Plasma Enhanced-Chemical Vapor Deposition of 2-Isopropenyl-2-Oxazoline to Promote the Adhesion between a Polyethylene Terephthalate Monofilament and the Rubber in a Tire
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasma Reactor and Parameters
2.3. Characterizations Techniques
2.4. Adhesion Studies
3. Results and Discussion
3.1. Thickness
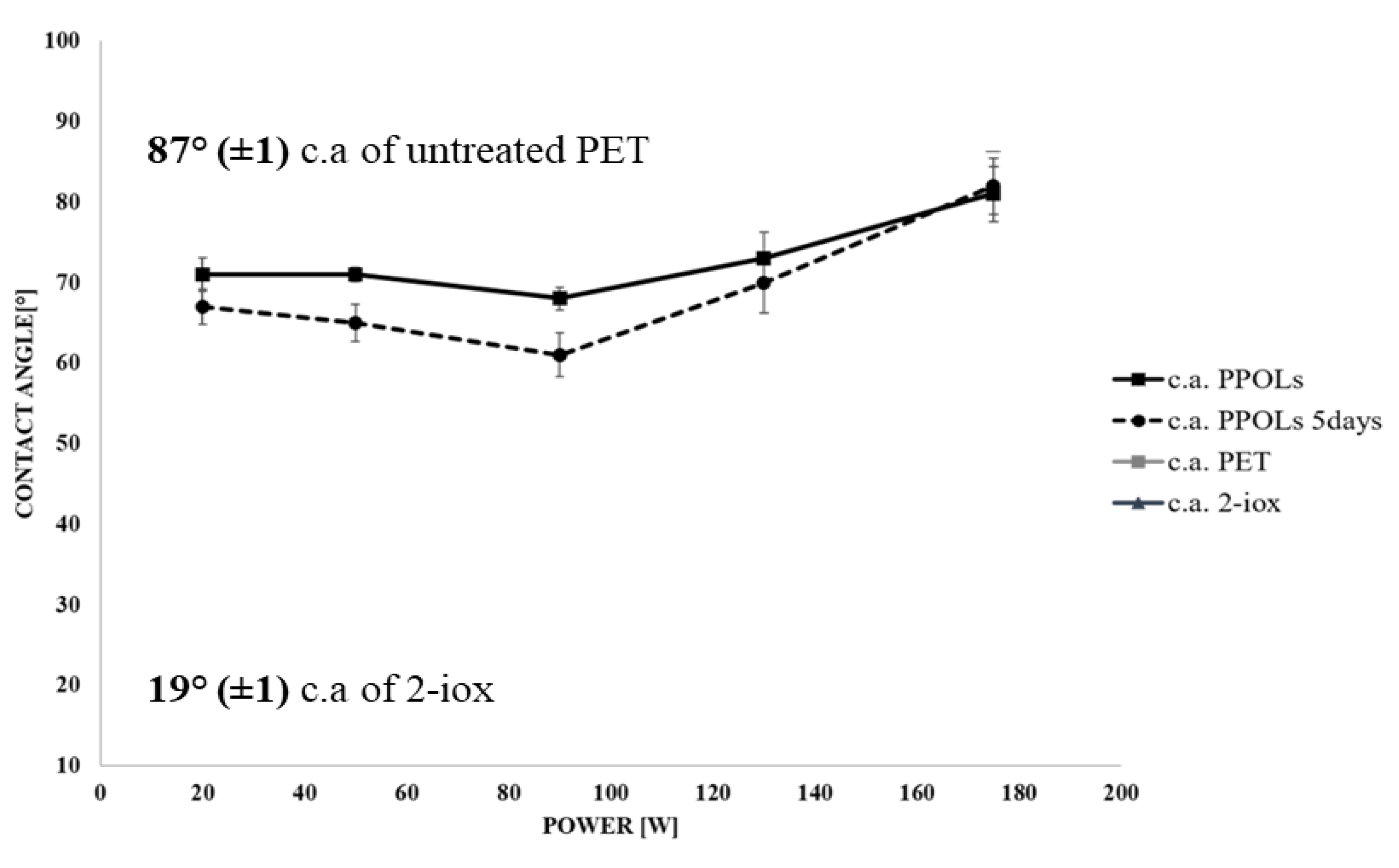
3.2. Wettability
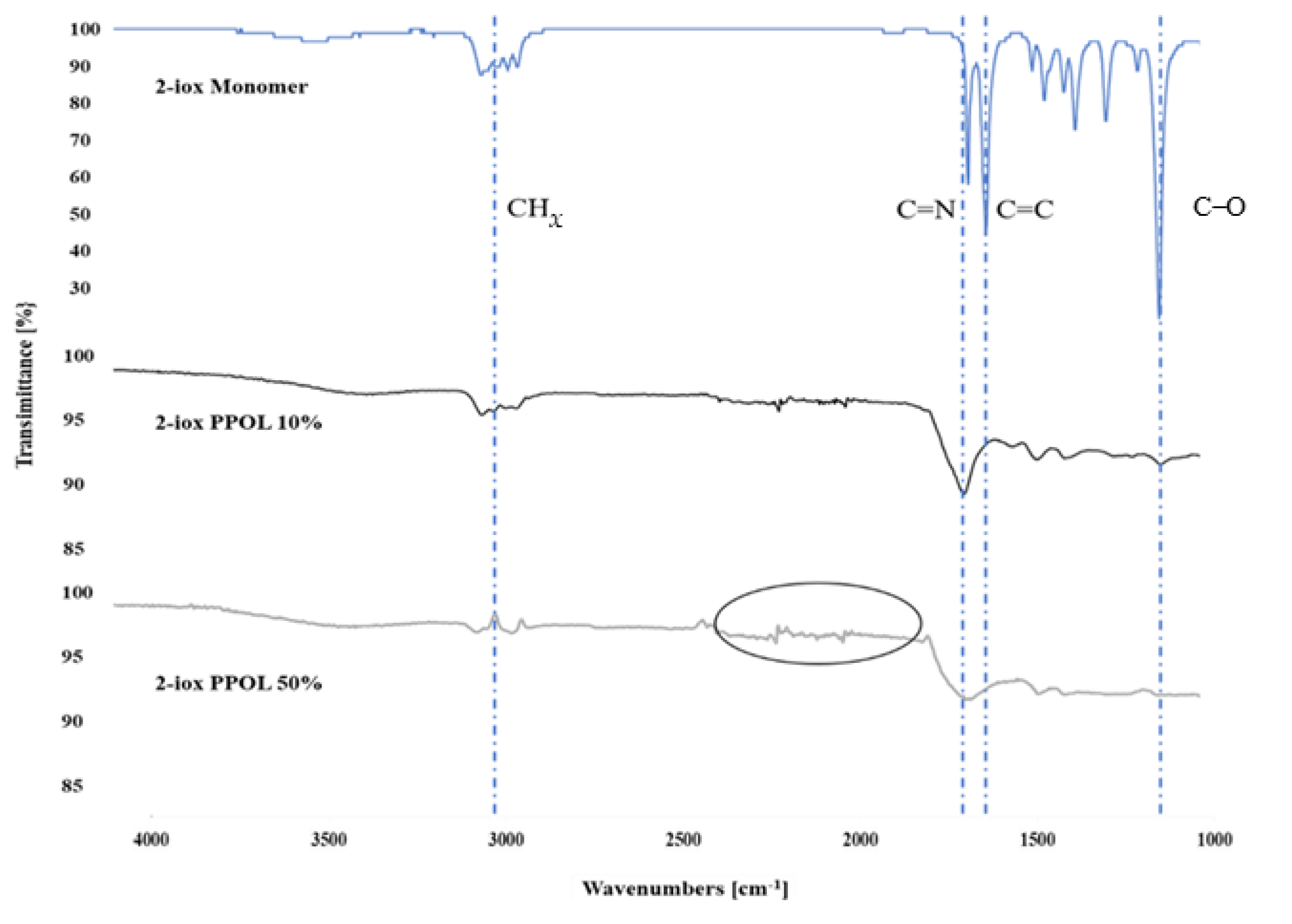
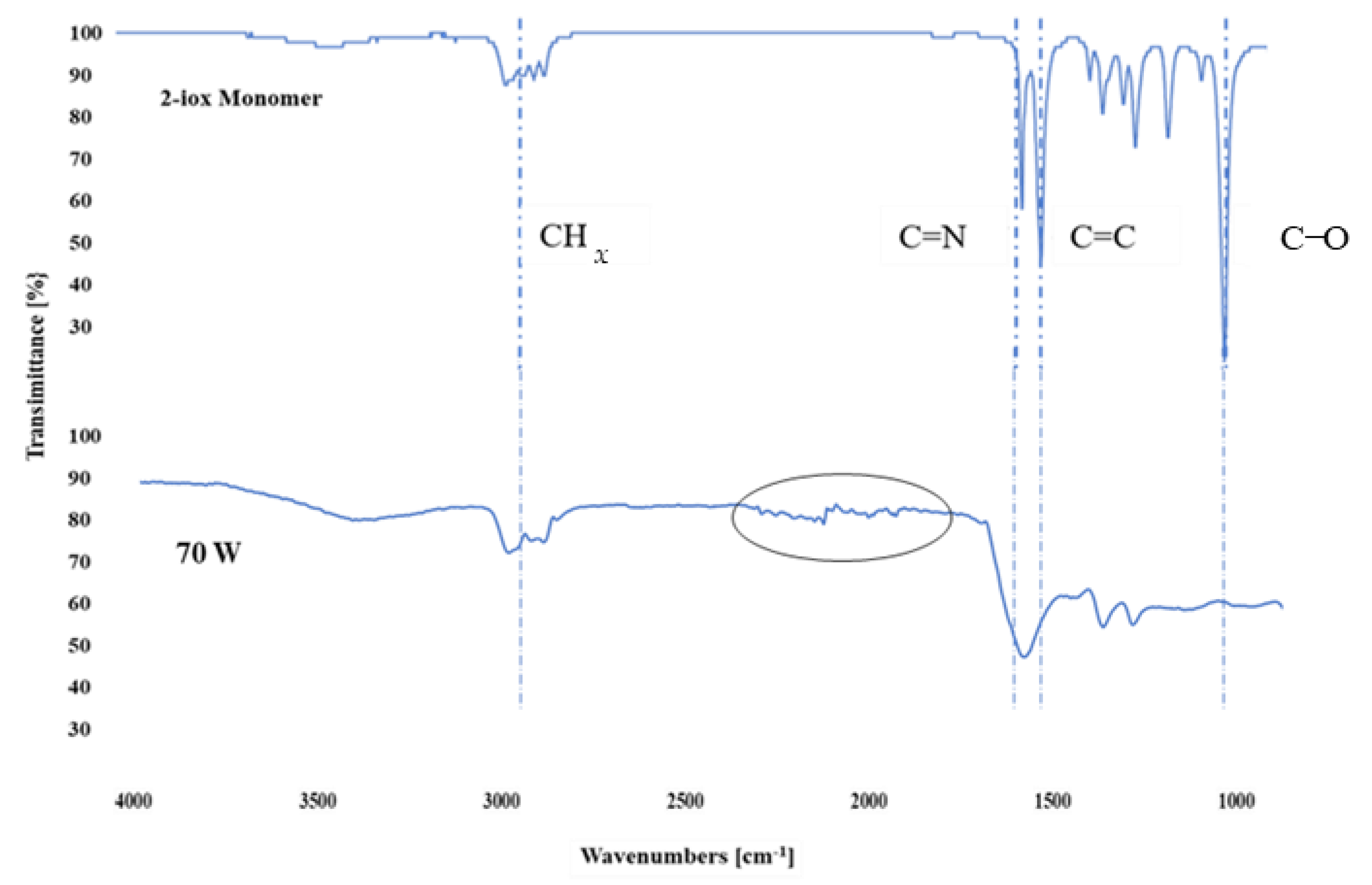
3.3. Chemical Characterizations
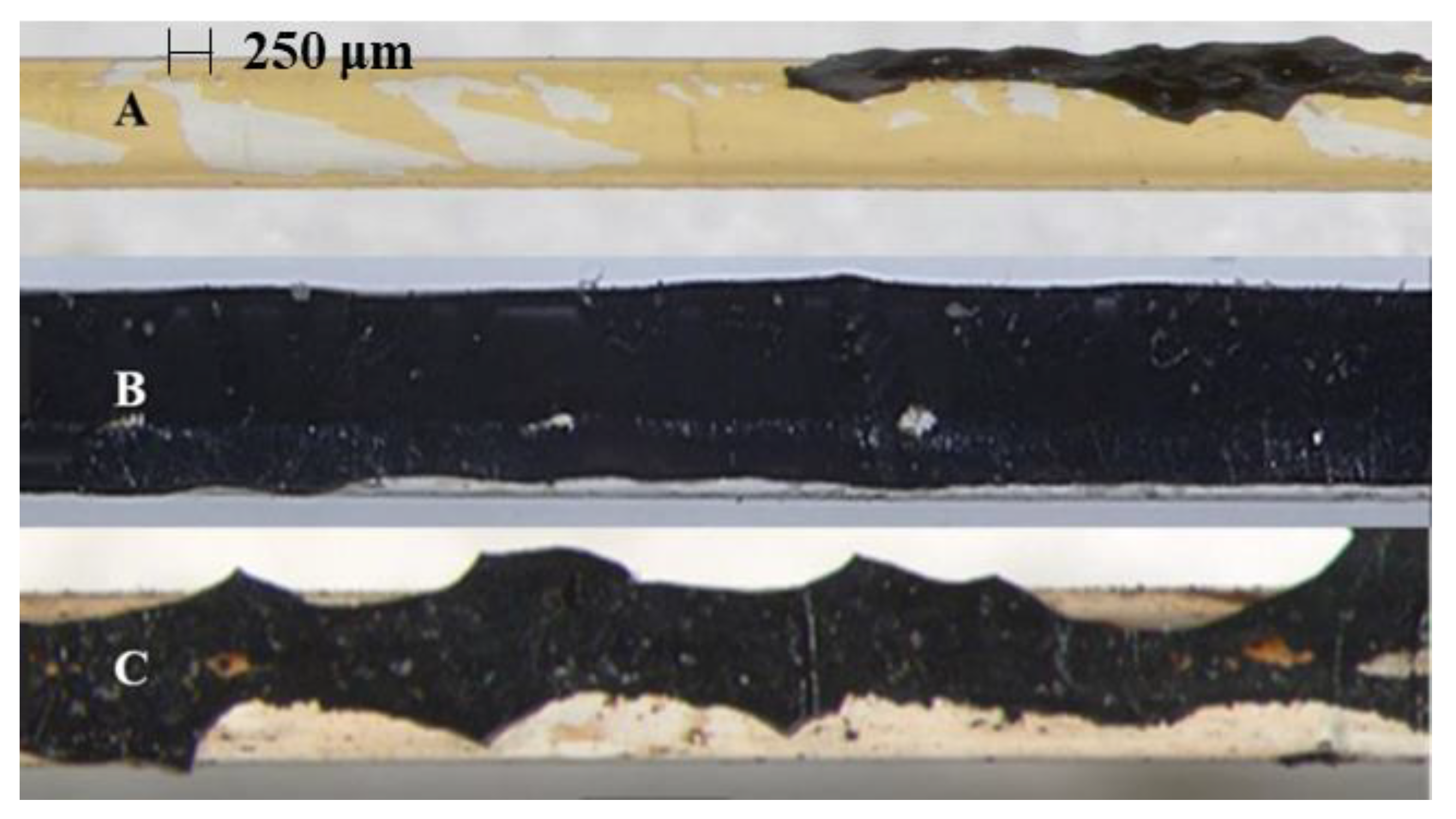
3.4. Adhesion Characterization
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leister, G. Passenger Car Tires and Wheels: Development—Manufacturing—Application; Springer: New York, NY, USA, 2018. [Google Scholar]
- Wootton, D. The Application of Textiles in Rubber; Smithers Rapra Publishing: Shrewsbury, UK, 2001. [Google Scholar]
- Gent, A.N.; Walter, J.D. Pneumatic Tire. In Mechanical Engineering Faculty Research; University of Akron: Akron, OH, USA, 2006; p. 864. [Google Scholar]
- Jefferson, S.C. EI DuPont De Nemours & CO. US Patent 3307966A, 7 March 1967. [Google Scholar]
- Jaššo, M.; Krump, H.; Hudec, I.; St’ahel’, P.; Kováčik, D.; Šíra, M. Coating of PET cords at atmospheric pressure plasma discharge in the presence of butadiene/nitrogen gas mixtures. Surf. Coat. Technol. 2006, 201, 57. [Google Scholar]
