Effect of Edible Carboxymethyl Chitosan-Gelatin Based Coating on the Quality and Nutritional Properties of Different Sweet Cherry Cultivars during Postharvest Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Plant Materials
2.1.2. Chemicals
2.2. Experimental Design
2.2.1. Preparation of AA-CaCl2-CMCS-GL Edible Coating Solution
2.2.2. Sweet Cherry Treatments and Storage Conditions
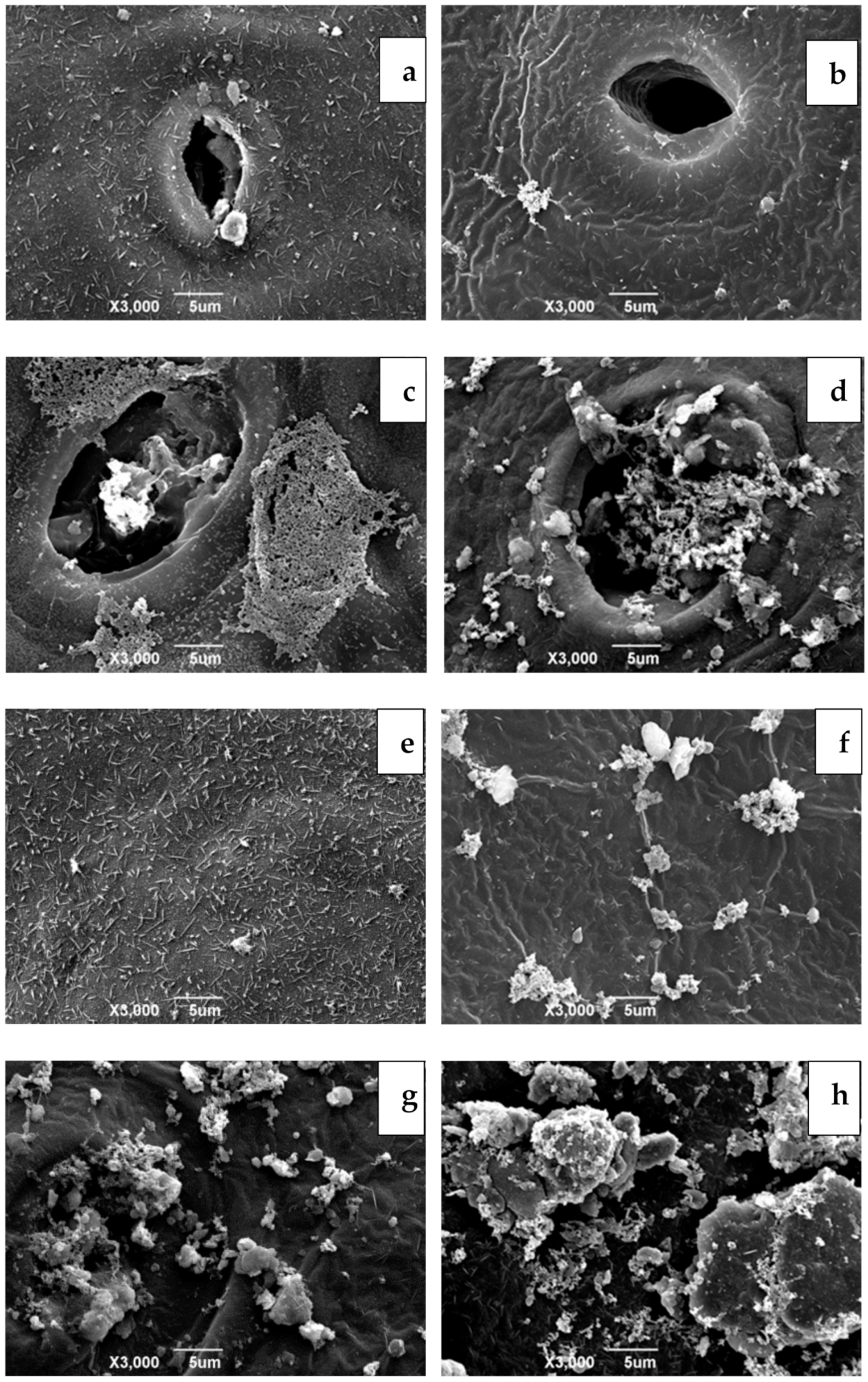
2.3. The Surface Microstructure Observation of Sweet Cherry Epidermis and Peduncle
2.4. Measurement of Fruit Decay Ratio, Weight Loss, and Fruit Firmness
2.5. Measurement of Skin Color Characteristics
2.6. Measurement of Peduncle Browning Incidence (PBI), Peduncle Moisture Content (PMC), and Peduncle Chlorophyll Content (PCC)
2.7. Measurement of Soluble Solids Content (SSC), Titratable Acidity (TA), and AA Content
2.8. Measurement of Total Phenolics Content (TPC), Total Anthocyanins Concentration (TAC), and Antioxidant Activity
2.9. Statistical Analysis
3. Results
3.1. Microstructure of Sweet Cherry Epidermis and Peduncle Surface at Harvest
3.2. Fruit Decay Ratio, Weight Loss, and Fruit Firmness
3.3. Skin Color Characteristics
3.4. PBI, PMC, and PCC
3.5. SSC, TA, and AA Content
3.6. TPC, TAC, and DPPH RSC
3.7. Correlations between Skin Color Characteristics, TPC, TAC, and DPPH RSC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blando, F.; Oomah, B.D. Sweet and sour cherries: Origin, distribution, nutritional composition and health benefits. Trends Food Sci. Technol. 2019, 86, 517–529. [Google Scholar] [CrossRef]
- Chockchaisawasdee, S.; Golding, J.B.; Vuong, Q.V.; Papoutsis, K.; Stathopoulos, C.E. Sweet cherry: Composition, postharvest preservation, processing and trends for its future use. Trends Food Sci. Technol. 2016, 55, 72–83. [Google Scholar] [CrossRef]
- Habib, M.; Bhat, M.; Dar, B.N.; Wani, A.A. Sweet cherries from farm to table: A review. Crit. Rev. Food Sci. 2017, 57, 1638–1649. [Google Scholar] [CrossRef]
- Correia, S.; Schouten, R.; Silva, A.P.; Goncalves, B. Factors Affecting Quality and Health Promoting Compounds during Growth and Postharvest Life of Sweet Cherry (Prunus avium L.). Front. Plant Sci. 2017, 8, 2166. [Google Scholar] [CrossRef] [PubMed]
- Tokatli, K.; Demirdoven, A. Effects of chitosan edible film coatings on the physicochemical and microbiological qualities of sweet cherry (Prunus avium L.). Sci. Hortic. Amst. 2020, 259, 7. [Google Scholar] [CrossRef]
- Petriccione, M.; De Sanctis, F.; Pasquariello, M.S.; Mastrobuoni, F.; Rega, P.; Scortichini, M.; Mencarelli, F. The effect of chitosan coating on the quality and nutraceutical traits of sweet cherry during postharvest life. Food Bioprocess Technol. 2015, 8, 394–408. [Google Scholar] [CrossRef]
- Abdipour, M.; Malekhossini, P.S.; Hosseinifarahi, M.; Radi, M. Integration of UV irradiation and chitosan coating: A powerful treatment for maintaining the postharvest quality of sweet cherry fruit. Sci. Hortic. Amst. 2020, 264, 8. [Google Scholar] [CrossRef]
- Zam, W. Effect of alginate and chitosan edible coating enriched with olive leaves extract on the shelf life of sweet cherries (Prunus avium L.). J. Food Qual. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Zimet, P.; Mombru, A.W.; Mombru, D.; Castro, A.; Pablo Villanueva, J.; Pardo, H.; Rufo, C. Physico-chemical and antilisterial properties of nisin-incorporated chitosan/carboxymethyl chitosan films. Carbohydr. Polym. 2019, 219, 334–343. [Google Scholar] [CrossRef]
- Bai, R.Y.; Zhang, X.; Yong, H.M.; Wang, X.C.; Liu, Y.P.; Liu, J. Development and characterization of antioxidant active packaging and intelligent Al3+-sensing films based on carboxymethyl chitosan and quercetin. Int. J. Biol. Macromol. 2019, 126, 1074–1084. [Google Scholar] [CrossRef]
- Dayarian, S.; Zamani, A.; Moheb, A.; Masoomi, M. Physico-mechanical properties of films of chitosan, carboxymethyl chitosan, and their blends. J. Polym. Environ. 2014, 22, 409–416. [Google Scholar] [CrossRef]
- Zhu, G.Q.; Wang, F.G.; Liu, Y.Y.; Gao, Q.C. Properties of water-soluble carboxymethyl chitosan film modified by hydrophobic poly(propylene glycol). Chem. Pap. 2013, 67, 423–428. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan and gelatin based biodegradable packaging films with UV-light protection. J. Photochem. Photobiol. B 2016, 163, 115–124. [Google Scholar] [CrossRef]
