Electroplating of Pure Aluminum from [HMIm][TFSI]–AlCl3 Room-Temperature Ionic Liquid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the IL
2.2. Electroplating and Electrochemical Experiments
2.3. Analytical Characterization
3. Results
3.1. Ionic Species in [HMIm][TFSI]–AlCl3 IL
3.2. Determination of the Deposition Potential and Reversibility
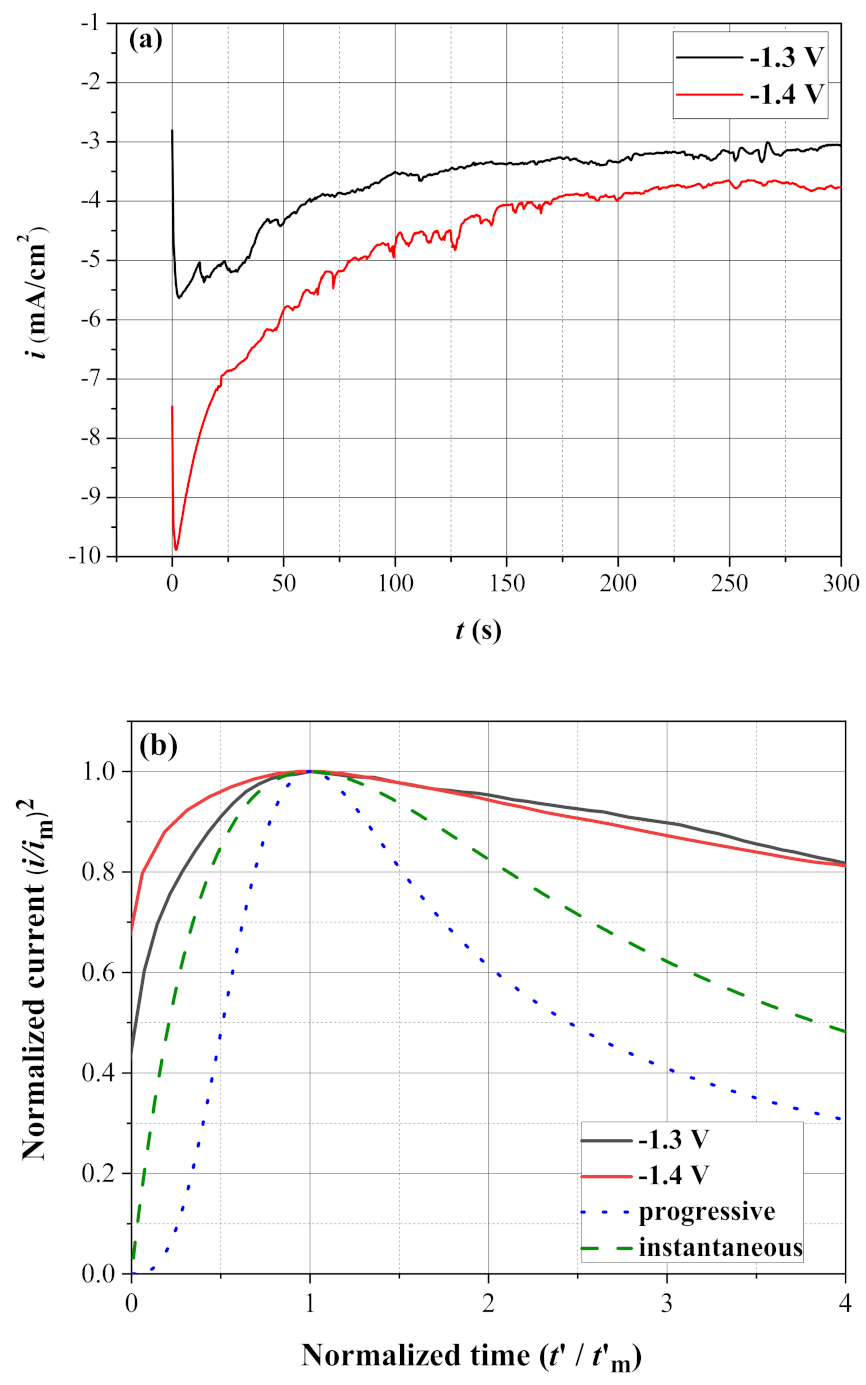
3.3. Nucleation and Growth
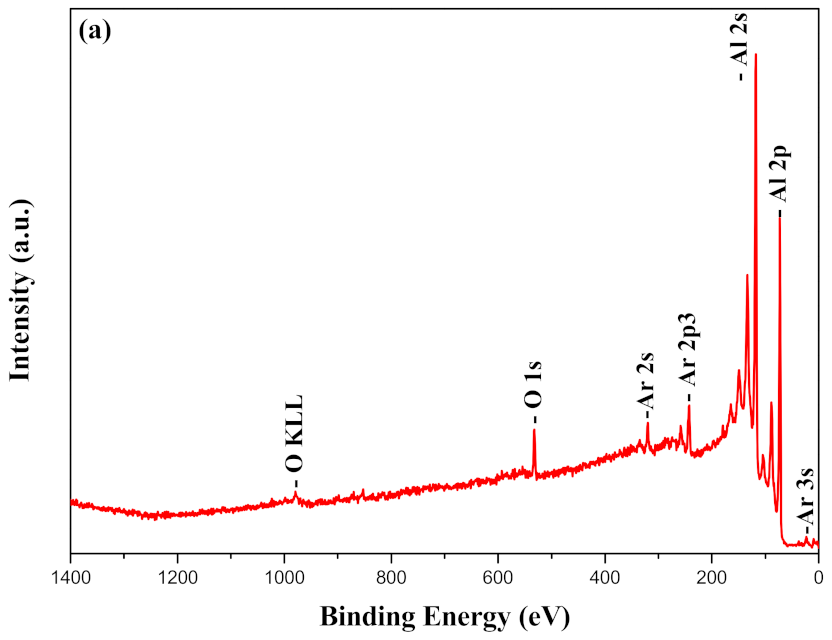
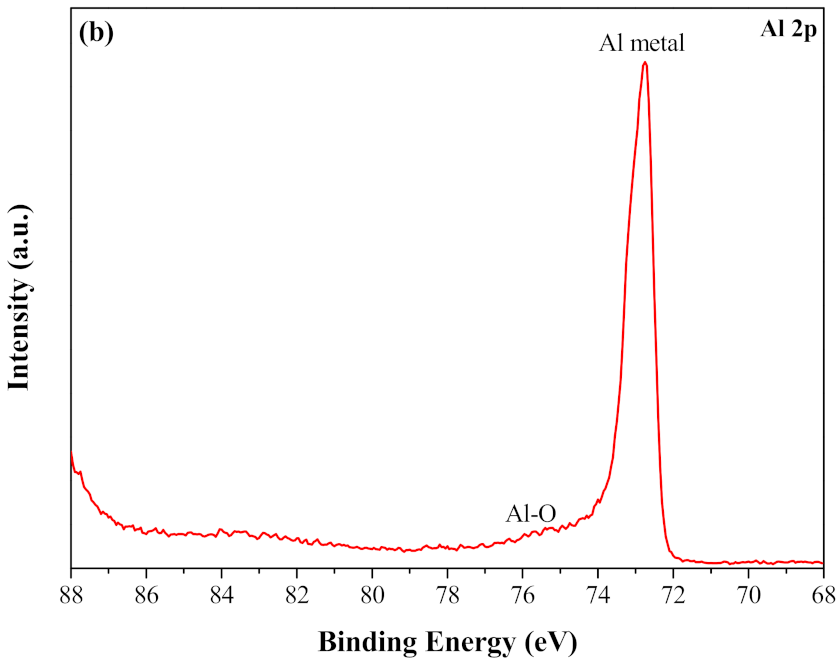
3.4. The Surface Chemistry of the Coating
3.5. The Crystal Structure of the Al Coating
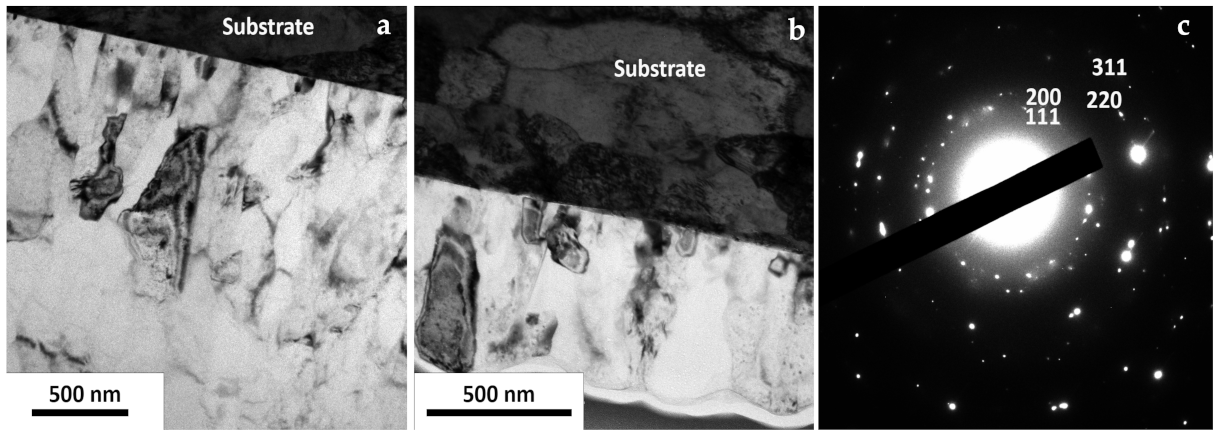
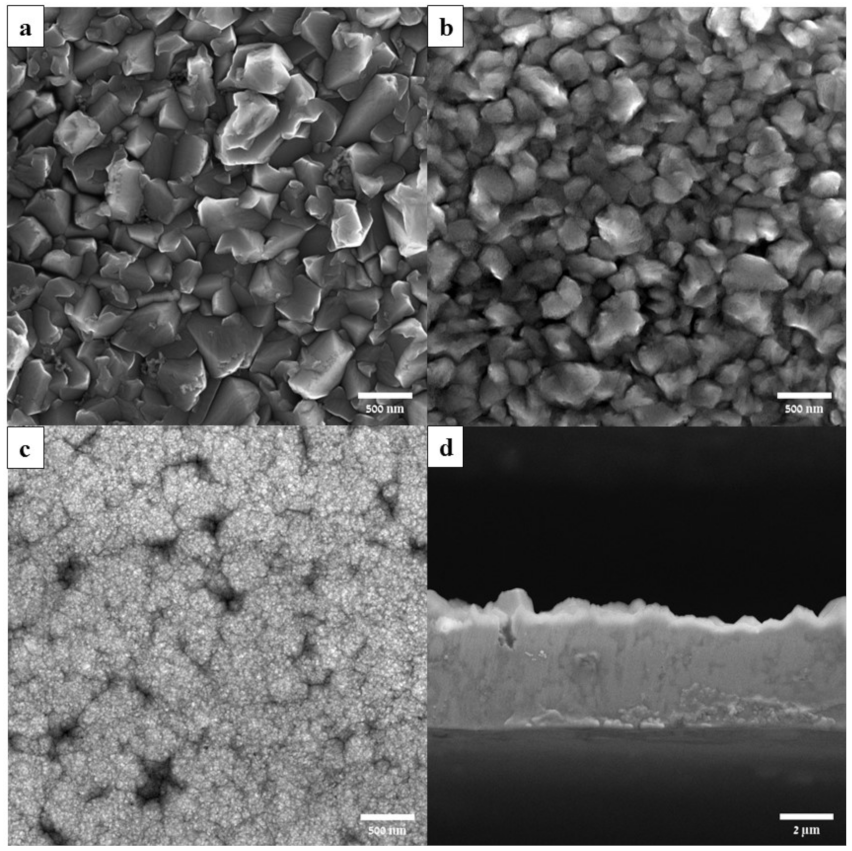
3.6. Surface Morphology of the Al Coating
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saevarsdottir, G.; Kvande, H.; Welch, B.J. Aluminum production in the times of climate change: The global challenge to reduce the carbon footprint and prevent carbon leakage. JOM 2020, 72, 296–308. [Google Scholar] [CrossRef]
- Cullen, J.M.; Allwood, J.M. Mapping the global flow of aluminum: From liquid aluminum to end-use goods. Environ. Sci. Technol. 2013, 47, 3057–3064. [Google Scholar] [CrossRef] [Green Version]
- Milovanoff, A.; Posen, I.D.; MacLean, H.L. Quantifying environmental impacts of primary aluminum ingot production and consumption: A trade-linked multilevel life cycle assessment. J. Ind. Ecol. 2021, 25, 67–78. [Google Scholar] [CrossRef]
- Maniam, K.K.; Paul, S.A. Review on the electrodeposition of aluminum and aluminum alloys in ionic liquids. Coatings 2021, 11, 80. [Google Scholar] [CrossRef]
- Eliyahu, D.; Gileadi, E.; Galun, E.; Eliaz, N. Atomic force microscope-based meniscus-confined three-dimensional electrodeposition. Adv. Mater. Technol. 2020, 5, 1900827. [Google Scholar] [CrossRef]
- Svetlizky, D.; Zheng, B.; Buta, T.; Zhou, Y.; Golan, O.; Breiman, U.; Haj-Ali, R.; Schoenung, J.M.; Lavernia, E.J.; Eliaz, N. Directed energy deposition of Al 5xxx alloy using Laser Engineered Net Shaping (LENS®). Mater. Des. 2020, 192, 108763. [Google Scholar] [CrossRef]
- Eliaz, N.; Fuks, N.; Geva, D.; Oren, S.; Shriki, N.; Vaknin, D.; Fishman, D.; Levi, O. Comparative quality control of titanium alloy Ti–6Al–4V, 17–4 PH stainless steel, and aluminum alloy 4047 either manufactured or repaired by Laser Engineered Net Shaping (LENS®). Materials 2020, 13, 4171. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N.; Gileadi, E. Physical Electrochemistry: Fundamentals, Techniques, and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2019; Ch. 17 & 18; ISBN 978-3-527-34139-9. [Google Scholar]
