Abstract
In this paper, the effect of rare earth Ce on the corrosion resistance of Al-20SiC composites treated with high-current pulsed electron beams is investigated, and the corresponding corrosion mechanism is proposed. The scanning electron microscope (SEM) results show that cracks arise on the surface of Al-20SiC composites prepared by pressureless sintering. After electron beam treatment, the pores on the surface are reduced because of the filling of Al liquid. After adding CeO2 to Al-20SiC composites, the wettability between Al and SiC phases is improved, thus realizing metallurgical bonding of the two phases, and microcracks generated after HCPEB treatment are significantly eliminated. Glancing X-ray diffraction (GIXRD) results show that after electron beam treatment, aluminum grains tend to grow more favorably with the stable and dense crystal plane of Al(111), thus improving corrosion resistance. The electrochemical test results show that the corrosion current density decreases by one order of magnitude with increase in the number of pulses because of rare earth Ce compared to the initial Al-20SiC composite specimens, indicating that the corrosion resistance of the Al-20SiC-0.3CeO2 composite is improved. This is because rare earth not only eliminates microcracks, but also changes the type of corrosion from localized to uniform, thus improving corrosion resistance. The Al-based composite material modified by electron beam and rare earth has many potential applications and development prospects.
1. Introduction
Al-SiC composite material, a type of Al-based composite material, has low density, high specific strength, excellent thermal conductivity, and dimensional stability characteristics. It is extensively used in aerospace applications, electronic integrated circuits, and packaging materials [1,2,3,4,5,6,7,8]. The performance of the Al matrix composite prepared by adding SiC to the aluminum matrix is similar to the performance of the second phase reinforcement and aluminum alloy matrix [9], which can meet the practical application requirements of high-strength Al alloys. The preparation of Al-SiC composite materials uses abundant raw materials and is inexpensive, with a low processing cost. The conventional methods for preparing Al-SiC composites include hot-press sintering, stirring, and powder metallurgy, with hot-press sintering having a high cost and low efficiency [10]. The structure of the material prepared by the stirring method is coarse, segregation is severe, and gas is easily introduced, which causes the quality of the casting to deteriorate [11]. The powder metallurgy method has the advantages of weak interfacial reaction, wide selection range of reinforcement, and low preparation cost, and is suitable for large-scale industrial production. However, a considerable amount of gas will be generated during the pressureless sintering process, which will cause the material to be loose and porous. Even if the subsequent pressing treatment will reduce some porosity, the ability to improve the pores is still limited [12]. Therefore, the pores, cracks, agglomerates, and other defects produced in the above-mentioned powder metallurgy preparation technology limit the wide application of Al-SiC composites, and a new solution to the above-mentioned problems is required.
The life of parts essentially depends on the surface properties of the material. Surface optimization can effectively improve the overall performance of the material. However, surface modification can achieve better surface quality, thereby increasing the service life of the material [13]. Recently, high-current pulsed electron beam technology (HCPEB), a surface modification micro-structure optimization technology, has attracted considerable attention because it is not affected by ion impurities, as ion beam technology is, and it has a much higher energy efficiency than the laser beam [14,15,16]. Ivanov et al. used a high-current pulsed electron beam to process hypoeutectic Al-Si alloy and found that because of the presence of the submicron Al-based solid solution formed by HCPEB irradiation and nano-scale structure at the grain boundary, the hardness of the surface layer increased by 3.8–4.2 times [17]. During the HCPEB treatment process, the surface can quickly melt and solidify in a short time (the cooling rate is as high as 107–109 K/s). Simultaneously, physical phenomena such as stress and shock waves are generated in the process [18,19,20,21]. During the modification process, the surface layer forms metastable phase structures such as nano-crystals, dislocations, and twins, which conventional surface treatment techniques fail to achieve [22]. Hutsaylyuk reported that in recent decades, electron beam processing technology has been widely used for surface modification of pure metals and their alloys. This technology has significantly improved the surface properties of materials, shows new potential applications, and is promoted for industrial applications [23].
Based on a related report, Cu-Cr alloys treated with high-current pulsed electron beam treatment prepared with the powder metallurgy technique produce fine-grained micro-structures, with excellent surface properties [24]. Hutsaylyuk used plasma to spray an oxide ceramic layer containing Cu and Ni on an Al alloy, which is localized in the pores of the oxide-ceramic layer and reduces its porosity and improves wear resistance [25]. However, there is no report on the use of HCPEB irradiation to treat Al-20SiC composites and to solve the defects, such as pores and cracks in the composites during the powder metallurgy process. The rare-earth element Ce is used to eliminate the above-mentioned defects. Because rare earths are beneficial elements, they can significantly eliminate micro-cracks and pores in the material and improve phase interface bonding Therefore, this study focuses on the micro-structure changes of Al-20SiC-0.3CeO2 composites before and after HCPEB irradiation and the effect of rare earth Ce on the electrochemical corrosion resistance of materials.
