Antifungal Hydroxypropyl Methylcellulose (HPMC)-Lipid Composite Edible Coatings and Modified Atmosphere Packaging (MAP) to Reduce Postharvest Decay and Improve Storability of ‘Mollar De Elche’ Pomegranates
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit
2.2. Formulation and Preparation of Antifungal Edible Coatings
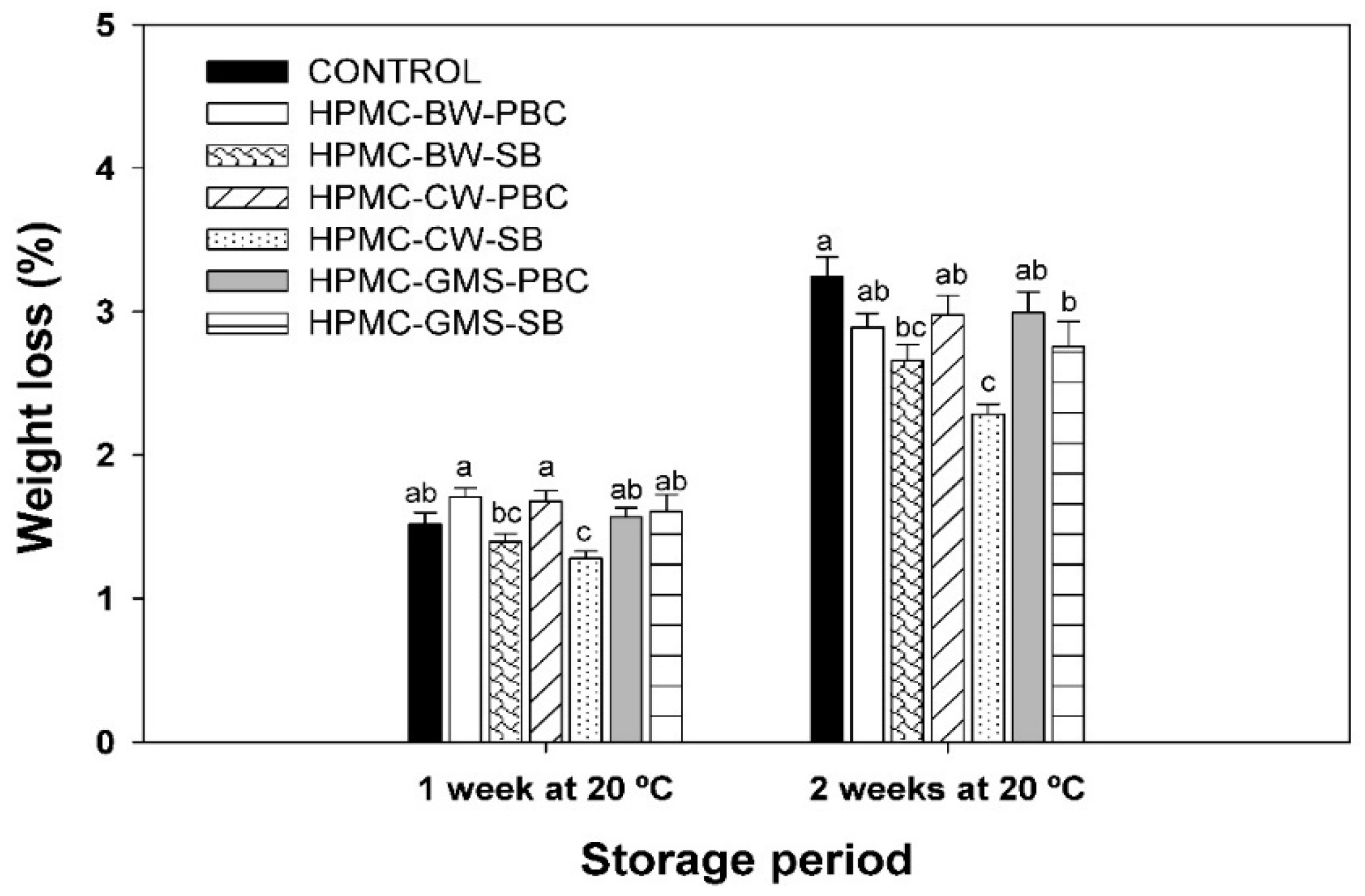
2.3. Experiment I. Effect of Fruit Coating on Weight Loss and Pomegranate Decay during Storage at 20 °C
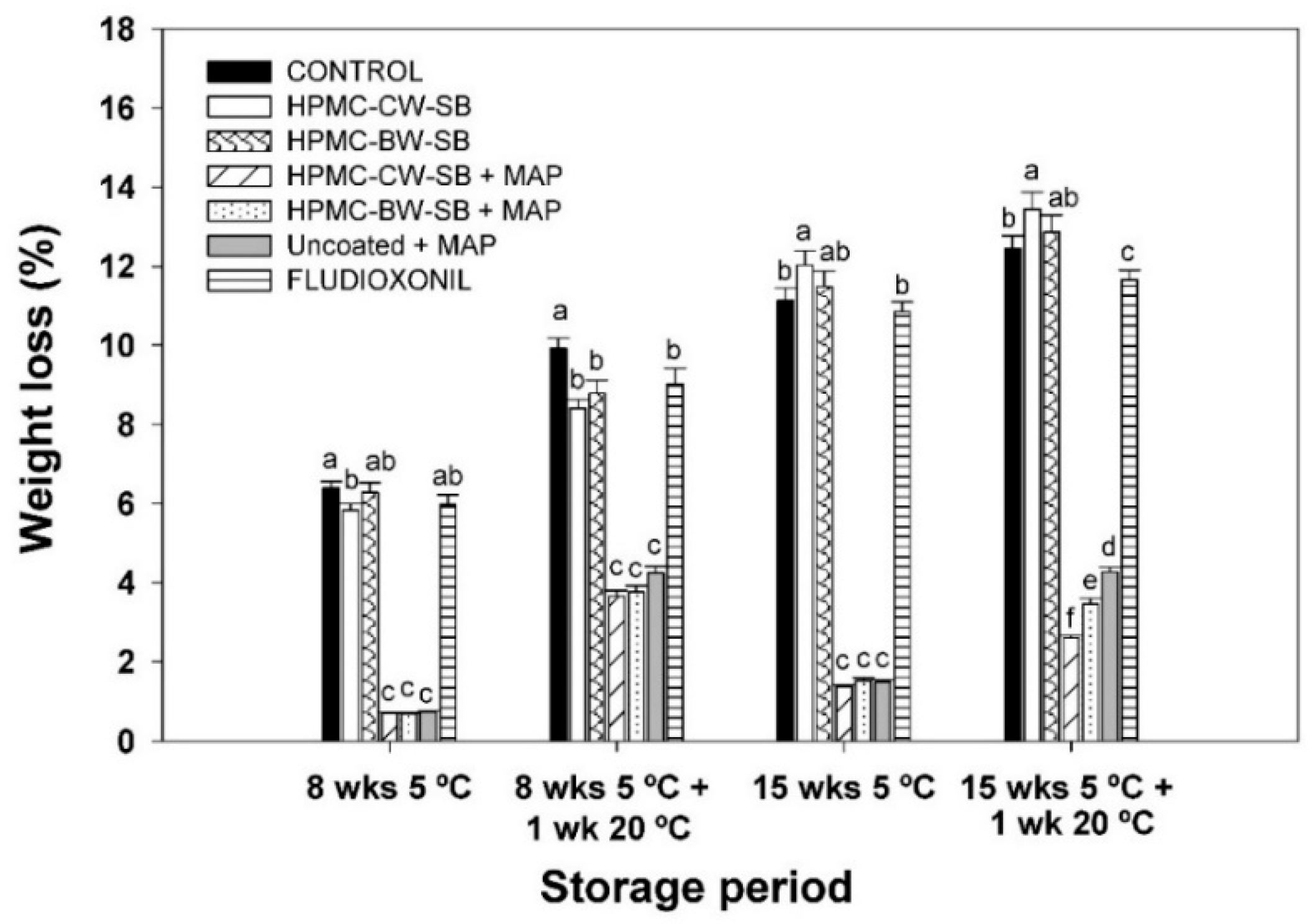
2.4. Experiment II. Effect of Coatings and MAP on Decay and Quality of Cold-Stored Pomegranates
2.5. Assessment of Fruit Quality
2.5.1. Weight Loss
2.5.2. Headspace Gas Concentration within MAP Bags
2.5.3. Rind Color
2.5.4. Juice Quality
2.5.5. Ethanol and Acetaldehyde Content
2.5.6. Physiological Disorders
2.5.7. Sensory Evaluation
2.6. Assessment of Fruit External and Internal Decay
2.7. Statistical Analysis
3. Results
3.1. Experiment I. Effect of Fruit Coating on Weight Loss and Pomegranate Decay during Storage at 20 °C
3.2. Experiment II. Effect of Coating and MAP on Decay and Quality of Cold-Stored Pomegranates
3.2.1. Weight Loss
3.2.2. Rind Color
3.2.3. Juice Quality and Ethanol and Acetaldehyde Content
3.2.4. External and Internal Physiological Disorders
3.2.5. Sensory Evaluation
3.2.6. External and Internal Fungal Decay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrara, G.; Selahvarzi, Y.; Ahmadpourmir, H.; Mazzeo, A.; Giancaspro, A. Production and growing regions. In The Pomegranate: Botany, Production and Uses; Sarkhosh, A., Yavari, A., Zamani, Z., Eds.; CABI: Osfordshire, UK, 2021; pp. 59–93. ISBN 9781789240764. [Google Scholar]
- Palou, L.; Taberner, V.; Guardado, A.; Del Río, M.A.; Montesinos-Herrero, C. Incidence and etiology of postharvest fungal diseases of pomegranate (Punica granatum cv. Mollar de Elche) in Spain. Phytopathol. Mediterr. 2013, 52, 478–489. [Google Scholar] [CrossRef]
- García-Alonso, M.; de Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Evaluation of the antioxidant properties of fruits. Food Chem. 2004, 84, 13–18. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Koyama, S.; Cobb, L.J.; Mehta, H.H.; Seeram, N.P.; Heber, D.; Pantuck, A.J.; Cohen, P. Pomegranate extract induces apoptosis in human prostate cancer cells by modulation of the IGF-IGFBP axis. Growth Horm. IGF Res. 2010, 20, 55–62. [Google Scholar] [CrossRef]
- Ben-Yehoshua, S.; Rodov, V. Transpiration and Water Stress. In Postharvest Physiology and Pathology of Vegetables; Bartz, J.A., Brecht, J.K., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2003; pp. 111–159. ISBN 9780429222269. [Google Scholar]
- Pareek, S.; Valero, D.; Serrano, M. Postharvest biology and technology of pomegranate. J. Sci. Food Agric. 2015, 95, 2360–2370. [Google Scholar] [CrossRef]
- Artés, F.; Marín, J.G.; Martínez, J.A. Controlled atmosphere storage of pomegranate. Eur. Food Res. Technol. 1996, 203, 33–37. [Google Scholar] [CrossRef]
- Caleb, O.J.; Opara, U.L.; Witthuhn, C.R. Modified atmosphere packaging of pomegranate fruit and arils: A review. Food Bioprocess Technol. 2012, 5, 15–30. [Google Scholar] [CrossRef]
- Elyatem, S.M.; Kader, A.A. Post-harvest physiology and storage behaviour of pomegranate fruits. Sci. Hortic. 1984, 24, 287–298. [Google Scholar] [CrossRef]
- Köksal, A.I. Research on the storage of pomegranate (cv. Gok Bahce) under different conditions. Acta Hortic. 1989, 258, 295–302. [Google Scholar] [CrossRef]
