Investigations on the Influence of Annealing on Microstructure and Mechanical Properties of Electrodeposited Ni-Mo and Ni-Mo-W Alloy Coatings
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
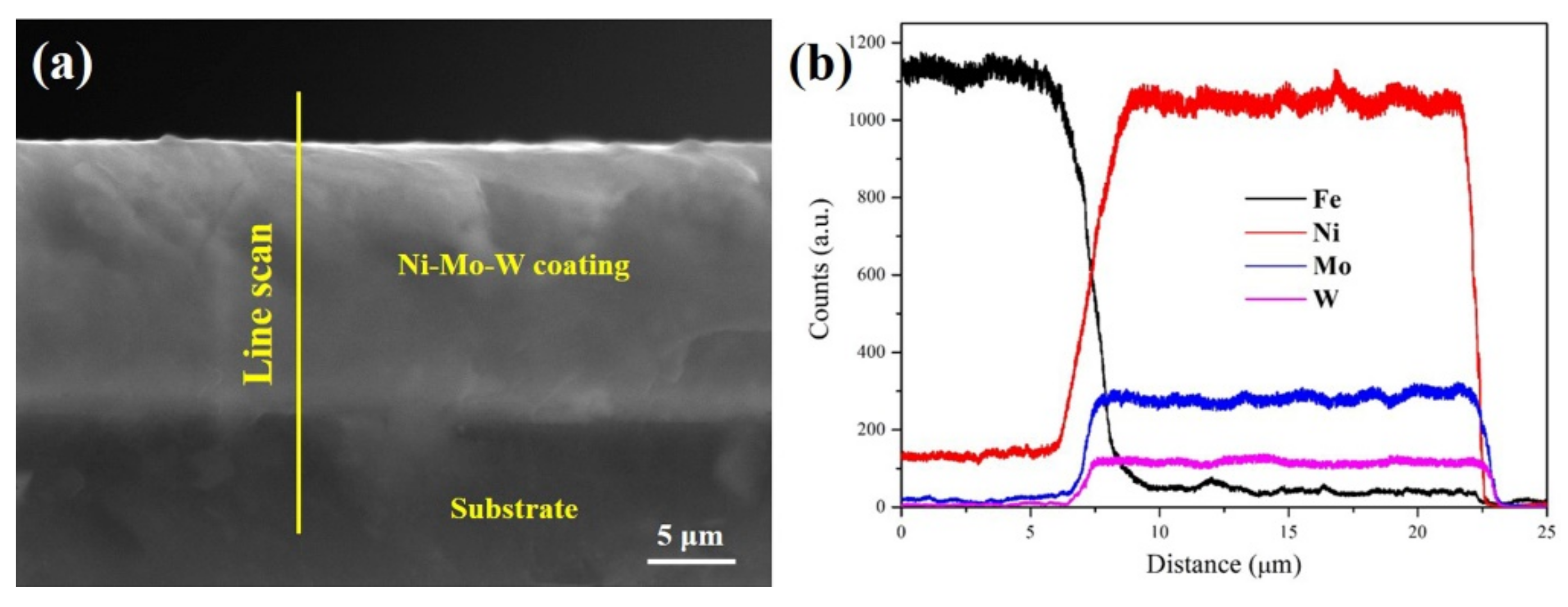
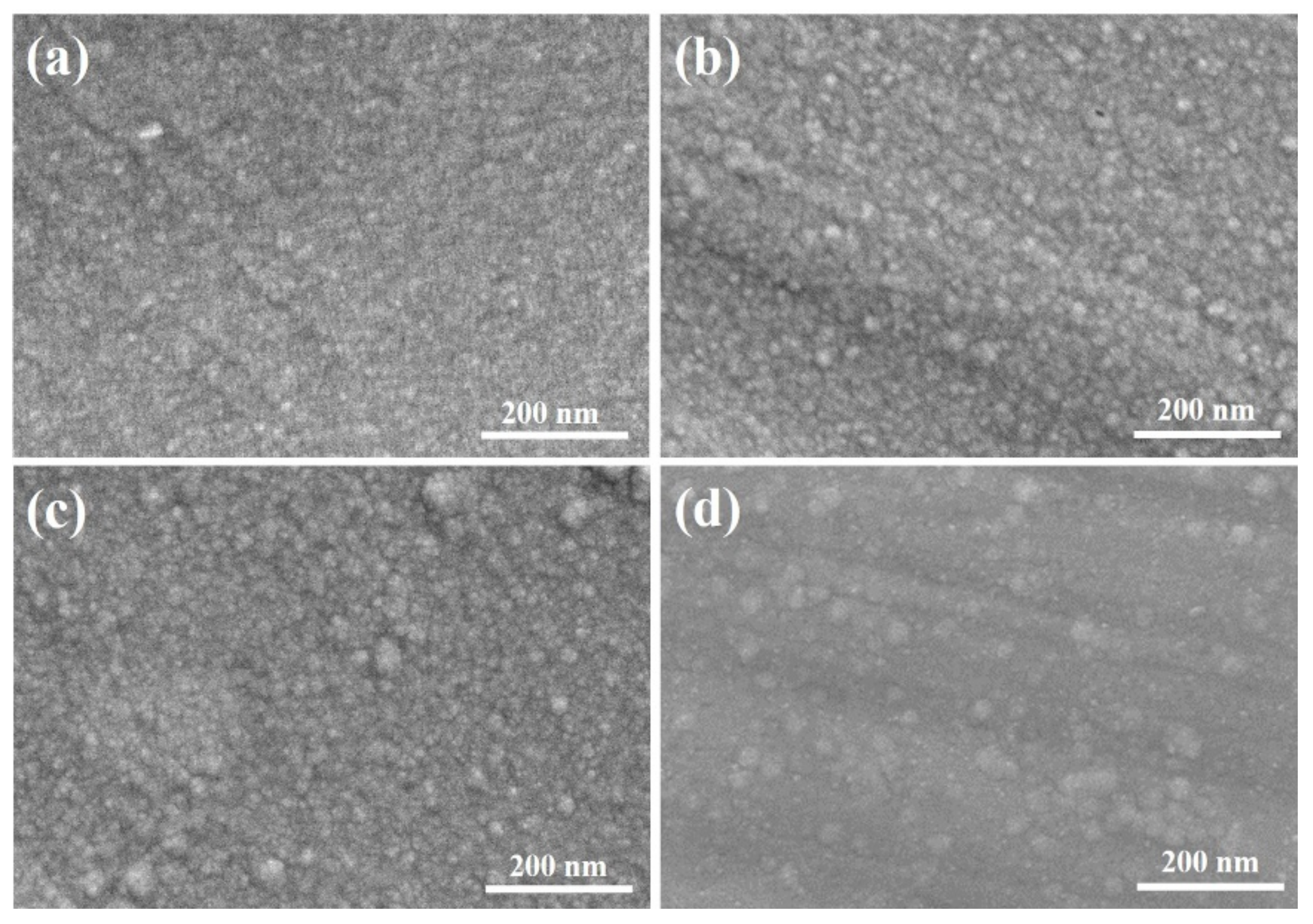
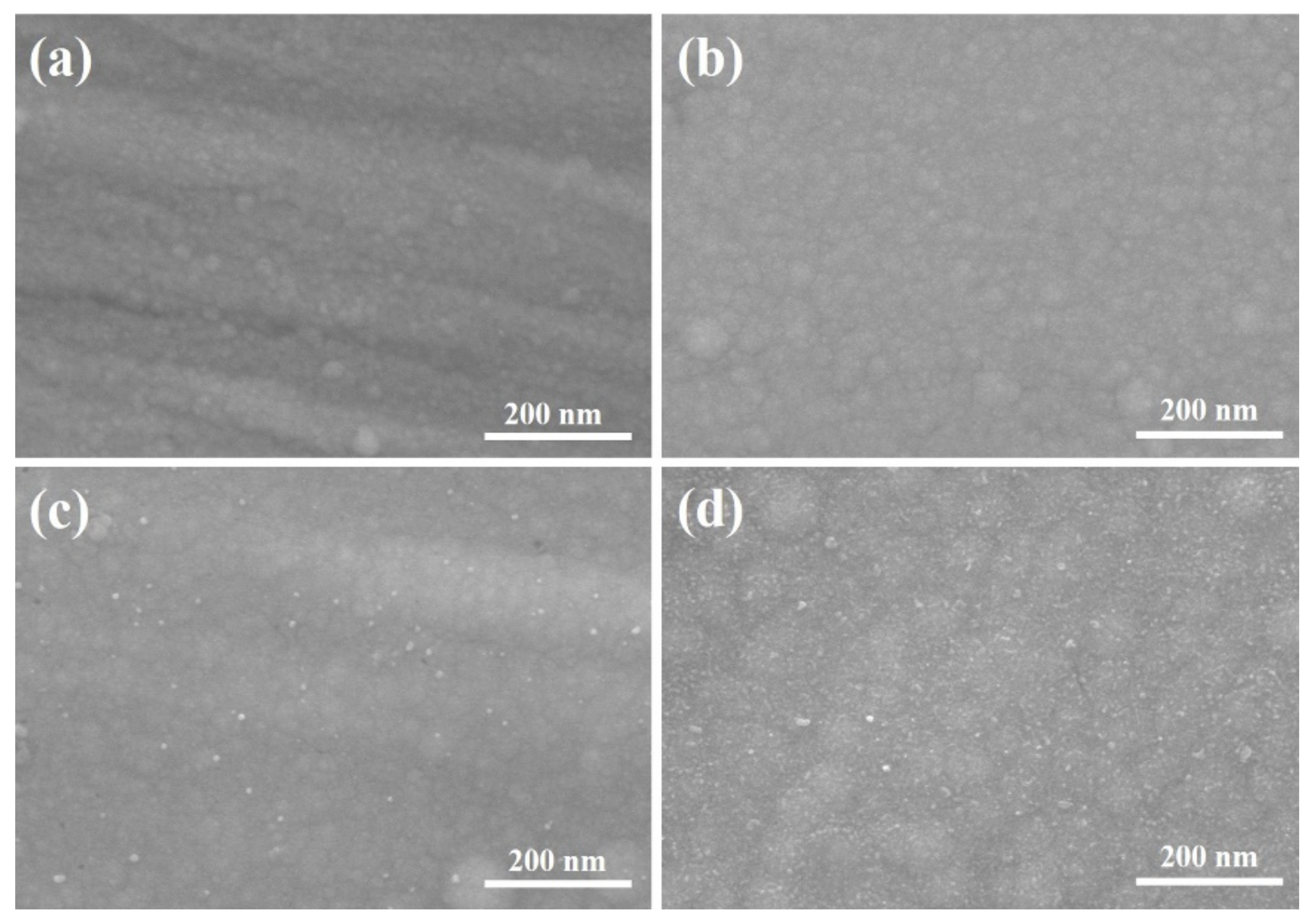
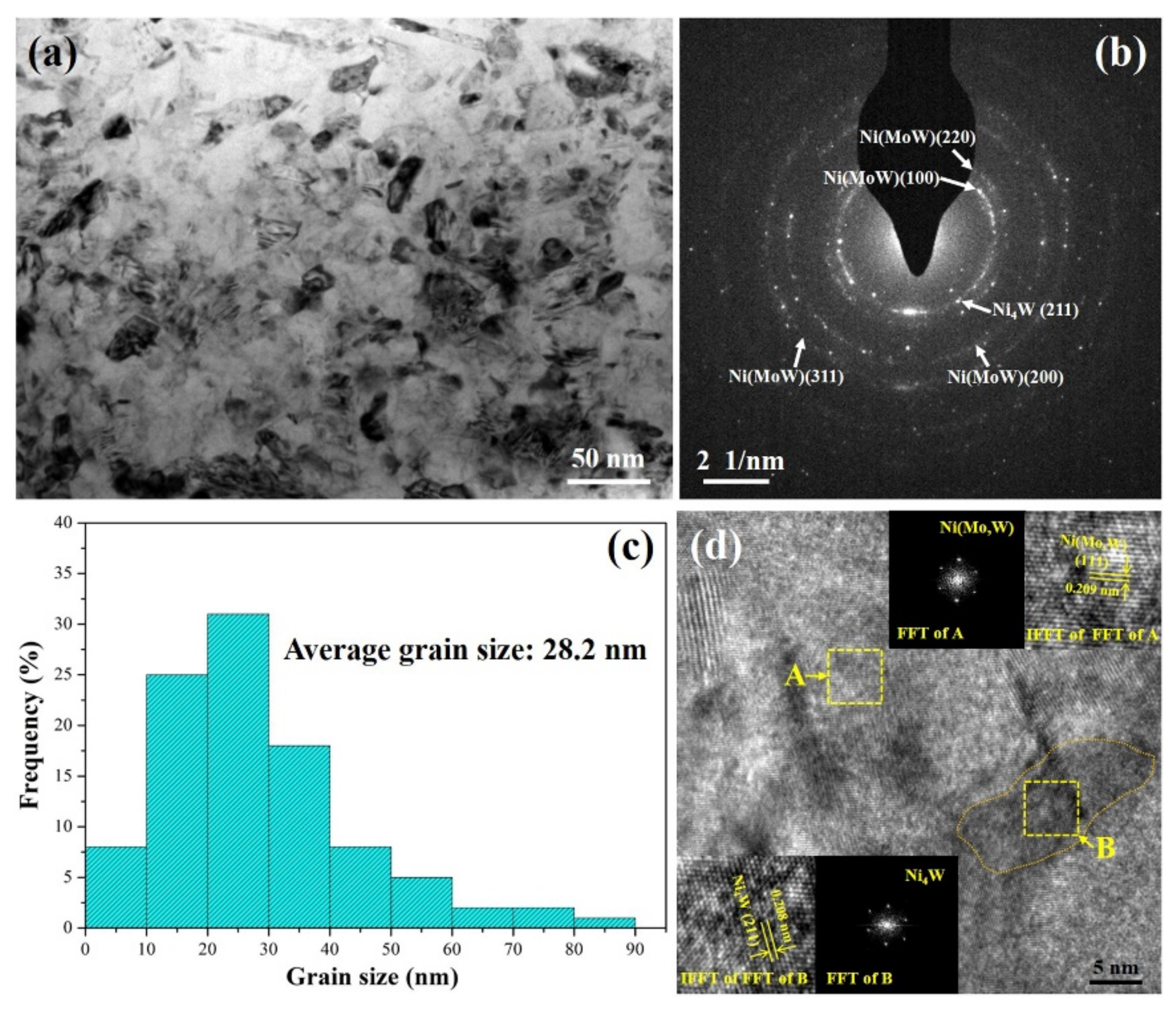
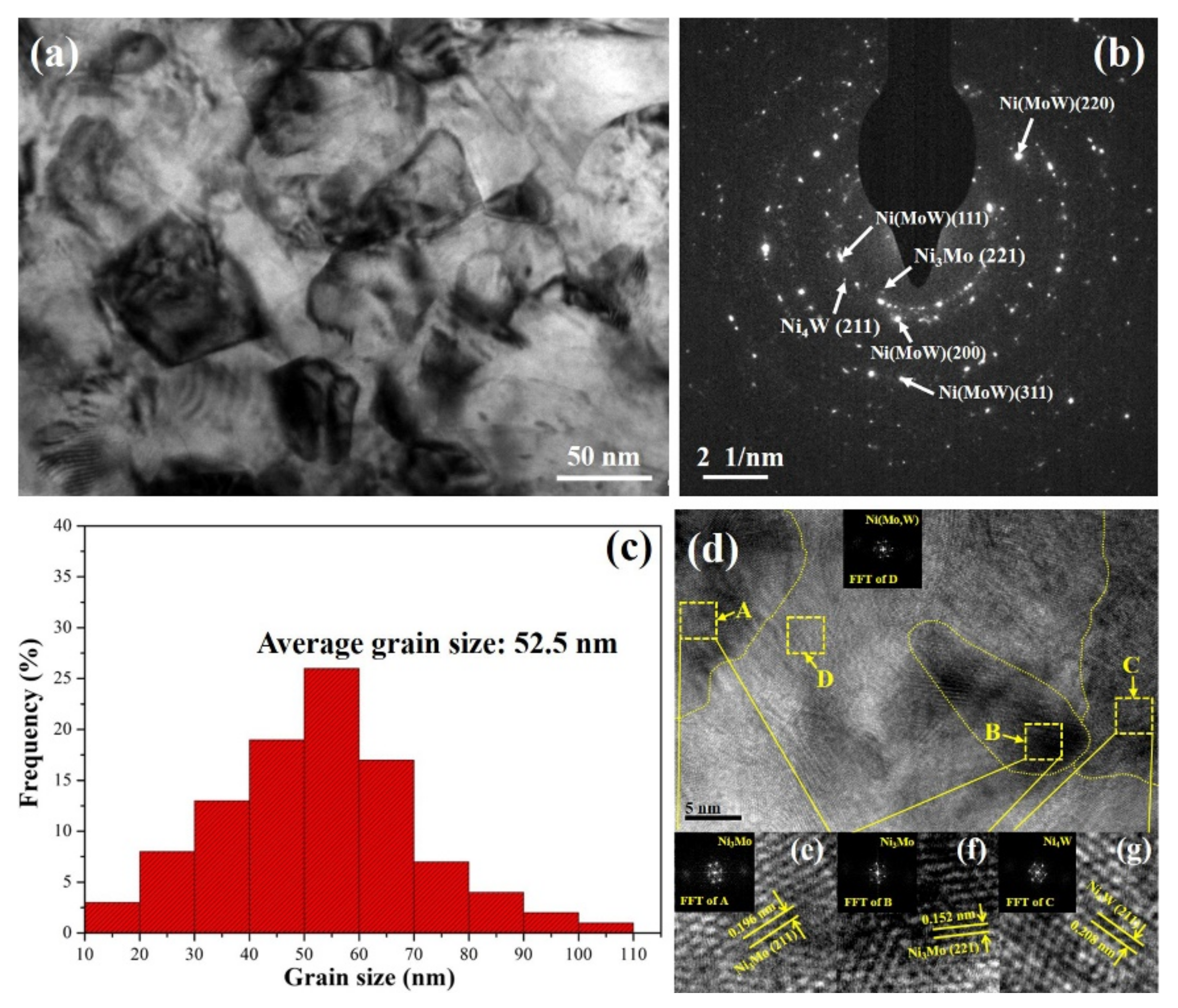
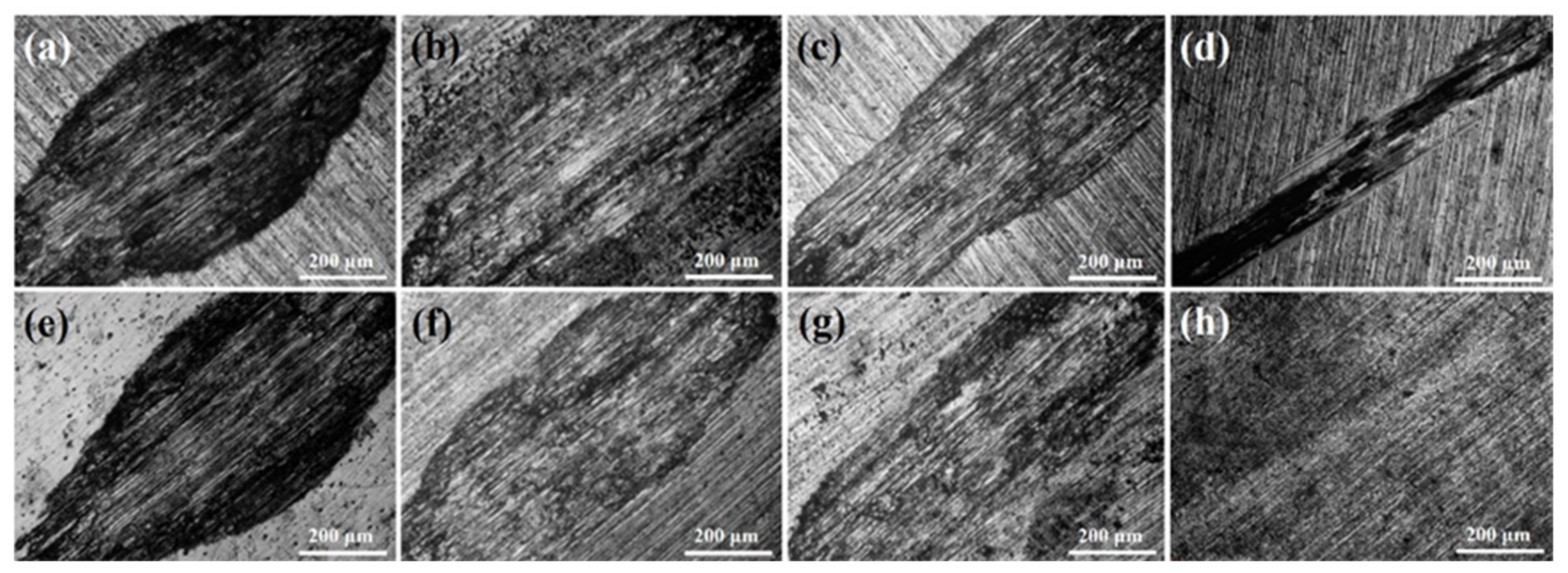
3.1. The Microstructure
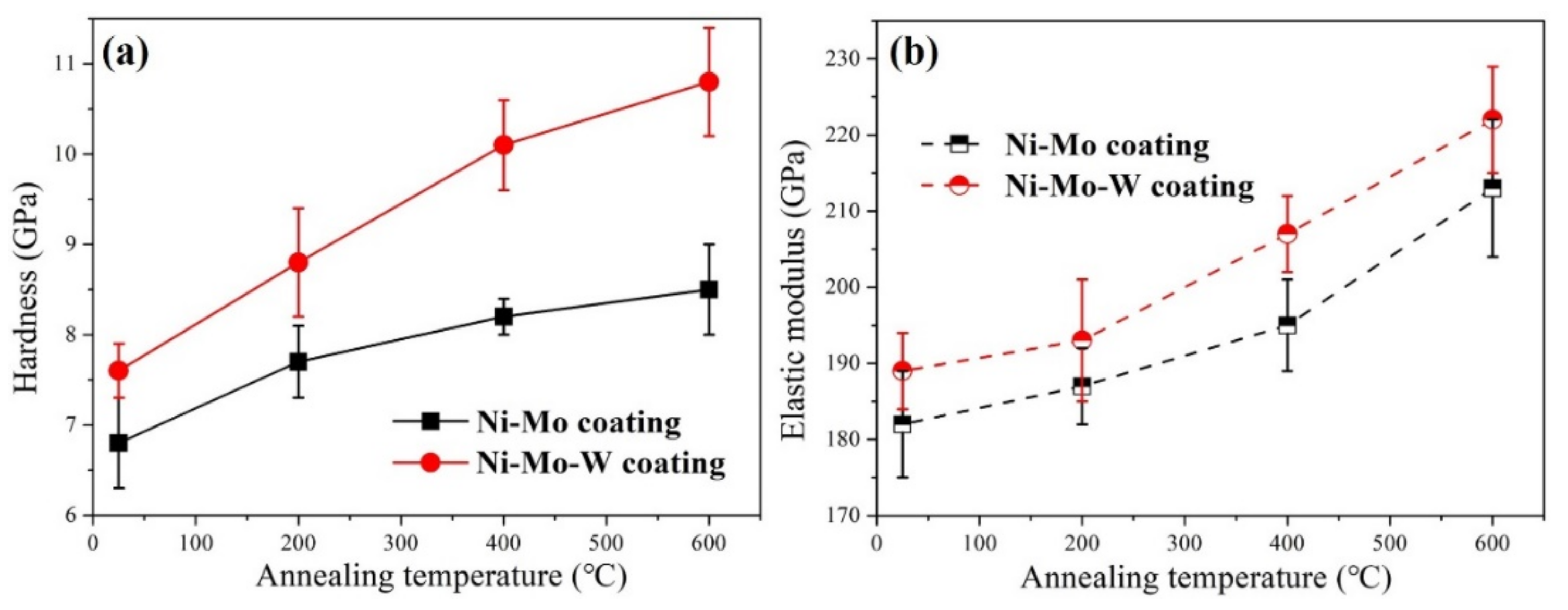
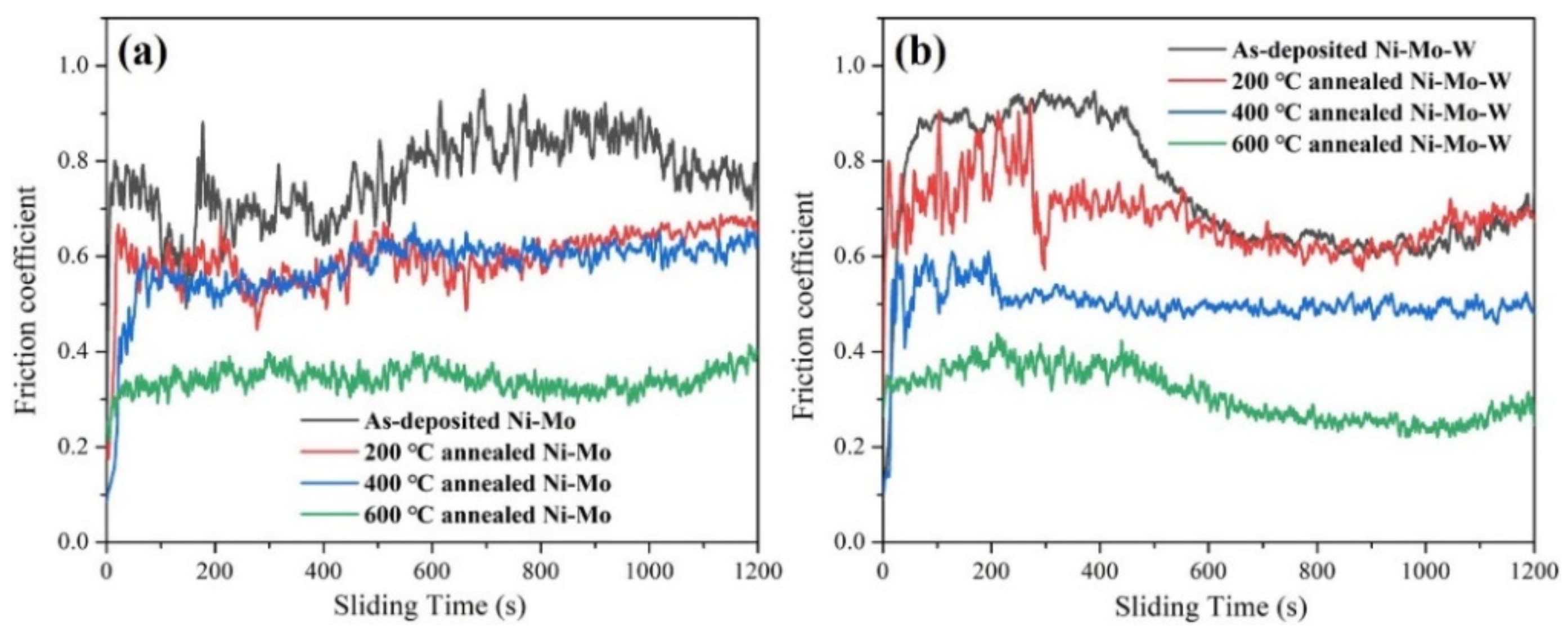
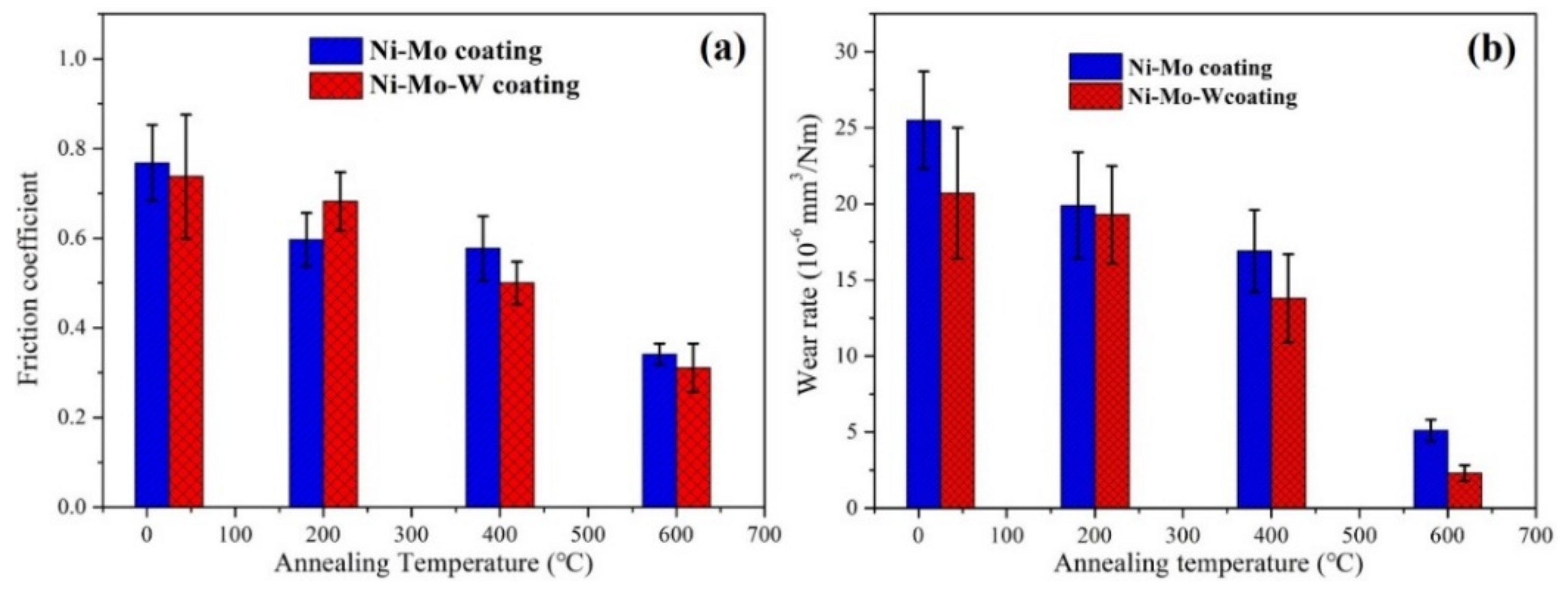
3.2. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, W.; Wang, K.; Wang, K.; Wang, P. Effects of jet rate on microstructure, microhardness, and wear behavior of jet electrodeposited Ni–SiC composites. Ceram. Int. 2018, 44, 7214–7220. [Google Scholar] [CrossRef]
- Lelevic, A.; Walsh, F.C. Electrodeposition of NiP alloy coatings: A review. Surf. Coat. Technol. 2019, 369, 198–220. [Google Scholar] [CrossRef]
- Shahzad, K.; Radwan, A.B.; Fayyaz, O.; Shakoor, R.A.; Uzma, M.; Umer, M.A.; Baig, M.N.; Raza, A. Effect of concentration of TiC on the properties of pulse electrodeposited Ni-P-TiC nanocomposite coatings. Ceram. Int. 2021, 47, 19123–19133. [Google Scholar] [CrossRef]
- Torabinejad, V.; Aliofkhazraei, M.; Rouhaghdam, A.S.; Allahyarzadeh, M.H. Tribological properties of Ni-Fe-Co multilayer coatings fabricated by pulse electrodeposition. Tribol. Int. 2017, 106, 34–40. [Google Scholar] [CrossRef]