2. Experiment
2.1. Preparation of Materials
The raw materials used in this experiment were commercial Al powder (purity 99.9 wt.%, size 50 µm), SiC powder (purity 99.9 wt.%, size 10.3 µm), and cerium oxide powder (purity 99.9%, size 8 µm). The powder metallurgy process was used to prepare Al-20SiC and Al-20SiC-0.3CeO2 composite materials. The specific preparation process was as follows. To prepare for ball milling, Al powder, SiC powder, cerium oxide powder (cerium oxide powder is not required for Al-20SiC composites in this step), and 1 wt.% hydroxypropyl methylcellulose (HPMC) binder, as well as zirconia agate balls, were weighed to place in the ball milling tank with a ball-to-material ratio of 3:1. Finally, the above ball mill mixture was mixed in a drum ball mill for 5 h. The mixed powder was then loaded into a groove in a steel mold for cold isostatic pressing to produce a raw billet with dimensions of 50 mm × 10 mm × 5 mm, with a holding pressure of 288 MPa for 3 min during the molding process. Next, the raw embryos were sintered in a tubular resistance furnace at 590 °C with a heating rate of 9 °C/min and held for 8 h to produce Al-20SiC-0.3CeO2 composites. Before the HCPEB treatment, the Al-20SiC and Al-20SiC-0.3CeO2 composite materials were cut into 10 mm × 10 mm × 5 mm samples with a metal cutting machine and then mechanically polished with different types of sandpaper (100#, 240#, 400#, 800#, 1500#, and 3000#) and diamond polishing paste (1 µm particle size) in sequence. The polished sample was ultrasonically cleaned in absolute ethanol for 7 min and then dried with a hairdryer before being subjected to electron beam treatment.
2.2. HCPEB Treatment
The surface modification of the material uses a HOPE-I type high-current pulsed electron beam processing device manufactured by the Dalian University of Technology (Liaoning, China). The corresponding process parameters are as follows: the pulse duration is 3 µs, the electron acceleration voltage is 24.5 kV, energy density is 2 J/cm2, vacuum degree is 6.5 × 10−3 Pa, and pulse times are 5, 15, and 25 times, respectively.
2.3. Micro-Structure Characterisation and Performance Analysis
The micro-structure of aluminum matrix composites before and after HCPEB modification was characterized using field-emission scanning electron microscopy (model TESCAN MIRA3, Tescan, Shanghai, China). The surface phase analysis was analyzed using a multifunctional X-ray diffractometer (model X’Pert PRO, PANalytical, Alemlo, The Netherlands). The test uses Cu target K radiation, corresponding to the X-ray characteristic wavelength λ = 0.001 Å, and graphite monochromator filtering; the scanning step is 0.05°, the acceleration voltage is 40 kV, the current is 40 mA, the scanning range is 10°–90°, and time per step is 0.8 min. The corrosion resistance of the Al-based composite material surface was measured using an electrochemical workstation (model CHI760E, China Chenhua Company, Shanghai, China) in a 5 wt.% NaCl aqueous solution. The electrochemical system adopts a three-electrode system, which includes a working (composite material sample), auxiliary (platinum electrode), and reference electrode (saturated calomel electrode). The scanning speed is 0.005 V/s, and the scanning range is open circuit potential ranging from −1.5 to 0 V. Microcrack density refers to the length of microcracks per unit (mm−1). It is used as a quantitative index to evaluate microcracks after electron beam treatment.
3. Results and Discussion
GIXRD Analysis
Figure 1 shows the Grazing Incidence X-ray Diffraction (GIXRD) patterns of Al-20SiC-0.3CeO2 composite samples before and after electron beam treatment. Figure 1a shows that, compared with the original sample, no new diffraction peaks are observed after high-current pulsed electron beam treatment, indicating that the sample with CeO2 has no phase change before and after electron beam treatment. Note that the CeO2 phase is not detected in the GIXRD pattern. This is primarily because the low rare earth content on the alloy surface does not reach the detection limit of the GIXRD instrument. To accurately measure the intensity of diffraction peaks, this study uses grazing incidence X-ray technology to measure Al diffraction peaks. Because of the small incidence angle of X-rays, the area of interaction between X-rays and the material is large, which improves the sensitivity of X-rays to the detection of surface information, and avoids strong signals from the substrate; furthermore, the intensity of diffraction peaks for the electron beams in the modified layer can be accurately measured. The GIXRD image in Figure 1a shows that as the number of pulses increases, the intensity of all Al diffraction peaks first increases and then decreases. The intensity of the Al diffraction peaks is strongest at the fifth pulse. The Al(220) and Al(311) diffraction peaks disappeared after 15 pulses, indicating that the high-current pulsed electron beam treatment changed the crystal orientation of the surface of the sample, and the Al grains were preferentially aligned along with the Al(111) and Al(200) crystal planes. The growth tendency is enhanced. The change of orientation of the material induced by the electron beam can be attributed to the following reasons. First, the rapid solidification process induced by the electron beam can change the crystal orientation. In fact, the precipitated phases generated after the nucleation of the surface-melting zone during the cooling process will grow along a specific crystal direction as the temperature gradient changes. Lonardelli [26] used neutron diffraction to study Ti alloys, where β-phase grains precipitated after melting in the surface region of the alloy grow along the gradient direction of temperature (perpendicular to the irradiation direction) and subsequently generate the α’martensite of specific orientation by the metastable selection. Second, the thermal stress generated by the rapid cooling process changes crystal orientation. Zou treated AISI 316L stainless steel surface with a high-current pulsed electron beam and discovered that severe plastic deformation occurred in the surface layer, and mechanical twins were activated in the crystal grains, which grew along the <1 1 1> direction close to the normal line of the sample [27]. Hao et al. treated AISI 316L stainless steel with a high-current pulsed electron beam and discovered that because of the plastic deformation during the modification process, the Al(111) crystal plane became the preferred direction of modification [28]. However, in this experiment, because no new phase is generated, the assumption that the phase transition changes crystal orientation is ruled out.