- Palou, L.; Kinay-Teksür, P.; Cao, S.; Karaoglanidis, G.; Vicent, A. Pomegranate, persimmon, and loquat. In Postharvest Pathology of Fresh Horticultural Produce; Palou, L., Smilanick, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 187–226. ISBN 9781138630833. [Google Scholar]
- Palou, L.; Vicent, A. Fungal pathogens causing postharvest decay of pomegranate fruit in Spain. Acta Hortic. 2019, 1254, 243–252. [Google Scholar] [CrossRef]
- Holland, D.; Hatib, K.; Bar-ya, I. Pomegranate: Botany, Horticulture, Breeding. Breed. Hortic. Rev. 2009, 35, 127–192. [Google Scholar]
- Tedford, E.; Adaskaveg, J.E.; Ott, A.J. Impact of Scholar (a new post-harvest fungicide) on the California pomegranate industry. Plant Health Prog. 2005, 1–3. [Google Scholar] [CrossRef]
- Mirabolfathy, M.; Groenewald, J.Z.; Crous, P.W. First report of Pilidiella granati causing dieback and fruit rot of pomegranate (Punica granatum) in Iran. Plant Dis. 2012, 96, 461. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Lennox, C.L.; Meitz-Hopkins, J.C.; Caleb, O.J.; Opara, U.L. Major diseases of pomegranate (Punica granatum L.), their causes and management—A review. Sci. Hortic. 2016, 211, 126–139. [Google Scholar] [CrossRef]
- Thomidis, T.; Exadaktylou, E. First report of Pilidiella granati on pomegranate with symptoms of crown rot in the Prefecture of Xanthi, Greece. Plant Dis. 2010, 95, 79. [Google Scholar] [CrossRef]
- Artés, F.; Villaescusa, R.; Tudela, A.J. Modified atmosphere packaging of pomegranate. Food Chem. Toxicol. 2000, 53, 1689–1699. [Google Scholar] [CrossRef]
- D’Aquino, S.; Palma, A.; Schirra, M.; Continella, A.; Tribulato, E.; La Malfa, S. Influence of film wrapping and fludioxonil application on quality of pomegranate fruit. Postharvest Biol. Technol. 2010, 55, 121–128. [Google Scholar] [CrossRef]
- Selcuk, N.; Erkan, M. Changes in phenolic compounds and antioxidant activity of sour-sweet pomegranates cv. “Hicaznar” during long-term storage under modified atmosphere packaging. Postharvest Biol. Technol. 2015, 109, 30–39. [Google Scholar] [CrossRef]
- Candir, E.; Ozdemir, A.E.; Aksoy, M.C. Effects of chitosan coating and modified atmosphere packaging on postharvest quality and bioactive compounds of pomegranate fruit cv. ‘Hicaznar’. Sci. Hortic. 2018, 235, 235–243. [Google Scholar] [CrossRef]
- Porat, R.; Kosto, I.; Daus, A. Bulk storage of “Wonderful” pomegranate fruit using modified atmosphere bags. Isr. J. Plant Sci. 2016, 63, 45–50. [Google Scholar] [CrossRef]
- Selcuk, N.; Erkan, M. Changes in antioxidant activity and postharvest quality of sweet pomegranates cv. Hicrannar under modified atmosphere packaging. Postharvest Biol. Technol. 2014, 92, 29–36. [Google Scholar] [CrossRef]
- Porat, R.; Weiss, B.; Fuchs, Y.; Sandman, A.; Ward, G.; Kosto, I. Keeping quality of pomegranate fruit during prolonged storage and transport by MAP: New developments and commercial applications. Acta Hortic. 2008, 804, 115–120. [Google Scholar] [CrossRef]
- Adaskaveg, J.E.; Förster, H. Management of gray mold of pomegranates caused by Botrytis cinerea using two reduced-risk fungicides, fludioxonil and fenhexamid. Phytopathology 2002, 93, S127. [Google Scholar]
- Porat, R.; Mayouni-Kirshenbaum, L.; Lichter, A.; Ezra, D.; Kosto, I. Reduction of crown rots development during storage of pomegranate fruit by preharvest and postharvest fungicidal treatments. Acta Hortic. 2015, 1089, 173–177. [Google Scholar] [CrossRef]
