Influence of the Physical Properties on the Antibacterial and Photocatalytic Behavior of Ag-Doped Indium Sulfide Film Deposited by Spray Pyrolysis
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structural and Morphological Analysis
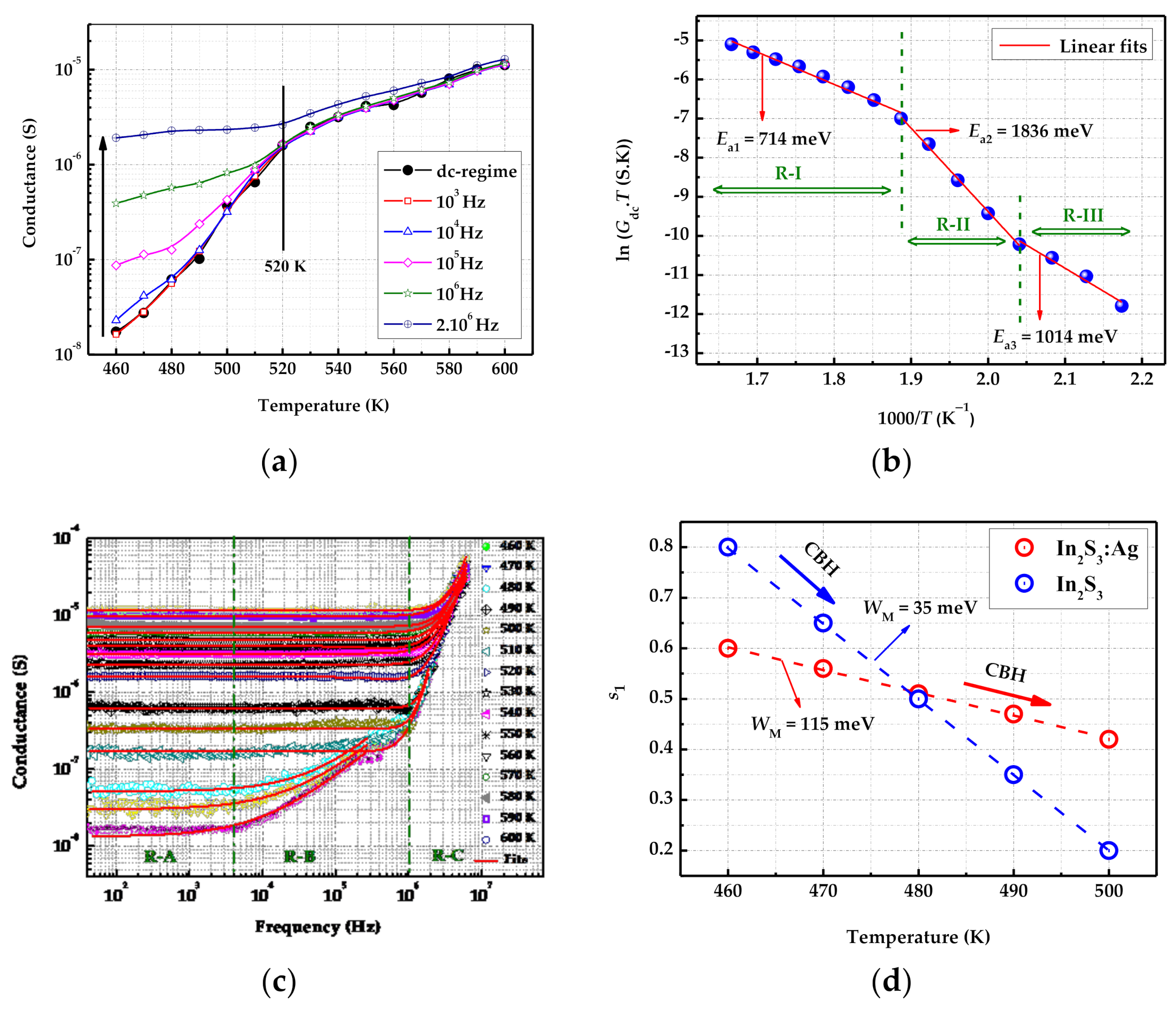
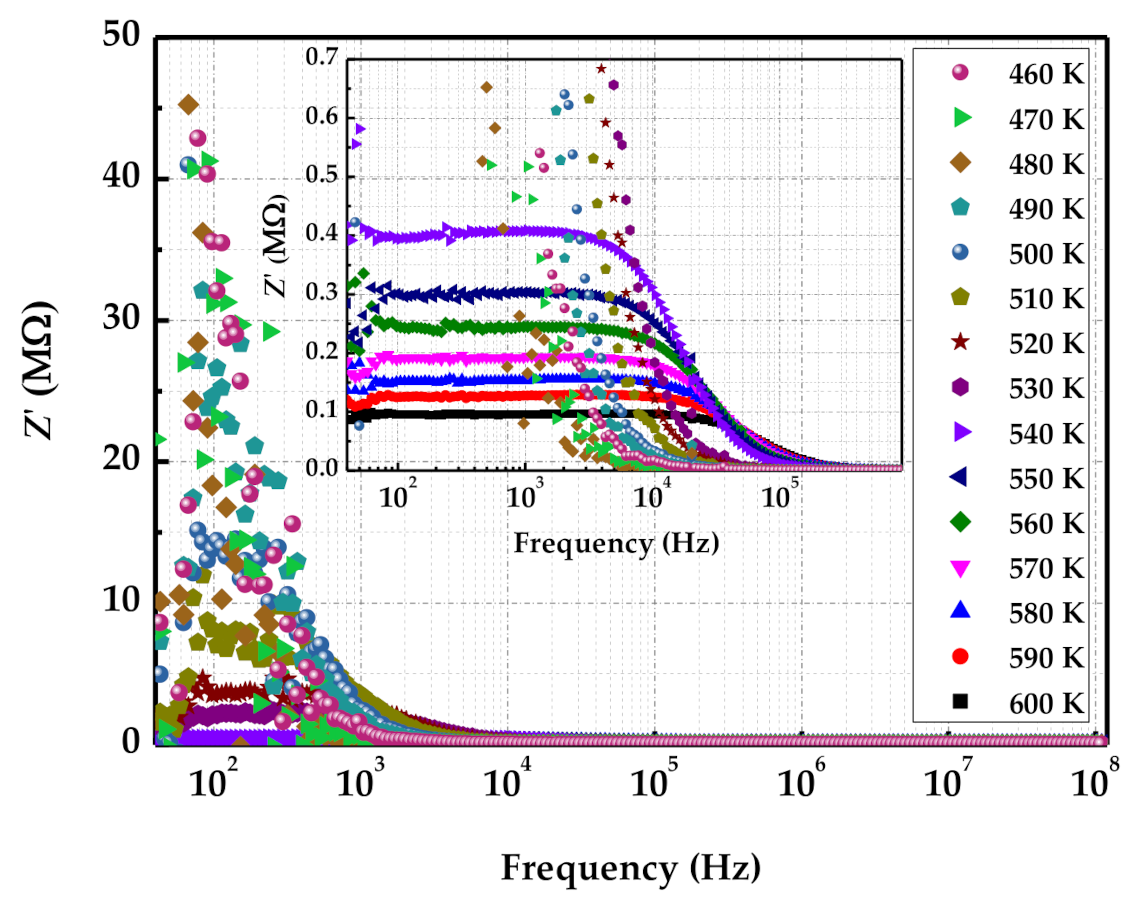
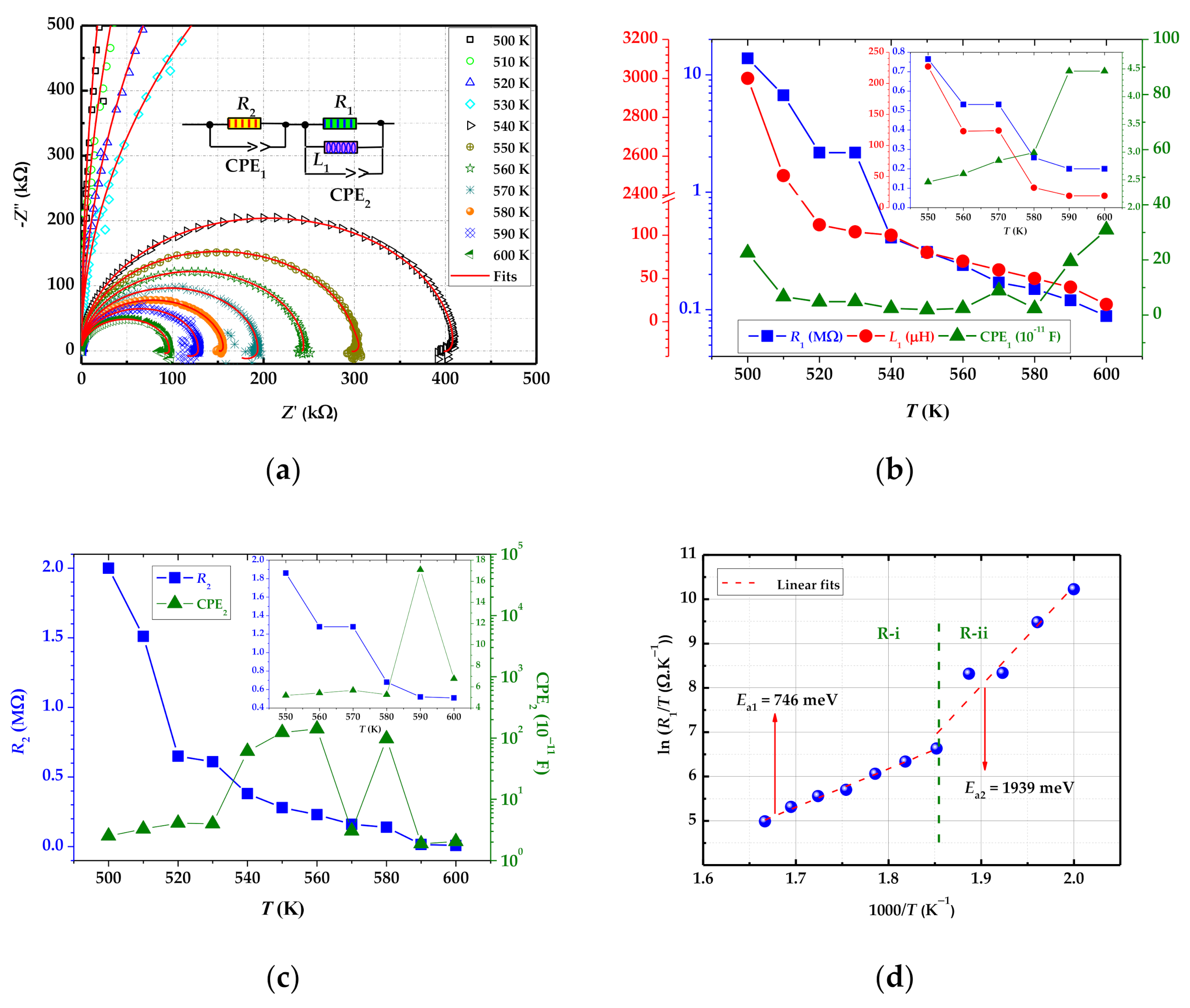
3.2. Electrical Properties
3.3. Photocatalytic Activity
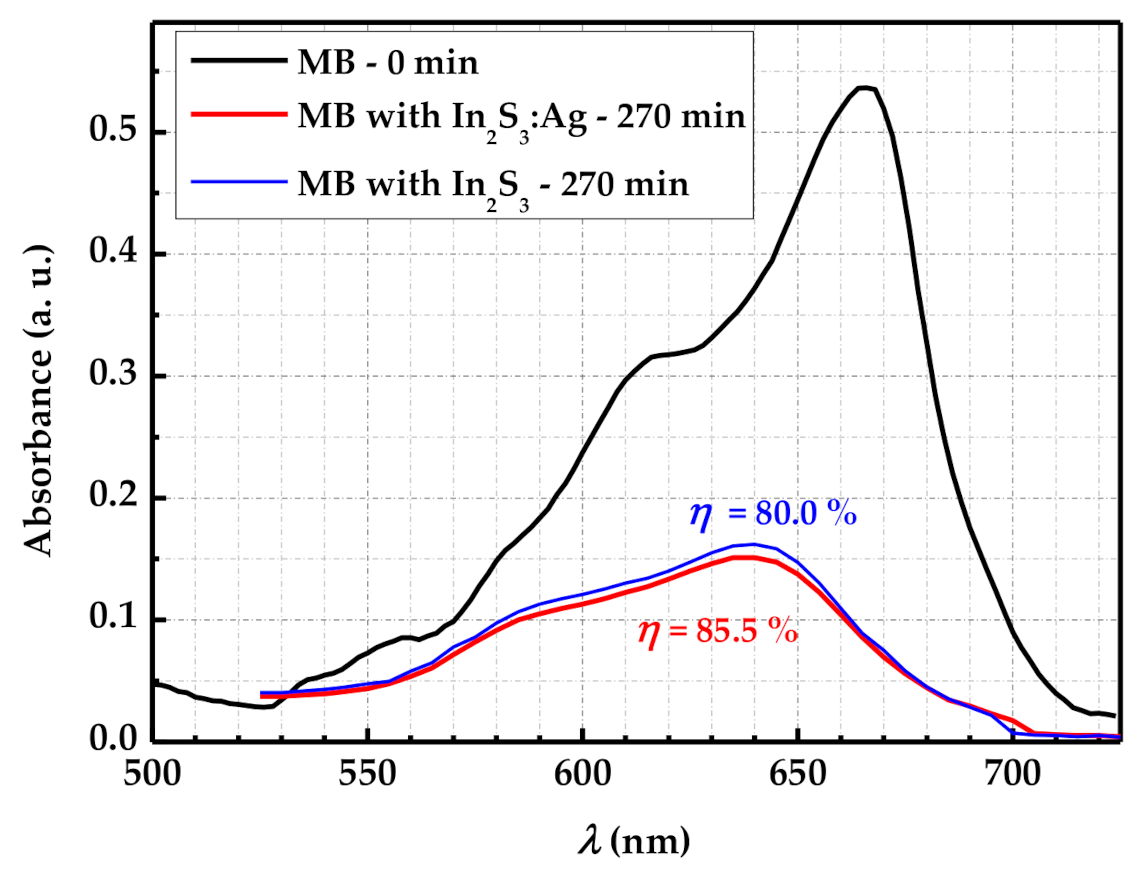
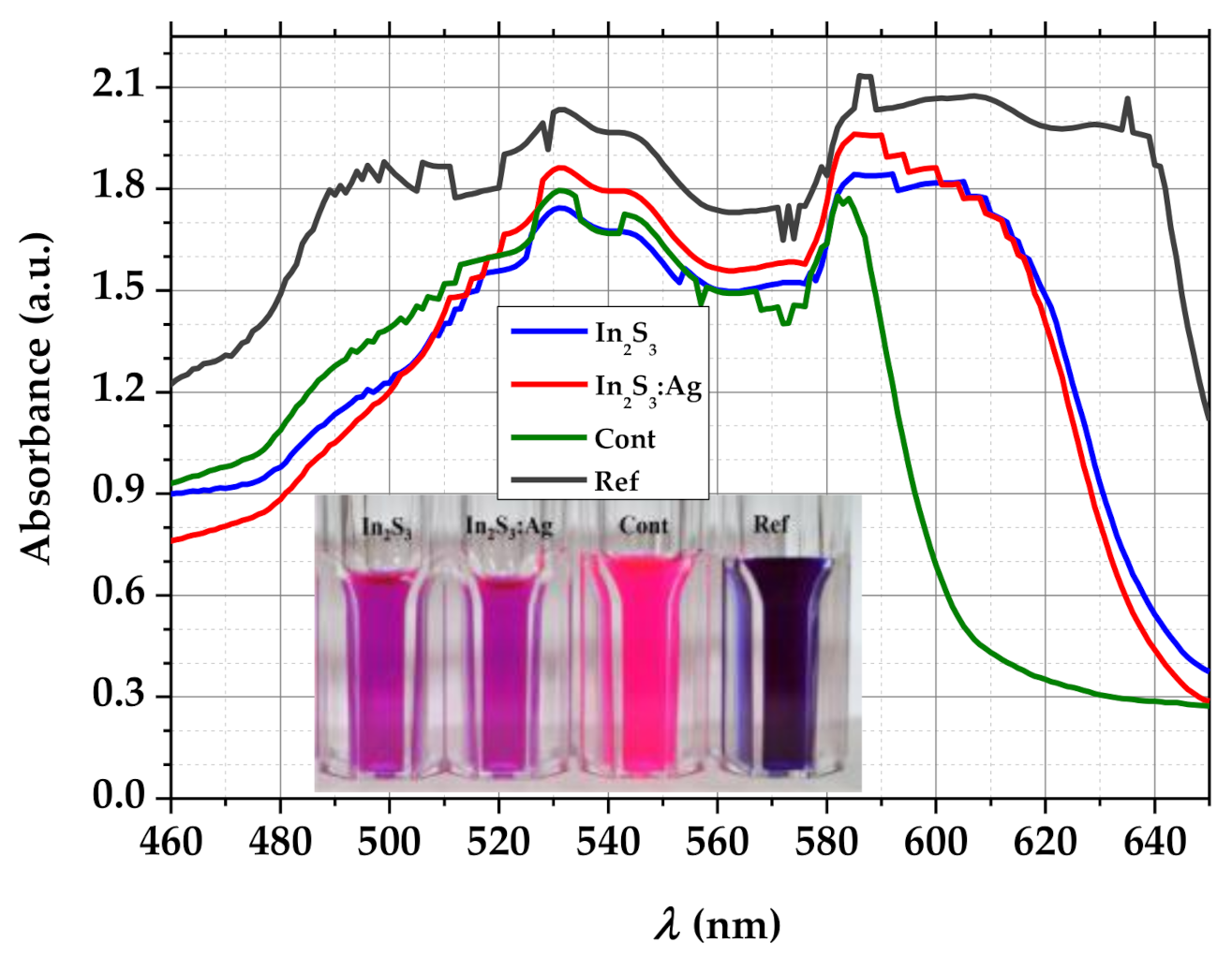
3.3.1. Absorption Analysis of MB Solution and Photodegradation Efficiency
3.3.2. Photodegradation Mechanism
3.4. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Cheng, M.; Liu, Z.; Zeng, G.; Zhong, H.; Chen, M.; Zhou, C.; Xiong, W.; Shao, B.; Song, B. Heterogeneous Fenton-like catalyst for treatment of rhamnolipid-solubilized hexadecane wastewater. Chemosphere 2019, 236, 124387. [Google Scholar] [CrossRef]
- Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Wang, R.; Wei, J.; Huang, C.; Xu, P.; Wan, J.; Zhang, C. Pyrolisis and reutilization of plant residues after phytoremediation of heavy metals contaminated sediments: For heavy metals, stabilization and dye absorption. Bioresour. Technol. 2018, 253, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Kuriakose, S.; Satpati, B.; Mohapatra, S. Enhanced photocatalytic activity of Co doped ZnO nanodisks and nanorods prepared by a facile wet chemical method. Phys. Chem. Chem. Phys. 2014, 16, 12741–12749. [Google Scholar] [CrossRef] [PubMed]
- Trandafilović, L.V.; Jovanović, D.J.; Zhang, X.; Ptasińska, S.; Dramićanin, M.D. Enhanced photocatalytic degradation of methylene blue and methyl orange by ZnO:Eu nanoparticles. Appl. Catal. B 2017, 203, 740–752. [Google Scholar] [CrossRef]
- Jiang, Z.-J.; Liu, C.-Y.; Sun, L.-W. Catalytic properties of silver nanoparticles supported on silica spheres. J. Phys. Chem. B 2005, 109, 1730–1735. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Du, Q.; Sun, J.; Jiao, Y.; Yang, G.; Wang, Z.; Xia, Y.; Zhang, W.; Wang, K.; et al. Adsorption of methylene blue from aqueous solution by graphene. Colloids Surf. B Biointerfaces 2012, 90, 197–203. [Google Scholar] [CrossRef]
- Rehman, M.S.U.; Kim, I.; Han, J.-I. Adsorption of methylene blue dye from aqueous solution by sugar extracted spent rice biomass. Carbohydr. Polym. 2012, 90, 1314–1322. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, B.; Dai, Y.; Qin, X.; Zhang, X.; Wang, P.; Liu, H.; Yu, J. Highly photocatalytic ZnO/In2O3 heteronanostructures synthesized by a coprecipitation method. J. Phys. Chem. C 2009, 113, 4612–4617. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, C.; Fu, Y.; Gao, J.; Huang, H.; Liu, Y.; Kang, Z. Construction of CDs/CdS photocatalysts for stable and efficient hydrogen production in water and seawater. Appl. Catal. B Environ. 2019, 242, 178–185. [Google Scholar] [CrossRef]