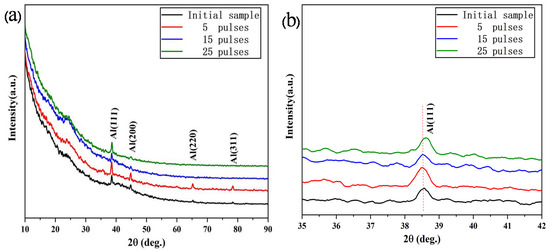
Figure 1.
GIXRD patterns of the Al-20SiC-0.3CeO2 sample before and after high-current pulsed electron beam (HCPEB) technology irradiation: (a) complete GIXRD pattern, (b) enlarged pattern of the lattice plane of Al(111).
Combining the above-mentioned analysis, the Al(111) and Al(200) crystal face preferential growth in this study is attributed to the rapid solidification effect induced by the electron beam and plastic deformation induced by the thermal stress generated during the electron beam treatment. Moreover, the Al(111) surface is close-packed. The surface has the smallest surface energy and good stability, which is conducive to improving the microstructure and performance [29]. In addition, Figure 1b (the magnified view of Figure 1a) shows that after 25 pulses, the diffraction peak shifts to a high angle, which is attributed to residual compressive stress in the modified layer. The residual compressive stress on the alloy surface reduces interplanar spacing and causes lattice distortion [30,31]. The effect of compressive stress is primarily attributed to the shift of the diffraction peaks to high angles. This phenomenon has been reported in the Al-Si alloy and nickel alloy systems [32,33]. Residual compressive stress contributes to improved corrosion resistance, which is confirmed in the following analysis of results. Increasing the residual compressive stress prevents Cl−1 penetration, while crack initiation is suppressed to an extent, and corrosion resistance is enhanced [34].
Figure 2 shows the surface micro-structure of the Al-20SiC composite before and after HCPEB treatment. Figure 2a(A) in the upper right corner is a magnified view of the yellow area in Figure 2a. Figure 2a(A) shows that the grey silicon carbide phase in the original structure is evenly distributed in the Al matrix, and the size of the SiC particles is ~10 μm. A certain amount of pore structure can be observed close to the SiC particles. The formation of this structure is attributed to the relatively high viscosity of liquid Al at a lower sintering temperature of 590 °C, which causes a poor flow of the Al liquid, which will make the material unable to complete the replenishment of the molten aluminum during the solidification process. Thus, the material shrinks to form a pore structure. After the electron beam treatment, the large-sized pores on the sample surface are reduced because of the filling of Al liquid; however, there are many micro-pores, as shown in Figure 2b–d. After the electron beam is irradiated, the surface of the sample is rapidly cooled at a rate of up to 108 K/S. The intense surface tension generated during the cooling process will cause the molten surface to rapidly shrink and form micro-pores. Compared with the original sample (see Figure 2a), the size of the micro-pores produced by the electron beam treatment is greatly reduced, particularly when the surface is irradiated with 15 and 25 pulses, and fewer micro-pores are observed on the surface. Because the continuously increasing energy provides a sufficient duration for the molten state after multiple irradiations, the previously generated micro-pores are refilled by the flowing liquid [35]. Moreover, Figure 2c shows that there are additional micro-cracks on the surface after 15 pulse treatments, because the energy of the electron beam is converted into heat when it irradiates the surface.
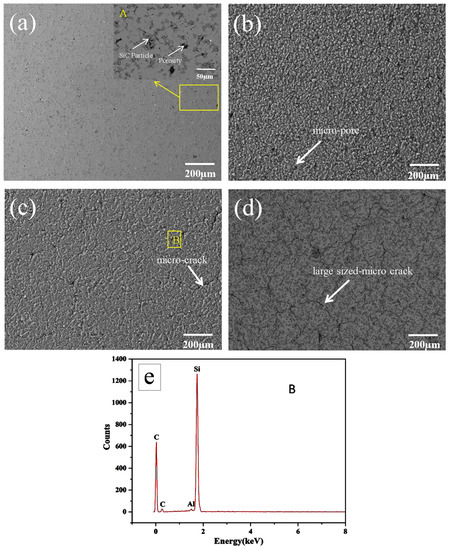
Figure 2.
Surface SEM images of Al-20SiC sample before and after HCPEB irradiation: (a) initial, (b) five pulses, (c) 15 pulses, (d) 25 pulses (BSE image), and (e) EDS results of the A region in Figure 2c.
In a short time, heat cannot be transferred to the matrix, and a larger thermal stress is concentrated on the surface. When the thermal stress exceeds the yield limit of the material, micro-cracks will form [36]. Because the Al matrix has high plasticity and the reinforced SiC phase has high brittleness, micro-cracks tend to form in the brittle phase, as shown in the EDS energy spectrum of Figure 2e. Figure 2d shows that the large-sized microcracks on the surface of the sample with 25 pulses are significantly increased compared to 5- and 15-pulsed samples (Figure 2b,c). The results show that with an increase in the number of pulses, tensile stress accumulates on the material surface. Stress promotes the initiation and propagation of microcracks, leading to an increase in microcracks.