- Peled, E.; Gileadi, E. The electrodeposition of aluminum from aromatic hydrocarbon. I. Composition of baths and the effect of additives. J. Electrochem. Soc. 1976, 123, 15–19. [Google Scholar] [CrossRef]
- Guinea, E.; Salicio-Paz, A.; Iriarte, A.; Grande, H.-J.; Medina, E.; García-Lecina, E. Robust aluminum electrodeposition from ionic liquid electrolytes containing light aromatic naphta as additive. ChemistryOpen 2019, 8, 1094–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuda, T.; Hussey, C.L. Electrochemistry of room-temperature ionic liquids and melts. In Modern Aspects of Electrochemistry; White, R., Ed.; Springer: New York, NY, USA, 2009; No. 45. [Google Scholar]
- Ispas, A.; Bund, A. Electrodeposition in ionic liquids. Electrochem. Soc. Interface 2014, 23, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Zein El Abedin, S.; Pölleth, M.; Meiss, S.A.; Janek, J.; Endres, F. Ionic liquids as green electrolytes for the electrodeposition of nanomaterials. Green Chem. 2007, 9, 549–553. [Google Scholar] [CrossRef]
- Tiago, G.A.O.; Matias, I.A.S.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. Application of ionic liquids in electrochemistry—Recent advances. Molecules 2020, 25, 5812. [Google Scholar] [CrossRef] [PubMed]
- Kar, M.; Plechkova, N.V.; Seddon, K.R.; Pringle, J.M.; MacFarlane, D.R. Ionic liquids—Further progress on the fundamental issues. Aust. J. Chem. 2019, 72, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P.; McKenzie, K.J. Application of ionic liquids to the electrodeposition of metals. Phys. Chem. Chem. Phys. 2006, 8, 4265–4279. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Chollier Brym, M.J.; Dubé, G.; Lasia, A.; Brisard, G.M. Electrodeposition of aluminium from ionic liquids: Part I—electrodeposition and surface morphology of aluminium from aluminium chloride (AlCl3)–1-ethyl-3-methylimidazolium chloride ([EMIm]Cl) ionic liquids. Surf. Coat. Technol. 2006, 201, 1–9. [Google Scholar] [CrossRef]
- Liu, Q.X.; Zein El Abedin, S.; Endres, F. Electrodeposition of nanocrystalline aluminum: Breakdown of imidazolium cations modifies the crystal size. J. Electrochem. Soc. 2008, 155, D357–D362. [Google Scholar] [CrossRef]
- Bakkar, A.; Neubert, V. A new method for practical electrodeposition of aluminium from ionic liquids. Electrochem. Commun. 2015, 51, 113–116. [Google Scholar] [CrossRef]
- Ispas, A.; Wolff, E.; Bund, A. An electrochemical quartz crystal microbalance study on electrodeposition of aluminum and aluminum-manganese alloys. J. Electrochem. Soc. 2017, 164, H5263–H5270. [Google Scholar] [CrossRef]