- Zhu, X.; Lin, Y.; Sun, Y.; Beard, M.C.; Yan, Y. Lead-halide perovskites for photocatalytic α-alkylation of aldehydes. J. Am. Chem. Soc. 2019, 141, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-H.; Dong, L.-Z.; Liu, J.; Li, S.-L.; Lan, Y.-Q. From molecular metal complex to metal-organic framework: The CO2 reduction photocatalysts with clear and tunable structure. Coord. Chem. Rev. 2019, 390, 86–126. [Google Scholar] [CrossRef]
- Zeng, D.; Zhou, T.; Ong, W.-J.; Wu, M.; Duan, X.; Xu, W.; Chen, Y.; Zhu, Y.-A.; Peng, D.-L. Sub-5 nm ultra-fine FeP nanodots as efficient co-catalysts modified porous g-C3N4 for precious-metal-free photocatalytic hydrogen evolution under visible light. ACS Appl. Mater. Interfaces 2019, 11, 5651–5660. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Saritha, K.; Ramakrishna Reddy, K.T.; Raveendranath Reddy, K.; Bychto, L.; Patryn, A.; Maliński, M.; Tivanov, M.S.; Gremenok, V.F. Optical properties of thermally evaporated In2S3 thin films measured using photoacoustic spectroscopy. Mater. Sci. Semicond. Process. 2017, 72, 4–8. [Google Scholar] [CrossRef]
- Buecheler, S.; Guettler, D.; Chirila, A.; Verma, R.; Müller, U.; Niesen, T.P.; Palm, J.; Tiwari, A.N. Ultrasonically sprayed indium sulfide buffer layers for Cu(In,Ga)(S,Se)2 thin-film solar cells. Thin Solid Films 2009, 517, 2312–2315. [Google Scholar] [CrossRef]
- Barreau, N.; Marsillac, S.; Albertini, D.; Bernède, J.C. Structural, optical and electrical properties of β-In2S3-3xO3x thin films obtained by PVD. Thin Solid Films 2002, 403, 331–334. [Google Scholar] [CrossRef]
- Pistor, P.; Merino Álvarez, J.M.; León, M.; di Michiel, M.; Schorr, S.; Klenka, R.; Lehmann, S. Structure reinvestigation of α-, β- and γ-In2S3. Acta. Cryst. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 410–415. [Google Scholar] [CrossRef]
- Bchiri, Y.; Tiss, B.; Bouguila, N.; Souissi, R.; Kraini, M.; Vázquez-Vázquez, C.; Khirouni, K.; Alaya, S. Electrical investigation of sprayed In2S3 film. Mater. Sci. Semicond. Process. 2021, 121, 105294. [Google Scholar] [CrossRef]
- Galarza-Gutierrez, U.; Albor-Aguilera, M.L.; Gonzalez-Trujillo, M.A.; Hernandez-Vasquez, C.; Ortega-Cardenas, J.A.; Flores-Marquez, J.M.; Cruz-Gandarilla, F.; Ramírez-Rosales, D.; Mendoza-Pérez, R.; Rueda-Morales, G. Incorporation of an efficient β-In2S3 thin film as window material into CdTe photovoltaic devices. Mat. Res. Express 2019, 6, 125510. [Google Scholar] [CrossRef]