- National Toxicology Program. Toxicology and Carcinogenesis Studies of Resorcinol (CAS No. 108-46-3) in F344 Rats and B6C3F1 Mice (Gavage Studies). Natl. Toxicol. Program Tech. Rep. Ser 1992, 403, 1–234. [Google Scholar]
- Cevahir, N.N.; Acar, A.E.; Bas, S. Kordsa Global Industry. WO Patent 2014091376A1, 19 June 2014. [Google Scholar]
- Parker, D.K.; Shuttleworth, D. The Goodyear Tire & Rubber Company. US Patent 5501880, 26 March 1996. [Google Scholar]
- Wennekes, W. Adhesion of RFL-Treated Cords to Rubber: New Insights into Interfacial Phenomena. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2008. [Google Scholar]
- Barni, R.; Zanini, S.; Riccardi, C. Diagnostics of reactive RF plasmas. Vacuum 2007, 82, 217–219. [Google Scholar] [CrossRef]
- Raffaele Addamo, A.; Riccardi, C.; Selli, E.; Barni, R.; Piselli, M.; Poletti, G.; Orsini, F. Characterization of plasma processing for polymers. Marcandalli Surf. Coat. Technol. 2003, 174, 886–890. [Google Scholar] [CrossRef]
- Zanini, S.; Riccardi, C.; Orlandi, M.; Grimoldi, E. Characterisation of SiOxCyHz thin films deposited by low-temperature PECVD. Vacuum 2007, 82, 290–293. [Google Scholar] [CrossRef]
- Zanini, S.; Orlandi, M.E.; Colombo, C.; Grimoldi, E.; Riccardi, C. Plasma-induced graft-polymerization of polyethylene glycol acrylate on polypropylene substrates. Eur. Phys. J. 2009, 54, 159–164. [Google Scholar] [CrossRef]