- Palou, L. Postharvest treatments with GRAS salts to control fresh fruit decay. Horticulturae 2018, 4, 46. [Google Scholar] [CrossRef]
- Pérez-Gago, M.B.; Palou, L. Antimicrobial packaging for fresh and fresh-cut fruits and vegetables. In Fresh-Cut Fruits and Vegetables: Technology, Physiology, and Safety; Pareek, S., Ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2016; pp. 1–9. ISBN 9781315370132. [Google Scholar]
- Valencia-Chamorro, S.A.; Palou, L.; Del Río, M.A.; Pérez-Gago, M.B. Antimicrobial edible films and coatings for fresh and minimally processed fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2011, 51, 872–900. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, C.; Pérez-Gago, M.B.; Monteiro, A.R.; Palou, L. Antifungal activity of food additives in vitro and as ingredients of hydroxypropyl methylcellulose-lipid edible coatings against Botrytis cinerea and Alternaria alternata on cherry tomato fruit. Int. J. Food Microbiol. 2013, 166, 391–398. [Google Scholar] [CrossRef]
- Guimarães, J.E.R.; de la Fuente, B.; Pérez-Gago, M.B.; Andradas, C.; Carbó, R.; Mattiuz, B.-H.; Palou, L. Antifungal activity of GRAS salts against Lasiodiplodia theobromae in vitro and as ingredients of hydroxypropyl methylcellulose-lipid composite edible coatings to control Diplodia stem-end rot and maintain postharvest quality of citrus fruit. Int. J. Food Microbiol. 2019, 301, 9–18. [Google Scholar] [CrossRef]
- Gunaydin, S.; Karaca, H.; Palou, L.; De La Fuente, B.; Pérez-Gago, M.B. Effect of hydroxypropyl methylcellulose-beeswax composite edible coatings formulated with or without antifungal agents on physicochemical properties of plums during cold storage. J. Food Qual. 2017, 8573549. [Google Scholar] [CrossRef]
- Karaca, H.; Pérez-Gago, M.B.; Taberner, V.; Palou, L. Evaluating food additives as antifungal agents against Monilinia fructicola in vitro and in hydroxypropyl methylcellulose-lipid composite edible coatings for plums. Int. J. Food Microbiol. 2014, 179, 72–79. [Google Scholar] [CrossRef]
- Martínez-Blay, V.; Pérez-Gago, M.B.; de la Fuente, B.; Carbó, R.; Palou, L. Edible coatings formulated with antifungal GRAS salts to control citrus anthracnose caused by Colletotrichum gloeosporioides and preserve postharvest fruit quality. Coatings 2020, 10, 730. [Google Scholar] [CrossRef]
- Navarro, M.L.; Navarro-Tarazaga, M.L.; Pérez-Gago, M.B.; Goodner, K.; Plotto, A. A new composite coating containing HPMC, beeswax, and shellac for ‘Valencia’ oranges and ‘Marisol’ tangerines. Proc. Fla. State Hort. Soc. 2007, 120, 228–234. [Google Scholar]
- Rodsamran, P.; Sothornvit, R.; Palou, L.; Pérez-Gago, M.B. Effect of polysaccharide-based edible coatings incorporated with sodium benzoate on the control of postharvest black spot of organic cherry tomatoes caused by Alternaria alternata. Acta Hortic. 2018, 1194, 241–247. [Google Scholar] [CrossRef]
- Valencia-Chamorro, S.A.; Pérez-Gago, M.B.; Del Río, M.A.; Palou, L. Effect of antifungal hydroxypropyl methylcellulose (HPMC)-lipid edible composite coatings on postharvest decay development and quality attributes of cold-stored ‘Valencia’ oranges. Postharvest Biol. Technol. 2009, 54, 72–79. [Google Scholar] [CrossRef]
- Valencia-Chamorro, S.A.; Pérez-Gago, M.B.; Del Río, M.A.; Palou, L. Effect of antifungal hydroxypropyl methylcellulose-lipid edible composite coatings on penicillium decay development and postharvest quality of cold-stored ‘Ortanique’ mandarins. J. Food Sci. 2010, 75, S418–S426. [Google Scholar] [CrossRef]