- Soni, P.; Raghuwanshi, M.; Wuerz, R.; Berghoff, B.; Knoch, J.; Raabe, D.; Cojocaru-Mirédin, O. Role of elemental intermixing at the In2S3/CIGSe heterojunction deposited using reactive RF magnetron sputtering. Sol. Energy Mater. Sol. Cells 2019, 195, 367–375. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Dong, X.; Zheng, N.; Ma, H.; Zhang, X. Fabrication of In2O3/In2S3 microsphere heterostructures for efficient and stable photocatalytic nitrogen fixation. Appl. Catal. B Environ. 2019, 257, 117932. [Google Scholar] [CrossRef]
- Uma, H.B.; Ananda, S.; Rai, V.R. Synthesis and characterization of In2S3 nanoparticles by electrochemical method: Evaluation of its performance in photo-assisted degradation of indigo carmine dye, antibacterial and antimitotic activity studies. Int. J. Nanotechnol. Appl. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Kamoun, N.; Belgacem, S.; Amlouk, M.; Bennaceur, R.; Bonnet, J.; Touhari, F.; Nouaoura, M.; Lassabatere, L. Structure, surface composition, and electronic properties of β-In2S3 and β-In2−xAlxS3. J. Appl. Phys. 2001, 29, 2766. [Google Scholar] [CrossRef]
- Faller, P.; Kienzler, K.; Krieger-Liszkay, A. Mechanism of Cd2+ toxicity: Cd2+ inhibits photoactivation of Photosystem II by competitive binding to the essential Ca2+ site. Biochimica Biophysica Acta (BBA) Bioenerg. 2005, 1706, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Barreau, N. Indium sulfide and relatives in the world of photovoltaics. Sol. Energy 2009, 83, 363–371. [Google Scholar] [CrossRef]
- Timoumi, A.; Bouzouita, H.; Kanzari, M.; Rezig, B. Fabrication and characterization of In2S3 thin films deposited by thermal evaporation technique. Thin Solid Films 2005, 480–481, 124–128. [Google Scholar] [CrossRef]
- Barreau, N.; Mokrani, A.; Couzinié-Devy, F.; Kessler, J. Bandgap properties of the indium sulfide thin-films grown by co-evaporation. Thin Solid Films 2009, 517, 2316–2319. [Google Scholar] [CrossRef]
- Allsop, N.A.; Schönmann, A.; Muffler, H.-J.; Bär, M.; Lux-Steiner, M.C.; Fisher, C.-H. Spray-ILGAR indium sulfide buffers for Cu(In,Ga)(S,Se)2 solar cells. Prog. Photovolt. Res. Appl. 2005, 13, 607–616. [Google Scholar] [CrossRef]
- Naghavi, N.; Spiering, S.; Powalla, M.; Cavana, B.; Lincot, D. High-efficiency copper indium gallium diselenide (CIGS) solar cells with indium sulfide buffer layers deposited by atomic layer chemical vapor deposition (ALCVD). Prog. Photovolt. Res. Appl. 2003, 11, 437–443. [Google Scholar] [CrossRef]
