Interfacial Structure and Physical Properties of High-Entropy Oxide Coatings Prepared via Atmospheric Plasma Spraying
Abstract
:1. Introduction
2. Experimental Method
2.1. Thermal Barrier Coating
2.2. Characterization
3. Results and Discussion
3.1. Phase Analysis
3.2. Microstructure
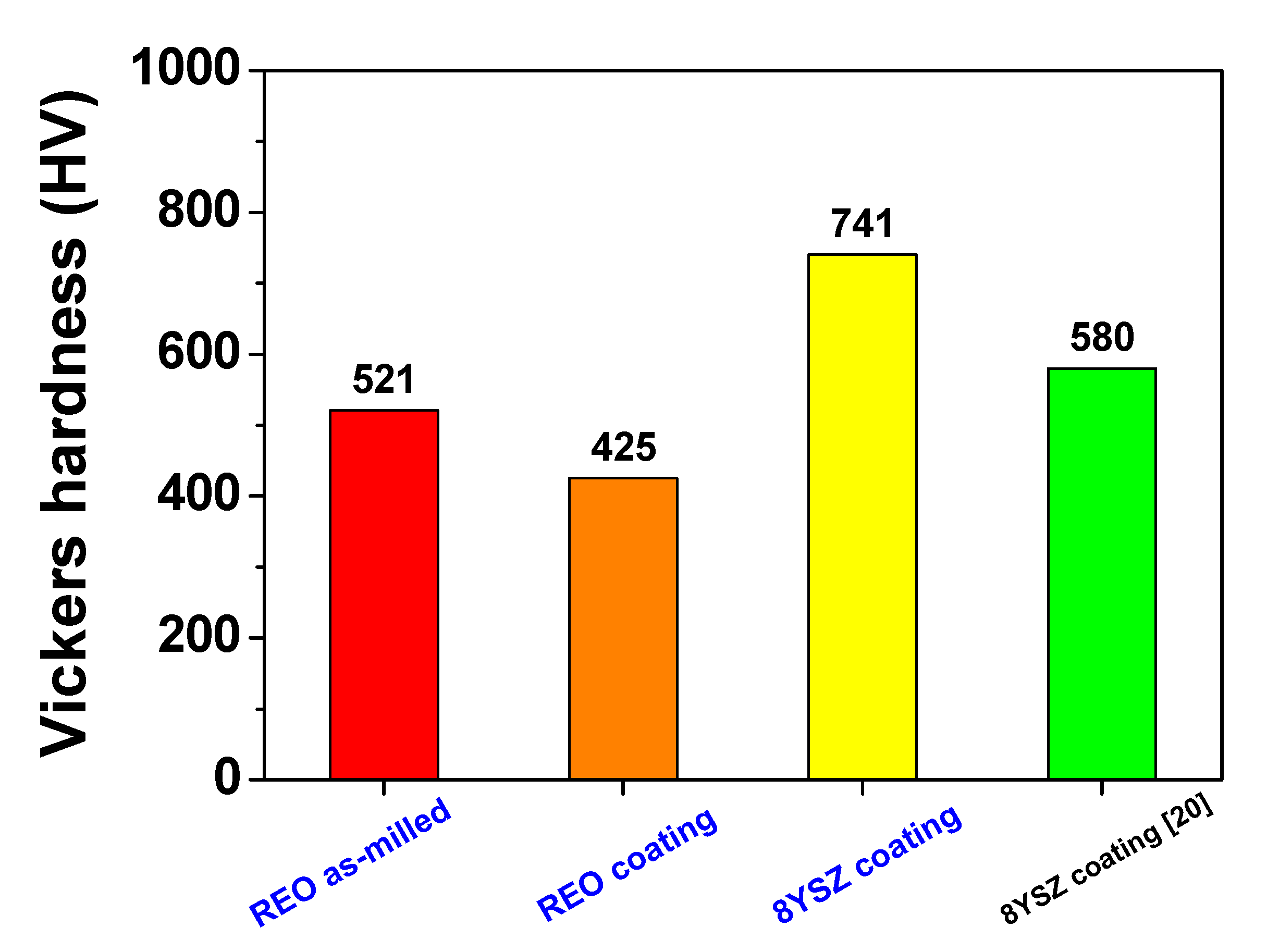
3.3. Microhardness
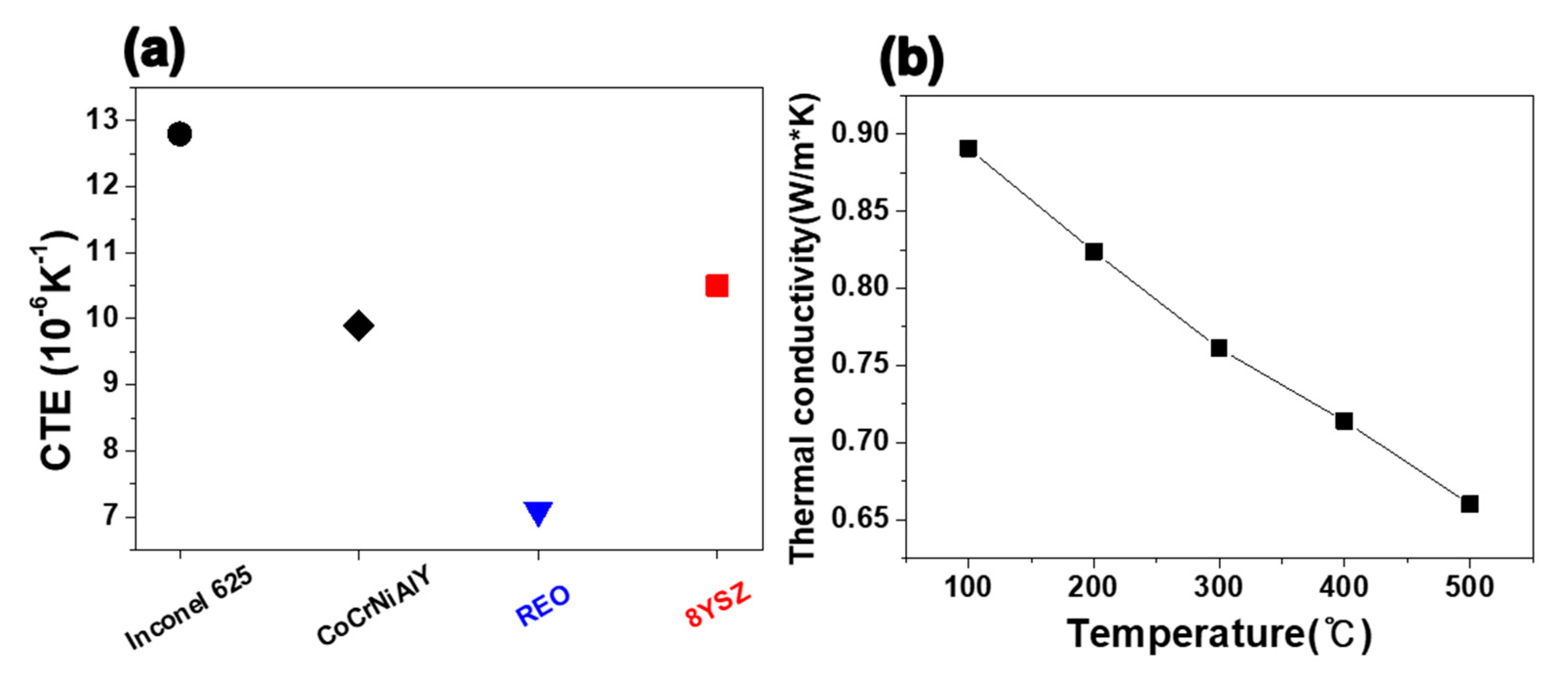
3.4. Thermal Behavior
3.5. Factors Affecting Coating Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nam, S.W. Single crystal blade for a gas turbine engine: A review of the characteristics and recent research trends for thermal barrier coating. Korean J. Met. Mater. 2015, 53, 833–843. [Google Scholar] [CrossRef]
- Lim, J.G.; Kim, M.K. Development of a durability evaluation model to optimize rare-earth-element-based thermal barrier coatings. Ceram. Int. 2019, 45, 19223–19236. [Google Scholar] [CrossRef]
- Song, D.; Song, T.; Paik, U.; Lyu, G.; Jung, Y.-G. Hot corrosion behavior in thermal barrier coatings with heterogeneous splat boundary. Corros. Sci. 2020, 163, 108225. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, X.; Chen, Y.; Xiao, P. Effect of superalloy substrate on the lifetime and interfacial toughness of electron beam physical vapor deposited thermal barrier coatings. Surf. Coat. Technol. 2019, 378, 124937. [Google Scholar] [CrossRef]
- Schulz, U.; Menzebach, M.; Leyens, C.; Yang, Y. Influence of substrate material on oxidation behavior and cyclic lifetime of EB-PVD TBC systems. Surf. Coat. Technol. 2001, 146–147, 117–123. [Google Scholar] [CrossRef]
- Haynes, J.A.; Ferber, M.K.; Porter, W.D. Thermal cycling behavior of plasma-sprayed thermal barrier coatings with various MCrAIX bond coats. J. Therm. Spray Technol. 2000, 9, 38–48. [Google Scholar] [CrossRef]
- Rabiei, A.; Evans, A.G. Failure mechanisms associated with the thermally grown oxide in plasma-sprayed thermal barrier coatings. Acta Mater. 2000, 48, 3963–3976. [Google Scholar] [CrossRef]
- Brandon, J.; Taylor, R. Thermal properties of ceria and yttria partially stabilized zirconia thermal barrier coatings. Surf. Coat. Technol. 1989, 39, 143–151. [Google Scholar] [CrossRef]
- Chen, L.B. Yttria-stabilized zirconia thermal barrier coatings—A review. Surf. Rev. Lett. 2006, 13, 535–544. [Google Scholar] [CrossRef]