- Böttcher, R.; Valitova, A.; Ispas, A.; Bund, A. Electrodeposition of aluminium from ionic liquids on high strength steel. Trans. IMF 2019, 97, 82–88. [Google Scholar] [CrossRef]
- Böttcher, R.; Mai, S.; Ispas, A.; Bund, A. Aluminum deposition and dissolution in [EMIm]Cl-based ionic liquids—Kinetics of charge-transfer and the rate-determining step. J. Electrochem. Soc. 2020, 167, 102516. [Google Scholar] [CrossRef]
- Böttcher, R.; Ispas, A.; Bund, A. Determination of transport parameters in [EMIm]Cl–based ionic liquids—Diffusion and electrical conductivity. Electrochim. Acta 2021, 366, 137370. [Google Scholar] [CrossRef]
- Yoshimura, K.; Miyahara, K.; Matsunaga, M. Electrodeposition of aluminum from ionic liquids. ECS Trans. 2009, 16, 449–452. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Theerthagiri, J.; Vikraman, D.; Yim, C.-J.; Hussain, S.; Sharma, R.; Maiyalagan, T.; Qin, J.; Kim, H.-S. Ionic liquid-based electrolytes for energy storage devices: A brief review on their limits and applications. Polymers 2020, 12, 918. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Zhang, K.; Wu, F.; Bai, Y.; Wu, C. Ionic liquid-based electrolytes for aluminum/magnesium/sodium-ion batteries. Energy Mater. Adv. 2021, 2021, 9204217. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Reichert, W.M.; Rogers, R.D. Crystal structures of imidazolium bis(trifluoromethanesulfonyl)imide ‘ionic liquid’ salts: The first organic salt with a cis-TFSI anion conformation. Dalton Trans. 2004, 2004, 2267–2271. [Google Scholar] [CrossRef]
- Bonhôte, P.; Dias, A.P.; Papageorgiou, N.; Kalyanasundaram, K.; Grätzel, M. Hydrophobic, highly conductive ambient-temperature molten salts. Inorg. Chem. 1996, 35, 1168–1178. [Google Scholar] [CrossRef]
- Hayley, A.E.; Andrea, G.B.; Douglas, R.M.; Greger, O.; Maria, F. Room temperature fast-ion conduction in imidazolium halide salts. J. Mater. Chem. 2001, 11, 3031–3036. [Google Scholar]
- Holbrey, J.D.; Seddon, K.R. The phase behaviour of 1-alkyl-3-methylimidazolium tetrafluoroborates; ionic liquids and ionic liquid crystals. J. Chem. Soc. Dalton Trans. 1999, 13, 2133–2139. [Google Scholar] [CrossRef]
- Paredes, X.; Queirós, C.S.G.P.; Santos, F.J.V.; Santos, A.F.; Santos, M.S.C.S.; Lourenço, M.J.V.; Nieto de Castro, C.A. Thermophysical properties of 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide, [C6mim][(CF3SO2)2N]—New data, reference data, and reference correlations. J. Phys. Chem. Ref. Data 2020, 49, 043101. [Google Scholar] [CrossRef]
- Nazet, A.; Sokolov, S.; Sonnleitner, T.; Makino, T.; Kanakubo, M.; Buchner, R. Densities, viscosities, and conductivities of the imidazolium ionic liquids [Emim][Ac], [Emim][FAP], [Bmim][BETI], [Bmim][FSI], [Hmim][TFSI], and [Omim][TFSI]. J. Chem. Eng. Data 2015, 60, 2400–2411. [Google Scholar] [CrossRef]