- Li, Z.; Tao, X.; Wu, Z.; Zhang, P.; Zhang, Z. Preparation of In2S3 nanopraricle by ultrasonic dispersion and its tribology property. Ultrason. Sonochem. 2009, 16, 221–224. [Google Scholar] [CrossRef]
- Lee, W.; Baek, S.; Mane, R.S.; Todkar, V.V.; Egorova, O.; Chae, W.-S.; Lee, S.-H.; Han, S.-H. Liquid phase deposition of amorphous In2S3 nanorods: Effect of annealing on phase change. Curr. Appl. Phys. 2009, 9, S62–S64. [Google Scholar] [CrossRef]
- Mari, B.; Mollar, M.; Soro, D.; Henriquez, R.; Schrebler, R.; Gómez, H. Electrodeposition of In2S3 thin films onto FTO substrate from DMSO solution. Int. J. Electrochen. Sci. 2013, 8, 3510–3523. [Google Scholar]
- Portillo-Vélez, N.S.; Bizarro, M. Sprayed pyrolyzed ZnO films with nanoflake and nanorod morphologies and their photocatalytic activity. J. Nanomater. 2016, 2016, 5981562. [Google Scholar] [CrossRef]
- Raj Mohamed, J.; Amalraj, L. Effect of precursor concentration on physical properties of nebulized spray deposited In2S3 thin films. J. Asian Ceram. Soc. 2016, 4, 357–366. [Google Scholar] [CrossRef]
- Zheng, Z.; Yu, J.; Cheng, S.; Lai, Y.; Zheng, Q.; Pan, D. Investigation of structural, optical and electrical properties of Cu doped β-In2S3 thin films. J. Mater. Sci. Mater. Electron. 2016, 27, 5810–5817. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, P.E.; Quiñones-Galván, J.G.; Marasamy, L.; Morales-Luna, M.; Santos-Cruz, J.; Arias-Cerón, J.S.; Zelaya-Angel, O.; de Moure-Flores, F. Optoelectronic properties of undoped and Al, B and Ga-doped In2S3 thin films grown by CBD on flexible PET/ITO substrates. Mater. Sci. Semicond. Process. 2019, 103, 104600. [Google Scholar] [CrossRef]
- Mathew, M.; Jayakrishnan, R.; Ratheesh Kumar, P.M.; Sudha Kartha, C.; Vijayakumar, K.P. Anomalous behavior of silver doped indium sulfide thin films. J. Appl. Phys. 2006, 100, 33504. [Google Scholar] [CrossRef]
- Ojha, D.P.; Poudel, M.B.; Kim, H.J. Investigation of electrochemical performance of a high surface area mesoporous Mn doped TiO2 nanoparticle for a supercapacitor. Mater. Lett. 2020, 264, 127363. [Google Scholar] [CrossRef]
- Poudel, M.B.; Karki, H.P.; Kim, H.J. Silver nanoparticles decorated molybdenum sulfide/tungstate oxide nanorods as high performance supercapacitor electrode. J. Energy Storage 2020, 32, 101693. [Google Scholar] [CrossRef]
- Mathew, M.; Sudha Kartha, C.; Vijayakumar, K.P. In2S3:Ag, an ideal buffer layer for thin film solar cells. J. Mater. Sci. Mater. Electron. 2009, 20, 294–298. [Google Scholar] [CrossRef]
- Lin, P.; Lin, S.; Cheng, S.; Ma, J.; Lai, Y.; Zhou, H.; Jia, H. Optical and electrical properties of Ag-doped In2S3 thin films prepared by thermal evaporation. Adv. Mater. Sci. Eng. 2014, 2014, 370861. [Google Scholar] [CrossRef]