- Torabinejad, V.; Aliofkhazraei, M.; Rouhaghdam, A.S.; Allahyarzadeh, M.H.; Kasama, T.; Alimadadi, H. Mechanical properties multilayer Ni-Fe and Ni-Fe-Al2O3 nanocomposite coating. Mater. Sci. Eng. A-Struct. 2017, 700, 448–456. [Google Scholar] [CrossRef]
- Allahyarzadeh, M.H.; Aliofkhazraei, M.; Rouhaghdam, A.S.; Alimadadi, H.; Torabinejad, V. Mechanical properties and load bearing capability of nanocrystalline nickel-tungsten multilayered coatings. Surf. Coat. Technol. 2020, 386, 125472. [Google Scholar] [CrossRef]
- Dilek, S.; Algül, H.; Akyol, A.; Alp, A.; Akbulut, H.; Uysal, M. Pulse electro co-deposition of submicron-sized TiC reinforced Ni-W coatings: Tribological and corrosion properties. J. Asian Ceram. Soc. 2021, 9, 673–685. [Google Scholar] [CrossRef]
- Wasekar, N.P.; Verulkar, S.; Vamsi, M.V.N.; Sundararajan, G. Influence of molybdenum on the mechanical properties, electrochemical corrosion and wear behavior of electrodeposited Ni-Mo alloy. Surf. Coat. Technol. 2019, 370, 298–310. [Google Scholar] [CrossRef]
- Mousavi, R.; Bahrololoom, M.E.; Deflorian, F. Preparation, corrosion, and wear resistance of Ni-Mo/Al composite coating reinforced with Al particles. Mat. Des. 2016, 110, 456–465. [Google Scholar] [CrossRef]
- Wasekar, N.P.; Sundararajan, G. Sliding wear behavior of electrodeposited Ni-W alloy and hard chrome coatings. Wear 2015, 342, 340–348. [Google Scholar] [CrossRef]
- Lv, J.; Wang, Z.; Liang, T.; Ken, S.; Miura, H. Effect of tungsten on microstructures of annealed electrodeposited Ni-W alloy and its corrosion resistance. Surf. Coat. Technol. 2018, 337, 516–524. [Google Scholar]