- Laribi, A.I.; Palou, L.; Intrigliolo, D.S.; Nortes, P.A.; Rojas-Argudo, C.; Taberner, V.; Bartual, J.; Pérez-Gago, M.B. Effect of sustained and regulated deficit irrigation on fruit quality of pomegranate cv. “Mollar de Elche” at harvest and during cold storage. Agric. Water Manag. 2013, 125, 61–70. [Google Scholar] [CrossRef]
- Palou, L.; Crisosto, C.H.; Garner, D. Combination of postharvest antifungal chemical treatments and controlled atmosphere storage to control gray mold and improve storability of “Wonderful” pomegranates. Postharvest Biol. Technol. 2007, 43, 133–142. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Effects of storage temperature and duration on physiological responses of pomegranate fruit. Ind. Crops Prod. 2013, 47, 300–309. [Google Scholar] [CrossRef]
- Fagundes, C.; Palou, L.; Monteiro, A.R.; Pérez-Gago, M.B. Hydroxypropyl methylcellulose-beeswax edible coatings formulated with antifungal food additives to reduce alternaria black spot and maintain postharvest quality of cold-stored cherry tomatoes. Sci. Hortic. 2015, 193, 249–257. [Google Scholar] [CrossRef]
- Fagundes, C.; Palou, L.; Monteiro, A.R.; Pérez-Gago, M.B. Effect of antifungal hydroxypropyl methylcellulose-beeswax edible coatings on gray mold development and quality attributes of cold-stored cherry tomato fruit. Postharvest Biol. Technol. 2014, 92, 1–8. [Google Scholar] [CrossRef]
- Pastor, C.; Sánchez-González, L.; Marcilla, A.; Chiralt, A.; Cháfer, M.; González-Martínez, C. Quality and safety of table grapes coated with hydroxypropylmethylcellulose edible coatings containing propolis extract. Postharvest Biol. Technol. 2011, 60, 64–70. [Google Scholar] [CrossRef]
- Valencia-Chamorro, S.A.; Palou, L.; Del Río, M.A.; Pérez-Gago, M.B. Inhibition of Penicillium digitatum and Penicillium italicum by hydroxypropyl methylcellulose-lipid edible composite films containing food additives with antifungal properties. J. Agric. Food Chem. 2008, 56, 11270–11278. [Google Scholar] [CrossRef]
- Valencia-Chamorro, S.A.; Pérez-Gago, M.B.; Del Río, M.A.; Palou, L. Curative and preventive activity of hydroxypropyl methylcellulose-lipid edible composite coatings containing antifungal food additives to control citrus postharvest green and blue molds. J. Agric. Food Chem. 2009, 57, 2770–2777. [Google Scholar] [CrossRef] [PubMed]
- Soto-Muñoz, L.; Taberner, V.; de la Fuente, B.; Jerbi, N.; Palou, L. Curative activity of postharvest GRAS salt treatments to control citrus sour rot caused by Geotrichum citri-aurantii. Int. J. Food Microbiol. 2020, 335, 108860. [Google Scholar] [CrossRef]
- Kinay-Teksur, P. Alternative technologies to control postharvest diseases of pomegranate. Stewart Postharv. Rev. 2015, 11. [Google Scholar] [CrossRef]
- Palou, L.; Pérez-Gago, M.B. GRAS salts as alternative low-toxicity chemicals for postharvest preservation of fresh horticultural products. In Postharvest Pathology. Next Generation Solutions to Reducing Losses and Enhancing Safety. Plant Pathology in the 21st Century; Spadaro, D., Droby, S., Gullino, M.L., Eds.; Springer: Cham, Switzerland, 2021; Volume 11, pp. 163–179. ISBN 9783030565299. [Google Scholar]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Effect of sodium caseinate on properties and ageing behaviour of corn starch based films. Food Hydrocoll. 2012, 29, 265–271. [Google Scholar] [CrossRef]