- Sankır, N.D.; Aydın, E.; Sankır, M. Impedance spectroscopy and dielectric properties of silver incorporated indium sulfide thin films. Int. J. Electrochem. Sci. 2014, 9, 3864–3875. [Google Scholar]
- Yan, L.L.; Ling, Y.J.; Ying, C.S.; Min, L.P. Influence of Ag and Sn incorporation in In2S3 thin films. Chin. Phys. 2015, 24, 78103. [Google Scholar] [CrossRef]
- Tiss, B.; Erouel, M.; Bouguila, N.; Kraini, M.; Khirouni, K. Effect of silver doping on structural and optical properties of In2S3 thin films fabricated by chemical pyrolysis. J. Alloy. Compd. 2019, 771, 60–66. [Google Scholar] [CrossRef]
- Zak, A.K.; Majid, W.H.A.; Abrishami, M.E.; Yousef, R. X-ray analysis of ZnO nanoparticles by Williamson–Hall and size-strain plot methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metallurgica 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Tiss, B.; Moualhi, Y.; Bouguila, N.; Erouel, M.; Kraini, M.; Alaya, S.; Aouida, S.; Vázquez–Vázquez, C.; Moura, C.; Cunha, L. Influence of silver doping on physical properties of sprayed In2S3 films for solar cells application. J. Mater. Sci. Mater Electron. 2021, 32, 4568–4580. [Google Scholar] [CrossRef]
- Moualhi, Y.; Rahmouni, H.; Gassoumi, M.; Khirouni, K. Summerfield scaling model and conduction processes defining the transport properties of silver substituted half doped (La–Ca) MnO3 ceramic. Ceram. Int. 2020, 46, 24710–24717. [Google Scholar] [CrossRef]
- Moualhi, Y.; M’nassri, R.; Nofal, M.M.; Rahmouni, H.; Selmi, A.; Gassoumi, M.; Chniba-Boudjada, N.; Khirouni, K.; Cheikrouhou, A. Magnetic properties and impedance spectroscopic analysis in Pr0.7Ca0.3Mn0.95Fe0.05O3 perovskite ceramic. J. Mater. Sci. Mater. Electron. 2020, 31, 21046–21058. [Google Scholar] [CrossRef]
- Hizi, W.; Rahmouni, H.; Gassoumi, M.; Khirouni, K.; Dhahri, S. Transport properties of La0.9Sr0.1MnO3 manganite. Eur. Phys. J. Plus 2020, 135, 456. [Google Scholar] [CrossRef]
- Mott, N.F. Conduction in non-crystalline materials. Philos. Mag. J. Theor. Exp. Appl. Phys. 1969, 19, 835–852. [Google Scholar] [CrossRef]
- Mott, N.F. Polarons. Mater. Res. Bull. 1978, 13, 1389–1394. [Google Scholar] [CrossRef]
- Mott, N.F. The origin of some ideas on non-crystalline materials. J. Non Cryst. Solids 1978, 28, 147–158. [Google Scholar] [CrossRef]
- Mott, N.F. Conduction in glasses containing transition metal ions. J. Non Cryst. Solids 1968, 1, 1–17. [Google Scholar] [CrossRef]
- Bruce, P.G. High and low frequency Jonscher behaviour of an ionically conducting glass. Solid State Ion. 1985, 15, 247–251. [Google Scholar] [CrossRef]