- Wang, H.; Liu, R.; Cheng, F.J.; Cao, Y.; Ding, G.F.; Zhao, X.L. Electrodepositing amorphous Ni-W alloys for MEMS. Microelectron. Eng. 2010, 87, 1901–1906. [Google Scholar] [CrossRef]
- Yin, D.; Marvel, C.J.; Cui, F.Y.; Vinci, R.P.; Harmer, M.P. Microstructure and fracture toughness of electrodeposited Ni-21 at.% W alloy thick films. Acta Mater. 2018, 143, 272–280. [Google Scholar] [CrossRef]
- Suresha, S.J.; Haj-Taieb, M.; Bade, K.; Aktaa, J.; Hemker, K.J. The influence of tungsten on the thermal stability and mechanical behavior of electrodeposited nickel MEMS structures. Scr. Mater. 2010, 63, 1141–1144. [Google Scholar] [CrossRef]
- Allahyarzadeh, M.H.; Aliofkhazraei, M.; Rezvanian, A.R.; Torabinejad, V.; Roughdam, A.R.S. Ni-W electrodeposited coatings: Characterization, properties and applications. Surf. Coat. Technol. 2016, 307, 978–1010. [Google Scholar] [CrossRef]
- Sim, G.D.; Krogstad, J.A.; Reddy, K.M.; Xie, K.Y.; Valentino, G.M.; Weihs, T.P.; Hemker, K.J. Nanotwinned metal MEMS films with unprecedented strength and stability. Sci. Adv. 2017, 3, e1700685. [Google Scholar] [CrossRef] [Green Version]
- Valentino, G.M.; Krogstad, J.A.; Weihs, T.P.; Hemker, K.J. Tailoring the coefficient of thermal expansion of ternary nickel alloys through compositional control and non-contact measurements. J. Alloys Compd. 2020, 833, 155024. [Google Scholar] [CrossRef]
- Mosayebi, S.; Rezaei, M.; Mahidashti, Z. Comparing corrosion behavior of Ni and Ni-Mo electroplated coatings in chloride mediums. Colloids Surf. A 2020, 594, 124654. [Google Scholar] [CrossRef]
