PLGA Coatings and PLGA Drug-Loading Coatings for Cardiac Stent Samples: Degradation Characteristics and Blood Compatibility
Abstract
:1. Introduction
2. Experimental Method
2.1. Preparation
2.2. Morphology and Thermal Property Test
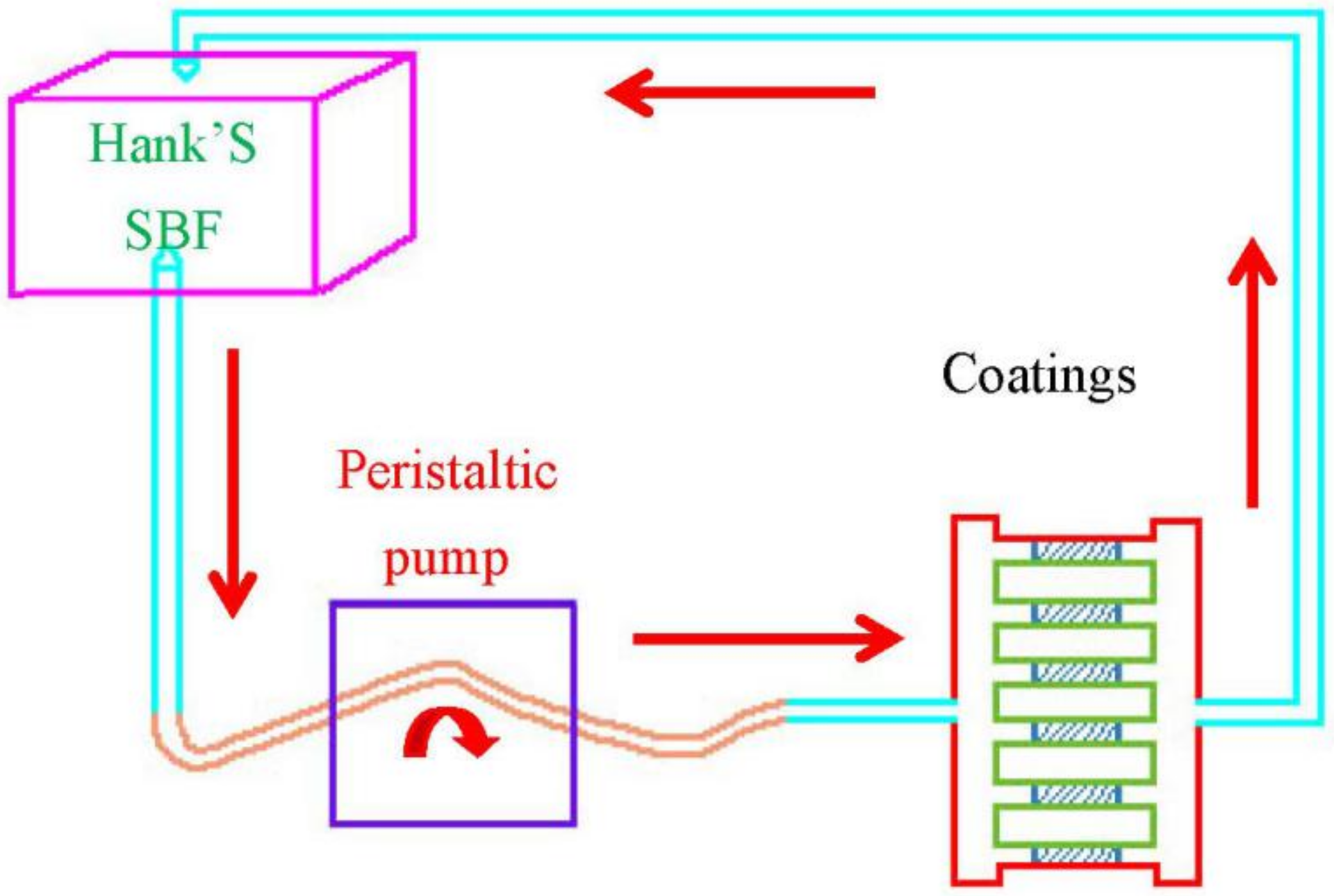
2.3. Degradation Characteristics