Figure 3 shows the surface micro-structure of the Al-20SiC-0.3CeO2 composite before and after HCPEB treatment. Figure 3a(C) is a partially magnified view of the yellow area, whereas Figure 2e shows the energy spectrum of Point D. Compared with the sample without CeO2, the original sample of Al-20SiC-0.3CeO2 has a smaller number of macro-pores, as shown in Figure 2a and Figure 3a.
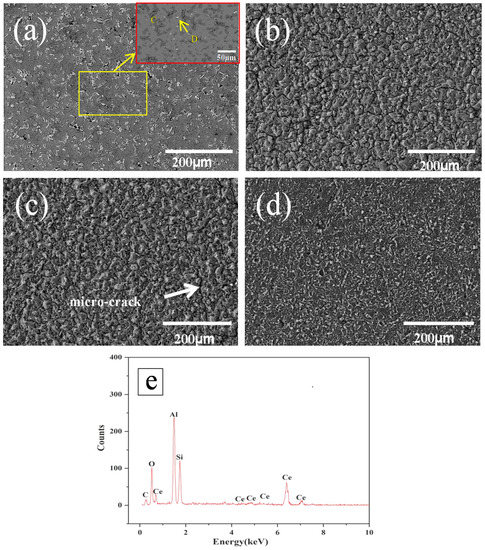
Figure 3.
Surface SEM images of Al-20SiC-0.3CeO2 sample before and after HCPEB irradiation: (a) initial, (b) 5 pulses, (c) 15 pulses, (d) 25 pulses, and (e) EDS results of the B point in Figure 3a.
This is primarily because the addition of CeO2 can significantly improve the wettability between the liquid-phase Al and solid-phase SiC, and therefore, relatively dense samples can be obtained. The white phase indicated by the point in Figure 3a was analyzed by SEM-EDS and indicated by the D point, which was found to be a Ce-rich intermetallic compound by SEM-EDS; the corresponding EDS results are shown in Figure 3e. Compared to the specimens without the addition of CeO2 (see Figure 2c,d), the surface micro-cracking was significantly reduced after the electron beam treatment, as shown in Figure 3c,d. The above phenomenon indicates that the addition of the rare-earth oxide CeO2 significantly improved the surface micro-cracking of the alloy. Based on a related study [36], rare-earth elements and their rare-earth oxides can eliminate micro-cracks generated in a molten cladding layer. The following is a detailed description of the mechanism of rare earth’s elimination of micro-cracks: CeO2 reacts with impurities in the re-melted layer of the high-current pulsed electron beam, weakening the local stress concentration of the brittle phase molten pool and reducing the sensitivity of micro-cracks, and thus suppresses the growth of micro-cracks and even eliminates them. Simultaneously, the surface tension is reduced such that the contact angle between Al and SiC becomes smaller, which aids in the elimination of pores. Besides, the addition of CeO2 helps to reduce the solid-liquid interface energy, improve the wettability of liquid aluminum and SiC, improve the bonding force of the metallurgical interface, reduce the probability of micro-cracks, and significantly improve the surface micro-structure.
The degree of surface micro-cracks (crack density) after HCPEB treatment is characterized through the measurement and calculation of crack length per unit area. Figure 4 shows the relationship between the micro-crack density and number of pulses. With an increase in the number of pulses, the crack density of samples with rare earth CeO2 and without rare earth CeO2 present an opposite trend. For the Ce-containing samples treated by HCPEB, the crack density decreased from 0.242 mm/mm2 for five pulses to 0.106 mm/mm2 for 25 pulses, indicating that the stress concentration decreases as the number of pulses increases. However, the crack density increase from 0.742 mm/mm2 for five pulses to 1.128 mm/mm2 for 25 pulses, indicating that the stress concentration increased with the number of pulses. Indeed, the change in the micro-cracks corresponds to the results in Figure 2; Figure 3, indicating that Ce reduces the stress concentration, reducing or even eliminating micro-cracks. The mechanism of rare earth’s elimination of micro-cracks caused by HCPEB treatment is summarized as follows: the presence of Ce significantly improves the surface micro-structure; at the same time, the stress concentration is reduced under the same pulse number; and finally, the micro-cracks are reduced.
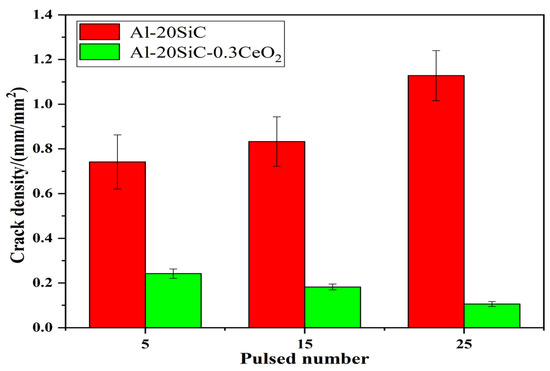
Figure 4.