- Allahyarzadeh, M.H.; Roozbehani, B.; Ashrafi, A.; Shadizadeh, S.R. Electrodeposition of high Mo content amorphous/nanocrystalline Ni-Mo alloys using 1-methyl-imidazolium chloride ionic liquid as an additive. Surf. Coat. Technol. 2011, 206, 137–142. [Google Scholar] [CrossRef]
- Liu, J.H.; Yan, J.X.; Pei, Z.L.; Gong, J.; Sun, C. Effects of Mo content on the grain size, hardness and anti-wear performance of electrodeposited nanocrystalline and amorphous Ni-Mo alloys. Surf. Coat. Technol. 2020, 404, 126476. [Google Scholar] [CrossRef]
- Laszczyńska, A.; Winiarski, J.; Szczygiel, B.; Szczygiel, I. Electrodeposition and characterization of Ni-Mo-ZrO2 composite coatings. Appl. Surf. Sci. 2016, 369, 224–231. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, S.; Fan, M.; Chen, Y.; Song, X.; Hao, J. Design and properties investigation of NiMo composite coating reinforced with duplex nanoparticles. Surf. Coat. Technol. 2019, 363, 51–60. [Google Scholar] [CrossRef]
- Alizadeh, M.; Cheshmpish, A. Electrodeposition of Ni-Mo/Al2O3 nano-composite coatings at various deposition current densities. Appl. Surf. Sci. 2019, 466, 433–440. [Google Scholar] [CrossRef]
- Liu, J.H.; Pei, Z.L.; Shi, W.B.; Liu, Y.D.; Gong, J.; Sun, C. Studies on preparation, microstructure, mechanical properties and corrosion resistance of Ni-Mo/micron-sized diamond composite coatings. Surf. Coat. Technol. 2020, 385, 125451. [Google Scholar] [CrossRef]