2.4. Platelet Adhesion Test
2.5. Dynamic Coagulation Time
3. Results and Discussion
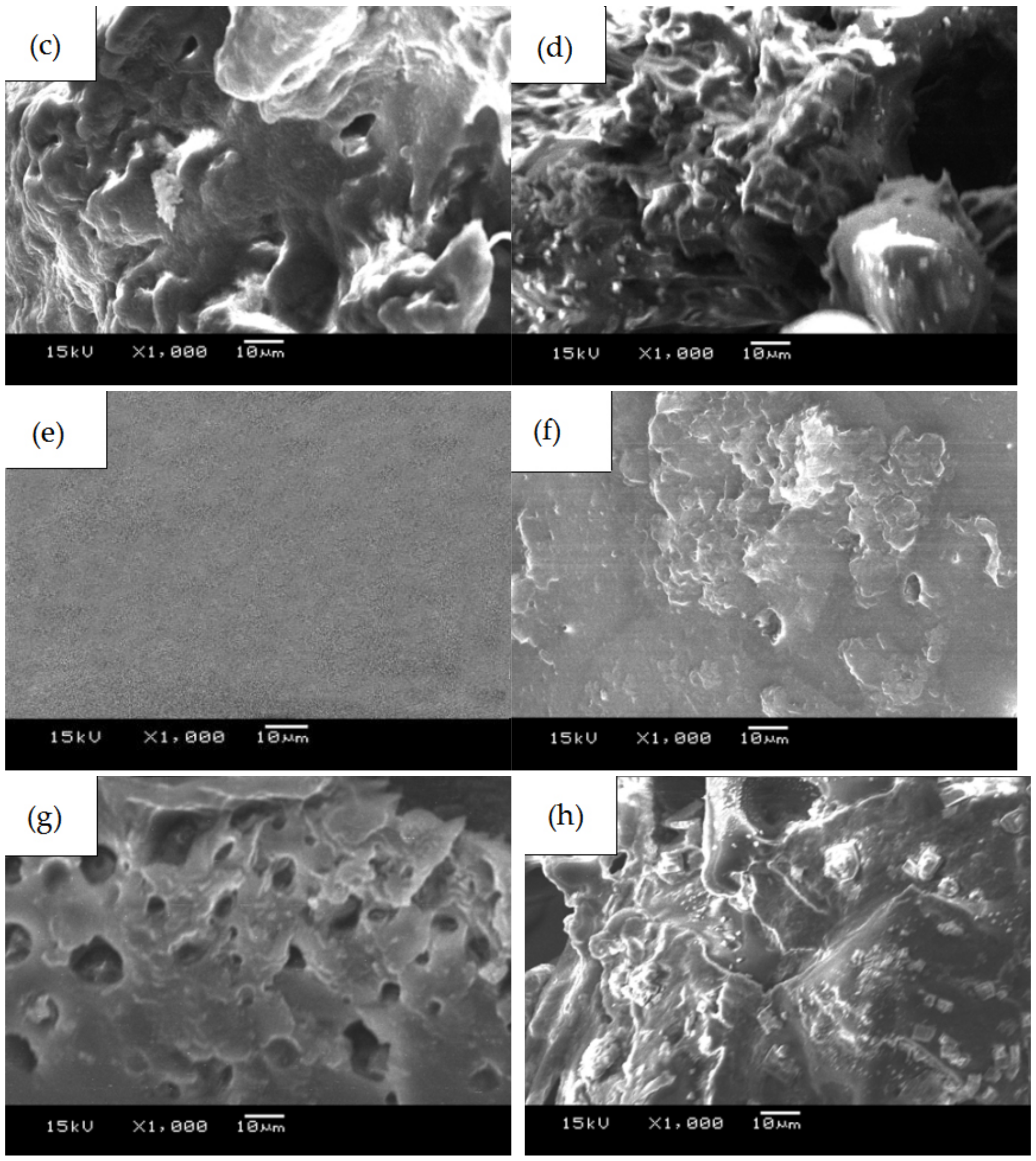
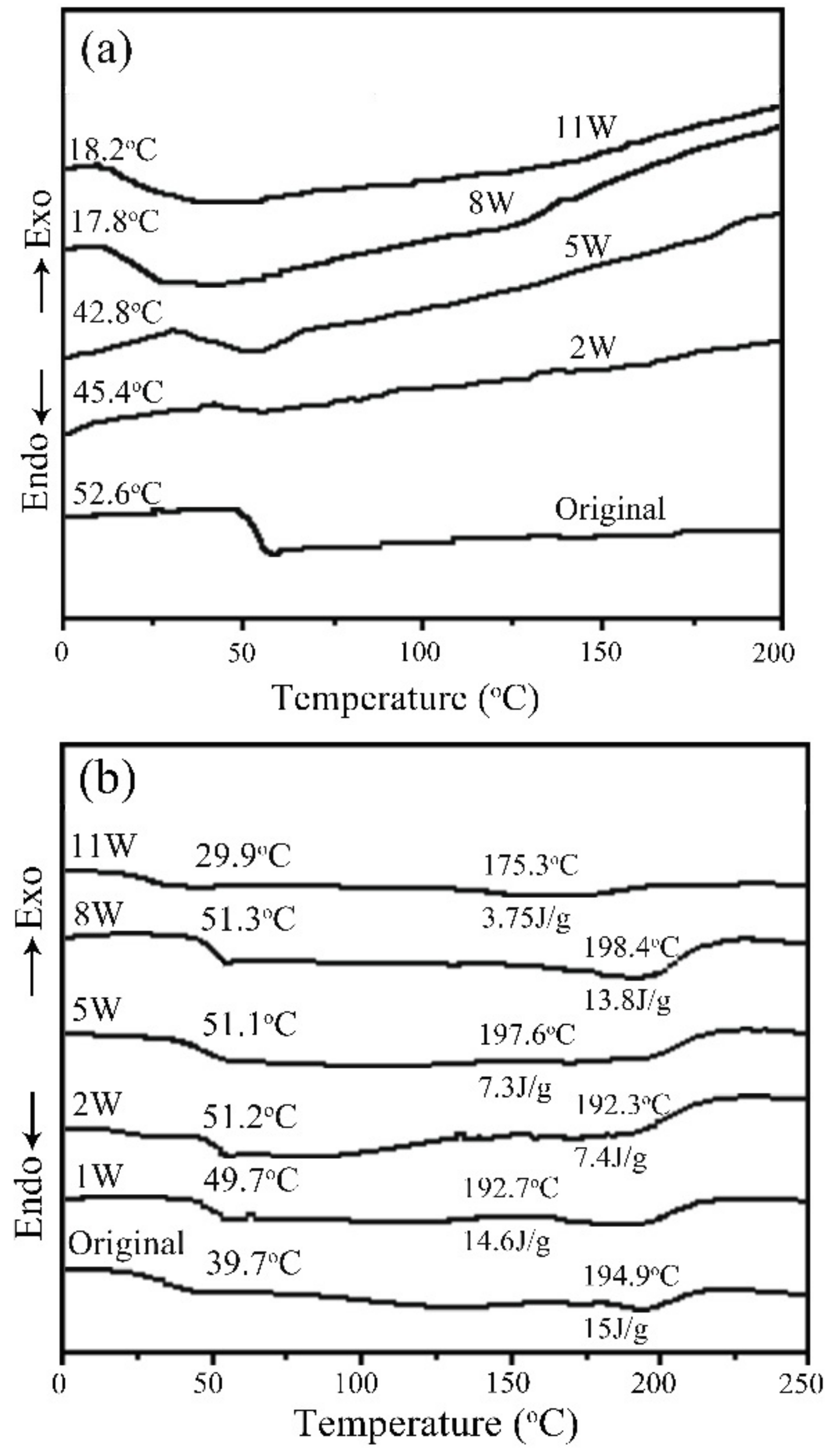
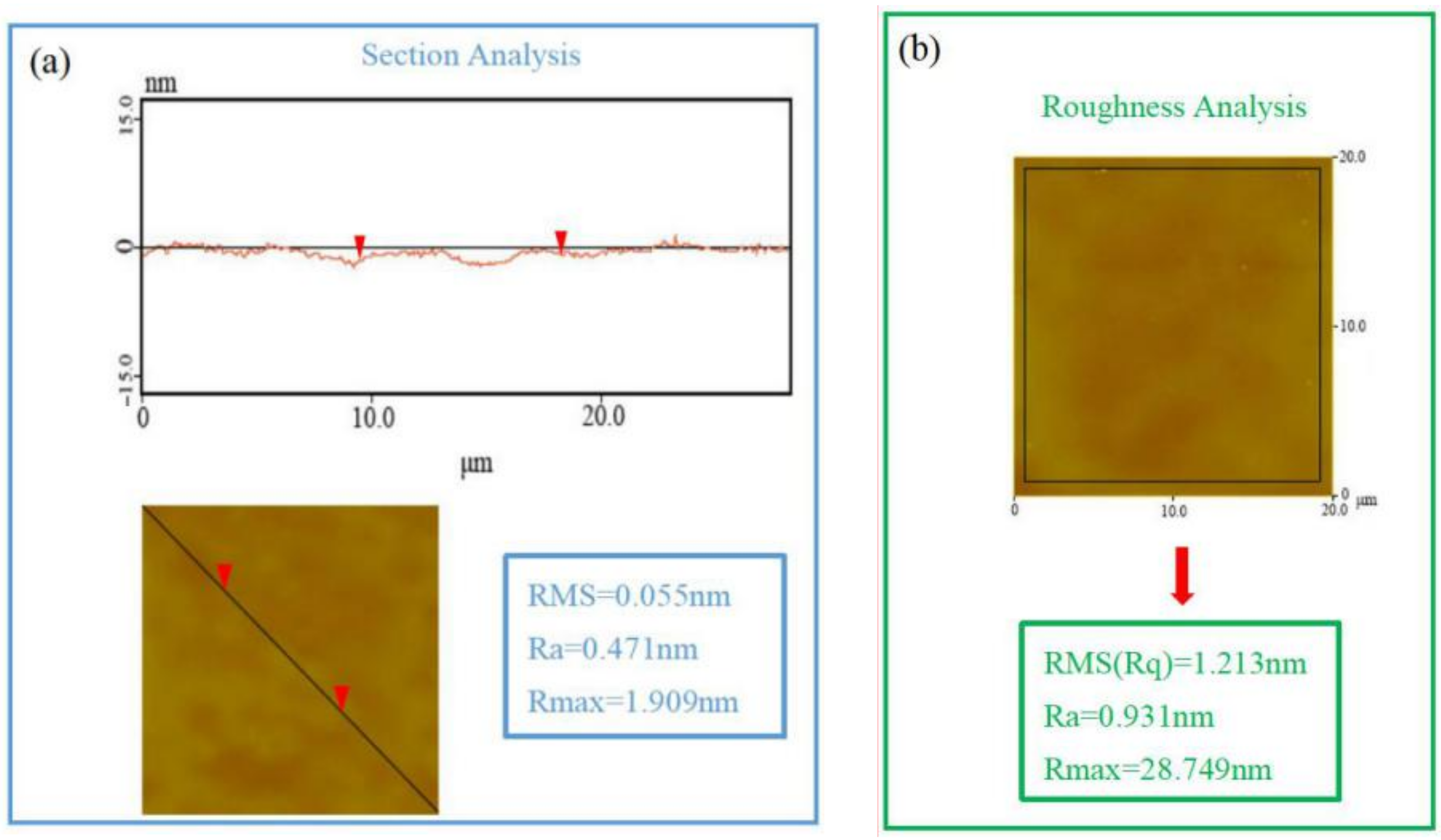
3.1. SEM, DSC, and XRD Measurements of PLGA Drug-Loading Coatings