- Dhomne, S.; Mahalle, A.M. Thermal barrier coating materials for SI engine. J. Mater. Res. Technol. 2019, 8, 1532–1537. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Liu, H.; Weng, Y. Study on thermal resistance performance of 8YSZ thermal barrier coatings. Int. J. Therm. Sci. 2017, 122, 12–25. [Google Scholar] [CrossRef]
- Jones, R.L. Some aspects of the hot corrosion of thermal barrier coatings. J. Therm. Spray Technol. 1997, 6, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Lakiza, S.M.; Grechanyuk, M.I.; Ruban, O.K.; Redko, V.P.; Glabay, M.S.; Myloserdov, O.B.; Dudnik, O.V.; Prokhorenko, S. Thermal barrier coatings: Current status, search, and analysis. Powder Metall. Met. Ceram. 2018, 57, 82–113. [Google Scholar] [CrossRef]
- Mehboob, G.; Liu, M.-J.; Xu, T.; Hussain, S.; Mehboob, G.; Tahir, A. A review on failure mechanism of thermal barrier coatings and strategies to extend their lifetime. Ceram. Int. 2020, 46, 8497–8521. [Google Scholar] [CrossRef]
- Clarke, D.R.; Oechsner, M.; Padture, N.P. Thermal-barrier coatings for more efficient gas-turbine engines. MRS Bull. 2012, 37, 891–898. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Li, F.; Liu, J.-X.; Hu, Q.; Bao, W.; Wu, Y.; Cao, X.; Xu, F.; Zhang, G.-J. High-entropy thermal barrier coating of rare-earth zirconate: A case study on (La0.2Nd0.2Sm0.2Eu0.2Gd0.2)2Zr2O7 prepared by atmospheric plasma spraying. J. Eur. Ceram. Soc. 2020, 40, 5731–5739. [Google Scholar] [CrossRef]
- Kim, S.-J.; Lee, W.-J.; Kwon, C.-S.; Lee, S.-M.; Oh, Y.-S.; Kim, H.-T.; Im, D.-S.; Kim, S. Phase formation and thermo-physical properties of GdO1.5-ZrO2 system for thermal barrier coating application. J. Korean Ceram. Soc. 2014, 51, 554–559. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Xiang, H.; Feng, Z. Theoretical investigation on mechanical and thermal properties of a promising thermal barrier material: Yb3Al5O12. J. Mater. Sci. Technol. 2014, 30, 631–638. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Z.; Xiang, H.; Dai, F.-Z.; Xu, W.; Sun, K.; Liu, J.; Zhou, Y. High entropy (Y0.2Yb0.2Lu0.2Eu0.2Er0.2)3Al5O12: A novel high temperature stable thermal barrier material. J. Mater. Sci. Technol. 2020, 48, 57–62. [Google Scholar] [CrossRef]
- Yang, P.; An, Y.; Yang, D.; Li, Y.; Chen, J. Structure, thermal properties and hot corrosion behaviors of Gd2Hf2O7 as a potential thermal barrier coating material. Ceram. Int. 2020, 46, 21367–21377. [Google Scholar] [CrossRef]
- Zhou, X.; He, L.; Cao, X.; Xu, Z.; Mu, R.; Sun, J.; Yuan, J.; Zou, B. La2(Zr0.7Ce0.3)2O7 thermal barrier coatings prepared by electron beam-physical vapor deposition that are resistant to high temperature attack by molten silicate. Corros. Sci. 2017, 115, 143–151. [Google Scholar] [CrossRef]