Crack density (crack length per unit area) against the pulsed number on the HCPEB-treated alloy surfaces.
Figure 5 shows the dynamic electrochemical polarization curves of Al-20SiC and Al-20SiC-0.3CeO2 composites. Table 1 shows the calculated corrosion current density and potentials using the Tafel curve extrapolation method. For the Al-20SiC composite material, the corrosion current density of the Al-20SiC metal surface treated by HCPEB increased by one order of magnitude compared to the current density of the untreated material, which shows that the corrosion resistance of the material surface is reduced after electron beam treatment (25 pulses, 1.691 × 10−4 µA cm−2; initial sample, 7.652 × 10−5 µA cm−2). This performance is primarily related to the micro-cracks produced after HCPEB treatment. The corrosion solution easily penetrates microcracks, which leads to an accelerated corrosion process on the alloy surface. Liang et al. [32] studied the corrosion resistance of the surface of hypereutectic Al-Si alloy treated with HCPEB and reported that NaCl corrosive liquid easily penetrates the micro-cracks, accelerates the corrosion process of the material surface, and decreases the corrosion resistance of the material. However, they can significantly eliminate micro-cracks after adding the rare earth Nd, thereby improving the corrosion resistance of the material. This study verified the use of rare earths to improve the phenomenon of micro-cracks. After 15 and 25 pulse treatments, the corrosion current density of Al-20SiC-0.3CeO2 composite material decreased by an order of magnitude. This shows that the existence of CeO2 eliminates the micro-cracks, such that the corrosion resistance of the material surface has been significantly improved, which is helpful for the application of aluminum-based composite materials in the field of corrosion resistance. The specific corrosion resistance mechanism will be further analyzed below.
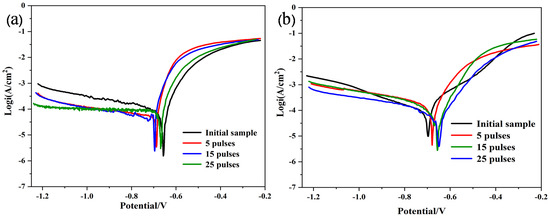
Figure 5.
CP curves of PM alloy samples before and after HCPEB irradiation in 5-wt.% NaCl solution. (a)Al-20SiC; (b)Al-20SiC-0.3CeO2.

Table 1.
Electrochemical parameters obtained in the corrosion measurements.
Figure 6 shows the corrosion morphology of the Al-20SiC composite material after being irradiated with a high-current pulsed electron beam without the addition of rare-earth elements. The figure shows that the brittle phase has exposed many grain boundaries of SiC particles after electrochemical corrosion, indicating that the grain boundaries of SiC particles have been corroded. Because of the elemental diffusion effect induced by the electron beam, Al will penetrate the brittle phase. Because the atoms at the grain boundaries deviate from the equilibrium position and have higher kinetic energy, there are additional defects such as holes, impurity atoms, and dislocations at the grain boundaries. Note that the Al atoms will diffuse along the grain boundaries of SiC particles rather than spreading to the inside. Next, the Al phase at the grain boundary corrodes under the corrosion of the NaCl solution, forming typical intergranular corrosion. Moreover, micro-cracks (yellow arrows) are reported within the grains, indicating that crack sources are formed in the grain boundaries. Because of the difference in chemical composition between the grain boundaries and grains, internal stress will be caused. Under the action of internal stress, micro-cracks propagate from the crack source at the grain boundary to the inside of the grain, resulting in the coexistence of intergranular corrosion and cracks. Based on the above analysis, the brittle phase has undergone intergranular corrosion, and the corroded material is the Al phase. This defect leads to the deterioration of corrosion resistance. The following factors are attributed to combining the corrosion morphology to explore the corrosion mechanism: After electron beam treatment, a large number of micro-cracks will appear on the surface of the alloy, allowing the NaCl solution to easily penetrate the micro-cracks and accelerate the corrosion process. There are few undissolved O2− ions in the micro-cracks; however, Cl− ions tend to be concentrated, which causes the metal to change from a passivated state to an activated state, and results in relatively low corrosion resistance of the alloy [37]. Moreover, the brittle phase will form a small-sized micro-porous structure after the electron beam treatment, and the alloy surface is subjected to residual stress. Consequently, micro-pores are easily used as corrosion initiation points, leading to the occurrence of pitting and stress corrosion. Therefore, the existence of the two structures is not conducive to surface corrosion resistance.
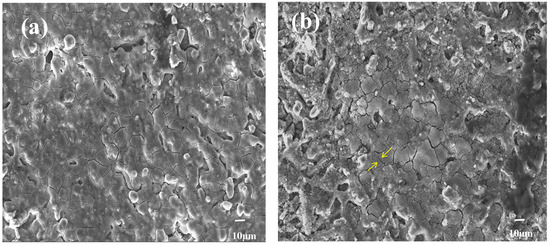
Figure 6.
SEM images of corrosion products for Al-20SiC composites after electrochemical test: (a) five pulses, (b) 15 pulses.