- Mguedla, R.; Ben Jazia Kharrat, A.; Moutia, N.; Khirouni, K.; Chniba-Boudjada, N.; Boujelben, W. Gd doping effect on structural, electrical and dielectric properties in HoCrO3 orthochromites for electric applications. J. Alloy. Compd. 2020, 836, 155186. [Google Scholar] [CrossRef]
- Elliott, S.R.A.C. Conduction in amorphous chalcogenide and pnictide semiconductors. Adv. Phys. 1987, 36, 135–217. [Google Scholar] [CrossRef]
- Tiss, B.; Bouguila, N.; Kraini, M.; Khirouni, K.; Vásquez-Vásquez, C.; Cunha, L.; Moura, C.; Alaya, S. Electrical transport of sprayed In2S3:Ag thin films. Mater. Sci. Semicond. Process. 2020, 114, 105080. [Google Scholar] [CrossRef]
- Chung, H. Transient Crack Growth in AISI 4340 Steel in Sodium Chloride Solution. Ph.D. Thesis, Ohio State University, Columbus, OH, USA, 1983. [Google Scholar]
- Sørensen, P.R.; Jacobsen, T. Conductivity, charge transfer and transport number—An ac-investigation of the polymer electrolyte LiSCN-poly (ethyleneoxide). Electrochimica acta 1982, 27, 1671–1675. [Google Scholar] [CrossRef]
- An, C.; Peng, S.; Sun, Y. Facile synthesis of sunlight-driven AgCl:Ag plasmonic nanophotocatalyst. Adv. Mater. 2010, 22, 2570–2574. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, Y.; Liu, Y.; Hao, Y.; Liu, R.; Zhao, D. Solution combustion synthesis and visible light-induced photocatalytic activity of mixed amorphous and crystalline MgAl2O4 nanopowders. Chem. Eng. J. 2011, 173, 750–759. [Google Scholar] [CrossRef]
- Castillo, N.C.; Heel, A.; Graule, T.; Pulgarin, C. Flame-assisted synthesis of nanoscale, amorphous and crystalline, spherical BiVO4 with visible-light photocatalytic activity. Appl. Catal. B Environ. 2010, 95, 335–347. [Google Scholar] [CrossRef]
- Wang, F.; Min, S.; Han, Y.; Feng, L. Visible-light-induced photocatalytic degradation of methylene blue with polyaniline-sensitized TiO2 composite photocatalysts. Superlattices Microstruct 2010, 48, 170–180. [Google Scholar] [CrossRef]
- Zhang, P.; Hong, R.Y.; Chen, Q.; Feng, W.G. On the electrical conductivity and photocatalytic activity of aluminum-doped zinc oxide. Powder Technol. 2014, 253, 360–367. [Google Scholar] [CrossRef]
- Li, H.; Xie, F.; Li, W.; Yang, H.; Snyders, R.; Chen, M.F.; Li, W.J. Preparation and photocatalytic activity of Ag2S/ZnS core-shell composites. Catal. Surv. Asia 2018, 22, 156–165. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Ye, Z.; Zhou, M.; Wang, H.; Ma, C.; Wang, D.; Huo, P.; Yan, Y. Fast electron transfer and enhanced visible light photocatalytic activity using multi-dimensional components of carbon quantum dots@3D daisy-like In2S3/single-wall carbon nanotubes. Appl. Catal. B Environ. 2017, 204, 224–238. [Google Scholar] [CrossRef]