- Haghighi, H.; De Leo, R.; Bedin, E.; Pfeifer, F.; Siesler, H.W.; Pulvirenti, A. Comparative analysis of blend and bilayer films based on chitosan and gelatin enriched with LAE (lauroyl arginate ethyl) with antimicrobial activity for food packaging applications. Food Packag. Shelf Life 2019, 19, 31–39. [Google Scholar] [CrossRef]
- Pereda, M.; Ponce, A.G.; Marcovich, N.E.; Ruseckaite, R.A.; Martucci, J.F. Chitosan-gelatin composites and bi-layer films with potential antimicrobial activity. Food Hydrocoll. 2011, 25, 1372–1381. [Google Scholar] [CrossRef]
- Riva, S.C.; Opara, U.O.; Fawole, O.A. Recent developments on postharvest application of edible coatings on stone fruit: A review. Sci. Hortic. Amst. 2020, 262, 10. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Anugrah, D.S.B.; Alexander, H.; Pramitasari, R.; Hudiyanti, D.; Sagita, C.P. A Review of Polysaccharide-Zinc Oxide Nanocomposites as Safe Coating for Fruits Preservation. Coatings 2020, 10, 988. [Google Scholar] [CrossRef]
- Angeletti, P.; Castagnasso, H.; Miceli, E.; Terminiello, L.; Concellon, A.; Chaves, A.; Vicente, A.R. Effect of preharvest calcium applications on postharvest quality, softening and cell wall degradation of two blueberry (Vaccinium corymbosum) varieties. Postharvest Biol. Technol. 2010, 58, 98–103. [Google Scholar] [CrossRef]
- Liu, K.D.; Yuan, C.C.; Chen, Y.; Li, H.L.; Liu, J.X. Combined effects of ascorbic acid and chitosan on the quality maintenance and shelf life of plums. Sci. Hortic. Amst. 2014, 176, 45–53. [Google Scholar] [CrossRef]
- Ozdemir, K.S.; Gokmen, V. Effect of chitosan-ascorbic acid coatings on the refrigerated storage stability of fresh-cut apples. Coatings 2019, 9, 503. [Google Scholar] [CrossRef]
- Chaiprasart, P.; Hansawasdi, C.; Pipattanawong, N. Effect of chitosan coating and calcium chloride treatment on postharvest qualities of strawberry fruit (Fragaria x ananassa). Acta Hortic. 2006, 337–342. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, D.H.H.; Nguyen, H.H. Combination effects of calcium chloride and nano-chitosan on the postharvest quality of strawberry (Fragaria x ananassa Duch.). Postharvest Biol. Technol. 2020, 162, 1–8. [Google Scholar] [CrossRef]
- Kou, X.H.; Guo, W.L.; Guo, R.Z.; Li, X.Y.; Xue, Z.H. Effects of chitosan, calcium chloride, and pullulan coating treatments on antioxidant activity in pear cv. “Huang guan” during storage. Food Bioprocess Technol. 2014, 7, 671–681. [Google Scholar] [CrossRef]
- Lin, L.; Wang, B.; Wang, M.; Cao, J.; Zhang, J.; Wu, Y.; Jiang, W. Effects of a chitosan-based coating with ascorbic acid on post-harvest quality and core browning of ’Yali’ pears (Pyrus bertschneideri Rehd.). J. Ence Food Agric. 2010, 88, 877–884. [Google Scholar] [CrossRef]
- Aleryani-Raqeeb, A.; Mahmud, T.M.M.; Omar, S.R.S.; Zaki, A.R.M. Effects of calcium infiltration and chitosan coating on storage life and quality characteristics during storage of papaya (Carica papaya L.). Int. J. Agric. Res. 2008, 3, 296–306. [Google Scholar] [CrossRef][Green Version]
- Sun, D.Q.; Liang, G.B.; Xie, J.H.; Lei, X.T.; Mo, Y.W. Improved preservation effects of litchi fruit by combining chitosan coating with ascorbic acid treatment during postharvest storage. Afr. J. Biotechnol. 2010, 9, 3272–3279. [Google Scholar] [CrossRef]
- Chong, J.X.; Lai, S.; Yang, H. Chitosan combined with calcium chloride impacts fresh-cut honeydew melon by stabilising nanostructures of sodium-carbonate-soluble pectin. Food Control 2015, 53, 195–205. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Hamdi, S. Use of almond gum and gum arabic as novel edible coating to delay postharvest ripening and to maitain sweet cherry (Prunus avium) quality during storage. J. Food Process Preserv. 2015, 39, 1499–1508. [Google Scholar] [CrossRef]
- Jafari, H.; Pirouzifard, M.; Khaledabad, M.A.; Almasi, H. Effect of chitin nanofiber on the morphological and physical properties of chitosan/silver nanoparticle bionanocomposite films. Int. J. Biol. Macromol. 2016, 92, 461–466. [Google Scholar] [CrossRef]