- Siliprandi, R.; Zanini, S.; Grimoldi, E.; Fumagalli, F.; Barni, R.; Riccardi, C. Atmospheric Pressure Plasma Discharge for Polysiloxane Thin Films Deposition and Comparison with Low Pressure Process. Plasma Chem. Plasma Process. 2011, 31, 353–372. [Google Scholar] [CrossRef]
- Zanini, S.; Massini, P.; Mietta, M.; Grimoldi, E.; Riccardi, C. Plasma treatments of PET meshes for fuel–water separation applications. J. Colloid Interface Sci. 2008, 322, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Biederman, H. Plasma Polymer Films; Imperial College Press: London, UK, 2004. [Google Scholar]
- Shuttleworth, D.; Mowdood, S.K.; Waddell, W.H.; Richards, J.L.; Ofstead, E.A.; Brenner, J.L. The Goodyear Tire & Rubber Company. EP0451425B1, 5 June 1996. [Google Scholar]
- Mzabi, S.L.; Siffer, F.G.A.; Bertemes, G.J.H.; Boes, C.E.F.; Gillick, J.G. The Goodyear Tire & Rubber Company. US Patent 20140374009 A1, 25 December 2014. [Google Scholar]
- Kagiya, T.; Matsuda, T. Selective Polymerization of 2-Isopropenyl-2-oxazoline and Cross-linking Reaction of the Polymers. Polym. J. 1972, 3, 307–314. [Google Scholar] [CrossRef]
- Chu, J.; Gillick, J.G. The Goodyear Tire & Rubber Company. US Patent 6703077B1, 9 March 2004. [Google Scholar]