- Fan, J.; Cui, S.; Chen, J.; Liu, K.; Han, Q. Electrochemical properties of pulse plating amorphous Ni-Mo-W alloy coating in alkaline medium. Rare Metal Mat. Eng. 2015, 44, 538–543. [Google Scholar]
- Allam, M.; Benaicha, M.; Dakhouche, A. Electrodeposition and characterization of NiMoW alloy as electrode material for hydrogen evolution in alkaline water electrolysis. Int. J. Hydrog. Energ. 2018, 43, 3394–3405. [Google Scholar] [CrossRef]
- Liu, J.H.; Li, W.H.; Pei, Z.L.; Gong, J.; Sun, C. Investigations on the structure and properties of nanocrystalline Ni-Mo alloy coatings. Mater. Charact. 2020, 167, 110532. [Google Scholar] [CrossRef]
- Wang, J.; Han, L.; Li, X.; Huang, Y.; Liu, Y.; Wang, Z. Temperature-dependent evolution of strength of nanocrystalline Ni(Mo) alloys at the Mo solubility limit. Mat. Sci. Eng. A-Struct. 2020, 786, 139326. [Google Scholar] [CrossRef]
- Kapoor, G.; Péter, L.; Fekete, É.; Lábár, J.L.; Gubicza, J. The influence of Mo addition on the microstructure and its thermal stability for electrodeposited Ni films. Mater. Charact. 2018, 145, 563–572. [Google Scholar] [CrossRef]
- Bigos, A.; Janusz-Skuza, M.; Szczerba, M.J.; Kot, M.; Zimowski, S.; Dębski, A.; Beltowska-Lehman, E. The effect of heat treatment on the microstructural changes in electrodeposited Ni-Mo coatings. J. Mater. Process. Technol. 2020, 276, 116397. [Google Scholar] [CrossRef]
- Liu, J.H.; Yan, J.X.; Liu, Y.D.; Li, W.H.; Gu, W.S.; Pei, Z.L.; Gong, J.; Sun, C. Impact of annealing temperature on the microstructure, microhardness, tribological properties and corrosion resistance of Ni-Mo/diamond composites. Appl. Surf. Sci. 2021, 541, 148367. [Google Scholar] [CrossRef]