- Feng, B.-B.; Wang, Y.; Jia, Q.; Huang, W.; Suo, H.-L.; Ma, W. Thermophysical properties of solution precursor plasma-sprayed La2Ce2O7 thermal barrier coatings. Rare Met. 2019, 38, 689–694. [Google Scholar] [CrossRef]
- Dong, Y.; Ren, K.; Lu, Y.; Wang, Q.; Liu, J.; Wang, Y. High-entropy environmental barrier coating for the ceramic matrix composites. J. Eur. Ceram. Soc. 2019, 39, 2574–2579. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, Y.; Li, W.; Liu, W.; Wu, Y.; Liu, F. Research progresses on ceramic materials of thermal barrier coatings on gas turbine. Coatings 2021, 11, 79. [Google Scholar] [CrossRef]
- Sharma, A. High entropy alloy coatings and technology. Coatings 2021, 11, 372. [Google Scholar] [CrossRef]
- Kim, J.H.; Na, Y.S. Tensile properties and serrated flow behavior of as-cast CoCrFeMnNi high-entropy alloy at room and elevated temperatures. Met. Mater. Int. 2019, 25, 296–303. [Google Scholar] [CrossRef]
- Kim, J.H.; Lim, K.R.; Won, J.W.; Na, Y.S.; Kim, H.-S. Mechanical properties and deformation twinning behavior of as-cast CoCrFeMnNi high-entropy alloy at low and high temperatures. Mater. Sci. Eng. A 2018, 712, 108–113. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiang, H.; Dai, F.-Z.; Peng, Z.; Zhou, Y. (TiZrHf)P2O7: An equimolar multicomponent or high entropy ceramic with good thermal stability and low thermal conductivity. J. Mater. Sci. Technol. 2019, 35, 2227–2231. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiang, H.; Dai, F.-Z.; Peng, Z.; Zhou, Y. (La0.2Ce0.2Nd0.2Sm0.2Eu0.2)2Zr2O7: A novel high-entropy ceramic with low thermal conductivity and sluggish grain growth rate. J. Mater. Sci. Technol. 2019, 35, 2647–2651. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.-P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, A.; Wang, Q.; Schiele, A.; Chellali, M.R.; Bhattacharya, S.S.; Wang, D.; Brezesinski, T.; Hahn, H.; Velasco, L.; Breitung, B. High-entropy oxides: Fundamental aspects and electrochemical properties. Adv. Mater. 2019, 31, e1806236. [Google Scholar] [CrossRef]
- Li, F.; Zhou, L.; Liu, J.-X.; Liang, Y.; Zhang, G.-J. High-entropy pyrochlores with low thermal conductivity for thermal barrier coating materials. J. Adv. Ceram. 2019, 8, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Ren, K.; Wang, Q.; Shao, G.; Zhao, X.; Wang, Y. Multicomponent high-entropy zirconates with comprehensive properties for advanced thermal barrier coating. Scr. Mater. 2020, 178, 382–386. [Google Scholar] [CrossRef]