- Zanini, S.; Zoia, L.; Della Pergola, R.; Riccardi, C. Pulsed plasma-polymerized 2-isopropenyl-2-oxazoline coatings: Chemical characterization and reactivity studies. Surf. Coat. Technol. 2018, 334, 173–181. [Google Scholar] [CrossRef]
- Siow, K.; Britcher, L.; Kumar, S.; Griesser, H. Plasma methods for the generation of chemically reactive surfaces for biomolecule immobilization and cell colonization-a review. Plasma Process. Polym. 2006, 3, 392–418. [Google Scholar] [CrossRef]
- Zanini, S.; Ziano, R.; Riccardi, C. Stable Poly(Acrylic Acid) Films from Acrylic Acid/Argon Plasmas: Influence of the Mixture Composition and the Reactor Geometry on the Thin Films Chemical Structures. Plasma Chem. Plasma Process. 2009, 29, 535. [Google Scholar] [CrossRef]
- Zanini, S.; Barni, R.; Della Pergola, R.; Riccardi, C. Modification of the PTFE wettability by oxygen plasma treatments: Influence of the operating parameters and investigation of the ageing behavior. J. Phys. 2014, 47, 325202. [Google Scholar] [CrossRef]
- Zanini, S.; Zoia, L.; Dell’Orto, E.C.; Natalello, A.; Villa, A.M.; Della Pergola, R.; Riccardi, C. Plasma polymerized 2-ethyl-2-oxazoline: Chemical characterization and study of the reactivity towards different chemical groups. Mater. Des. 2016, 108, 791–800. [Google Scholar] [CrossRef]