- Aday, M.S.; Caner, C. Understanding the effects of various edible coatings on the storability of fresh cherry. Packag. Technol. Sci. 2010, 23, 441–456. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Alburquerque, N.; Valverde, J.M.; Guillén, F.; Castillo, S.; Valero, D.; Serrano, M. Postharvest sweet cherry quality and safety maintenance by Aloe vera treatment: A new edible coating. Postharvest Biol. Technol. 2006, 39, 93–100. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Dong, Y. Pre-harvest application of harpin beta protein improves fruit on-tree and storage quality attributes of ’Lapins’ and ’Regina’ sweet cherry (Prunus avium L.). Sci. Hortic. Amst. 2020, 263. [Google Scholar] [CrossRef]
- Pasquariello, M.S.; Di Patre, D.; Mastrobuoni, F.; Zampella, L.; Scortichini, M.; Petriccione, M. Influence of postharvest chitosan treatment on enzymatic browning and antioxidant enzyme activity in sweet cherry fruit. Postharvest Biol. Technol. 2015, 109, 45–56. [Google Scholar] [CrossRef]
- Faniadis, D.; Drogoudi, P.D.; Vasilakakis, M. Effects of cultivar, orchard elevation, and storage on fruit quality characters of sweet cherry (Prunus avium L.). Sci. Hortic. Amst. 2010, 125, 301–304. [Google Scholar] [CrossRef]
- Lara, I.; Belge, B.; Goulao, L.F. The fruit cuticle as a modulator of postharvest quality. Postharvest Biol. Technol. 2014, 87, 103–112. [Google Scholar] [CrossRef]
- Martin, L.B.B.; Rose, J.K.C. There’s more than one way to skin a fruit: Formation and functions of fruit cuticles. J. Exp. Bot. 2014, 65, 4639–4651. [Google Scholar] [CrossRef]
- Athoo, T.O.; Winkler, A.; Knoche, M. Pedicel transpiration in sweet cherry fruit: Mechanisms, pathways, and factors. J. Am. Soc. Hortic. Sci. 2015, 140, 136–143. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, X.B.; Long, L.E. The effect of postharvest calcium application in hydro-cooling water on tissue calcium content, biochemical changes, and quality attributes of sweet cherry fruit. Food Chem. 2014, 160, 22–30. [Google Scholar] [CrossRef]
- Aglar, E.; Ozturk, B.; Guler, S.K.; Karakaya, O.; Uzun, S.; Saracoglu, O. Effect of modified atmosphere packaging and ’Parka’ treatments on fruit quality characteristics of sweet cherry fruits (Prunus avium L. ’0900 Ziraat’) during cold storage and shelf life. Sci. Hortic. Amst. 2017, 222, 162–168. [Google Scholar] [CrossRef]
- Mackinney, G. Absorption of light by chlorphyll solutions. J. Biol. Chem. 1941, 140, 315–322. [Google Scholar] [CrossRef]
- Diaz-Mula, H.M.; Serrano, M.; Valero, D. Alginate coatings preserve fruit quality and bioactive compounds during storage of sweet cherry fruit. Food Bioprocess Technol. 2012, 5, 2990–2997. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M.; Metheney, P. Consumer acceptance of ‘Brooks’ and ‘Bing’ cherries is mainly dependent on fruit SSC and visual skin color. Postharvest Biol. Technol. 2003, 28, 159–167. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Andres-Lacueva, C. Polyphenols and health: Current state and progress. J. Agric. Food Chem. 2012, 60, 8773–8775. [Google Scholar] [CrossRef]
- Alique, R.; Zamorano, J.P.; Martínez, M.A.; Alonso, J. Effect of heat and cold treatments on respiratory metabolism and shelf-life of sweet cherry, type picota cv “Ambrunés”. Postharvest Biol. Technol. 2005, 35, 153–165. [Google Scholar] [CrossRef]
- Wani, A.A.; Singh, P.; Gul, K.; Wani, M.H.; Langowski, H.C. Sweet cherry (Prunus avium): Critical factors affecting the composition and shelf life. Food Packag. Shelf Life 2014, 1, 86–99. [Google Scholar] [CrossRef]
- Powrie, W.D.; Skura, B.J. Modified Atmosphere Packaging of Fruits and Vegetables; Springer US: New York City, NY, USA, 1991. [Google Scholar]
- Conway, W.S.; Gross, K.C.; Boyer, C.D.; Sams, C.E. Inhibition of Penicillium expansum polygalacturonase activity by increased apple cell wall calcium. Phytopathology 1988, 78, 1052–1055. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Koushesh Saba, M.; Emamifar, A. Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Dong, F.; Wang, X.L. Guar gum and ginseng extract coatings maintain the quality of sweet cherry. Lwt-Food Sci. Technol. 2018, 89, 117–122. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C. Micro- and nanoscale characterization of hydrophobic and hydrophilic leaf surfaces. Nanotechnology 2006, 17, 2758. [Google Scholar] [CrossRef]