- Matsakidou, A.; Tsimidou, M.Z.; Kiosseoglou, V. Storage behavior of caseinate-based films incorporating maize germ oil bodies. Food Res. Int. 2019, 116, 1031–1040. [Google Scholar] [CrossRef]
- Kamper, S.L.; Fennema, O. Water vapor permeability of an edible, fatty acid, bilayer Film. J. Food Sci. 1984, 49, 1482–1485. [Google Scholar] [CrossRef]
- Shellhammer, T.H.; Krochta, J.M. Whey protein emulsion film performance as affected by lipid type and amount. J. Food Sci. 1997, 62, 390–394. [Google Scholar] [CrossRef]
- Laribi, A.I.; Palou, L.; Taberner, V.; Pérez-Gago, M.B. Modified atmosphere packaging to extend cold storage of pomegranate cv. ‘Mollar de Elche’. In Proceedings of the International Conference of Agricultural Engineering—CIGR-AgEng 2012: Agriculture and Engineering for a Healthier Life, Valencia, Spain, 8–12 July 2012; p. C-0781. [Google Scholar]
- Selçuk, N.; Erkan, M. Impact of passive modified atmosphere packaging on physicochemical properties, bioactive compounds, and quality attributes of sweet pomegranates. Turk. J. Agric. For. 2016, 40, 475–488. [Google Scholar] [CrossRef]
- Chater, J.M.; Yavari, A.; Sarkhosh, A.; Jia, Z.; Merhaut, D.J.; Preece, J.E.; Cossio, F.; Qin, G.; Liu, C.; Li, J.; et al. World pomegranate cultivars. In The Pomegranate: Botany, Production and Uses; Sarkhosh, A., Yavari, A., Zamani, Z., Eds.; CABI: Osfordshire, UK, 2021; pp. 157–195. ISBN 9781789240764. [Google Scholar]
- Ghafir, S.A.M.; Ibrahim, I.Z.; Zaied, S.A.; Abusrewel, G.S. Response of local variety “Shlefy” pomegranate fruits to packaging and cold storage. Acta Hortic. 2010, 877, 427–431. [Google Scholar] [CrossRef]
- Ayhan, Z.; Eştürk, O. Overall quality and shelf life of minimally processed and modified atmosphere packaged “ready-to-eat” pomegranate arils. J. Food Sci. 2009, 74, C399–C405. [Google Scholar] [CrossRef] [PubMed]
- Watkins, C.B. Responses of horticultural commodities to high carbon dioxide as related to modified atmosphere packaging. HortTechnology 2000, 10, 501–506. [Google Scholar] [CrossRef]
| Coating | Biopolymer | Lipid | Plasticizer | Emulsifier | GRAS Salt | ||||
|---|---|---|---|---|---|---|---|---|---|
| HPMC | BW | CW | GMS | Gly | SA | DATEM + LEC | PBC | SB | |
| HPMC-BW-PBC | 2 | 0.8 | - | - | 0.4 | 0.4 | - | 2 | - |
| HPMC-BW-SB | 2 | 0.8 | - | - | 0.4 | 0.4 | - | - | 2 |
| HPMC-CW-PBC | 2 | - | 0.8 | - | 0.4 | 0.4 | - | 2 | - |
| HPMC-CW-SB | 2 | - | 0.8 | - | 0.4 | 0.4 | - | - | 2 |
| HPMC-GMS-PBC | 2 | - | - | 0.44 | 0.3 | - | 0.43 + 0.43 | 2 | - |
| HPMC-GMS-SB | 2 | - | - | 0.44 | 0.3 | - | 0.43 + 0.43 | - | 2 |
| External Decay | Treatment 3 | Storage Period | |||
|---|---|---|---|---|---|
| 4 Weeks | 8 Weeks | ||||
| % Incidence | Severity Index (0–4) | % Incidence | Severity Index (0–4) | ||
| Crown Decay 1 | CONTROL | 5.00 ± 2.89 a | 2.00 ± 1.15 a | 35.00 ± 6.45 a | 3.13 ± 0.13 a |
| HPMC-BW-PBC | 5.00 ± 2.89 a | 1.75 ± 1.03 a | 37.50 ± 7.50 a | 3.38 ± 0.36 a | |
| HPMC-BW-SB | 2.50 ± 2.50 a | 0.75 ± 0.75 a | 15.00 ± 6.45 bc | 1.75 ± 0.63 a | |
| HPMC-CW-PBC | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 15.00 ± 5.00 abc | 2.83 ± 0.44 a | |
| HPMC-CW-SB | 5.00 ± 2.89 a | 1.50 ± 0.87 a | 12.50 ± 4.79 bc | 2.63 ± 0.94 a | |
| HPMC-GMS-PBC | 2.50 ± 2.50 a | 0.50 ± 0.50 a | 25.00 ± 6.45 ab | 2.48 ± 0.22 a | |
| HPMC-GMS-SB | 2.50 ± 2.50 a | 1.00 ± 1.00 a | 7.50 ± 4.79 c | 2.00 ± 1.15 a | |
