K.; Rabenecker, P. Trace detection of oxygen—ionic liquids in gas sensor design. Talanta 2013, 116, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yang, X.; Shu, Y.; Chen, X.; Wang, J. Ionic liquid-based slab optical waveguide sensor for the detection of ammonia in human breath. J. Colloid Interface Sci. 2018, 512, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Perez de Vargas-Sansalvador, I.M.; Erenas, M.M.; Diamond, D.; Quilty, B.; Capitan-Vallvey, L.F. Water based-ionic liquid carbon dioxide sensor for applications in the food industry. Sens. Actuators B Chem. 2017, 253, 302–309. [Google Scholar] [CrossRef]
- Behera, K.; Pandey, S.; Kadyan, A.; Pandey, S. Ionic Liquid-Based Optical and Electrochemical Carbon Dioxide Sensors. Sensors 2015, 15, 30487–30503. [Google Scholar] [CrossRef] [PubMed]
- Kapurch, C.J.; Abcejo, A.S.; Pasternak, J.J. The relationship between end-expired carbon dioxide tension and severity of venous air embolism during sitting neurosurgical procedures—A contemporary analysis. J. Clin. Anesth. 2018, 51, 49–54. [Google Scholar] [CrossRef]
- Laporte-Uribe, J.A. The role of dissolved carbon dioxide in both the decline in rumen pH and nutritional diseases in ruminants. Anim. Feed Sci. Technol. 2016, 219, 268–279. [Google Scholar] [CrossRef]
- Lin, B.; Benjamin, N.I. Determinants of industrial carbon dioxide emissions growth in Shanghai: A quantile analysis. J. Clean. Prod. 2019, 217, 776–786. [Google Scholar] [CrossRef]
- Yu, M.; Sediq, A.S.; Zhang, S.; Nejadnik, M.R.; Every, H.A.; Jiskoot, W.; Witkamp, G.-J. Towards the development of a supercritical carbon dioxide spray process to coat solid protein particles. J. Supercrit. Fluids 2018, 141, 49–59. [Google Scholar] [CrossRef]
- Puligundla, P.; Jung, J.; Ko, S. Carbon dioxide sensors for intelligent food packaging applications. Food Control 2012, 25, 328–333. [Google Scholar] [CrossRef]
- Soriano, A.N.; Doma, B.T.; Li, M.-H. Solubility of Carbon Dioxide in 1-Ethyl-3-methylimidazolium Tetrafluoroborate. J. Chem. Eng. Data 2008, 53, 2550–2555. [Google Scholar] [CrossRef]
- Wu, J.; Yin, M.-J.; Seefeldt, K.; Dani, A.; Guterman, R.; Yuan, J.; Zhang, A.P.; Tam, H.-Y. In Situ μ-printed optical fiber-tip CO2 sensor using a photocrosslinkable poly(ionic liquid). Sens. Actuators B Chem. 2018, 259, 833–839. [Google Scholar] [CrossRef]
- Revsbech, N.P.; Garcia-Robledo, E.; Sveegaard, S.; Andersen, M.H.; Gothelf, K.V.; Larsen, L.H. Amperometic microsensor for measurement of gaseous and dissolved CO2. Sens. Actuators B Chem. 2019, 283, 349–354. [Google Scholar] [CrossRef]
- Aki, S.N.V.K.; Mellein, B.R.; Saurer, E.M.; Brennecke, J.F. High-Pressure Phase Behavior of Carbon Dioxide with Imidazolium-Based Ionic Liquids. J. Phys. Chem. B 2004, 108, 20355–20365. [Google Scholar] [CrossRef]
- Jacquemin, J.; Costa Gomes, M.F.; Husson, P.; Majer, V. Solubility of carbon dioxide, ethane, methane, oxygen, nitrogen, hydrogen, argon, and carbon monoxide in 1-butyl-3-methylimidazolium tetrafluoroborate between temperatures 283 K and 343 K and at pressures close to atmospheric. J. Chem. Thermodyn. 2006, 38, 490–502. [Google Scholar] [CrossRef]
- Doganata, S.; Coskun, S.; Oeztuerk, Y.; Celikoglu, M. Investigation of interaction of carbon dioxide with ionic liquid filled photonic crystal fiber. Optoelectron. Adv. Mater. Rapid Commun. 2016, 10, 354–357. [Google Scholar]
- Oter, O.; Ertekin, K.; Derinkuyu, S. Ratiometric sensing of CO2 in ionic liquid modified ethyl cellulose matrix. Talanta 2008, 76, 557–563. [Google Scholar] [CrossRef]
- Oter, O.; Ertekin, K.; Topkaya, D.; Alp, S. Emission-based optical carbon dioxide sensing with HPTS in green chemistry reagents: Room-temperature ionic liquids. Anal. Bioanal. Chem. 2006, 386, 1225–1234. [Google Scholar] [CrossRef]
- Fernández-Ramos, M.D.; Aguayo-López, M.L.; Pérez de Vargas-Sansalvador, I.; Capitán-Vallvey, L.F. Ionic liquids on optical sensors for gaseous carbon dioxide. Anal. Bioanal. Chem. 2018, 410, 5931–5939. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef]
- Hodgkinson, J.; Smith, R.; Ho, W.O.; Saffell, J.R.; Tatam, R.P. Non-dispersive infra-red (NDIR) measurement of carbon dioxide at 4.2μm in a compact and optically efficient sensor. Sens. Actuators B Chem. 2013, 186, 580–588. [Google Scholar] [CrossRef]