- Koch, K.; Ensikat, H.-J. The hydrophobic coatings of plant surfaces: Epicuticular wax crystals and their morphologies, crystallinity and molecular self-assembly. Micron (Oxf. Engl. 1993) 2008, 39, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, F.; Lai, S.; Wang, H.; Yang, H. Impact of soybean protein isolate-chitosan edible coating on the softening of apricot fruit during storage. Lwt-Food Sci. Technol. 2018, 96, 604–611. [Google Scholar] [CrossRef]
- Neda Maftoonazad, H.S.R.M.M. Shelf-life extension of peaches through sodium alginate and methyl cellulose edible coatings. Int. J. Food Sci. Technol. 2008, 43, 951–957. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G. Effect of alginate edible coating on quality and antioxidant properties in sweet cherry during postharvest storage. Ital. J. Food Sci. 2015, 27, 173–180. [Google Scholar]
- Goncalves, B.; Silva, A.P.; Moutinho-Pereira, J.; Bacelar, E.; Rosa, E.; Meyer, A.S. Effect of ripeness and postharvest storage on the evolution of colour and anthocyanins in cherries (Prunus avium L.). Food Chem. 2007, 103, 976–984. [Google Scholar] [CrossRef]
- Kumar, P.; Sethi, S.; Sharma, R.R.; Srivastav, M.; Varghese, E. Effect of chitosan coating on postharvest life and quality of plum during storage at low temperature. Sci. Hortic. Amst. 2017, 226, 104–109. [Google Scholar] [CrossRef]
- Linke, M.; Herppich, W.B.; Geyer, M. Green peduncles may indicate postharvest freshness of sweet cherries. Postharvest Biol. Technol. 2010, 58, 135–141. [Google Scholar] [CrossRef]
- Knoche, M.; Athoo, T.O.; Winkler, A.; Bruggenwirth, M. Postharvest osmotic dehydration of pedicels of sweet cherry fruit. Postharvest Biol. Technol. 2015, 108, 86–90. [Google Scholar] [CrossRef]
- Smith, E.D.; Whiting, M.D. The pedicel’s role in pestharvest weight loss of two sweet cherry cultivars. Acta Hortic. 2011, 903, 935–939. [Google Scholar] [CrossRef]
- Wallace, G.; Fry, S.C. Action of diverse peroxidases and laccases on six cell wall-related phenolic compounds. Phytochemistry 1999, 52, 769–773. [Google Scholar] [CrossRef]
- Adiletta, G.; Zampella, L.; Coletta, C.; Petriccione, M. Chitosan coating to preserve the qualitative traits and improve antioxidant system in fresh figs (Ficus carica L.). Agric. Basel 2019, 9, 84. [Google Scholar] [CrossRef]
- Hazrati, S.; Kashkooli, A.B.; Habibzadeh, F.; Tahmasebi-Sarvestani, Z.; Sadeghi, A.R. Evaluation of Aloe vera Gel as an alternative edible coating for peach fruits during cold storage period. Gesunde Pflanz. 2017, 69, 131–137. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry antioxidants: From farm to table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef]
- Nair, M.S.; Saxena, A.; Kaur, C. Effect of chitosan and alginate based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.). Food Chem. 2018, 240, 245–252. [Google Scholar] [CrossRef]
- Carolina Formica-Oliveira, A.; Benito Martinez-Hernandez, G.; Diaz-Lopez, V.; Artes, F.; Artes-Hernandez, F. Effects of UV-B and UV-C combination on phenolic compounds biosynthesis in fresh-cut carrots. Postharvest Biol. Technol. 2017, 127, 99–104. [Google Scholar] [CrossRef]

| Cultivar (Abbreviation) | Types of Maturity | Harvest Date | Skin Color | Pulp Color | Average Fruit Weight (g) |
|---|---|---|---|---|---|
| Red Light (RL) | Precocious | 27 May | Fuchsia | Red | 5.16 ± 0.15 |
| Ranier (RN) | Mid-ripening | 6 June | Yellow-red | Light yellow | 4.35 ± 0.12 |
| Red Agate (RA) | Mid-ripening | 6 June | Fuchsia | Fuchsia | 5.98 ± 0.14 |
| Friendship (FS) | Serotinous | 15 June | Crimson | Red | 7.86 ± 0.19 |
| Serial Number | Cultivar | Treatment | Abbreviation |
|---|---|---|---|
| 1 | RL | Tap water | RL-C |
| 2 | AA-CaCl2-CMCS-GL coating | RL-T | |
| 3 | RN | Tap water | RN-C |
| 4 | AA-CaCl2-CMCS-GL coating | RN-T | |
| 5 | RA | Tap water | RA-C |
| 6 | AA-CaCl2-CMCS-GL coating | RA-T | |
| 7 | FS | Tap water | FS-C |
| 8 | AA-CaCl2-CMCS-GL coating | FS-T |
| Characteristic | Storage Time (Days) | RL-C | RL-T | RN-C | RN-T | RA-C | RA-T | FS-C | FS-T |
|---|---|---|---|---|---|---|---|---|---|
| Fruit decay ratio (%) | 0 | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a |
| 6 | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | |
| 12 | 1.7 ± 0.6 a | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | |
| 18 | 8.7 ± 1.2 a | 1.3 ± 0.6 b | 4.3 ± 1.2 c | 0 ± 0 d | 1.3 ± 0.6 b | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | |
| 24 | 16.3 ± 1.5 a | 4.7 ± 0.6 b | 10.7 ± 2.5 c | 4.3 ± 1.5 b | 10.3 ± 2.1 c | 1.7 ± 0.6 d | 1.7 ± 0.6 d | 0 ± 0 d | |
| 30 | 35.0 ± 1.0 a | 11.3 ± 1.2 b | 31.7 ± 1.5 c | 8.7 ± 2.1 bd | 29.0 ± 4.0 c | 2.7 ± 0.6 e | 6.7 ± 1.2 d | 1.3 ± 0.6 e | |
| 33 | 63.0 ± 2.6 a | 15.3 ± 0.6 b | 58.7 ± 3.1 a | 14.3 ± 1.5 b | 59.7 ± 5.7 a | 5.3 ± 0.6 c | 17.7 ± 1.5 b | 3.0 ± 1.0 c | |
| Weight loss (%) | 0 | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a |
| 6 | 2.23 ± 0.15 a | 2.07 ± 0.15 ab | 2.04 ± 0.13 ab | 1.58 ± 0.26 c | 1.81 ± 0.13 bc | 1.78 ± 0.19 bc | 2.71 ± 0.14 d | 1.98 ± 0.31 ab | |
| 12 | 4.98 ± 0.24 a | 2.88 ± 0.10 b | 4.82 ± 0.29 a | 2.94 ± 0.06 b | 3.80 ± 0.23 c | 2.60 ± 0.20 b | 4.92 ± 0.20 a | 2.83 ± 0.32 b | |
| 18 | 5.44 ± 0.26 a | 3.47 ± 0.25 b | 6.69 ± 0.14 c | 4.24 ± 0.14 d | 5.25 ± 0.24 a | 3.40 ± 0.18 b | 7.97 ± 0.21 d | 5.19 ± 0.20 a | |
| 24 | 6.82 ± 0.27 a | 4.85 ± 0.22 b | 8.84 ± 0.17 c | 5.62 ± 0.13 d | 6.47 ± 0.24 a | 4.17 ± 0.24 e | 9.86 ± 0.28 f | 6.42 ± 0.24 a | |
| 30 | 9.03 ± 0.25 a | 5.87 ± 0.19 b | 9.86 ± 0.24 c | 7.04 ± 0.27 d | 8.01 ± 0.14 e | 4.73 ± 0.26 f | 11.72 ± 0.23 g | 7.45 ± 0.30 d | |
| 33 | 11.51 ± 0.30 a | 7.39 ± 0.24 b | 13.14 ± 0.23 c | 8.50 ± 0.23 d | 10.75 ± 0.34 e | 5.46 ± 0.30 f | 14.89 ± 0.33 g | 9.44 ± 0.25 h | |
| Fruit firmness (N) | 0 | 19.49 ± 0.75 a | 19.56 ± 0.71 a | 19.65 ± 0.71 a | 19.81 ± 0.59 a | 20.73 ± 0.77 b | 21.11 ± 0.66 b | 23.87 ± 0.68 c | 23.53 ± 0.61 c |
| 6 | 18.95 ± 0.62 a | 19.22 ± 0.68 a | 19.26 ± 0.64 a | 19.51 ± 0.69 a | 20.15 ± 0.77 b | 20.97 ± 0.69 c | 22.68 ± 0.71 d | 23.18 ± 0.64 d | |
| 12 | 17.91 ± 0.74 a | 19.06 ± 0.71 b | 18.31 ± 0.69 a | 18.97 ± 0.71 b | 19.29 ± 0.87 b | 20.87 ± 0.53 c | 21.55 ± 0.64 d | 22.55 ± 0.71 e | |
| 18 | 17.04 ± 0.71 a | 18.56 ± 0.65 b | 17.70 ± 0.65 c | 18.59 ± 0.67 b | 17.65 ± 0.81 c | 20.64 ± 0.58 d | 19.83 ± 0.74 e | 21.35 ± 0.62 f | |
| 24 | 15.27 ± 1.57 a | 16.96 ± 0.62 b | 17.32 ± 0.61 b | 17.75 ± 0.56 c | 15.85 ± 0.54 a | 20.48 ± 0.87 d | 19.10 ± 0.57 e | 20.15 ± 0.59 d | |
| 30 | 12.23 ± 0.65 a | 16.02 ± 0.58 b | 16.33 ± 0.69 b | 17.18 ± 0.61 c | 14.96 ± 0.68 d | 20.05 ± 0.81 e | 18.61 ± 0.59 f | 19.29 ± 0.54 g | |
| 33 | 10.39 ± 0.58 a | 15.25 ± 0.59 b | 13.82 ± 0.59 c | 16.49 ± 0.58 d | 11.89 ± 0.69 e | 19.69 ± 0.53 f | 16.22 ± 0.52 d | 17.97 ± 0.59 g |
| Characteristic | Storage Time (Days) | RL-C | RL-T | RN-C | RN-T | RA-C | RA-T | FS-C | FS-T |
|---|---|---|---|---|---|---|---|---|---|
| L* | 0 | 28.76 ± 2.51 a | 30.23 ± 2.61 b | 62.95 ± 2.38 c | 64.12 ± 2.31 d | 32.29 ± 1.25 e | 35.64 ± 0.94 f | 33.72 ± 2.77 g | 35.96 ± 2.58 f |
| 6 | 26.35 ± 2.68 a | 28.52 ± 2.34 b | 60.81 ± 2.85 c | 62.04 ± 2.25 d | 31.80 ± 1.07 e | 35.05 ± 0.82 f | 31.29 ± 2.65 e | 33.65 ± 2.36 g | |
| 12 | 25.19 ± 2.36 a | 27.83 ± 2.17 b | 58.23 ± 2.38 c | 60.28 ± 2.11 d | 31.29 ± 1.18 e | 34.65 ± 1.17 f | 29.32 ± 2.58 g | 31.22 ± 2.18 e | |
| 18 | 23.32 ± 1.96 a | 26.49 ± 2.28 b | 56.53 ± 2.18 c | 59.03 ± 2.08 d | 30.73 ± 1.14 e | 33.97 ± 1.09 f | 26.70 ± 2.33 b | 30.09 ± 2.11 e | |
| 24 | 21.16 ± 1.36 a | 24.97 ± 2.33 b | 55.03 ± 2.06 c | 56.94 ± 2.31 d | 28.06 ± 1.04 e | 33.34 ± 1.09 f | 25.32 ± 2.46 b | 29.68 ± 2.23 g | |
| 30 | 19.62 ± 1.83 a | 22.78 ± 2.42 b | 52.42 ± 2.31 c | 54.52 ± 2.18 d | 26.54 ± 1.03 e | 32.98 ± 1.07 f | 23.28 ± 2.39 b | 28.26 ± 2.15 g | |
| 33 | 16.94 ± 1.21 a | 20.29 ± 1.96 b | 50.03 ± 2.15 c | 52.49 ± 2.22 d | 22.43 ± 1.05 e | 31.85 ± 1.03 f | 21.03 ± 2.12 g | 26.72 ± 2.33 h | |
| Chroma | 0 | 20.76 ± 1.56 a | 21.05 ± 1.62 a | 41.19 ± 1.80 c | 41.17 ± 1.77 c | 38.56 ± 1.05 d | 40.64 ± 0.86 c | 34.73 ± 2.88 b | 35.99 ± 2.45 e |
| 6 | 21.89 ± 1.75 a | 21.44 ± 1.58 b | 40.30 ± 1.53 c | 40.78 ± 1.61 c | 36.53 ± 1.07 d | 38.04 ± 1.02 e | 35.05 ± 2.39 a | 36.08 ± 2.08 d | |
| 12 | 25.19 ± 2.36 a | 27.83 ± 2.17 a | 38.57 ± 1.31 b | 40.05 ± 1.66 c | 34.86 ± 0.89 d | 37.65 ± 0.97 e | 32.50 ± 2.41 f | 34.81 ± 2.60 d | |
| 18 | 20.96 ± 1.59 a | 20.52 ± 1.44 a | 35.34 ± 1.62 b | 38.58 ± 1.31 c | 33.79 ± 1.14 d | 37.97 ± 1.04 e | 30.35 ± 2.36 f | 33.44 ± 2.22 d | |