Figure 7 shows the typical morphology and energy spectrum of the Al-20SiC-0.3CeO2 alloy after electrochemical testing before and after electron beam irradiation. In Figure 6a, ‘petal-like’ cracks can be seen in the local area. Combined with the energy spectrum analysis of Figure 7d, it is found that the surface of the material is corroded from the brittle phase SiC. Figure 7b,c, along with the analysis of the corrosion results in Figure 6, show that as the number of pulses increases, the Al matrix composites with the addition of rare-earth elements have a relatively more complete surface with a better protective layer, good micro-structure, and greatly enhanced corrosion resistance compared to those without the addition of rare-earth elements. The improvement in corrosion resistance can be attributed to the following points: after adding rare-earth elements, the micro-structure of the alloy surface improves, the surface is relatively dense and flat, and the corrosive liquid is insulated from the substrate, which is beneficial to reducing pitting corrosion of the alloy surface and improves the passivation performance of the alloy [38]. Rare-earth elements not only eliminate micro-cracking but also change the form of corrosion, transforming local corrosion into uniform corrosion and improving corrosion resistance. As per electrochemical theory, first, Cl− corrodes the oxide layer on the outer surface of Al alloy in NaCl. It then forms a micro-couple with the substrate after destroying the surface film. Over time, the solid solution formed by the rare-earth elements in the Al matrix forms an inhibitory oxidation-hydroxide protective film on the surface of the cathode, which inhibits the corrosion activity in the cathode region and improves the corrosion resistance of the alloy. Moreover, the presence of rare-earth elements can significantly change the corrosion behavior of the substrate. Furthermore, because of the effect of rare earth eliminating micro-cracks, it prevents NaCl corrosion liquid from entering the alloy through micro-cracks, inhibits pitting corrosion, and improves the corrosion resistance of the alloy surface after electron beam treatment [36]. Furthermore, with the increase in residual compressive stress, the invasion of chloride ion is blocked, the crack initiation is restrained to an extent, and the corrosion resistance is enhanced.
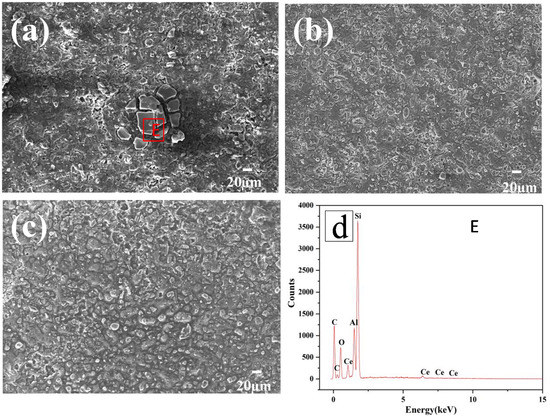
Figure 7.
Typical SEM image of Al-20SiC-0.3CeO2 sample after electrochemical test: (a) 5 pulses, (b) 15 pulses, (c) 25 pulses, and (d) EDS C region results.
4. Conclusions
The analysis of GIXRD results shows that the relative intensity of the diffraction peaks has changed, which can be attributed to the thermal stress generated during the rapid cooling process.
The SEM results show that the rapid melting and solidification effect of the electron beam causes the molten aluminum to fill and reduce the pores.
The scanning electron microscope results show that the existence of the rare-earth element Ce eliminates thermal stress in the electron beam during the cooling process, inhibiting the generation of micro-cracks.
The results of the electrochemical test and its corrosion morphology show that after adding rare-earth elements, the corrosion resistance is improved, primarily because of the elimination of micro-cracks. The corrosion resistance of samples treated by HCPEB treated without adding rare-earth elements decreases because of the generation of micro-cracks.
Author Contributions
Conceptualization, Y.S. and B.G.; methodology, Y.S.; software, Y.S.; validation, Y.S., B.G. and L.H.; formal analysis, Y.S.; investigation, Y.S., Y.Z. and K.L.; resources, B.G.; data curation, Y.S. and L.H.; writing—original draft preparation, Y.S. and B.G.; writing—review and editing, Y.S.; visualization, Y.S.; supervision, B.G.; project administration, B.G.; funding acquisition, B.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (51671052), the Fundamental Research Funds for the Central Universities (N182502042), and the Liao Ning Revitalization Talents Program (XLYC1902105).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data can be requested from the corresponding author at request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, Q.Y.; Wang, F.; Qiu, X.P.; An, D.; He, Z.Y.; Zhang, Q.F.; Xie, Z.P. Effffects of La and Ce on microstructure and properties of SiC/Al composites. Ceram. Int. 2020, 46, 1232–1235. [Google Scholar] [CrossRef]