3.2. Degradation Characteristics of PLGA Drug-Loading Coatings
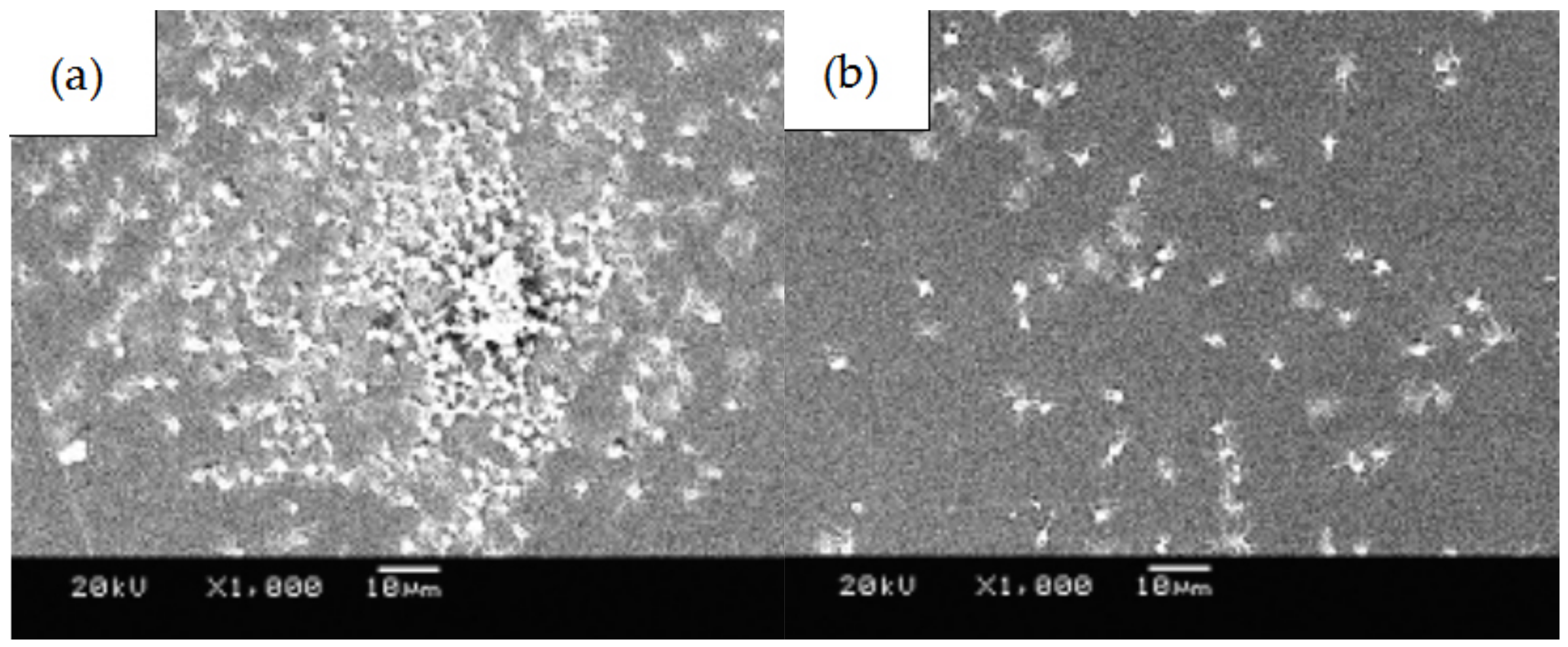
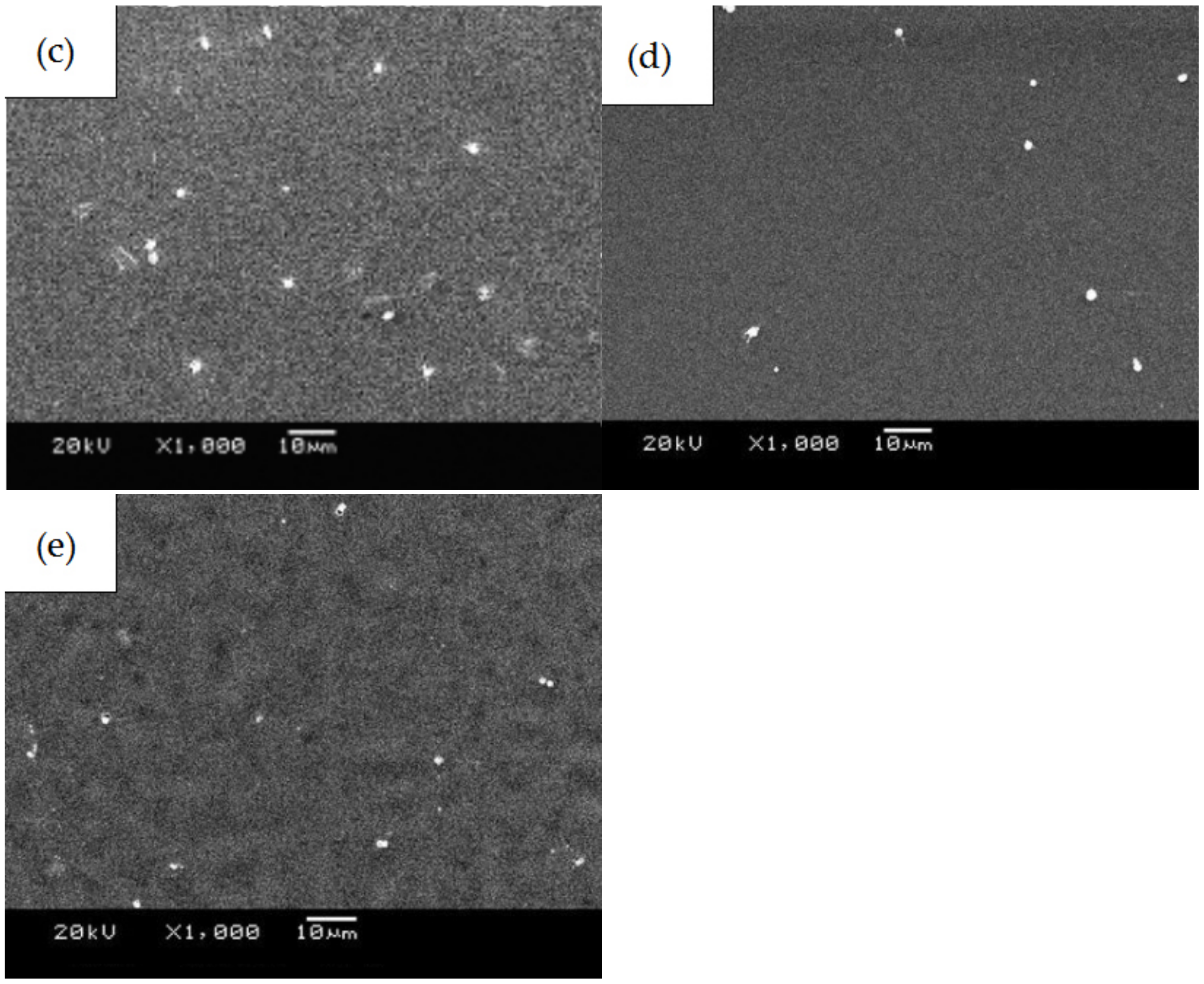
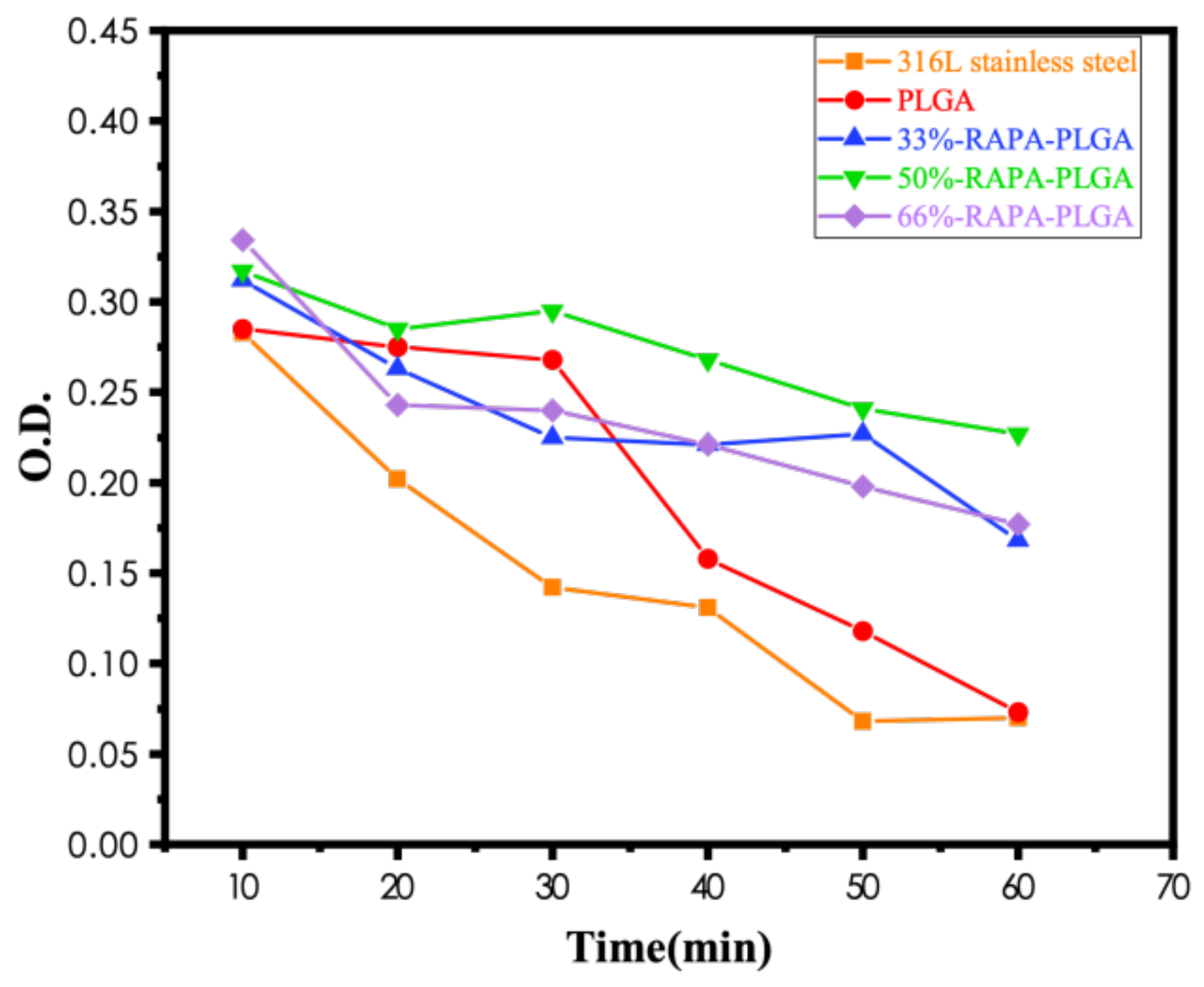
3.3. Blood Compatibility Analyses of PLGA Drug-Loading Coatings
4. Conclusions
- (1)
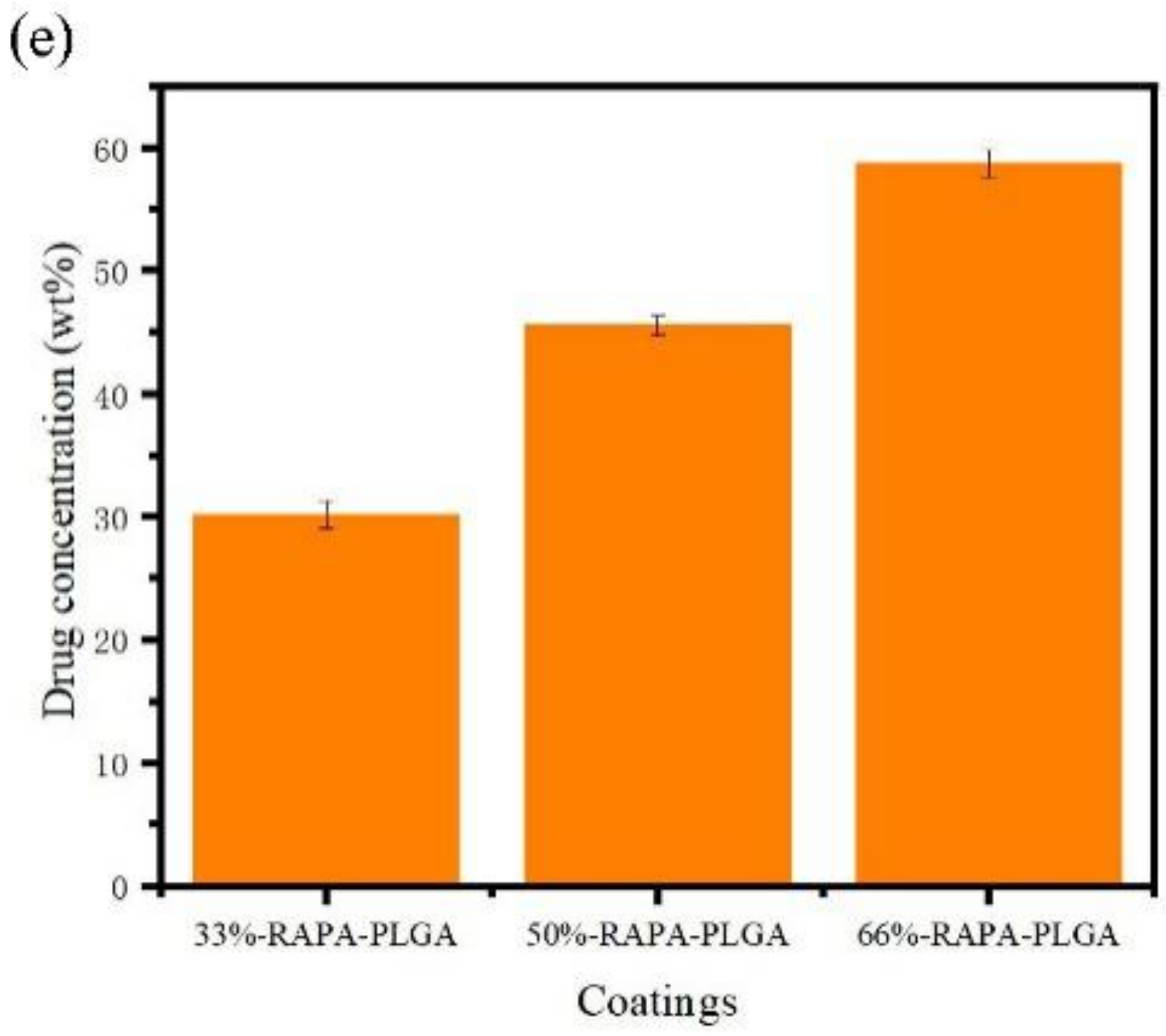