- Liu, S.; Liedel, C.; Tarakina, N.V.; Ostic, N.C.; Akcora, P. Dynamics of ionic liquids in the presence of polymer-grafted nanoparticles. Nanoscale 2019, 11, 19832–19841. [Google Scholar] [CrossRef] [PubMed]
- Łachwa, J.; Morgado, P.; Esperança, J.M.S.S.; Guedes, H.J.R.; Canongia Lopes, J.N.; Rebelo, L.P.N. Fluid-phase behavior of {1-Hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imide, [C6mim][NTf2], + C2−C8n-alcohol} mixtures: liquid−liquid equilibrium and excess volumes. J. Chem. Eng. Data 2006, 51, 2215–2221. [Google Scholar] [CrossRef]
- Abbott, A.P.; Harris, R.C.; Hsieh, Y.-T.; Ryder, K.S.; Sun, I.-W. Aluminium electrodeposition under ambient conditions. Phys. Chem. Chem. Phys. 2014, 16, 14675–14681. [Google Scholar] [CrossRef]
- Barrado, E.; Rodriguez, J.A.; Hernández, P.; Castrillejo, Y. Electrochemical behavior of copper species in the 1-buthyl-3-methyl-imidazolium chloride (BMIMCl) ionic liquid on a Pt electrode. J. Electroanal. Chem. 2016, 768, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Galiński, M.; Lewandowski, A.; Stępniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Torriero, A.A.J.; Sunarso, J.; Howlett, P.C. Critical evaluation of reference systems for voltammetric measurements in ionic liquids. Electrochim. Acta 2012, 82, 60–68. [Google Scholar] [CrossRef]
- Yamato, Y.; Katayama, Y.; Miura, T. Effects of the interaction between ionic liquids and redox couples on their reaction entropies. J. Electrochem. Soc. 2013, 160, H309–H314. [Google Scholar] [CrossRef]
- Damodaran, K. Recent NMR studies of ionic liquids. Annu. Rep. NMR Spectrosc. 2016, 88, 215–244. [Google Scholar]
- Kaupilla, T.J.; Kostiainen, R. Ambient mass spectrometry in the analysis of compounds of low polarity. Anal. Methods 2017, 9, 4936–4953. [Google Scholar] [CrossRef]
- Steckel, A.; Schlosser, G. An organic chemist’s guide to electrospray mass spectrometric structure elucidation. Molecules 2019, 24, 611. [Google Scholar] [CrossRef] [Green Version]
- Babij, N.R.; McCusker, E.O.; Whiteker, G.T.; Canturk, B.; Choy, N.; Creemer, L.C.; De Amicis, C.V.; Hewlett, N.M.; Johnson, P.L.; Knobelsdorf, J.A.; et al. NMR chemical shifts of trace impurities: Industrially preferred solvents used in process and green chemistry. Org. Process Res. Dev. 2016, 20, 661–667. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]