- Sharifi, E.M.; Karimzadeh, F.; Enayati, M.H. Fabrication and evaluation of mechanical and tribological properties of boron carbide reinforced aluminum matrix nanocomposites. Mater. Des. 2011, 32, 63–3271. [Google Scholar]
- Feng, P.F.; Liang, G.Q.; Zhang, J.F. Ultrasonic vibration-assisted scratch characteristic-cs of silicon carbide-reinforced aluminum matrix composites. Ceram. Int. 2014, 40, 10817–10823. [Google Scholar] [CrossRef]
- Li, G.J.; Zhang, X.; Fan, Q.B.; Wang, L.L.; Zhang, H.M.; Wang, F.C. Simulation of damage and failure processes of interpenetrating SiC/Al composites subjected to dynamic compressive loading. Acta Mater. 2014, 78, 190–202. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, X.Y.; Wu, G.H. Interfacial microstructure of SiCp/Al composite produced by the pressureless infiltration technique. Ceram. Int. 2013, 39, l4893–l4897. [Google Scholar] [CrossRef]
- Wang, L.L.; Fan, Q.B.; Li, G.J.; Zhang, H.M.; Wang, F.C. Experimental observation and numerical simulation of SiC3D/Al interpenetrating phase composite material subjected to a three-point bending load. Comput. Mater. Sci. 2014, 95, 408–413. [Google Scholar] [CrossRef]
- Rodrguez-Reyes, M.; Pech-Canul, I.; Parras-Medécigo, E.E.; Gorokhovsky, A. Effect of Mg loss on the kinetics of pressureless infiltration in the processing of Al-Si-Mg/SiCp composites. Mater. Lett. 2003, 57, 2081–2089. [Google Scholar] [CrossRef]
- Shaga, A.; Shen, P.; Guo, R.F.; Jiang, Q.C. Effects of oxide addition on the microstructure and mechanical properties of lamellar SiC scaffolds and Al-Si-Mg/SiC composites prepared by freeze casting and pressureless infiltration. Ceram. Int. 2016, 42, 9653–9659. [Google Scholar] [CrossRef]
- Shen, P.; Wang, Y.; Ren, L.H.; Li, S.X.; Liu, Y.H.; Jiang, Q.C. Influence of SiC surface polarity on the wettability and reactivity in an Al/SiC system. Appl. Surf. Sci. 2015, 355, 930–938. [Google Scholar] [CrossRef]
- Guo, S.Q.; Hirosaki, N.; Yamamoto, Y.; Nishimura, T.; Mitomo, M. Improvement of high-temperature strength of hot-pressed sintering silicon nitride with Lu2O3 addition. Scr. Mater. 2001, 45, 867–874. [Google Scholar] [CrossRef]
- Zhang, L.J.; Qiu, F.; Wang, J.G.; Jiang, Q.C. High strength and good ductility at elevated temperature of nano-SiCp/Al2014 composites fabricated by semi-solid stir casting combined with hot extrusion. Mater. Sci. Eng. A 2015, 626, 338–341. [Google Scholar] [CrossRef]
- Fang, Q.; Kang, Z.X. An investigation on morphology and structure of Cu–Cr alloy powders prepared by mechanical milling and alloying. Powder Technol. 2015, 270, 104–111. [Google Scholar] [CrossRef]
- Li, Y.C.; Chen, C.; Deng, R.X.; Feng, X.M.; Shen, Y.F. Microstructure evolution of Cr coatings on Cu substrates prepared by mechanical alloying method. Powder Technol. 2014, 268, 165–172. [Google Scholar] [CrossRef]
- Grosdidier, T.; Zou, J.X.; Stein, N.; Boulanger, C.; Hao, S.Z.; Dong, C. Texture modification, grain refinement and improved hardness/corrosion balance of a FeAl alloy by pulsed electron beam surface treatment in the “heating mode”. Scr. Mater. 2008, 58, 1058–1061. [Google Scholar] [CrossRef]
- Zou, J.X.; Grosdidier, T.D.; Zhang, K.M.; Dong, C. Mechanisms of nanostructure and metastable phase formations in the surface melted layers of a HCPEB-treated D2 steel. Acta Mater. 2006, 54, 5409–5419. [Google Scholar] [CrossRef]
- Sun, Y.; Li, K.; Gao, B.; Sun, P.Y.; Fu, H.Y.; Liu, Z.; Yin, J.T. Study on microstructure and wear resistance of Zr-17Nb alloy irradiated by high current pulsed electron beam. Rev. Adv. Mater. Sci. 2020, 59, 514–522. [Google Scholar] [CrossRef]
- Ivanov, Y.F.; Gromov, V.E.; Konovalov, S.V.; Zagulyaev, D.V.; Petrikova, E.A.; Semin, A.P. Modification of structure and surface properties of hypoeutectic silumin by intense pulse electron beams. Prog. Phys. Met. 2018, 19, 195–222. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Z.X.; Zhao, L.; Guan, Q.F.; Zhao, L.; Liu, Y.H. Microstructures and corrosion resistance of Zircaloy-4 after surface alloying with copper by high-current pulsed electron beam. Appl. Surf. Sci. 2020, 501, 144222–144230. [Google Scholar] [CrossRef]
- Yan, P.; Zou, J.X.; Zhang, C.L.; Grosdidier, T. Surface modifications of a cold rolled 2024 Al alloy by high current pulsed electron beams. Appl. Surf. Sci. 2020, 504, 144382–144396. [Google Scholar] [CrossRef]