- Dong, S.; Feng, J.; Fan, M.; Pi, Y.; Hu, L.; Han, X.; Liu, M.; Sun, J.; Sun, J. Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: A review. RSC Adv. 2015, 5, 14610–14630. [Google Scholar] [CrossRef]
- Wan, Y.; Chen, J.; Zhan, J.; Ma, Y. Facile synthesis of mesoporous NiCo2O4 fibers with enhanced photocatalytic performance for the degradation of methyl red under visible light irradiation. J. Environ. Chem. Eng. 2018, 6, 6079–6087. [Google Scholar] [CrossRef]
- Zhao, Y.; Kuai, L.; Geng, B. Low-cost and highly efficient composite visible light-driven Ag–AgBr/γ-Al2O3 plasmonic photocatalyst for degrading organic pollutants. Catal. Sci. Technol. 2012, 2, 1269–1274. [Google Scholar] [CrossRef]
- Jiang, Z.; Xie, J.; Jiang, D.; Yan, Z.; Jing, J.; Liu, D. Enhanced adsorption of hydroxyl contained/anionic dyes on non functionalized Ni@SiO2 core–shell nanoparticles: Kinetic and thermodynamic profile. Appl. Surf. Sci. 2014, 292, 301–310. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.; Song, Z.; Wang, H.; Xie, Y. Photocatalytic degradation of β-O-4 lignin model compound by In2S3 nanoparticles under visible light irradiation. Bioenergy Res. 2018, 11, 166–173. [Google Scholar] [CrossRef]
- Sharma, R.K.; Chouryal, Y.N.; Chaudhari, S.; Saravanakumar, J.; Dey, S.R.; Ghosh, P. Adsorption-driven catalytic and photocatalytic activity of phase tuned In2S3 nanocrystals synthesized via ionic liquids. ACS Appl. Mater. Interfaces 2017, 9, 11651–11661. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, W.; Gao, W. Tuning photocatalytic activity of In2S3 broadband spectrum photocatalyst based on morphology. Appl. Surf. Sci. 2016, 368, 288–297. [Google Scholar] [CrossRef]
- Vallejo, W.; DÍaz-Uribe, C.; Rios, K. Methylene blue photocatalytic degradation under visible irradiation on In2S3 synthesized by chemical bath deposition. Adv. Phys. Chem. 2017, 2017, 6358601. [Google Scholar] [CrossRef]
- Štengl, V.; Opluštil, F.; Němec, T. In 3+-doped TiO2 and TiO2/In2S3 nanocomposite for photocatalytic and stoichiometric degradations. Photochem. Photobiol. 2012, 88, 265–276. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, Y.; Su, C.; Gao, Y.; Tian, W.; Yang, X. Fabrication of magnetic and recyclable In2S3/ZnFe2O4 nanocomposites for visible light photocatalytic activity enhancement. Mater. Res. Express 2020, 7, 1–12. [Google Scholar] [CrossRef]
- Nefzi, C.; Souli, M.; Castilla, M.L.D.; García, J.M.; Kamoun-Turki, N. CFTS-3/In2S3/SnO2:F heterojunction structure as eco-friendly photocatalytic candidate for removing organic pollutants. Arab. J. Chem. 2020, 13, 6366–6378. [Google Scholar] [CrossRef]