- Vamsi, M.V.N.; Wasekar, N.P.; Sundararajan, G. Influence of heat treatment on microstructure and mechanical properties of pulse electrodeposited Ni-W alloy coatings. Surf. Coat. Technol. 2017, 319, 403–414. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Zmitrowicz, A. Wear patterns and laws of wear-a review. Theor. Appl. Mech. 2006, 44, 219–253. [Google Scholar]
- Sun, S.; Bairachna, T.; Podlaha, E.J. Induced codeposition behavior of electrodeposited NiMoW alloys. J. Electrochem. Soc. 2013, 160, D434–D440. [Google Scholar] [CrossRef]
- Rupert, T.J.; Trelewicz, J.R.; Schuh, C.A. Grain boundary relaxation strengthening of nanocrystalline Ni-W alloys. J. Mater. Res. 2012, 27, 1285–1294. [Google Scholar] [CrossRef]
- Detor, A.J.; Schuh, C.A. Tailoring and patterning the grain size of nanocrystalline alloys. Acta Mater. 2007, 55, 371–379. [Google Scholar] [CrossRef]
- Schuh, C.A.; Nieh, T.G.; Iwasaki, H. The effect of solid solution W additions on the mechanical properties of nanocrystalline Ni. Acta Mater. 2003, 51, 431–443. [Google Scholar] [CrossRef]
- Yamasaki, T.; Schloβmacher, P.; Ehrlich, K.; Ogino, Y. Formation of amorphous electrodeposited Ni-W alloys and their nanocrystallization. Nanostruct. Mater. 1998, 10, 375–388. [Google Scholar] [CrossRef]
- Szajewski, B.A.; Crone, J.C.; Knap, J. Analytic model for the Orowan dislocation-precipitate bypass mechanism. Materialia 2020, 11, 100671. [Google Scholar] [CrossRef]