| Wound Decay 2 | CONTROL | 15.00 ± 6.45 a | 2.38 ± 0.80 a | 40.00 ± 12.25 a | 2.70 ± 0.57 a |
| HPMC-BW-PBC | 5.00 ± 5.00 a | 0.63 ± 0.63 a | 27.5.0 ± 6.29 a | 2.81 ± 0.64 a | |
| HPMC-BW-SB | 20.00 ± 8.16 a | 1.31 ± 0.45 a | 35.00 ± 6.45 a | 2.55 ± 0.12 a | |
| HPMC-CW-PBC | 2.50 ± 2.50 a | 0.75 ± 0.75 a | 12.50 ± 4.79 a | 2.38 ± 0.85 a | |
| HPMC-CW-SB | 20.00 ± 4.08 a | 1.71 ± 0.29 a | 35.00 ± 6.45 a | 3.04 ± 0.38 a | |
| HPMC-GMS-PBC | 10.00 ± 4.08 a | 1.38 ± 0.47 a | 22.50 ± 4.79 a | 2.67 ± 0.58 a | |
| HPMC-GMS-SB | 12.50 ± 7.50 a | 0.96 ± 0.60 a | 37.50 ± 7.50 a | 2.37 ± 0.32 a | |
| Treatment | Storage Time | |||||
|---|---|---|---|---|---|---|
| 30 Days | 60 Days | 90 Days | ||||
| O2 | CO2 | O2 | CO2 | O2 | CO2 | |
| HPMC-CW-SB + MAP | 18.43 a | 3.63 a | 18.44 a | 4.50 a | 18.93 a | 3.37 a |
| HPMC-BW-SB + MAP | 19.53 a | 2.63 a | 19.50 a | 3.02 a | 19.40 a | 2.87 a |
| Uncoated + MAP | 18.20 a | 4.00 a | 18.07 a | 4.67 a | 18.97 a | 3.33 a |
| Storage Time | Treatment | Rind Color | ||||
|---|---|---|---|---|---|---|
| L* | a* | b* | C* | h° | ||
| 8 weeks at 5 °C + 1 week at 20 °C | CONTROL | 65.83 ± 1.34 c | 15.40 ± 1.12 a | 37.95 ± 0.53 cd | 41.65 ± 0.36 bc | 66.92 ± 1.84 a |
| HPMC-CW-SB | 68.07 ± 1.33 bc | 14.69 ± 1.24 a | 40.58 ± 0.31 a | 43.41 ± 1.05 a | 70.26 ± 1.63 a | |
| HPMC-BW-SB | 69.78 ± 1.25 ab | 12.64 ± 1.08 a | 40.42 ± 0.32 ab | 42.76 ± 0.25 ab | 72.86 ± 1.47 a | |
| HPMC-CW-SB + MAP | 69.85 ± 0.97 ab | 12.99 ± 0.88 a | 39.12 ± 0.28 bc | 41.47 ± 0.38 c | 71.83 ± 1.17 a | |
| HPMC-BW-SB + MAP | 71.47 ± 1.25 ab | 10.41 ± 0.96 a | 37.49 ± 0.53 d | 39.65 ± 0.41 d | 74.54 ± 1.41 a | |
| Uncoated + MAP | 71.78 ± 1.35 a | 11.77 ± 1.21 a | 39.06 ± 0.33 bc | 41.32 ± 0.29 c | 73.42 ± 1.70 a | |
| FLUDIOXONIL | 69.53 ± 1.29 ab | 14.10 ± 1.27 a | 38.82 ± 0.38 cd | 41.55 ± 0.32 c | 70.04 ± 1.83 a | |
| 15 weeks at 5 °C + 1 week at 20 °C | CONTROL | 69.65 ± 1.11 c | 10.87 ± 0.65 a | 41.33 ± 0.42 ab | 43.45 ± 0.26 a | 75.33 ± 0.90 c |
| HPMC-CW-SB | 71.22 ± 0.82 bc | 11.04 ± 0.98 a | 41.87 ± 0.29 a | 43.62 ± 0.23 a | 75.31 ± 1.30 c | |
| HPMC-BW-SB | 71.49 ± 0.72 bc | 9.98 ± 0.57 ab | 41.95 ± 0.42 a | 43.43 ± 0.37 a | 76.71 ± 0.76 bc | |
| HPMC-CW-SB + MAP | 70.91 ± 0.70 c | 10.02 ± 0.48 ab | 41.09 ± 0.34 ab | 43.05 ± 0.27 a | 75.86 ± 0.77 bc | |
| HPMC-BW-SB + MAP | 73.31 ± 0.55 a | 7.92 ± 0.37 bc | 40.34 ± 0.33 bc | 41.23 ± 0.36 b | 78.54 ± 0.57 ab | |
| Uncoated + MAP | 73.15 ± 0.64 a | 7.22 ± 0.40 c | 39.31 ±0.34 c | 40.51 ± 0.28 b | 79.62 ± 0.58 a | |
| FLUDIOXONIL | 73.25 ± 0.43 a | 7.97 ± 0.45 bc | 39.18 ± 0.57 c | 40.54 ± 0.39 b | 79.98 ± 0.57 a | |
| Storage Time | Treatments | Juice Quality | |||||
|---|---|---|---|---|---|---|---|
| SSC (° Brix) | TA (% Citric Acid) | pH | MI | Acetaldehyde (mg/100 mL) | Ethanol (mg/100 mL) | ||
| At harvest | - | 15.90 ± 0.03 | 0.23 ± 0.01 | 4.16 ± 0.05 | 69.13 ± 0.58 | 0.18 ± 0.03 | 0.52 ± 0.10 |
| 8 weeks at 5 °C + 1 week at 20 °C | CONTROL | 15.15 ± 0.10 b | 0.18 ± 0.00 b | 5.10 ± 0.02 a | 82.88 ± 0.95 b | 0.23 ± 0.02 a | 1.69 ± 0.25 c |
| HPMC-CW-SB | 15.28 ± 0.12 b | 0.18 ± 0.00 b | 5.10 ± 0.04 a | 83.03 ± 0.43 b | 0.27 ± 0.02 a | 1.63 ± 0.31 c | |
| HPMC-BW-SB | 15.92 ± 0.22 ab | 0.18 ± 0.00 b | 5.08 ± 0.03 a | 90.58 ± 2.63 a | 0.21 ± 0.02 a | 0.85 ± 0.34 c | |
| HPMC-CW-SB + MAP | 16.42 ± 0.41 a | 0.18 ± 0.00 b | 5.01 ± 0.04 ab | 90.82 ± 1.95 a | 0.09 ± 0.04 b | 4.33 ± 0.79 a | |
| HPMC-BW-SB + MAP | 15.15 ± 0.13 b | 0.18 ± 0.00 b | 4.93 ± 0.01 bc | 84.36 ± 2.59 ab | 0.23 ± 0.02 a | 3.22 ± 1.05 ab | |