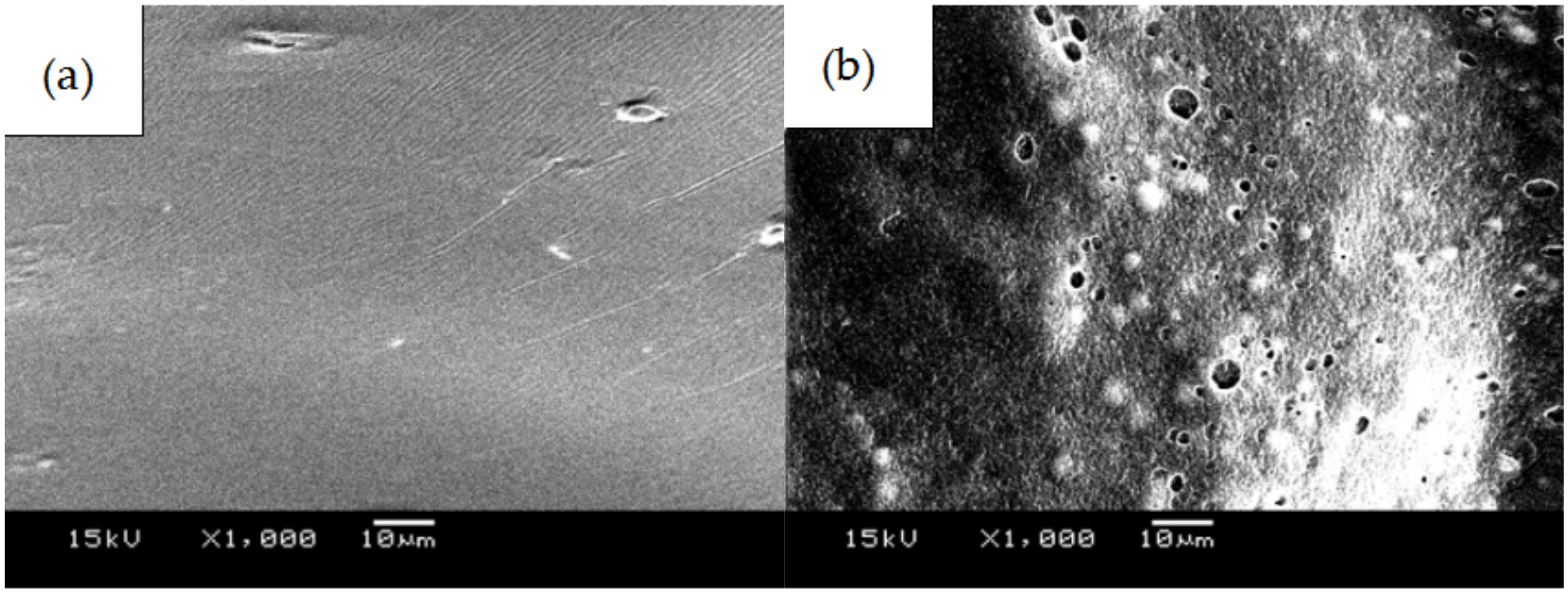
- PLGA and PLGA coatings with RAPA levels of 33%, 50%, and 66% were successfully prepared on the surface of 316 L stainless steel. SEM results demonstrated that when the RAPA level was 33%, the drug-loading coating morphology was smooth and