- Bracco, G.; Holst, B. Surface Science Techniques; Springer: Berlin/Heidelberg, Germany, 2013; Volume 51, p. 663. [Google Scholar]
- Finn, R.; McCuan, J.; Wente, H.C. Thomas Young’s Surface Tension Diagram: Its History, Legacy, and Irreconcilabilities. J. Math. Fluid Mech. 2012, 14, 445. [Google Scholar] [CrossRef]
- Olsztyńska-Janus, S.; Czarnecki, M.A. Effect of elevated temperature and UV radiation on molecular structure of linoleic acid by ATR-IR and two-dimensional correlation spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 238, 118436. [Google Scholar] [CrossRef] [PubMed]
- Daccà, A.; Gemme, G.; Parodi, R. Techniche di Spettroscopia Elettronica per L’Analisi di Superfici; Istituto Nazionale di Fisica Nucleare: Rome, Italy, 1997; Volume 97, p. 14. [Google Scholar]
- Kim, K.-S.; Aravas, N. Elastoplastic analysis of the peel test. Int. J. Solids Struct. 1988, 24, 417–435. [Google Scholar] [CrossRef]
- Macgregor, M.; Cavallaro, A.; Vasilev, K. Antibiofouling Properties of Plasma-Deposited Oxazoline-Based Thin Films. J. Mater. Chem. B. 2015, 3, 10. [Google Scholar]


| Power (W) | Thickness (nm) |
|---|---|
| 20 | 235 ± 20 |
| 50 | 357 ± 18 |
| 90 | 364 ± 21 |
| 130 | 503 ± 25 |
| 175 | 448 ± 18 |
| Duty Cycle (%) | Effective Power (W) (W × d.c.) | Thickness (nm) |
|---|---|---|
| 10% | 18 | 202 ± 10 |
| 30% | 53 | 334 ± 16 |