| Uncoated + MAP | 15.77 ± 0.47 ab | 0.20 ± 0.01 a | 4.87 ± 0.04 cd | 80.40 ± 3.92 b | 0.21 ± 0.02 a | 1.83 ± 0.10 bc | |
| FLUDIOXONIL | 15.52 ± 0.13 b | 0.20 ± 0.00 a | 4.79 ± 0.01 d | 78.60 ± 0.73 b | 0.23 ± 0.02 a | 1.44 ± 0.11 c | |
| 15 weeks at 5 °C + 1 week at 20 °C | CONTROL | 15.07 ± 0.25 a | 0.17 ± 0.01 a | 4.71 ± 0.02 a | 86.95 ± 4.17 a | 3.06 ± 0.12 cd | 27.37 ± 0.85 bc |
| HPMC-CW-SB | 15.10 ± 0.05 a | 0.17 ± 0.01 a | 4.80 ± 0.05 a | 90.99 ± 2.43 a | 3.88 ± 0.06 ab | 26.22 ± 0.28 cd | |
| HPMC-BW-SB | 15.17 ± 0.07 a | 0.18 ± 0.01 a | 4.71 ± 0.09 a | 84.48 ± 2.92 a | 4.28 ± 0.35 a | 30.55 ± 1.25 b | |
| HPMC-CW-SB + MAP | 15.33 ± 0.12 a | 0.16 ± 0.01 a | 4.87 ± 0.03 a | 93.58 ± 3.54 a | 2.77 ± 0.23 cd | 23.24 ± 1.77 de | |
| HPMC-BW-SB + MAP | 14.67 ± 0.32 a | 0.16 ± 0.01 a | 4.91 ± 0.06 a | 92.85 ± 3.36 a | 3.45 ± 0.23 bc | 21.31 ± 0.83 e | |
| Uncoated + MAP | 15.23 ± 0.27 a | 0.16 ± 0.00 a | 4.75 ± 0.08 a | 92.76 ± 2.12 a | 2.53 ± 0.07 d | 29.74 ± 3.04 bc | |
| FLUDIOXONIL | 15.07 ± 0.13 a | 0.17 ± 0.00 a | 4.86 ± 0.05 a | 89.37 ± 2.76 a | 3.81 ± 0.38 ab | 35.61 ± 0.92 a | |
| Storage Time | Treatments 1 | Rind Sinking 2 | External Pitting/Browning 2 | Pitting of Teguments 3 | Browning of Arils 3 |
|---|---|---|---|---|---|
| Severity (0–3) | Severity (0–3) | Severity (0–3) | Severity (0–3) | ||
| 8 weeks at 5 °C + 1 week at 20 °C | CONTROL | 1.23 ± 0.08 a | 0.83 ± 0.08 a | 0.00 ± 0.00 a | 0.08 ± 0.02 a |
| HPMC-CW-SB | 1.08 ± 0.33 a | 0.85 ± 0.31 a | 0.04 ± 0.04 a | 0.02 ± 0.02 a | |
| HPMC-BW-SB | 1.10 ± 0.13 a | 0.48 ± 0.06 a | 0.00 ± 0.00 a | 0.08 ± 0.02 a | |
| HPMC-CW-SB + MAP | 0.29 ± 0.10 b | 0.35 ± 0.09 a | 0.04 ± 0.04 a | 0.13 ± 0.10 a | |
| HPMC-BW-SB + MAP | 0.47 ± 0.08 b | 0.90 ± 0.08 a | 0.19 ± 0.04 a | 0.08 ± 0.02 a | |
| Uncoated + MAP | 0.42 ± 0.13 b | 0.31 ± 0.16 a | 0.10 ± 0.02 a | 0.08 ± 0.04 a | |
| FLUDIOXONIL | 1.09 ± 0.16 a | 0.88 ± 0.18 a | 0.10 ± 0.08 a | 0.13 ± 0.00 a | |
| 15 weeks at 5 °C + 1 week at 20 °C | CONTROL | 2.00 ± 0.23 a | 1.52 ± 0.06 a | 2.02 ± 0.13 a | 1.08 ± 0.18 a |
| HPMC-CW-SB | 1.96 ± 0.11 a | 1.54 ± 0.08 a | 1.81 ± 0.07 a | 1.15 ± 0.25 a | |
| HPMC-BW-SB | 1.96 ± 0.09 a | 1.54 ± 0.12 a | 1.90 ± 0.15 a | 0.77 ± 0.11 abc | |
| HPMC-CW-SB + MAP | 0.98 ± 0.02 b | 0.94 ± 0.13 b | 1.67 ± 0.11 a | 0.48 ± 0.08 bc | |
| HPMC-BW-SB + MAP | 1.19 ± 0.10 b | 1.65 ± 0.13 a | 1.88 ± 0.10 a | 0.81 ± 0.06 ab | |
| Uncoated + MAP | 1.17 ± 0.15 b | 0.73 ± 0.17 b | 1.56 ± 0.04 a | 0.38 ± 0.11 c | |
| FLUDIOXONIL | 1.96 ± 0.06 a | 1.54 ± 0.06 a | 1.94 ± 0.00 a | 0.94 ± 0.04 a |
| Storage Time | Treatments 1 | Sensory Analysis | ||
|---|---|---|---|---|
| Flavor 2 (1–9 Scale) | Off Flavor 3 (1–5 Scale) | Visual Quality 4 (1–3 Scale) | ||
| 8 weeks at 5 °C + 1 week at 20 °C | CONTROL | 6.38 ± 0.27 a | 1.27 ± 0.12 a | 1.27 ± 0.15 d |
| HPMC-CW-SB | 7.15 ± 0.32 a | 1.15 ± 0.10 a | 1.27 ± 0.12 d | |
| HPMC-BW-SB | 7.62 ± 0.24 a | 1.13 ± 0.09 a | 1.75 ± 0.23 c | |
| HPMC-CW-SB + MAP | 7.29 ± 0.38 a | 1.20 ± 0.11 a | 2.40 ± 0.25 b | |
| HPMC-BW-SB + MAP | 7.64 ± 0.47 a | 1.27 ± 0.12 a | 1.67 ± 0.41 c | |
| Uncoated + MAP | 7.75 ± 0.30 a | 1.20 ± 0.11 a | 3.00 ± 0.00 a | |
| FLUDIOXONIL | 6.61 ± 0.40 a | 1.31 ± 0.13 a | 2.57 ± 0.32 b | |
| 15 weeks at 5 °C + 1 week at 20 °C | CONTROL | 5.00 ± 0.28 ab | 1.79 ± 0.21 a | 1.60 ± 0.16 c |
| HPMC-CW-SB | 4.42 ± 0.38 b | 2.77 ± 0.34 a | 1.00 ± 0.07 d | |