flat, which was similar to that of the pure PLGA coating. DSC results indicated the melting peak was observed upon the addition of RAPA, and the melting heat of the coating increased with drug level increases, and the drug existed in a microcrystalline form.
- (2)
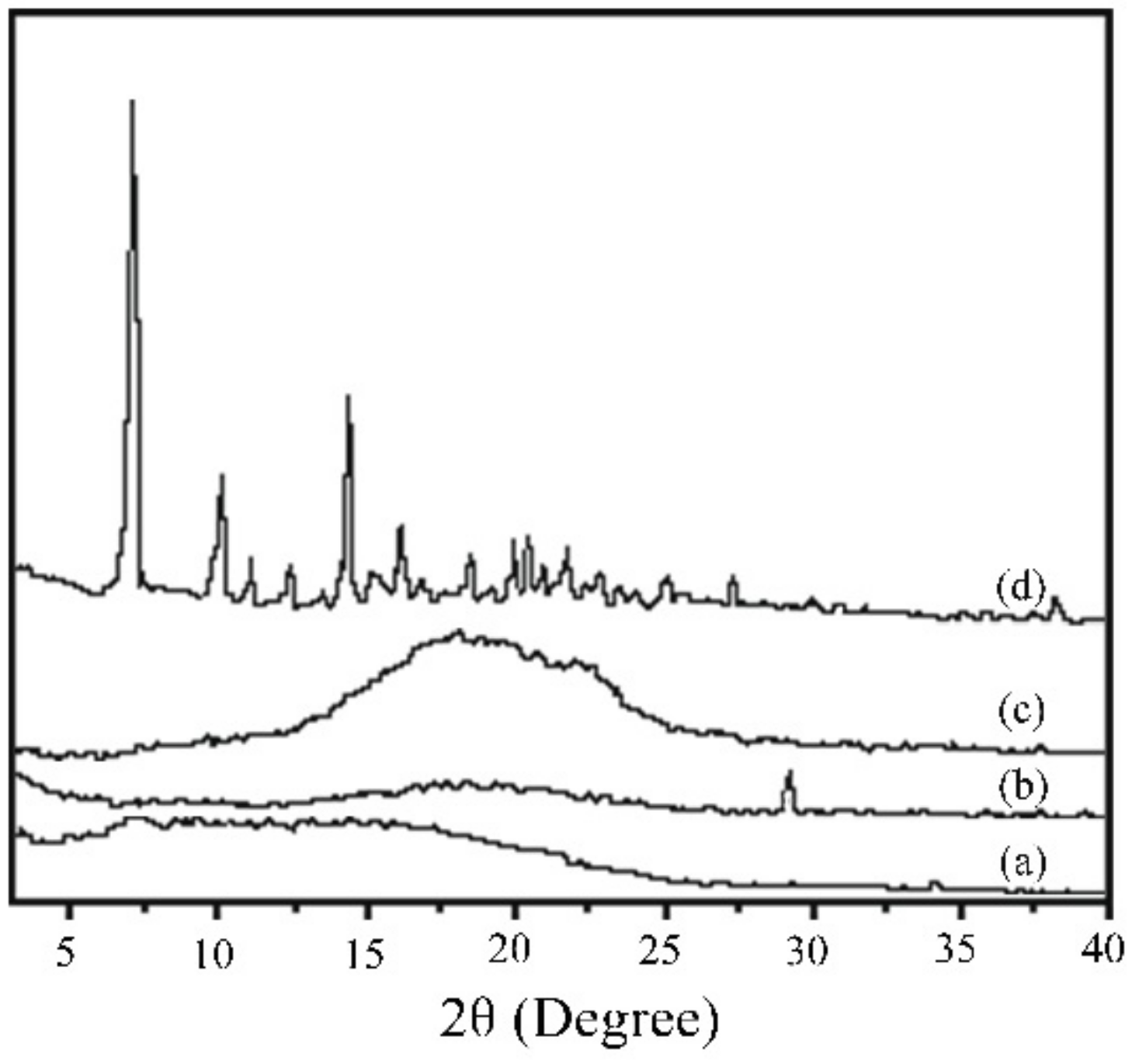
- No characteristic peaks from the drug crystal were observed in the XRD results, and the drug-loading coating was still amorphous. RAPA addition decreased the Tg of the PLGA coating, accompanied by partial crystallization that reduced the degradation rate of the drug loading coating.