| 24 | 19.32 ± 1.78 a | 19.57 ± 1.36 a | 32.77 ± 1.49 b | 36.54 ± 1.43 c | 32.86 ± 1.04 b | 37.34 ± 1.19 d | 29.32 ± 2.17 f | 31.90 ± 2.11 g | |
| 30 | 17.47 ± 1.29 a | 18.38 ± 1.38 b | 29.67 ± 1.42 c | 35.59 ± 1.20 d | 31.75 ± 0.83 e | 36.98 ± 0.97 f | 27.81 ± 2.31 g | 30.92 ± 1.97 h | |
| 33 | 13.66 ± 0.30 a | 16.99 ± 1.53 b | 26.37 ± 1.24 c | 32.17 ± 1.34 d | 28.45 ± 1.05 e | 34.85 ± 1.03 f | 26.65 ± 2.18 c | 28.92 ± 2.08 e | |
| Hue angle | 0 | 18.47 ± 2.65 a | 19.06 ± 2.99 a | 60.73 ± 2.21 b | 59.97 ± 2.05 b | 25.48 ± 0.89 c | 27.37 ± 1.21 d | 23.46 ± 3.29 e | 24.19 ± 3.01 e |
| 6 | 16.97 ± 2.24 a | 18.18 ± 2.59 b | 57.98 ± 1.94 c | 58.46 ± 2.22 c | 23.33 ± 0.63 d | 26.37 ± 1.03 e | 21.12 ± 3.24 f | 21.99 ± 3.03 g | |
| 12 | 15.83 ± 2.20 a | 16.57 ± 2.55 a | 58.48 ± 2.05 b | 56.86 ± 1.84 c | 22.56 ± 1.12 d | 25.93 ± 0.79 e | 21.14 ± 3.41 f | 21.40 ± 2.75 f | |
| 18 | 14.77 ± 1.89 a | 16.92 ± 2.32 b | 58.95 ± 2.94 c | 57.43 ± 2.15 d | 20.45 ± 1.03 e | 25.21 ± 0.91 f | 20.42 ± 2.86 e | 20.52 ± 2.64 e | |
| 24 | 14.71 ± 1.79 a | 16.16 ± 2.21 b | 60.09 ± 3.10 c | 58.75 ± 1.86 d | 18.59 ± 0.89 e | 24.32 ± 1.03 f | 19.30 ± 3.26 e | 18.81 ± 2.12 e | |
| 30 | 14.25 ± 1.67 a | 16.52 ± 2.22 b | 59.11 ± 3.15 c | 60.26 ± 2.05 d | 18.17 ± 0.93 e | 23.74 ± 0.97 f | 18.09 ± 3.30 e | 18.26 ± 2.05 e | |
| 33 | 14.81 ± 2.24 a | 15.84 ± 2.13 b | 60.15 ± 3.23 c | 61.37 ± 2.55 d | 16.37 ± 0.86 b | 22.49 ± 0.84 e | 16.41 ± 2.96 b | 17.41 ± 2.02 f |
| Characteristic | Storage Time (Days) | RL-C | RL-T | RN-C | RN-T | RA-C | RA-T | FS-C | FS-T |
|---|---|---|---|---|---|---|---|---|---|
| PBI (%) | 0 | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a |
| 6 | 13.7 ± 2.1 a | 5.3 ± 1.5 bc | 12.3 ± 2.1 ad | 0 ± 0 e | 10.7 ± 1.5 d | 4.3 ± 1.5 c | 7.3 ± 1.5 b | 0 ± 0 e | |
| 12 | 29.0 ± 2.6 a | 12.7 ± 2.1 b | 24.3 ± 1.5 c | 3.7 ± 0.6 d | 24.7 ± 2.5 c | 9.3 ± 2.5 b | 19.0 ± 2.0 e | 2.7 ± 0.6 d | |
| 18 | 54.3 ± 2.5 a | 22.3 ± 3.1 b | 44.7 ± 2.1 c | 8.0 ± 1.0 d | 50.0 ± 2.6 e | 15.0 ± 2.0 f | 36.7 ± 2.1 g | 5.7 ± 1.2 d | |
| 24 | 79.7 ± 3.1 a | 30.3 ± 2.5 b | 57.3 ± 3.1 c | 14.3 ± 1.2 d | 63.0 ± 3.6 e | 19.0 ± 2.6 f | 49.3 ± 2.3 g | 13.7 ± 2.1 d | |
| 30 | 85.0 ± 3.6 a | 48.0 ± 3.0 b | 74.7 ± 3.8 c | 21.3 ± 1.5 d | 85.0 ± 3.0 a | 25.3 ± 3.1 d | 69.3 ± 1.5 e | 20.7 ± 1.5 d | |
| 33 | 99.3 ± 1.2 a | 64.7 ± 2.5 b | 88.0 ± 3.0 c | 30.7 ± 1.5 de | 95.0 ± 2.6 f | 33.0 ± 2.6 e | 72.0 ± 2.0 g | 27.0 ± 2.0 d | |
| PMC (%) | 0 | 73.2 ± 0.7 a | 73.1 ± 0.6 a | 76.0 ± 0.4 b | 75.8 ± 0.4 b | 76.4 ± 1.0 b | 76.7 ± 1.5 b | 73.6 ± 1.1 a | 73.9 ± 0.4 a |
| 6 | 68.3 ± 0.9 a | 71.0 ± 0.2 b | 70.8 ± 0.6 b | 74.9 ± 0.6 c | 73.0 ± 0.6 d | 76.2 ± 1.8 c | 70.6 ± 0.8 b | 72.7 ± 0.4 d | |
| 12 | 62.8 ± 0.7 a | 68.8 ± 0.9 b | 67.5 ± 1.3 c | 73.3 ± 0.6 d | 67.9 ± 0.4 b | 76.0 ± 0.7 e | 66.3 ± 0.5 c | 70.2 ± 0.6 f | |
| 18 | 55.9 ± 1.1 a | 66.6 ± 0.8 b | 65.6 ± 0.9 b | 71.1 ± 0.8 c | 65.5 ± 0.9 b | 75.7 ± 0.6 d | 63.4 ± 0.8 e | 69.0 ± 0.8 f | |
| 24 | 49.7 ± 0.7 a | 63.7 ± 1.0 b | 62.4 ± 1.1 b | 69.3 ± 0.5 c | 58.4 ± 1.2 d | 74.9 ± 0.5 e | 58.6 ± 1.1 d | 67.6 ± 0.6 f | |
| 30 | 44.5 ± 1.2 a | 59.6 ± 0.9 b | 58.7 ± 0.8 b | 66.7 ± 0.4 c | 53.0 ± 1.6 d | 74.0 ± 0.7 e | 56.5 ± 0.9 f | 64.3 ± 0.8 g | |
| 33 | 30.5 ± 0.8 a | 51.1 ± 1.3 b | 43.4 ± 1.0 c | 58.6 ± 0.8 d | 36.4 ± 1.2 e | 69.3 ± 0.8 f | 48.5 ± 0.7 g | 56.7 ± 0.5 h | |
| PCC (mg g−1) | 0 | 0.464 ± 0.015 a | 0.467 ± 0.011 ab | 0.475 ± 0.015 ab | 0.479 ± 0.011 ab | 0.460 ± 0.009 a | 0.469 ± 0.007 ab | 0.483 ± 0.004 ab | 0.490 ± 0.006 b |
| 6 | 0.430 ± 0.016 ab | 0.448 ± 0.019 abc | 0.440 ± 0.017 abc | 0.462 ± 0.009 c | 0.425 ± 0.007 a | 0.443 ± 0.013 abc | 0.437 ± 0.013 abc | 0.458 ± 0.008 bc | |
| 12 | 0.390 ± 0.012 a | 0.424 ± 0.019 bc | 0.418 ± 0.011 bc | 0.433 ± 0.004 c | 0.401 ± 0.005 ab | 0.423 ± 0.007 bc | 0.414 ± 0.007 bc | 0.435 ± 0.007 c | |
| 18 | 0.351 ± 0.007 a | 0.410 ± 0.014 bc | 0.394 ± 0.015 bcd | 0.426 ± 0.007 d | 0.379 ± 0.011 e | 0.401 ± 0.004 bcd | 0.393 ± 0.008 bd | 0.416 ± 0.008 ce | |
| 24 | 0.311 ± 0.006 a | 0.363 ± 0.005 bcd | 0.356 ± 0.037 bc | 0.386 ± 0.006 cd | 0.347 ± 0.003 b | 0.379 ± 0.006 bcd | 0.357 ± 0.011 bc | 0.396 ± 0.011 d | |