| HPMC-BW-SB | 5.42 ± 0.32 a | 1.77 ± 0.30 a | 2.36 ± 0.19 ab | |
| HPMC-CW-SB + MAP | 5.92 ± 0.36 a | 1.86 ± 0.42 a | 1.93 ± 0.16 bc | |
| HPMC-BW-SB + MAP | 5.69 ± 0.36 a | 1.57 ± 0.17 a | 1.71 ± 0.19 c | |
| Uncoated + MAP | 5.71 ± 0.22 a | 1.71 ± 0.29 a | 2.57 ± 0.17 a | |
| FLUDIOXONIL | 5.83 ± 0.27 a | 1.79 ± 0.24 a | 1.50 ± 0.14 cd | |
| Storage Time | Treatment 1 | Storage Period | |||
|---|---|---|---|---|---|
| External Decay | Internal Decay | ||||
| % Incidence | Severity Index (0–4) 2 | % Incidence | Severity Index (0–4) 3 | ||
| 8 weeks at 5 °C + 1 week at 20 °C | CONTROL | 4.17 ± 2.08 a | 0.67 ± 0.33 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| HPMC-CW-SB | 2.08 ± 2.08 a | 0.67 ± 0.67 a | 2.08 ± 2.08 a | 0.67 ± 0.67 a | |
| HPMC-BW-SB | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | |
| HPMC-CW-SB + MAP | 4.17 ± 4.17 a | 1.00 ± 0.50 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | |
| HPMC-BW-SB + MAP | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | |
| Uncoated + MAP | 6.25 ± 3.60 a | 0.83 ± 0.44 a | 4.17 ± 2.08 a | 0.00 ± 0.00 a | |
| FLUDIOXONIL | 4.17 ± 2.08 a | 1.33 ± 0.88 a | 4.17 ± 2.08 a | 1.00 ± 0.58 a | |
| 15 weeks at 5 °C + 1 week at 20 °C | CONTROL | 35.42 ± 5.51 a | 1.41 ± 0.21 a | 22.92 ± 7.51 a | 1.78 ± 0.20 a |
| HPMC-CW-SB | 20.83 ± 9.08 ab | 1.17 ± 0.17 a | 10.42 ± 4.17 a | 1.17 ± 0.17 a | |
| HPMC-BW-SB | 16.67 ± 2.08 abc | 1.39 ± 0.06 a | 8.33 ± 4.17 a | 1.00 ± 0.58 a | |
| HPMC-CW-SB + MAP | 2.08 ± 2.08 d | 1.00 ± 0.58 a | 2.08 ± 2.08 a | 0.33 ± 0.33 a | |
| HPMC-BW-SB + MAP | 18.75 ± 6.25 abc | 1.17 ± 0.08 a | 14.58 ± 7.51 a | 1.06 ± 0.53 a | |
| Uncoated + MAP | 8.33 ± 2.08 bcd | 1.33 ± 0.33 a | 2.08 ± 2.08 a | 0.67 ± 0.67 a | |
| FLUDIOXONIL | 6.25 ± 3.61 cd | 1.17 ± 0.60 a | 6.25 ± 0.00 a | 2.00 ± 0.58 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Millo, B.; Martínez-Blay, V.; Pérez-Gago, M.B.; Argente-Sanchis, M.; Grimal, A.; Baraldi, E.; Palou, L. Antifungal Hydroxypropyl Methylcellulose (HPMC)-Lipid Composite Edible Coatings and Modified Atmosphere Packaging (MAP) to Reduce Postharvest Decay and Improve Storability of ‘Mollar De Elche’ Pomegranates. Coatings 2021, 11, 308. https://doi.org/10.3390/coatings11030308
Di Millo B, Martínez-Blay V, Pérez-Gago MB, Argente-Sanchis M, Grimal A, Baraldi E, Palou L. Antifungal Hydroxypropyl Methylcellulose (HPMC)-Lipid Composite Edible Coatings and Modified Atmosphere Packaging (MAP) to Reduce Postharvest Decay and Improve Storability of ‘Mollar De Elche’ Pomegranates. Coatings. 2021; 11(3):308. https://doi.org/10.3390/coatings11030308
Chicago/Turabian StyleDi Millo, Bruno, Victoria Martínez-Blay, María B. Pérez-Gago, Maricruz Argente-Sanchis, Amparo Grimal, Elena Baraldi, and Lluís Palou. 2021. "Antifungal Hydroxypropyl Methylcellulose (HPMC)-Lipid Composite Edible Coatings and Modified Atmosphere Packaging (MAP) to Reduce Postharvest Decay and Improve Storability of ‘Mollar De Elche’ Pomegranates" Coatings 11, no. 3: 308. https://doi.org/10.3390/coatings11030308
APA StyleDi Millo, B., Martínez-Blay, V., Pérez-Gago, M. B., Argente-Sanchis, M., Grimal, A., Baraldi, E., & Palou, L. (2021). Antifungal Hydroxypropyl Methylcellulose (HPMC)-Lipid Composite Edible Coatings and Modified Atmosphere Packaging (MAP) to Reduce Postharvest Decay and Improve Storability of ‘Mollar De Elche’ Pomegranates. Coatings, 11(3), 308. https://doi.org/10.3390/coatings11030308