- (3)
- The number of platelets adhered to the PLGA drug-loading coating surface was significantly less than for the PLGA coating, and there was no deformation or pseudopodia. The coagulation time of the drug-loading coating was longer, and the activation degree of the coagulation factor minimized when the RAPA content was 50%. The microphase separation structure was generated by mixing RAPA and PLGA, which effectively increased the critical tension and reduced the free energy of the crystal plane to improve the blood compatibility of the coating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sofogianni, A. Lipoprotein-associated Phospholipase A2 and Coronary Heart Disease. Curr. Pharm. Design. 2018, 24, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.L.; Qiao, X.; Ren, S.F.; Zhao, Z.P. Effect of severe stenosis or occlusion of small vessel on coronary artery after cardiac stent implantation on cardiac function. Clin. Res. Pract. 2018, 13, 131–137. [Google Scholar]
- Mostaed, E.; Sikora, J.; Malgorzata, D.; Jaroslaw, W. Zinc-based alloys for degradable vascular stent applications. Acta Biomater. 2018, 71, 1–23. [Google Scholar] [CrossRef]
- Katayama, Y.; Kubo, T.; Akasaka, T.; Aoki, H. TCTAP A-085 Vascular Response to Drug-eluting Stent with Biodegradable Versus Durable Polymer. J. Am. Coll. Cardiol. 2017, 13, S47. [Google Scholar] [CrossRef]
- Francesca, B.M.; Francesco, B.; Carlo, T.; Ornella, L. Coronary stents and vascular response to implantation: Literature review. Pragmat. Obs. Res. 2017, 8, 137. [Google Scholar]
- Chalony, C.; Aguilar, L.E.; Chan, H.P. Drug free anti-cell proliferative and anti-platelet adhesion coatings for vascular stents via polymeric electrospun fibers. Mater. Lett. 2021, 291, 1295–1302. [Google Scholar] [CrossRef]
- Wu, T.; Chen, X.; Fan, D.Z.; Pang, X.L. Development and application of metalmaterials in terms of vascular stents. Bio-Med. Mater. Eng. 2015, 25, 628–635. [Google Scholar]
- Liang, C.; Hao, J.; Wang, H.; Li, B.; Xia, D. Preparation and Research Progress of Contact-Induced Surface of Metal Implants. Acta Metall Sin 2017, 13, 21–28. [Google Scholar]
- Togha, M.; Jahanshahi, M.; Alizadeh, L.; Jahromi, S.R.; Ghaemi, A. Rapamycin Augments Immunomodulatory Properties of Bone Marrow-Derived Mesenchymal Stem Cells in Experimental Autoimmune Encephalomyelitis. Mol. Neurobiol. 2017, 54, 2445–2457. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.F.; Fu, Q.Y.; Liu, J.; Cardiology, D.O. Advances in Coronary Drug-eluting Stent Restenosis. Adv. Cardiovasc. Dis. 2017, 371, 968–974. [Google Scholar]
- Hilliard, S.; Baldinozzi, G.; Friedrich, D.; Kressman, S.; Strub, H.; Artero, V. Mesoporous thin film WO3 photoanode for photoelectrochemical water splitting: A sol-gel dip coating approach. Sustain. Energy Fuels. 2017, 1, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Bakhsheshi-Rad, R.H.; Hamzah, E.; George, J.; Safaa, D.; Saud, N. Fabrication and characterisation of novel ZnO/MWCNT duplex coatings deposited on Mg alloy by PVD coupled with dip-coatings techniques. J. Alloys Compd. 2017, 728, 4478–4486. [Google Scholar] [CrossRef]
- Xia, F.; Li, C.; Ma, C.; Li, Q.; Xing, H. Effect of pulse current density on microstructure and wear property of Ni-TiN nanocoatings deposited via pulse electrodeposition. Appl. Surf. Sci. 2021, 538, 148139. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, D.; Ma, Z. Effects of duty cycle and pulse frequency on microstructures and properties of electrodeposited Ni-Co-SiC nanocoatings. Ceram. Int. 2020, 46, 12128–12137. [Google Scholar] [CrossRef]
- Xia, F.; Li, Q.; Ma, C.; Liu, W.; Ma, Z. Preparation and wear properties of Ni/TiN-SiC nanocoatingss obtained by pulse current electrodeposition. Ceram. Int. 2020, 46, 7961–7969. [Google Scholar] [CrossRef]
- Sidewinder, O.A.; Prikhozhdenko, E.S.; Schurov, I. Patterned Drug-Eluting coatingss for Tracheal Stents Based on PLA, PLGA, and PCL for the Granulation Formation Reduction: In Vivo Studies. Pharmaceutics 2021, 13, 119–126. [Google Scholar]
- Qi, H.; Heise, S.; Zhou, J.; Schuhladen, K.; Lu, T. Electrophoretic Deposition of Bioadaptive Drug Delivery coatingss on Magnesium Alloy for Bone Repair. Acs. Appl. Mater. Interfaces 2019, 11, 35–42. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.X.; Fan, W.; Wang, Q.X.; Li, S.R. Effects of different stents coated with rapamycin on serum adiponectin, GDF-15 and Cys-C levels in patients with true coronary bifurcation lesions. Chin. J. Health. Insp. 2017, 27, 87–90. (In Chinese) [Google Scholar]
- Tabatabaeian, K.; Simayee, M.; Shojaie, A.F.; Mashayekhi, F.; Hadavi, M. The Effect of Silica coatings on the Drug Release Profile and Biocompatibility of Nano-MOF-5. J. Sci. Islamic Repub. Iran 2020, 31, 371–377. [Google Scholar]