- Urabe, T.; Tanaka, M.; Kumakura, S.; Tsugoshi, T. Study on chemical speciation in aluminum chloride solution by ESI-Q-MS. J. Mass Spectrom. 2007, 42, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Hellman, H.; Laitinen, R.S.; Kaila, L.; Jalonen, J.; Hietapelto, V.; Jokela, J.; Sarpola, A.; Ramo, J. Identification of hydrolysis products of FeCl3·6H2O by ESI-MS. J. Mass Spectrom. 2006, 41, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Zarzana, C.A.; Groenewold, G.S.; Benson, M.T.; Delmore, J.; Tsuda, T.; Hagiwara, R. Iron fluoroanions and their clusters by electrospray ionization of a fluorinating ionic liquid. J. Am. Soc. Mass Spectrom. 2015, 26, 1559–1569. [Google Scholar] [CrossRef]
- Kruve, A.; Kaupmees, K.; Liigand, J.; Oss, M.; Leito, I. Sodium adduct formation efficiency in ESI source. J. Mass Spectrom. 2013, 48, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Creuziger, A.; Stafford, G.; Hussey, C.L. Anodic dissolution of aluminum in the aluminum chloride-1-Ethyl-3-methylimidazolium chloride ionic liquid. J. Electrochem. Soc. 2016, 163, H1186–H1194. [Google Scholar] [CrossRef] [Green Version]
- Böttcher, R.; Ispas, A.; Bund, A. Anodic dissolution of aluminum and anodic passivation in [EMIm]Cl-based ionic liquids. Electrochem. Commun. 2020, 115, 106720. [Google Scholar] [CrossRef]
- Heinze, J.; Rasche, A.; Pagels, M.; Geschke, B. On the origin of the so-called nucleation loop during electropolymerization of conducting polymers. J. Phys. Chem. B 2007, 111, 989–997. [Google Scholar] [CrossRef]
- Yang, M.; Hu, Z. Electrodeposition of bismuth onto glassy carbon electrodes from nitrate solutions. J. Electroanal. Chem. 2005, 583, 46–55. [Google Scholar] [CrossRef]
- Andrieux, C.P.; Merz, A.; Saveant, J.M. Dissociative electron transfer. Autocatalysis and kinetic characteristics of the electrochemical reductive cleavage of the carbon halogen bond in alkyl halides giving rise to stable radicals and carbanions. J. Am. Chem. Soc. 1985, 107, 6097–6103. [Google Scholar] [CrossRef]
- Houmam, A.; Hamed, E.M. Application of the dissociative electron transfer theory and its extension to the case of in-cage interactions in the electrochemical reduction of arene sulfonyl chlorides. Phys. Chem. Chem. Phys. 2012, 14, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Bard, J.A.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley and Sons: New York, NY, USA, 2001. [Google Scholar]
- Scharifker, B.; Hills, G. Theoretical and experimental studies of multiple nucleation. Electrochim. Acta 1983, 28, 879–889. [Google Scholar] [CrossRef]
- Scharifker, B.R.; Mostany, J.; Palomar-Pardavé, M.; González, I. On the theory of the potentiostatic current transient for diffusion-controlled three-dimensional electrocrystallization processes. J. Electrochem. Soc. 1999, 146, 1005–1012. [Google Scholar] [CrossRef]