- Fernández-Ramos, M.D.; Aguayo-López, M.L.; de los Reyes-Berbel, E.; Santoyo-González, F.; Capitán-Vallvey, L.F. NIR optical carbon dioxide gas sensor based on simple azaBODIPY pH indicators. Analyst 2019, 144, 3870–3877. [Google Scholar] [CrossRef] [PubMed]
- Honeycutt, W.T.; Ley, M.T.; Materer, N.F. Precision and Limits of Detection for Selected Commercially Available, Low-Cost Carbon Dioxide and Methane Gas Sensors. Sensors 2019, 19, 3157. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Gatterer, K.; Bitschnau, B.; Klimant, I. Preparation and Characterization of Chromium(III)-Activated Yttrium Aluminum Borate: A New Thermographic Phosphor for Optical Sensing and Imaging at Ambient Temperatures. J. Phys. Chem. C 2010, 114, 9118–9124. [Google Scholar] [CrossRef] [PubMed]
- Jokic, T.; Borisov, S.M.; Saf, R.; Nielsen, D.A.; Kühl, M.; Klimant, I. Highly Photostable Near-Infrared Fluorescent pH Indicators and Sensors Based on BF2-Chelated Tetraarylazadipyrromethene Dyes. Anal. Chem. 2012, 84, 6723–6730. [Google Scholar] [CrossRef]
- Gorman, A.; Killoran, J.; O’Shea, C.; Kenna, T.; Gallagher, W.M.; O’Shea, D.F. In Vitro Demonstration of the Heavy-Atom Effect for Photodynamic Therapy. J. Am. Chem. Soc. 2004, 126, 10619–10631. [Google Scholar] [CrossRef]
- Capitán-Vallvey, L.F.; Fernández Ramos, M.D.; Avidad, R.; Deheidel, M.K.A. Determination of the pesticide morestan by means of a single-use phosphorimetric sensor. Anal. Chim. Acta 2001, 440, 131–141. [Google Scholar] [CrossRef][Green Version]
- Mills, A.; Lepre, A.; Wild, L. Breath-by-breath measurement of carbon dioxide using a plastic film optical sensor. Sens. Actuators B 1997, 39, 419–425. [Google Scholar] [CrossRef]
- Aguayo-López, M.L.; Capitán-Vallvey, L.F.; Fernández-Ramos, M.D. Optical sensor for carbon dioxide gas determination, characterization and improvements. Talanta 2014, 126, 196–201. [Google Scholar] [CrossRef]
- Mills, A.; Chang, Q.; McMurray, N. Equilibrium Studies on Colorimetric Plastic Film Sensors for Carbon Dioxide. Anal. Chem. 1992, 64, 1383–1389. [Google Scholar] [CrossRef]
- Fernández-Ramos, M.D.; Moreno-Puche, F.; Escobedo, P.; García-López, P.A.; Capitán-Vallvey, L.F.; Martínez-Olmos, A. Optical portable instrument for the determination of CO2 in indoor environments. Talanta 2020, 208, 120387. [Google Scholar] [CrossRef] [PubMed]
- Marazuela, M.D.; Moreno Bondi, M.C.; Orellana, G. Enhanced performance of a fibre-optic luminescence CO2 sensor using carbonic anhydrase. Sens. Actuators B Chem. 1995, 29, 126–131. [Google Scholar] [CrossRef]
- Neurauter, G.; Klimant, I.; Wolfbeis, O.S. Microsecond lifetime-based optical carbon dioxide sensor using luminescence resonance energy transfer. Anal. Chim. Acta 1999, 382, 67–75. [Google Scholar] [CrossRef]
- Mills, A.; Chang, Q. Fluorescence plastic thin-film sensor for carbon dioxide. Analyst 1993, 118, 839–843. [Google Scholar] [CrossRef]
- Borisov, S.M.; Waldhier, M.C.; Klimant, I.; Wolfbeis, O.S. Optical Carbon Dioxide Sensors Based on Silicone-Encapsulated Room-Temperature Ionic Liquids. Chem. Mater. 2007, 19, 6187–6194. [Google Scholar] [CrossRef]
- Gehlen, M.H. The centenary of the Stern-Volmer equation of fluorescence quenching: From the single line plot to the SV quenching map. J. Photochem. Photobiol. C: Photochem. Rev. 2020, 42, 100338. [Google Scholar] [CrossRef]
- Nakamura, N.; Amao, Y. Optical sensor for carbon dioxide combining colorimetric change of a pH indicator and a reference luminescent dye. Anal. Bioanal. Chem. 2003, 376, 642–646. [Google Scholar] [CrossRef]
- ISO. Capability of detection. Methodology in the linear and non-linear cases. In ISO 11843-5:2008. Capability of Detection. Part 5: Methodology in the Linear and Non-Linear Calibration Cases; International Standardization Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Aydogdu, S.; Ertekin, K.; Suslu, A.; Ozdemir, M.; Celik, E.; Cocen, U. Optical CO2 Sensing with Ionic Liquid Doped Electrospun Nanofibers. J. Fluoresc. 2011, 21, 607–613. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).