- Nijhoff, F.; Stella, P.R.; Troost, M.S.; Belkacemi, A.; Nathoe, H.M.; Voskuil, M. Comparative assessment of the antirestenotic efficacy of two paclitaxel drug-eluting balloons with different coatings in the treatment of in-stent restenosis. Clin. Res. Cardiol. 2016, 105, 401–411. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Hubert, M.; Pinnamaneni, S.; Tao, L.; Zhao, J.; Sharif, S.; Ramakrishnan, R.K.; Nazarenko, S. Effect of Moisture Sorption on Free Volume and Relaxation of Spray Dried Dispersions: Relation to Drug Recrystallization—ScienceDirect. J. Pharm. Sci. 2020, 109, 1050–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.H.; Mueannoom, W.; Gaisford, S.; Kett, V.L. Investigation into the effect of varying l-leucine concentration on the product characteristics of spray-dried liposome powders. J. Pharm. Pharmacol. 2012, 64, 1412–1424. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Tripathi, S.; Tripathi, P.K. Invasomes: Potential vesicular systems for transdermal delivery of drug molecules. J. Drug. Deliv. Sci. Tec. 2020, 61, 102166. [Google Scholar] [CrossRef]
- Liu, X.; Zhen, Z.; Jing, L.; Xi, T.; Zheng, Y.; Guan, S. Multifunctional MgF2/Polydopamine coatings on Mg Alloy for Vascular Stent Application. J. Mater. Sci. Technol. 2015, 31, 733–743. [Google Scholar] [CrossRef]
- Guinesi, L.S.; Cavalheiro, D.T.G. The use of DSC curves to determine the acetylation degree of chitin/Chitosan samples. Thermochim. Acta 2006, 444, 128–133. [Google Scholar] [CrossRef]
- Eskew, M.W.; Benight, A.S. Ligand binding constants for human serum albumin evaluated by ratiometric analysis of DSC thermograms. Anal. Biochem. 2021, 628, 114293. [Google Scholar] [CrossRef]
- Biran, R.; Pond, D. Heparin coatingss for improving blood compatibility of medical devices. Adv. Drug Deliv. Rev. 2016, 26, 12–23. [Google Scholar]
- Ayyar, M.; Mani, M.P.; Jaganathan, S.K.; Rathanasamy, R. Preparation, characterization and blood compatibility assessment of a novel electrospun nanocomposite comprising polyurethane and ayurvedic-indhulekha oil for tissue engineering applications. Biomed. Tech. 2017, 3, 245–253. [Google Scholar] [CrossRef]
- Vassallo, V.; Stellavato, A.; Cimini, D.; Pirozzi, A.V.A.; Alfano, A.; Cammarota, M.; Balato, G.; D’Addona, A.; Ruosi, C.; Schiraldi, C. Unsulfated biotechnological chondroitin by itself as well as in combination with high molecular weight hyaluronan improves the inflammation profile in osteoarthritis in vitro model. J. Cell. Biochem. 2021, 122, 1021–1036. [Google Scholar] [CrossRef]
- Russo, R.; Vassallo, V.; Stellavato, A.; Valletta, M.; Cimini, D.; Pedone, P.V.; Schiraldi, C.; Chambery, A. Differential Secretome Profiling of Human Osteoarthritic Synoviocytes Treated with Biotechnological Unsulfated and Marine Sulfated Chondroitins. Int. J. Mol. Sci. 2020, 21, 3746. [Google Scholar] [CrossRef]
- Abe, K.; Kataoka, K.; Sekiguchi, M.; Okano, T.; Shimada, A. Transmission and scanning electron microscopic analysis of rat platelets adhered to HEMA-St ABA type block copolymer surface with microphase separated structure. Blood Vessel 1985, 16, 149–158. [Google Scholar] [CrossRef]
- Catauro, M.; Tranquillo, E.; Dal Poggetto, G.; Naviglio, S.; Barrino, F. Antibacterial properties of sol-gel biomaterials with different percentages of PEG or PCL. Macromol. Symp. 2020, 389, 1900056. [Google Scholar] [CrossRef]






| Time | PLGA | 33%-RAPA-PLGA | ||
|---|---|---|---|---|
| (Weeks) | Remaining Weight (%) | pH | Remaining Weight (%) | pH |
| 0 | 100 | 7.5 | 100 | 7.6 |
| 1 | 90.3 | 7.4 | 88.2 | 7.5 |
| 2 | 88.7 | 7.4 | 85.8 | 7.5 |
| 5 | 87.6 | 7.4 | 87.1 | 7.4 |
| 8 | 83.5 | 5.5 | 84.6 | 5.8 |
| 11 | 43.3 | 5.0 | 80.5 | 5.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Z.; Ma, C.; Zhang, H. PLGA Coatings and PLGA Drug-Loading Coatings for Cardiac Stent Samples: Degradation Characteristics and Blood Compatibility. Coatings 2021, 11, 1427. https://doi.org/10.3390/coatings11111427
Jia Z, Ma C, Zhang H. PLGA Coatings and PLGA Drug-Loading Coatings for Cardiac Stent Samples: Degradation Characteristics and Blood Compatibility. Coatings. 2021; 11(11):1427. https://doi.org/10.3390/coatings11111427
Chicago/Turabian StyleJia, Ziyang, Chunyang Ma, and Hongbin Zhang. 2021. "PLGA Coatings and PLGA Drug-Loading Coatings for Cardiac Stent Samples: Degradation Characteristics and Blood Compatibility" Coatings 11, no. 11: 1427. https://doi.org/10.3390/coatings11111427
APA StyleJia, Z., Ma, C., & Zhang, H. (2021). PLGA Coatings and PLGA Drug-Loading Coatings for Cardiac Stent Samples: Degradation Characteristics and Blood Compatibility. Coatings, 11(11), 1427. https://doi.org/10.3390/coatings11111427