- Gao, B.; Hu, L.; Li, S.W.; Hao, Y.; Zhang, Y.D.; Tu, G.F.; Grosdidier, T. Study on the nanostructure formation mechanism of hypereutectic Al–17.5Si alloy induced by high current pulsed electron beam. Appl. Surf. Sci. 2015, 346, 147–157. [Google Scholar] [CrossRef]
- Ivanov, Y.F.; Zaguliaev, D.V.; Glezer, A.M.; Gromov, V.E.; Abaturova, A.A.; Leonov, A.A.; Semin, A.P.; Sundeev, R.V. Changes in surface structure and mechanical characteristics of Al–5 wt% Si alloy after irradiation by electron beam. Mater. Lett. 2020, 275, 128105–128108. [Google Scholar] [CrossRef]
- Lv, P.; Sun, X.; Cai, J. Microstructure and high temperature oxidation resistance of nickel base alloy GH4169 irradiated by high current pulsed electron beam. Surf. Coat. Technol. 2017, 309, 401–409. [Google Scholar] [CrossRef]
- Valkov, S.; Ormanova, M.; Petrov, P. Electron-beam surface treatment of metals and alloys: Techniques and trends. Metals 2020, 10, 1219. [Google Scholar] [CrossRef]
- Lamperti, A.; Ossi, P.M.; Rotshtein, V.P. Microstructure and properties of Cu-Cr powder metallurgical alloy induced by high-current pulsed electron beam. Surf. Coat. Technol. 2006, 200, 6373–6377. [Google Scholar] [CrossRef]
- Hutsaylyuk, V.; Student, M.; Posuvailo, V.; Student, O.; Sirak, Y.; Hvozdets’kyi, V.; Maruschak, P.; Veselivska, H. The properties of oxide-ceramic layers with Cu and Ni inclusions synthesizing by PEO method on top of the gas-spraying coatings on aluminium alloys. Vacuum 2020, 179, 109514. [Google Scholar] [CrossRef]
- Lonardelli, I.; Gey, N.; Wenk, H.R. In situ observation of texture evolution during α→β and β→α Phase transformations in titanium alloys investigated by neutron diffraction. Acta Mater. 2007, 55, 5718–5727. [Google Scholar] [CrossRef]
- Zou, J.X.; Zhang, K.M.; Grosdidier, T.; Dong, C.; Qin, Y.; Hao, S.Z.; Yang, D.Z. Orientation-dependent deformation on 316L stainless steel induced by high-current pulsed electron beam irradiation. Mater. Sci. Eng. A 2008, 483–484, 302–305. [Google Scholar] [CrossRef]
- Hao, S.Z.; Wu, P.; Zou, J.X.; Grosdidier, T.; Dong, C. Microstructure evolution occurring in the modified surface of 316L stainless steel under high current pulsed electron beam treatment. Appl. Surf. Sci. 2007, 253, 5349–5354. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Teng, Y.Y.; Zhang, M.X.; Zhu, S.L. Density functional theory study of surface energies of Al (001), (110) and (111). Corros. Sci. Prot. Technol. 2005, 17, 47–49. [Google Scholar]
- Gao, B.; Li, K.; Xing, P.F. Formation of a Double-Layer Ultrafifine Crystal Structure for High-Current Pulsed Electron Beam-Treated Al-20Si-5Mg Alloy. Coatings 2019, 9, 413. [Google Scholar] [CrossRef]
- Hao, S.Z.; Zhang, X.D.; Mei, X.X.; Grosdidier, T.; Dong, C. Surface Treatment of DZ4 Directionally Solidified Nickel-based Superalloy by High Current Pulsed Electron Beam. Mater. Lett. 2008, 62, 414–417. [Google Scholar] [CrossRef]
- He, J.D.; Gao, B.; Hu, L.; Zhu, G.L. Effects of HCPEB on Morphology and Hardness of Al-Si Alloy with Rare Earth Element. Surf. Technol. 2017, 46, 153–158. [Google Scholar]
- Proskurovsky, D.I.; Rotshtein, V.P.; Ozur, G.E. Physical foundations for surface treatment of materials with low energy, high current electron beams. Surf. Coat. Technol. 2000, 125, 49–56. [Google Scholar] [CrossRef]
- Jiang, C.Y.; Wang, C.Y.; Luo, K.Y.; Lu, J.Z. Effects of Laser Shock Layer Number and Cl−1 Concentration on Anticorrosion Behaviors of AM50 Mg Alloys. Chin. J. Lasers 2018, 45, 1–9. [Google Scholar]
- Hao, S.Z.; Yao, S.; Guan, J.F.; Wu, A.; Zhong, M.P.; Dong, C. Surface treatment of aluminum by high current pulsed electron beam. Curr. Appl. Phys. 2001, 1, 203–208. [Google Scholar] [CrossRef]
- Hu, L.; Gao, B.; Zhu, G.L.; Hao, Y.; Sun, S.C.; Tu, G.F. The effect of neodymium on the microcracks generated on the Al–17.5 Si alloy surface treated by high current pulsed electron beam. Appl. Surf. Sci. 2016, 364, 490–497. [Google Scholar] [CrossRef]
- Liu, H.X.; Wang, C.Q.; Zhang, X.W.; Jiang, Y.H.; Cai, C.X.; Tang, S.J. Improving the corrosion resistance and mechanical property of 45 steel surface by laser cladding with Ni6oCuMoW alloy powder. Surf. Coat. Technol. 2013, 228, S296–S300. [Google Scholar] [CrossRef]
- Liu, Y.R.; Zhang, K.M.; Zou, J.X.; Liu, D.K.; Zhang, T.C. Effect of the high current pulsed electron beam treatment on the surface microstructure and corrosion resistance of a Mg-4Sm alloy. J. Alloy. Compd. 2018, 741, 65–75. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).