- Wright, A.J.; Wang, Q.; Ko, S.-T.; Chung, K.M.; Chen, R.; Luo, J. Size disorder as a descriptor for predicting reduced thermal conductivity in medium- and high-entropy pyrochlore oxides. Scr. Mater. 2020, 181, 76–81. [Google Scholar] [CrossRef]
- Clarke, D.R.; Phillpot, S.R. Thermal barrier coating materials. Mater. Today 2005, 8, 22–29. [Google Scholar] [CrossRef]
- Thakare, J.G.; Pandey, C.; Mahapatra, M.M.; Mulik, R.S. Thermal barrier coatings—A state of the art review. Met. Mater. Int. 2020. [Google Scholar] [CrossRef]
- Fauchais, P. Understanding plasma spraying. J. Phys. D Appl. Phys. 2004, 37, R86–R108. [Google Scholar] [CrossRef]
- Xie, L.; Ma, X.; Jordan, E.H.; Padture, N.P.; Xiao, D.T.; Gell, M. Deposition mechanisms of thermal barrier coatings in the solution precursor plasma spray process. Surf. Coat. Technol. 2004, 177–178, 103–107. [Google Scholar] [CrossRef]
- Fauchais, P.; Vardelle, M.; Goutier, S. Atmospheric plasma spraying evolution since the sixties through modeling, measurements and sensors. Plasma Chem. Plasma Process. 2017, 37, 601–626. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, H.; Xiang, H.; Dai, F.-Z.; Wang, X.; Xu, W.; Sun, K.; Peng, Z.; Zhou, Y. High-entropy (Y0.2Nd0.2Sm0.2Eu0.2Er0.2)AlO3: A promising thermal/environmental barrier material for oxide/oxide composites. J. Mater. Sci. Technol. 2020, 47, 45–51. [Google Scholar] [CrossRef]
- Ren, X.; Tian, Z.; Zhang, J.; Wang, J. Equiatomic quaternary (Y1/4Ho1/4Er1/4Yb1/4)2SiO5 silicate: A perspective multifunctional thermal and environmental barrier coating material. Scr. Mater. 2019, 168, 47–50. [Google Scholar] [CrossRef]
- Capitelli, M.; Ficocelli, E.; Molinari, E. Equilibrium Compositions and Thermodynamic Properties of Mixed Plasmas: He-N2, AR-N2, and Xe-Ne at One Atmosphere between 5000 K and 35,000 K; Editrice Adriatica Bari: Bari, Italy, 1971. [Google Scholar]
- Pateyron, B.; Elchinger, M.-F.; Delluc, G.; Fauchais, P. Thermodynamic and transport properties of Ar-H2 and Ar-He plasma gases used for spraying at atmospheric pressure. I: Properties of the mixtures. Plasma Chem. Plasma Process. 1992, 12, 421–448. [Google Scholar] [CrossRef]