- Budevski, E.; Staikov, G.; Lorenz, W.J. Electrochemical Phase Formation and Growth: An Introduction to the Initial Stages of Metal Deposition; VCH: Weinheim, Germany, 1996. [Google Scholar]
- Barrosse-Antle, L.E.; Bond, A.M.; Compton, R.G.; O’Mahony, A.M.; Rogers, E.I.; Silvester, D.S. Voltammetry in room temperature ionic liquids: Comparisons and contrasts with conventional electrochemical solvents. Chem. Asian J. 2010, 5, 202–230. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N.; Eliyahu, M. Electrochemical processes of nucleation and growth of hydroxyapatite on titanium supported by real-time electrochemical atomic force microscopy. J. Biomed. Mater. Res. A 2007, 80, 621–634. [Google Scholar] [CrossRef]
- Grujicic, D.; Pesic, B. Electrodeposition of copper: The nucleation mechanisms. Electrochim. Acta 2002, 47, 2901–2912. [Google Scholar] [CrossRef]
- Carlin, R.T.; De Long, H.C.; Fuller, J.; Trulove, P.C. Microelectrode evaluation of transition metal-aluminum alloy electrodepositions in chloroaluminate ionic liquids. J. Electrochem. Soc. 1998, 145, 1598–1607. [Google Scholar] [CrossRef]
- Lee, J.-J.; Miller, B.; Shi, X.; Kalish, R.; Wheeler, K.A. Aluminum deposition and nucleation on nitrogen-incorporated tetrahedral amorphous carbon electrodes in ambient temperature chloroaluminate melts. J. Electrochem. Soc. 2000, 147, 3370–3376. [Google Scholar] [CrossRef]
- Jiang, T.; Chollier Brym, M.J.; Dubé, G.; Lasia, A.; Brisard, G.M. Electrodeposition of aluminium from ionic liquids: Part II—studies on the electrodeposition of aluminum from aluminum chloride (AlCl3)—Trimethylphenylammonium chloride (TMPAC) ionic liquids. Surf. Coat. Technol. 2006, 201, 10–18. [Google Scholar] [CrossRef]
- Yong, Z.; Suojiang, Z.; Xingmei, L.; Qian, W.; Yong, Z.; Lian, L. Low-temperature electrodeposition of aluminium from Lewis acidic 1-Allyl-3 methylimidazolium chloroaluminate ionic liquids. Chin. J. Chem. Eng. 2012, 20, 130–139. [Google Scholar]
- Sherstyuk, O.V.; Pron’kin, S.N.; Chuvilin, A.L.; Salanov, A.N.; Savinova, E.R.; Tsirlina, G.A.; Petrii, O.A. Platinum electrodeposits on glassy carbon: The formation mechanism, morphology, and adsorption properties. Russ. J. Electrochem. 2000, 36, 741–751. [Google Scholar] [CrossRef]
- Theivaprakasam, S.; Girard, G.; Howlett, P.; Forsyth, M.; Mitra, S.; MacFarlane, D. Passivation behaviour of aluminium current collector in ionic liquid alkyl carbonate (hybrid) electrolytes. npj Mater. Degrad. 2018, 2, 13. [Google Scholar]
- Ohring, M. Engineering Materials Science; Academic Press: London, UK, 1995; Ch. 7; pp. 299–370. [Google Scholar]