| 30 | 0.264 ± 0.003 a | 0.302 ± 0.010 bc | 0.314 ± 0.011 cd | 0.342 ± 0.003 e | 0.295 ± 0.007 b | 0.327 ± 0.007 de | 0.324 ± 0.007 d | 0.363 ± 0.006 f | |
| 33 | 0.219 ± 0.003 a | 0.277 ± 0.008 b | 0.259 ± 0.004 c | 0.309 ± 0.006 d | 0.247 ± 0.008 c | 0.296 ± 0.005 e | 0.286 ± 0.004 be | 0.335 ± 0.008 f |
| Characteristic | PBI | PMC | PCC |
|---|---|---|---|
| PBI | 1 | - | - |
| PMC | −0.923 ** | 1 | - |
| PCC | −0.901 ** | 0.875 ** | 1 |
| Characteristic | Storage Time (Days) | RL-C | RL-T | RN-C | RN-T | RA-C | RA-T | FS-C | FS-T |
|---|---|---|---|---|---|---|---|---|---|
| SSC (%) | 0 | 15.36 ± 0.19 a | 15.40 ± 0.08 a | 16.62 ± 0.30 b | 16.56 ± 0.11 b | 11.20 ± 0.36 c | 11.40 ± 0.17 c | 13.45 ± 0.21 d | 13.36 ± 0.19 d |
| 6 | 18.49 ± 0.23 a | 17.55 ± 0.19 b | 17.49 ± 0.16 b | 17.35 ± 0.22 b | 12.09 ± 0.23 c | 11.55 ± 0.13 d | 13.85 ± 0.11 e | 13.70 ± 0.12 e | |
| 12 | 20.42 ± 0.27 a | 20.06 ± 0.51 a | 18.86 ± 0.44 b | 18.60 ± 0.21 b | 12.57 ± 0.26 c | 12.05 ± 0.21 c | 14.50 ± 0.22 d | 14.38 ± 0.16 d | |
| 18 | 22.36 ± 0.15 a | 21.39 ± 0.10 b | 20.81 ± 0.23 c | 19.94 ± 0.19 d | 13.58 ± 0.27 e | 12.55 ± 0.20 f | 14.97 ± 0.15 g | 14.81 ± 0.14 g | |
| 24 | 25.57 ± 0.24 a | 24.57 ± 0.24 b | 22.73 ± 0.35 c | 20.85 ± 0.09 d | 14.17 ± 0.14 e | 12.99 ± 0.26 f | 15.68 ± 0.31 g | 15.21 ± 0.18 h | |
| 30 | 27.48 ± 0.27 a | 25.98 ± 0.26 b | 23.88 ± 0.06 c | 21.98 ± 0.14 d | 14.97 ± 0.15 e | 13.75 ± 0.01 f | 16.27 ± 0.22 g | 15.61 ± 0.22 h | |
| 33 | 29.48 ± 0.21 a | 27.25 ± 0.38 b | 24.67 ± 0.14 c | 23.05 ± 0.18 d | 15.46 ± 0.41 e | 13.83 ± 0.19 f | 16.77 ± 0.22 g | 16.33 ± 0.20 h | |
| TA (g·kg−1) | 0 | 22.64 ± 0.24 a | 22.94 ± 0.27 a | 17.75 ± 0.12 b | 17.88 ± 0.14 b | 15.20 ± 0.14 c | 15.00 ± 0.12 c | 20.27 ± 0.24 d | 20.48 ± 0.21 d |
| 6 | 18.65 ± 0.11 a | 20.59 ± 0.12 b | 14.15 ± 0.20 c | 15.79 ± 0.13 d | 12.15 ± 0.28 e | 14.64 ± 0.21 f | 16.27 ± 0.13 g | 19.76 ± 0.17 h | |
| 12 | 16.26 ± 0. 18 a | 19.31 ± 0.15 b | 12.98 ± 0.12 c | 14.76 ± 0.12 d | 10.49 ± 0.07 e | 13.77 ± 0.27 f | 14.32 ± 0.21 g | 17.12 ± 0.15 h | |
| 18 | 15.75 ± 0. 20 a | 17.25 ± 0.12 b | 9.38 ± 0.22 c | 12.56 ± 0.18 d | 10.20 ± 0.31 e | 12.89 ± 0.21 d | 11.17 ± 0.11 f | 13.95 ± 0.18 g | |
| 24 | 13.70 ± 0.14 a | 14.86 ± 0.14 b | 8.73 ± 0.13 c | 11.31 ± 0.10 d | 8.74 ± 0.10 c | 12.02 ± 0.17 e | 9.79 ± 0.14 f | 12.71 ± 0.27 g | |
| 30 | 12.40 ± 0.18 a | 13.57 ± 0.19 b | 8.58 ± 0.14 c | 10.01 ± 0.14 d | 7.93 ± 0.11 e | 11.15 ± 0.20 f | 9.03 ± 0.11 g | 11.79 ± 0.13 h | |
| 33 | 11.00 ± 0.08 a | 12.40 ± 0.24 b | 8.19 ± 0.15 c | 9.66 ± 0.19 d | 5.73 ± 0.10 e | 9.96 ± 0.27 d | 8.28 ± 0.16 c | 11.03 ± 0.33 a | |
| AA (mg·kg−1) | 0 | 175.4 ± 1.0 a | 175.1 ± 1.3 a | 130.0 ± 1.5 b | 127.3 ± 1.3 c | 142.5 ± 1.1 d | 146.5 ± 1.4 e | 201.4 ± 1.5 f | 198.5 ± 1.7 g |
| 6 | 152.1 ± 1.3 a | 152.3 ± 1.8 a | 111.4 ± 1.4 b | 118.7 ± 1.9 c | 125.8 ± 1.3 d | 129.9 ± 1.2 e | 181.7 ± 1.4 f | 184.0 ± 1.4 f | |
| 12 | 137.5 ± 1.3 a | 144.9 ± 1.8 b | 98.6 ± 2.0 c | 115.6 ± 1.9 d | 118.1 ± 1.2 d | 127.2 ± 1.8 e | 161.1 ± 1.3 f | 175.2 ± 1.3 g | |
| 18 | 117.4 ± 1.1 a | 132.4 ± 1.5 b | 91.8 ± 1.6 c | 102.5 ± 1.3 d | 120.8 ± 1.1 d | 120.8 ± 1.5 e | 145.0 ± 1.5 f | 164.4 ± 1.3 g | |
| 24 | 104.4 ± 1.0 a | 124.3 ± 1.4 b | 81.6 ± 1.7 c | 96.1 ± 1.5 d | 96.7 ± 0.9 d | 116.6 ± 1.5 e | 122.4 ± 1.4 b | 153.2 ± 1.2 f | |
| 30 | 93.9 ± 1.4 a | 112.0 ± 1.1 b | 70.8 ± 1.1 c | 84.1 ± 1.9 d | 80.8 ± 1.7 e | 107.9 ± 1.7 f | 114.2 ± 1.4 b | 144.6 ± 1.6 g | |
| 33 | 70.4 ± 1.6 a | 93.9 ± 1.5 b | 55.0 ± 1.2 c | 69.2 ± 1.5 a | 62.1 ± 2.0 d | 94.4 ± 1.1 b | 93.3 ± 1.2 b | 118.6 ± 1.8 e |
| Characteristic | Storage Time (Days) | RL-C | RL-T | RN-C | RN-T | RA-C | RA-T | FS-C | FS-T |
|---|---|---|---|---|---|---|---|---|---|
| TPC (mg·g−1) | 0 | 22.03 ± 0.54 a | 22.23 ± 0.30 a | 12.20 ± 0.33 b | 12.11 ± 0.14 b | 20.56 ± 0.46 c | 20.55 ± 0.26 c | 18.07 ± 0.31 d | 18.14 ± 0.21 d |
| 6 | 21.06 ± 0.23 a | 21.16 ± 0.41 a | 11.69 ± 0.17 b | 11.89 ± 0.27 b | 19.17 ± 0.55 c | 19.90 ± 0.57 c | 16.79 ± 0.32 d | 17.19 ± 0.36 d | |
| 12 | 19.75 ± 0.33 a | 20.28 ± 0.38 a | 10.39 ± 0.31 b | 11.18 ± 0.26 c | 17.83 ± 0.52 d | 18.56 ± 0.36 d | 15.18 ± 0.26 e | 16.19 ± 0.28 f | |
| 18 | 17.68 ± 0.34 a | 19.53 ± 0.40 b | 9.14 ± 0.18 c | 10.69 ± 0.29 d | 16.46 ± 0.48 e | 18.38 ± 0.30 f | 13.97 ± 0.22 g | 15.26 ± 0.29 h | |