- Li, T.; Liu, Y.; Liu, B.; Guo, W.; Xu, L. Microstructure and wear behavior of FeCoCrNiMo0.2 high entropy coatings prepared by air plasma spray and the high velocity oxy-fuel spray processes. Coatings 2017, 7, 151. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, R.; Shoja-Razavi, R.; Mozafarinia, R.; Jamali, H. Laser glazing of plasma-sprayed nanostructured yttria stabilized zirconia thermal barrier coatings. Ceram. Int. 2013, 39, 9483–9490. [Google Scholar] [CrossRef]
- Lima, R.S.; Kucuk, A.; Berndt, C. Integrity of nanostructured partially stabilized zirconia after plasma spray processing. Mater. Sci. Eng. A 2001, 313, 75–82. [Google Scholar] [CrossRef]
- Lee, J.-H.; Tsai, P.-C.; Chang, C.-L. Microstructure and thermal cyclic performance of laser-glazed plasma-sprayed ceria–yttria-stabilized zirconia thermal barrier coatings. Surf. Coat. Technol. 2008, 202, 5607–5612. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Sun, X.G.; He, J.Q.; Pan, Z.Y.; Wang, C.H. Thermal shock behavior of 8YSZ and double-ceramic-layer La2Zr2O7/8YSZ thermal barrier coatings fabricated by atmospheric plasma spraying. Ceram. Int. 2012, 38, 3595–3606. [Google Scholar] [CrossRef]
- Chang, F.; Zhou, K.; Tong, X.; Xu, L.; Zhang, X.; Liu, M. Microstructure and thermal shock resistance of the peg-nail structured TBCs treated by selective laser modification. Appl. Surf. Sci. 2014, 317, 598–606. [Google Scholar] [CrossRef]
- Wu, J.; Guo, H.-B.; Zhou, L.; Wang, L.; Gong, S.-K. Microstructure and thermal properties of plasma sprayed thermal barrier coatings from nanostructured YSZ. J. Therm. Spray Technol. 2010, 19, 1186–1194. [Google Scholar] [CrossRef]
- Myoung, S.-W.; Kim, J.-H.; Lee, W.-R.; Jung, Y.-G.; Lee, K.-S.; Paik, U. Microstructure design and mechanical properties of thermal barrier coatings with layered top and bond coats. Surf. Coat. Technol. 2010, 205, 1229–1235. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, J.; Shao, F.; Zhong, X.; Zhao, H.; Zhuang, Y.; Ni, J.; Tao, S. Thermal stability of ysz coatings deposited by plasma spray–Physical vapor deposition. Coatings 2019, 9, 464. [Google Scholar] [CrossRef] [Green Version]
- Ang, A.S.M.; Berndt, C.C.; Sesso, M.L.; Anupam, A.; Sathiyamoorthi, P.; Kottada, R.S.; Murty, B.S. Plasma-sprayed high entropy alloys: Microstructure and properties of AlCoCrFeNi and MnCoCrFeNi. Met. Mater. Trans. A 2015, 46, 791–800. [Google Scholar] [CrossRef]
- Cao, X. Application of rare earths in thermal barrier coating. J. Mater. Sci. Technol. 2007, 23, 15–35. [Google Scholar]
- Davis, J. Handbook of Thermal Spray Technology; AMS: Materials Park, OH, USA, 2004. [Google Scholar]