- De Jong, M.; Chen, W.; Angsten, T.; Jain, A.; Notestine, R.; Gamst, A.; Sluiter, M.; Ande, C.K.; van der Zwaag, S.; Plata, J.J.; et al. Charting the complete elastic properties of inorganic crystalline compounds. Sci. Data 2015, 2, 150009. [Google Scholar] [CrossRef] [Green Version]
- Leyland, A.; Matthews, A. On the significance of the H/E ratio in wear control: A nanocomposite coating approach to optimised tribological behavior. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Zhai, W.; Bai, L.; Zhou, R.; Fan, X.; Kang, G.; Liu, Y.; Zhou, K. Recent progress on wear-resistant materials: Designs, properties, and applications. Adv. Sci. 2021, 8, 2003739. [Google Scholar] [CrossRef]
- Vamsi, M.V.N.; Wasekar, N.P.; Sundararajan, G. Sliding wear of as-deposited and heat-treated nanocrystalline nickel-tungsten alloy coatings. Wear 2018, 412, 136–143. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Walsh, F.C.; Zangari, G.; Köçkar, H.; Alper, M.; Rizal, C.; Magagnin, L.; Protsenko, V.; Arunachalam, R.; Rezvanian, A.; et al. Development of electrodeposited multilayer coatings: A review of fabrication, microstructure, properties and applications. Appl. Surf. Sci. Adv. 2021, 6, 100141. [Google Scholar] [CrossRef]
- Woydt, M.; Skopp, A.; Dorfel, I.; Witke, K. Wear engineering oxides/antiwear oxides. Tribol. Trans. 1999, 42, 21–31. [Google Scholar] [CrossRef]


| Coatings | Na2WO4·2H2O Concentration | Ni (at.%) | Mo (at.%) | W (at.%) |
|---|---|---|---|---|
| Ni-Mo | 0 g/L | 81.9 | 18.1 | 0 |