| 24 | 15.47 ± 0.54 a | 19.08 ± 0.28 b | 8.41 ± 0.25 c | 10.09 ± 0.22 d | 14.63 ± 0.51 e | 17.48 ± 0.10 f | 12.18 ± 0.25 g | 14.15 ± 0.24 e | |
| 30 | 13.61 ± 0.34 a | 18.44 ± 0.21 b | 7.20 ± 0.24 c | 9.21 ± 0.25 d | 13.87 ± 0.48 a | 17.00 ± 0.35 e | 10.93 ± 0.28 f | 12.86 ± 0.22 g | |
| 33 | 11.56 ± 0.29 a | 17.40 ± 0.22 b | 5.74 ± 0.30 c | 7.86 ± 0.25 d | 11.20 ± 0.35 a | 16.59 ± 0.46 e | 9.60 ± 0.29 f | 11.46 ± 0.29 a | |
| TAC (mg·g−1) | 0 | 1.85 ± 0.02 a | 1.84 ± 0.01 a | 0.06 ± 0.00 b | 0.06 ± 0.01 b | 1.05 ± 0.04 c | 1.05 ± 0.02 c | 0.97 ± 0.02 d | 0.97 ± 0.02 d |
| 6 | 2.06 ± 0.02 a | 2.06 ± 0.02 a | 0.05 ± 0.01 b | 0.06 ± 0.01 b | 0.95 ± 0.02 c | 1.03 ± 0.02 d | 0.84 ± 0.01 e | 0.87 ± 0.00 f | |
| 12 | 1.77 ± 0.02 a | 1.82 ± 0.02 b | 0.04 ± 0.00 c | 0.05 ± 0.00 c | 0.76 ± 0.00 d | 0.99 ± 0.02 e | 0.76 ± 0.01 d | 0.79 ± 0.01 d | |
| 18 | 1.56 ± 0.01 a | 1.70 ± 0.02 b | 0.03 ± 0.00 c | 0.04 ± 0.01 c | 0.70 ± 0.01 d | 0.92 ± 0.03 e | 0.67 ± 0.02 d | 0.71 ± 0.01 f | |
| 24 | 1.36 ± 0.02 a | 1.50 ± 0.02 b | 0.02 ± 0.00 c | 0.04 ± 0.00 c | 0.65 ± 0.03 d | 0.92 ± 0.01 e | 0.62 ± 0.01 d | 0.70 ± 0.01 f | |
| 30 | 1.21 ± 0.02 a | 1.34 ± 0.01 b | 0.02 ± 0.00 c | 0.03 ± 0.00 c | 0.57 ± 0.02 d | 0.90 ± 0.01 e | 0.56 ± 0.01 d | 0.62 ± 0.01 f | |
| 33 | 1.07 ± 0.02 a | 1.26 ± 0.02 b | 0.01 ± 0.00 c | 0.02 ± 0.00 c | 0.47 ± 0.03 d | 0.90 ± 0.01 e | 0.50 ± 0.01 f | 0.54 ± 0.01 g | |
| DPPH RSC (mg·g−1) | 0 | 41.17 ± 0.23 a | 41.09 ± 0.18 a | 26.06 ± 0.38 b | 26.24 ± 0.29 b | 39.83 ± 0.73 c | 38.86 ± 0.25 d | 33.66 ± 0.13 e | 33.53 ± 0.25 e |
| 6 | 38.57 ± 0.21 a | 39.50 ± 0.28 b | 23.36 ± 0.39 c | 25.63 ± 0.41 d | 34.47 ± 0.48 e | 37.52 ± 0.18 f | 30.50 ± 0.27 g | 31.15 ± 0.40 g | |
| 12 | 33.42 ± 0.30 a | 38.59 ± 0.14 b | 20.20 ± 0.53 c | 24.11 ± 0.42 d | 29.78 ± 0.48 e | 36.32 ± 0.43 f | 25.73 ± 0.31 g | 28.70 ± 0.35 h | |
| 18 | 26.76 ± 0.55 a | 37.60 ± 0.35 b | 15.66 ± 0.57 c | 23.16 ± 0.26 d | 23.39 ± 0.60 d | 34.26 ± 0.15 e | 19.76 ± 0.34 f | 24.78 ± 0.36 g | |
| 24 | 20.03 ± 0.24 a | 36.28 ± 0.24 b | 12.32 ± 0.46 c | 22.40 ± 0.57 d | 19.89 ± 0.32 e | 31.54 ± 0.21 f | 15.88 ± 0.32 g | 22.69 ± 0.41 h | |
| 30 | 15.54 ± 0.39 a | 35.50 ± 0.33 b | 8.61 ± 0.15 c | 21.10 ± 0.43 d | 15.79 ± 0.55 a | 30.31 ± 0.62 e | 10.24 ± 0.42 f | 19.37 ± 0.42 g | |
| 33 | 9.95 ± 0.46 a | 34.32 ± 0.38 b | 3.48 ± 0.19 c | 19.29 ± 0.41 d | 6.06 ± 0.32 e | 28.38 ± 0.37 f | 5.55 ± 0.23 e | 14.75 ± 0.50 g |
| Characteristic | L* | Chroma | Hue Angle | TPC | TAC | DPPH RSC |
|---|---|---|---|---|---|---|
| L* | 1 | - | - | - | - | - |
| Chroma | 0.655 ** | 1 | - | - | - | - |
| Hue angle | 0.972 ** | 0.536 ** | 1 | - | - | - |
| TPC | −0.533 ** | −0.195 | −0.661 ** | 1 | - | - |
| TAC | −0.730 ** | −0.622 ** | −0.775 ** | 0.866 ** | 1 | - |
| DPPH RSC | −0.154 | 0.041 | −0.301 * | 0.893 ** | 0.661 ** | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-L.; Cui, Q.-L.; Wang, Y.; Shi, F.; Fan, H.; Zhang, Y.-Q.; Lai, S.-T.; Li, Z.-H.; Li, L.; Sun, Y.-K. Effect of Edible Carboxymethyl Chitosan-Gelatin Based Coating on the Quality and Nutritional Properties of Different Sweet Cherry Cultivars during Postharvest Storage. Coatings 2021, 11, 396. https://doi.org/10.3390/coatings11040396
Zhang Y-L, Cui Q-L, Wang Y, Shi F, Fan H, Zhang Y-Q, Lai S-T, Li Z-H, Li L, Sun Y-K. Effect of Edible Carboxymethyl Chitosan-Gelatin Based Coating on the Quality and Nutritional Properties of Different Sweet Cherry Cultivars during Postharvest Storage. Coatings. 2021; 11(4):396. https://doi.org/10.3390/coatings11040396
Chicago/Turabian StyleZhang, Yu-Lei, Qing-Liang Cui, Yu Wang, Fei Shi, Hua Fan, Yan-Qing Zhang, Si-Tong Lai, Ze-Hui Li, Lang Li, and Yi-Ke Sun. 2021. "Effect of Edible Carboxymethyl Chitosan-Gelatin Based Coating on the Quality and Nutritional Properties of Different Sweet Cherry Cultivars during Postharvest Storage" Coatings 11, no. 4: 396. https://doi.org/10.3390/coatings11040396
APA StyleZhang, Y.-L., Cui, Q.-L., Wang, Y., Shi, F., Fan, H., Zhang, Y.-Q., Lai, S.-T., Li, Z.-H., Li, L., & Sun, Y.-K. (2021). Effect of Edible Carboxymethyl Chitosan-Gelatin Based Coating on the Quality and Nutritional Properties of Different Sweet Cherry Cultivars during Postharvest Storage. Coatings, 11(4), 396. https://doi.org/10.3390/coatings11040396