- Meshi, L.; Cherns, D.; Griffiths, I.; Khongphetsak, S.; Gott, A.; Liu, C.; Denchitcharoen, S.; Shields, P.; Wang, W.; Campion, R.P.; et al. The reduction of threading dislocations in GaN using a GaN nanocolumn interlayer. Phys. Stat. Sol. (c). 2008, 5, 1645–1647. [Google Scholar] [CrossRef]
- Stark, A.; Behrend, P.; Braun, O.; Müller, A.; Ranke, J.; Ondruschk, B.; Bernd Jastorff, B. Purity specification methods for ionic liquids. GreenChem. 2008, 10, 1152–1161. [Google Scholar] [CrossRef]
- Andanson, J.-M.; Meng, X.; Traïkia, M.; Husson, P. Quantification of the impact of water as an impurity on standard physico-chemical properties of ionic liquids. J. Chem. Thermodyn. 2016, 94, 169–176. [Google Scholar] [CrossRef]
- Trémillon, B.; Letisse, G. Proprietes en solution dans le tetrachloroaluminate de sodium fondu I. systemes “acide-base”. J. Electroanal. Chem. Interfacial Electrochem. 1968, 17, 371–386. [Google Scholar] [CrossRef]
- Shi, M.; Jiang, J.; Zhao, H. Electrodeposition of aluminum in the 1-Ethyl-3-Methylimidazolium Tetrachloroaluminate ionic liquid. Electrochem 2021, 2, 185–196. [Google Scholar] [CrossRef]
- Berretti, E.; Giaccherini, A.; Martinuzzi, S.M.; Innocenti, M.; Schubert, T.J.S.; Stiemke, F.M.; Caporali, S. Aluminium electrodeposition from ionic liquid: Effect of deposition temperature and sonication. Materials 2016, 9, 719. [Google Scholar] [CrossRef]
- Sakhalkar, M.; Aduri, P.; Lande, S.; Chandra, S. Single-step synthesis of novel chloroaluminate ionic liquid for green Friedel–Crafts alkylation reaction. Clean Technol. Environ. Policy 2020, 22, 59–71. [Google Scholar] [CrossRef]
- Cvijović, M.; Kilibarda, V.; Jelikić-Stankov, M.; Lazarević, I.; Jakovljević, I.; Joksović, L.; Đurđević, P. ESI-MS study of speciation in hydrolyzed aluminum chloride solutions. J. Braz. Chem. Soc. 2012, 23, 1087–1097. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Yoshii, K.; Jiang, X.; Sun, X.G.; Tsuda, T.; Mehio, N.; Dai, S. An AlCl3 based ionic liquid with a neutral substituted pyridine ligand for electrochemical deposition of aluminum. Electrochim. Acta 2015, 160, 82–88. [Google Scholar] [CrossRef]
- Brausch, N.; Metlen, A.; Wasserscheid, P. New, highly acidic ionic liquid systems and their application in the carbonylation of toluene. Chem. Commun. 2004, 13, 1552–1553. [Google Scholar] [CrossRef] [PubMed]
- Bakkar, A.; Neubert, V. Electrodeposition and corrosion characterisation of micro- and nano-crystalline aluminium from AlCl3/1-ethyl-3-methylimidazolium chloride ionic liquid. Electrochim. Acta 2013, 103, 211–218. [Google Scholar] [CrossRef]