| 50% | 88 | 417 ± 21 |
| 70% | 123 | 426 ±25 |
| 100% | 175 | 448 ± 24 |
| Element | C | O | N | |||
|---|---|---|---|---|---|---|
| Sample | C–C C=C | C–O | O–C=O | C–O–C | C=O | – |
| Pristine | 83.4% | 16.6% | – | |||
| 74.1% | 5.7% | 3.6% | 8.4% | 8.2% | – | |
| 10% | 73.9% | 12.5% | 13.6% | |||
| 47.1% | 19.2% | 7.6% | 6.3% | 6.2% | ||
| 50% | 76.7% | 10.7% | 12.6% | |||
| 53.0% | 17.3% | 6.4% | 5.3% | 5.4% | ||
| 175 W | 77.9% | 10.2% | 10.9% | |||
| 55.1% | 18.7% | 4.1% | 5.1% | 5.1% | ||
| PPOL | CO/C% |
|---|---|
| 10% d.c. | 26% |
| 50% d.c. | 22% |
| Plasma Regime | PPOL | PE-CVD (2-iox) | Epoxy + RFL | Avg. Peel Force | De.st. |
|---|---|---|---|---|---|
| Continuous | 50 W | V | V | 36.9 N | 4.2 |
| Continuous | 50 W | V | X | 22.9 N | 1.9 |
| Continuous | 90 W | V | V | 33.6 N | 5.8 |
| Continuous | 90 W | V | X | 23.1 N | 1.7 |
| Pulsed | 10% | V | X | 19.2 N | 2.3 |
| Pulsed | 10% | V | V | 14.8 N | 3.0 |
| Pulsed | 50% | V | X | 55.3 N | 5.9 |
| Pulsed | 50% | V | V | 15.3 N | 2.3 |
| – | EPOXY+ISOCYANATE | V | 52.9 N | 4.7 | |
| PET | UNTREATED | X | 11.3 N | 2.9 | |
| Covering | Ar Activation | PE-CVD | Epoxy + RFL | Avg. Peel Force | De.st. | Coverage |
|---|---|---|---|---|---|---|
| 50% PPOL | X | V | X | 6.3 N | 0.5 | 1 |
| 50% PPOL | V | V | X | 14.3 N | 1.7 | 4 |