| Ni-Mo-W | 20 g/L | 75.4 | 16.9 | 7.7 |
| Annealing Statements | Coatings | H/E |
|---|---|---|
| As-deposited | Ni-Mo | 0.03736 ± 0.00435 |
| Ni-Mo-W | 0.04021 ± 0.00272 | |
| 200 °C annealed | Ni-Mo | 0.04118 ± 0.00333 |
| Ni-Mo-W | 0.04560 ± 0.00521 | |
| >400 °C annealed | Ni-Mo | >0.04205 ± 0.00239 |
| Ni-Mo-W | 0.04879 ± 0.00368 | |
| 600 °C annealed | Ni-Mo | 0.03991 ± 0.00421 |
| Ni-Mo-W | 0.04865 ± 0.00437 |
| Selected Locations | O (at.%) | Ni (at.%) | Mo (at.%) | W (at.%) | Fe (at.%) | Cr (at.%) |
|---|---|---|---|---|---|---|
| 1 | 17.1 | 39.8 | 7.2 | 4.6 | 30.9 | 0.4 |
| 2 | 21.5 | 42.4 | 7.7 | 3.8 | 24.3 | 0.3 |
| 3 | 55.2 | 22.7 | 8.7 | 7.0 | 6.3 | 0.1 |
| 4 | 70.2 | 16.5 | 9.4 | 2.1 | 1.8 | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Si, W.; Wang, Y.; Dai, S.; Shu, D. Investigations on the Influence of Annealing on Microstructure and Mechanical Properties of Electrodeposited Ni-Mo and Ni-Mo-W Alloy Coatings. Coatings 2021, 11, 1428. https://doi.org/10.3390/coatings11111428
Zhang C, Si W, Wang Y, Dai S, Shu D. Investigations on the Influence of Annealing on Microstructure and Mechanical Properties of Electrodeposited Ni-Mo and Ni-Mo-W Alloy Coatings. Coatings. 2021; 11(11):1428. https://doi.org/10.3390/coatings11111428
Chicago/Turabian StyleZhang, Chao, Wudong Si, Yin Wang, Sichao Dai, and Da Shu. 2021. "Investigations on the Influence of Annealing on Microstructure and Mechanical Properties of Electrodeposited Ni-Mo and Ni-Mo-W Alloy Coatings" Coatings 11, no. 11: 1428. https://doi.org/10.3390/coatings11111428