- Kim, S.-W.; Kim, J.H. In-situ observations of deformation twins and crack propagation in a CoCrFeNiMn high-entropy alloy. Mater. Sci. Eng. A 2018, 718, 321–325. [Google Scholar] [CrossRef]
- Weyant, C.; Almer, J.; Faber, K. Through-thickness determination of phase composition and residual stresses in thermal barrier coatings using high-energy X-rays. Acta Mater. 2010, 58, 943–951. [Google Scholar] [CrossRef]
- Weng, W.-X.; Zheng, Z.-H.; Li, Q. Cracking evolution of atmospheric plasma-sprayed YSZ thermal barrier coatings subjected to isothermal heat treatment. Surf. Coat. Technol. 2020, 402, 125924. [Google Scholar] [CrossRef]
- Thompson, J.; Clyne, T. The effect of heat treatment on the stiffness of zirconia top coats in plasma-sprayed TBCs. Acta Mater. 2001, 49, 1565–1575. [Google Scholar] [CrossRef]
- Nakajima, R.; Katori, H.; Ito, K.; Arai, M.; Suidzu, T. Numerical simulation on internal stress evolution based on formation of thermally grown oxide in thermal barrier coatings. Eng. Res. Express 2020, 2, 025037. [Google Scholar] [CrossRef]
- Doleker, K.M.; Ahlatci, H.; Karaoglanli, A.C. Investigation of isothermal oxidation behavior of thermal barrier coatings (TBCs) consisting of YSZ and multilayered YSZ/Gd2Zr2O7 ceramic layers. Oxid. Met. 2017, 88, 109–119. [Google Scholar] [CrossRef]
- Mahalingam, S.; Yunus, S.M.; Manap, A.; Afandi, N.M.; Zainuddin, R.A.; Kadir, N.F. Crack propagation and effect of mixed oxides on TGO growth in thick La–Gd–YSZ thermal barrier coating. Coatings 2019, 9, 719. [Google Scholar] [CrossRef] [Green Version]
- Adomako, N.K.; Kim, J.H.; Hyun, Y.T. High-temperature oxidation behaviour of low-entropy alloy to medium- and high-entropy alloys. J. Therm. Anal. Calorim. 2018, 133, 13–26. [Google Scholar] [CrossRef]
- Cheng, B.; Zhang, Y.-M.; Yang, N.; Zhang, M.; Chen, L.; Yang, G.-J.; Li, C.-X.; Li, C.-J. Sintering-induced delamination of thermal barrier coatings by gradient thermal cyclic test. J. Am. Ceram. Soc. 2017, 100, 1820–1830. [Google Scholar] [CrossRef]
- Lima, R.S.; Marple, B. Nanostructured YSZ thermal barrier coatings engineered to counteract sintering effects. Mater. Sci. Eng. A 2008, 485, 182–193. [Google Scholar] [CrossRef] [Green Version]