| CLASSICAL | EPOXY+ISOCYANATE | V | 19.7 N | 1.3 | 3 | |
| PET | UNTREATED | X | 4.2 N | 0.2 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaifami, C.M.; Zanini, S.; Zoia, L.; Riccardi, C. Plasma Enhanced-Chemical Vapor Deposition of 2-Isopropenyl-2-Oxazoline to Promote the Adhesion between a Polyethylene Terephthalate Monofilament and the Rubber in a Tire. Coatings 2021, 11, 708. https://doi.org/10.3390/coatings11060708
Gaifami CM, Zanini S, Zoia L, Riccardi C. Plasma Enhanced-Chemical Vapor Deposition of 2-Isopropenyl-2-Oxazoline to Promote the Adhesion between a Polyethylene Terephthalate Monofilament and the Rubber in a Tire. Coatings. 2021; 11(6):708. https://doi.org/10.3390/coatings11060708
Chicago/Turabian StyleGaifami, Carlo Maria, Stefano Zanini, Luca Zoia, and Claudia Riccardi. 2021. "Plasma Enhanced-Chemical Vapor Deposition of 2-Isopropenyl-2-Oxazoline to Promote the Adhesion between a Polyethylene Terephthalate Monofilament and the Rubber in a Tire" Coatings 11, no. 6: 708. https://doi.org/10.3390/coatings11060708
APA StyleGaifami, C. M., Zanini, S., Zoia, L., & Riccardi, C. (2021). Plasma Enhanced-Chemical Vapor Deposition of 2-Isopropenyl-2-Oxazoline to Promote the Adhesion between a Polyethylene Terephthalate Monofilament and the Rubber in a Tire. Coatings, 11(6), 708. https://doi.org/10.3390/coatings11060708