- CellTiter-Blue Cell Viability Assay. Available online: https://worldwide.promega.com/-/media/files/resources/protocols/technical-bulletins/101/celltiter-blue-cell-viability-assay-protocol.pdf?la=en (accessed on 30 January 2021).
- Guo, B.L.; Han, P.; Guo, L.C.; Cao, Y.Q.; Li, A.D.; Kong, J.Z.; Zhai, H.F.; Wu, D. The antibacterial activity of ta-doped ZnO nanoparticles. Nanoscale Res. Lett. 2015, 10, 336. [Google Scholar] [CrossRef]
- Qiu, H.; Fang, S.; Huang, G.; Bi, J. A novel application of In2S3 for visible-light-driven photocatalytic inactivation of bacteria: Kinetics, stability, toxicity and mechanism. Environ. Res. 2020, 190, 110018. [Google Scholar] [CrossRef]
- Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A.H.; Su, B. Antibacterial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress. Nat. Commun. 2020, 11, 1626–1630. [Google Scholar] [CrossRef]




| Samples | Composition (at.%) | ||
|---|---|---|---|
| Ag | In | S | |
| In2S3 | 0.0 | 35.0 | 51.8 |
| In2S3:Ag | 0.6 | 34.9 | 51.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiss, B.; Moualhi, Y.; Bouguila, N.; Kraini, M.; Alaya, S.; Croitoru, C.; Ghiuta, I.; Cristea, D.; Patroi, D.; Moura, C.; et al. Influence of the Physical Properties on the Antibacterial and Photocatalytic Behavior of Ag-Doped Indium Sulfide Film Deposited by Spray Pyrolysis. Coatings 2021, 11, 370. https://doi.org/10.3390/coatings11040370
Tiss B, Moualhi Y, Bouguila N, Kraini M, Alaya S, Croitoru C, Ghiuta I, Cristea D, Patroi D, Moura C, et al. Influence of the Physical Properties on the Antibacterial and Photocatalytic Behavior of Ag-Doped Indium Sulfide Film Deposited by Spray Pyrolysis. Coatings. 2021; 11(4):370. https://doi.org/10.3390/coatings11040370
Chicago/Turabian StyleTiss, Belgacem, Youssef Moualhi, Noureddine Bouguila, Mabrouk Kraini, Sahbi Alaya, Catalin Croitoru, Ioana Ghiuta, Daniel Cristea, Delia Patroi, Cacilda Moura, and et al. 2021. "Influence of the Physical Properties on the Antibacterial and Photocatalytic Behavior of Ag-Doped Indium Sulfide Film Deposited by Spray Pyrolysis" Coatings 11, no. 4: 370. https://doi.org/10.3390/coatings11040370
APA StyleTiss, B., Moualhi, Y., Bouguila, N., Kraini, M., Alaya, S., Croitoru, C., Ghiuta, I., Cristea, D., Patroi, D., Moura, C., & Cunha, L. (2021). Influence of the Physical Properties on the Antibacterial and Photocatalytic Behavior of Ag-Doped Indium Sulfide Film Deposited by Spray Pyrolysis. Coatings, 11(4), 370. https://doi.org/10.3390/coatings11040370