- Karaoglanli, A.C.; Oge, M.; Doleker, K.M.; Hotamis, M. Comparison of tribological properties of HVOF sprayed coatings with different composition. Surf. Coat. Technol. 2017, 318, 299–308. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Yang, J.; Li, D.; Zhong, X.; Zhao, H.; Shao, F.; Tao, S. Thermal aging behavior of axial suspension plasma-sprayed yttria-stabilized zirconia (YSZ) thermal barrier coatings. J. Therm. Spray Technol. 2015, 24, 338–347. [Google Scholar] [CrossRef]
- Ren, K.; Wang, Q.; Cao, Y.; Shao, G.; Wang, Y. Multicomponent rare-earth cerate and zirconocerate ceramics for thermal barrier coating materials. J. Eur. Ceram. Soc. 2021, 41, 1720–1725. [Google Scholar] [CrossRef]
- Clarke, D. Materials selection guidelines for low thermal conductivity thermal barrier coatings. Surf. Coat. Technol. 2003, 163–164, 67–74. [Google Scholar] [CrossRef]
- Braun, J.L.; Rost, C.M.; Lim, M.; Giri, A.; Olson, D.H.; Kotsonis, G.N.; Stan, G.; Brenner, D.W.; Maria, J.; Hopkins, P.E. Charge-induced disorder controls the thermal conductivity of entropy-stabilized oxides. Wiley Online Libr. 2018, 30, e1805004. [Google Scholar] [CrossRef] [PubMed]
- Schlichting, K.W.; Padture, N.P.; Klemens, P.G. Thermal conductivity of dense and porous yttria-stabilized zirconia. J. Mater. Sci. 2001, 36, 3003–3010. [Google Scholar] [CrossRef]
- Hu, L.; Wang, C.-A.; Hu, Z.; Lu, S.; Sun, C.; Huang, Y. Porous yttria-stabilized zirconia ceramics with ultra-low thermal conductivity. Part II: Temperature dependence of thermophysical properties. J. Mater. Sci. 2010, 46, 623–628. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, H.; Xiang, H.; Dai, F.-Z.; Wang, X.; Xu, W.; Sun, K.; Peng, Z.; Zhou, Y. High entropy defective fluorite structured rare-earth niobates and tantalates for thermal barrier applications. J. Adv. Ceram. 2020, 9, 303–311. [Google Scholar] [CrossRef]
- Guo, H.; Vaßen, R.; Stöver, D. Atmospheric plasma sprayed thick thermal barrier coatings with high segmentation crack density. Surf. Coat. Technol. 2004, 186, 353–363. [Google Scholar] [CrossRef]
- Kulkarni, A.; Vaidya, A.; Goland, A.; Sampath, S.; Herman, H. Processing effects on porosity-property correlations in plasma sprayed yttria-stabilized zirconia coatings. Mater. Sci. Eng. A 2003, 359, 100–111. [Google Scholar] [CrossRef]
- Portinha, A.; Teixeira, V.; Carneiro, J.; Beghi, M.; Bottani, C.E.; Franco, N.; Vassen, R.; Stoever, D.; Sequeira, A. Residual stresses and elastic modulus of thermal barrier coatings graded in porosity. Surf. Coat. Technol. 2004, 188–189, 120–128. [Google Scholar] [CrossRef]
- Aruna, S.; Balaji, N.; Rajam, K. Phase transformation and wear studies of plasma sprayed yttria stabilized zirconia coatings containing various mol% of yttria. Mater. Charact. 2011, 62, 697–705. [Google Scholar] [CrossRef]
- Miller, R.; Garlick, R.; Smialek, J. Phase distributions in plasma-sprayed zirconia-yttria. Am. Ceram. Soc. Bull. 1983, 62, 1355–1358. [Google Scholar]
- Hannink, R.H.J.; Garvie, R.C. Sub-eutectoid aged Mg-PSZ alloy with enhanced thermal up-shock resistance. J. Mater. Sci. 1982, 17, 2637–2643. [Google Scholar] [CrossRef]
- Helminiak, M. Factors Affecting the Lifetime of Thick Air Plasma Sprayed Thermal Barrier Coatings. Master’s Thesis, University of Pittsburgh, Pittsburgh, PA, USA, 2010. [Google Scholar]
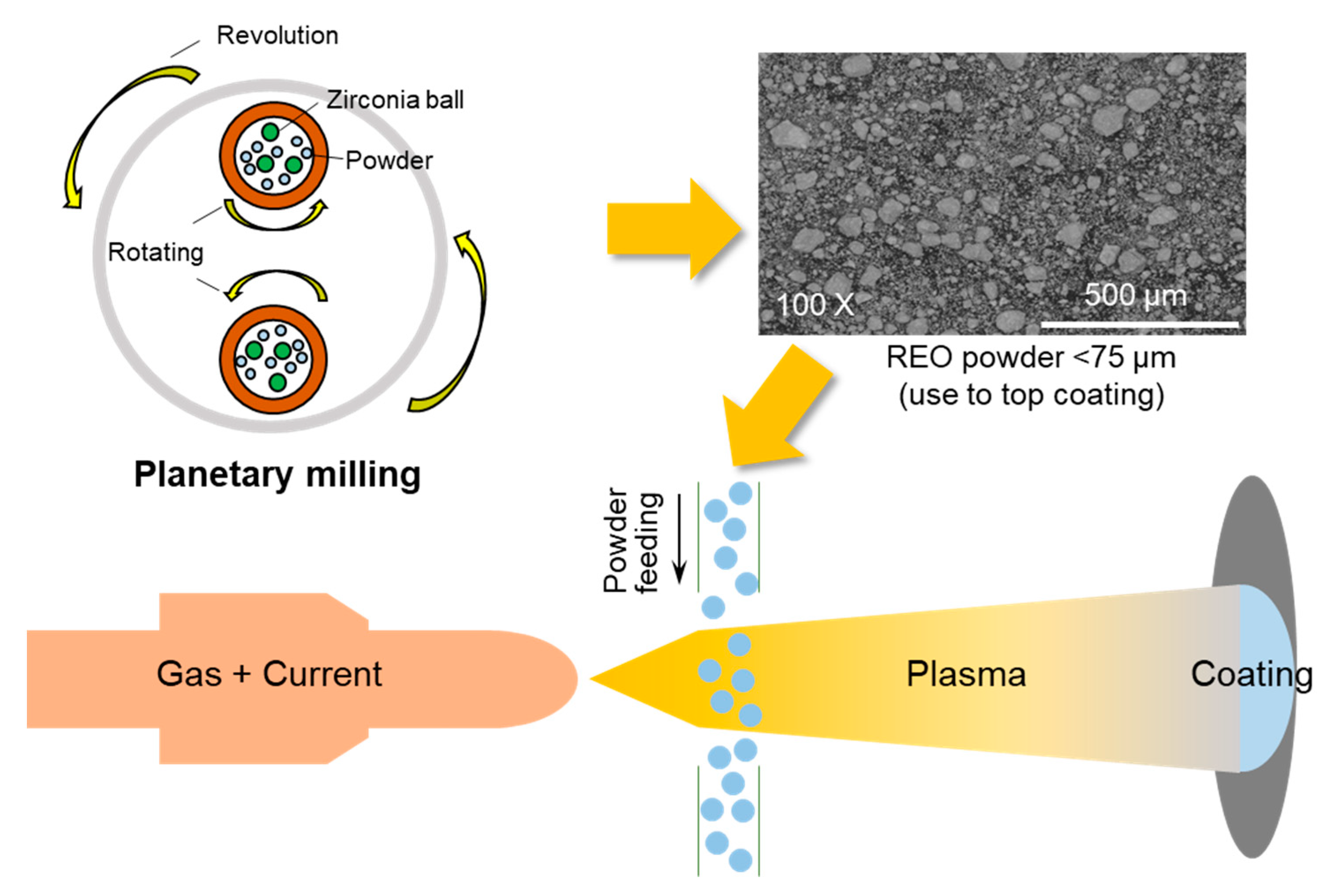

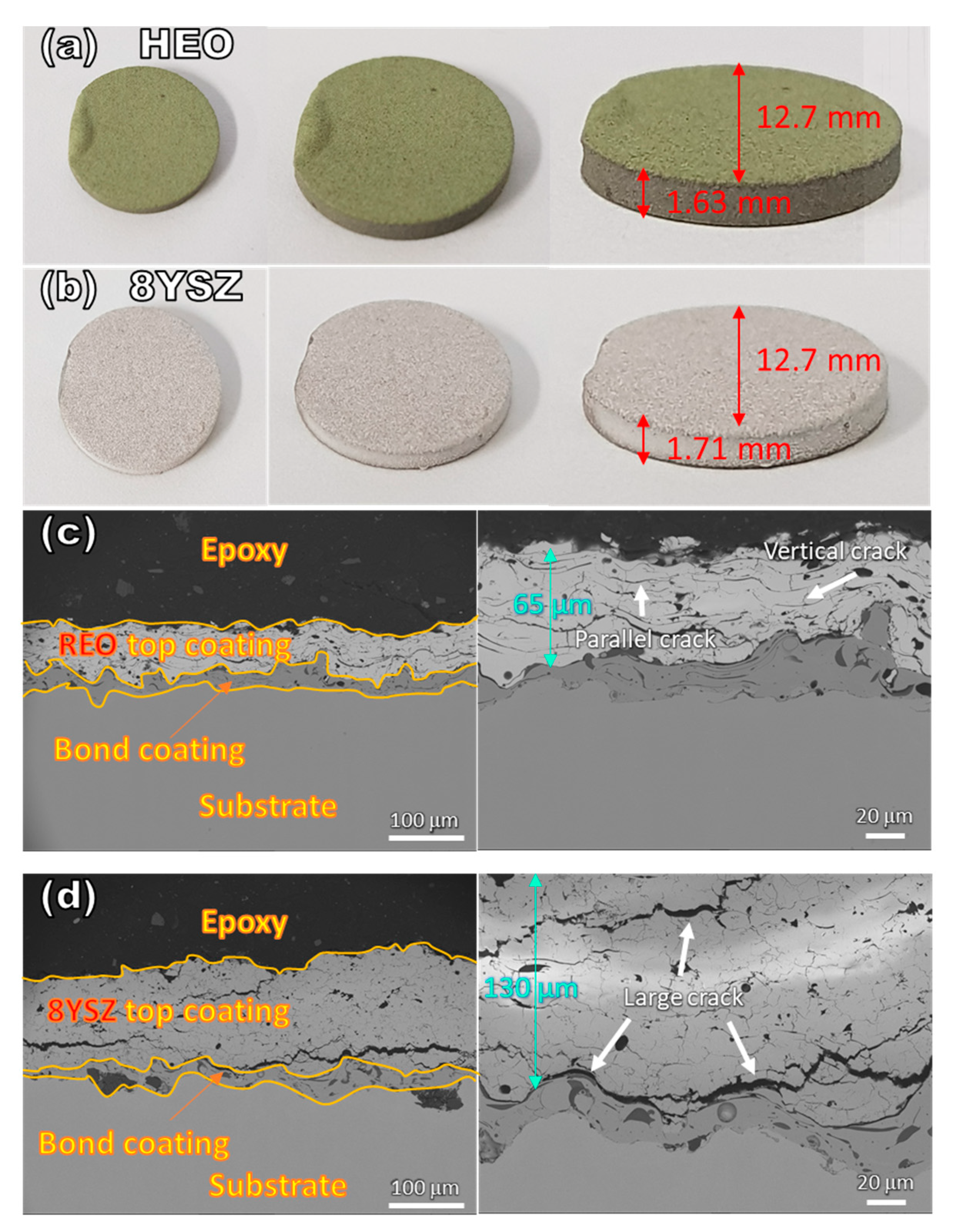
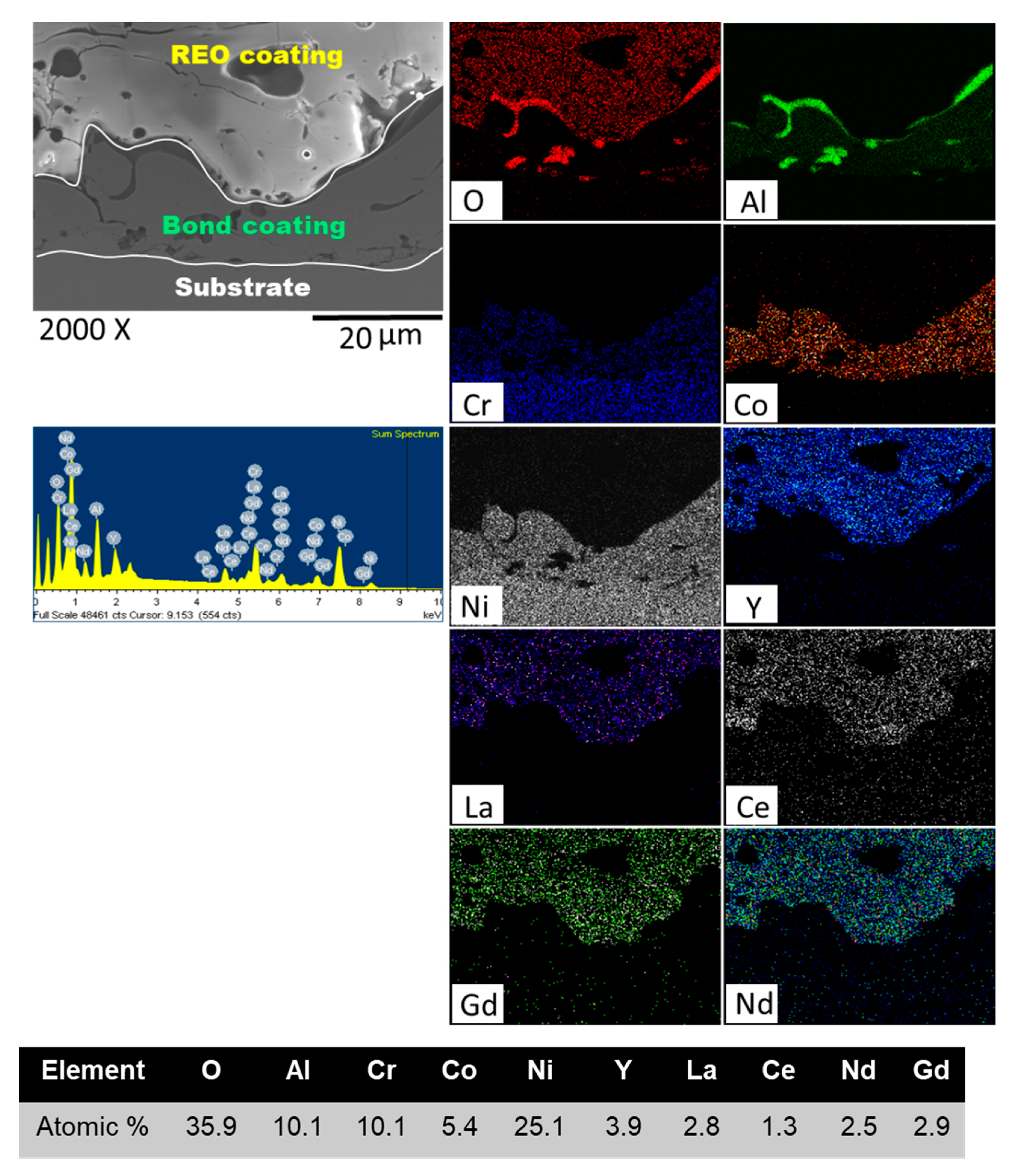
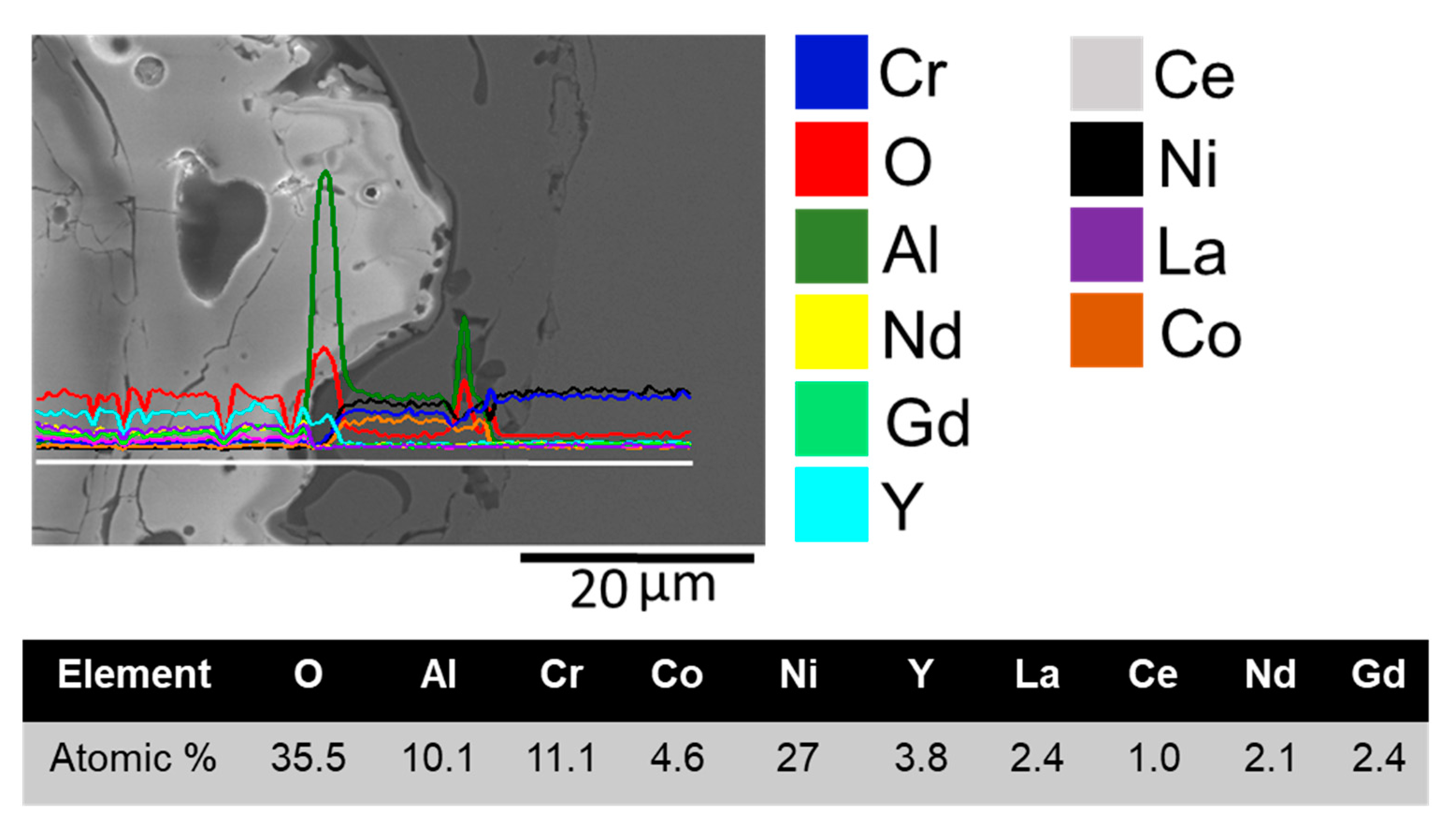
| Precursor | Space Group | Valence | Ionic Radius (nm) |
|---|---|---|---|
| CeO2 | fluorite (Fm3m) | 4+ | 0.97 |
| Y2O3 | bixbyite (Ia3) | 3+ | 0.9 |
| La2O3 | trigonal (P3m1) | 3+ | 1.1 |
| Nd2O3 | trigonal (P3m1) | 3+ | 1.048 |
| Gd2O3 | cubic (Ia3) | 3+ | 0.938 |
| Parameters | Value |
|---|---|
| Powder feed rate (g/min) | 20 |
| Gas flow (L/min) | Hydrogen (22.5) |
| Argon (100) | |
| Gun speed (mm/s) | 1000 |
| Gun to work distance (mm) | 100 |
| Thickness after 1 pass (µm) | 6.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, T.-s.; Adomako, N.K.; Ashong, A.-n.; Kim, Y.-k.; Yang, S.-m.; Kim, J.-h. Interfacial Structure and Physical Properties of High-Entropy Oxide Coatings Prepared via Atmospheric Plasma Spraying. Coatings 2021, 11, 755. https://doi.org/10.3390/coatings11070755
Park T-s, Adomako NK, Ashong A-n, Kim Y-k, Yang S-m, Kim J-h. Interfacial Structure and Physical Properties of High-Entropy Oxide Coatings Prepared via Atmospheric Plasma Spraying. Coatings. 2021; 11(7):755. https://doi.org/10.3390/coatings11070755
Chicago/Turabian StylePark, Tae-sung, Nana Kwabena Adomako, Andrews-nsiah Ashong, Young-kuk Kim, Seung-min Yang, and Jeoung-han Kim. 2021. "Interfacial Structure and Physical Properties of High-Entropy Oxide Coatings Prepared via Atmospheric Plasma Spraying" Coatings 11, no. 7: 755. https://doi.org/10.3390/coatings11070755
APA StylePark, T.-s., Adomako, N. K., Ashong, A.-n., Kim, Y.-k., Yang, S.-m., & Kim, J.-h. (2021). Interfacial Structure and Physical Properties of High-Entropy Oxide Coatings Prepared via Atmospheric Plasma Spraying. Coatings, 11(7), 755. https://doi.org/10.3390/coatings11070755