- Zhang, L.P.; Ge, Z.W.; Yu, X.J.; Zhao, Z.D.; Dong, Y.H. Al electrodeposition from chloroaluminate ionic liquid. Canad. Metall. Quart. 2013, 52, 398–404. [Google Scholar] [CrossRef]
- Sandford, C.; Edwards, M.A.; Klunder, K.J.; Hickey, D.P.; Li, M.; Barman, K.; Sigman, M.S.; White, H.S.; Minteer, S.D. A synthetic chemist’s guide to electroanalytical tools for studying reaction mechanisms. Chem. Sci. 2019, 10, 6404–6422. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, R.S.; Shain, I. Theory of stationary electrode polarography: Single scan and cyclic methods applied to reversible, irreversible, and kinetic systems. Anal. Chem. 1964, 36, 706–723. [Google Scholar] [CrossRef]
- Sisso, O.; Dor, S.; Eliyahu, D.; Sabatani, E.; Eliaz, N. Corrosion inhibition of copper in ferric chloride solution with organic inhibitors. npj Mater. Degrad. 2020, 4, 38. [Google Scholar] [CrossRef]
- Elwell, D.; Rao, G.M. Mechanism of electrodeposition of silicon from K2SiF6–flinak. Electrochim. Acta 1982, 27, 673–676. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, J.; Luo, H.; Tsay, C.; Jiang, H.; Lin, Y.-H.; Schroeder, M.A.; Guo, J. Synthesis and electrochemical properties of an aluminum hexafluorophosphate electrolyte. J. Phys. Chem. Lett. 2021, 12, 5903–5908. [Google Scholar] [CrossRef]
- Baik, S.-I.; Duhin, A.; Phillips, P.J.; Klie, R.F.; Gileadi, E.; Seidman, D.N.; Eliaz, N. Atomic-scale structural and chemical study of columnar and multilayer Re–Ni electrodeposited thermal barrier coating. Adv. Eng. Mater. 2016, 18, 1133–1144. [Google Scholar] [CrossRef]
- Cohen Sagiv, M.; Eliaz, N.; Gileadi, E. Incorporation of iridium into electrodeposited rhenium–nickel alloys. Electrochim. Acta 2013, 88, 240–250. [Google Scholar] [CrossRef]
- Hegde, A.C.; Venkatakrishna, K.; Eliaz, N. Electrodeposition of Zn–Ni, Zn–Fe and Zn–Ni–Fe alloys. Surf. Coat. Technol. 2010, 205, 2031–2041. [Google Scholar] [CrossRef]
- Eliaz, N.; Venkatakrishna, K.; Hegde, A.C. Electroplating and characterization of Zn–Ni, Zn–Co and Zn–Ni–Co alloys. Surf. Coat. Technol. 2010, 205, 1969–1978. [Google Scholar] [CrossRef]
- Rafailović, L.D.; Gammer, C.; Ebner, C.; Rentenberger, C.; Jovanović, A.Z.; Pašti, I.A.; Skorodumova, N.V.; Karnthaler, H.P. High density of genuine growth twins in electrodeposited aluminum. Sci. Adv. 2019, 5, eaax3894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Shinde, P.S.; Reddy, R.G. Diffusion coefficient and nucleation density studies on electrochemical deposition of aluminum from chloroaluminate ionic liquid electrolytes. J. Electroanal. Chem. 2021, 895, 115363. [Google Scholar] [CrossRef]

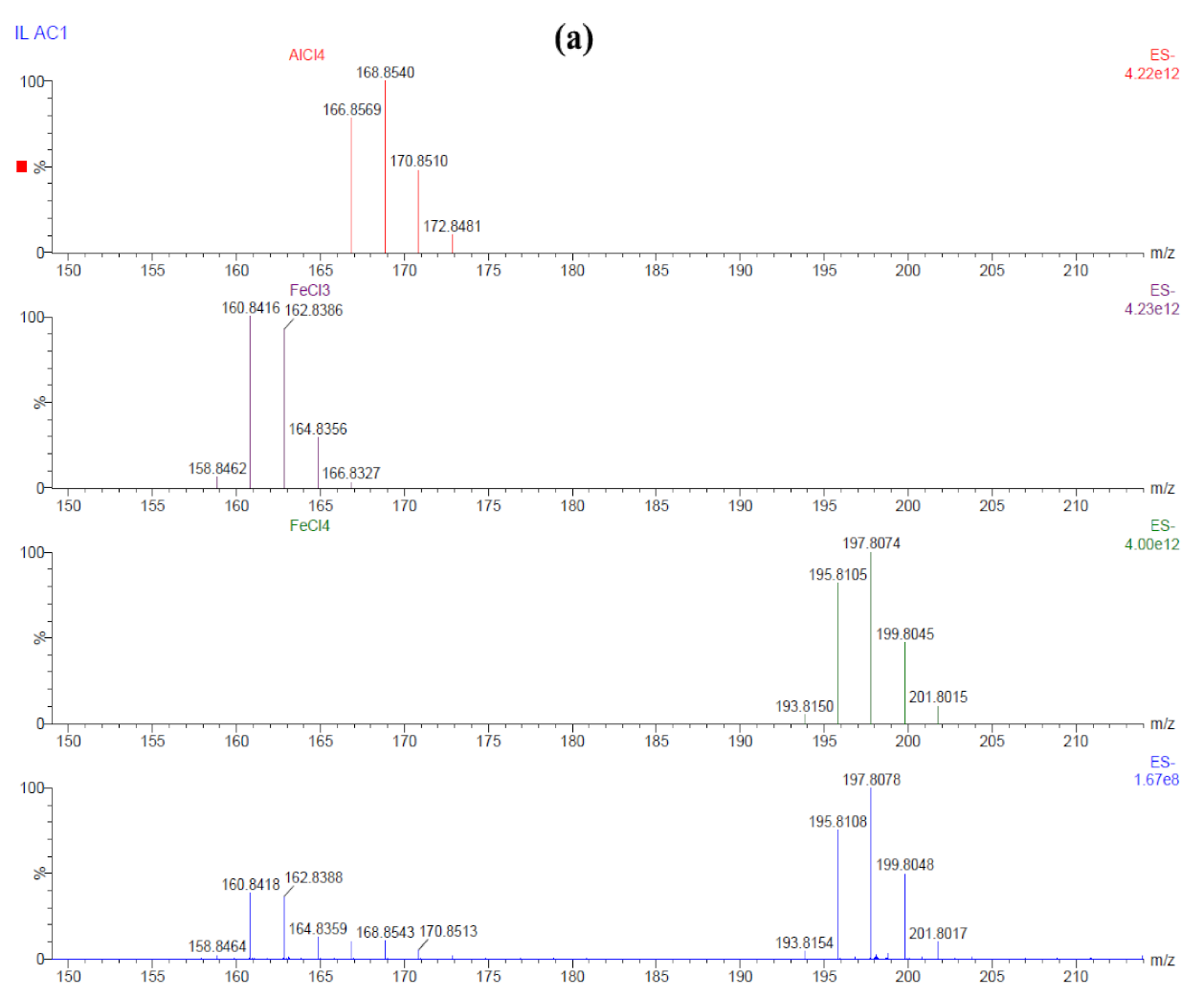
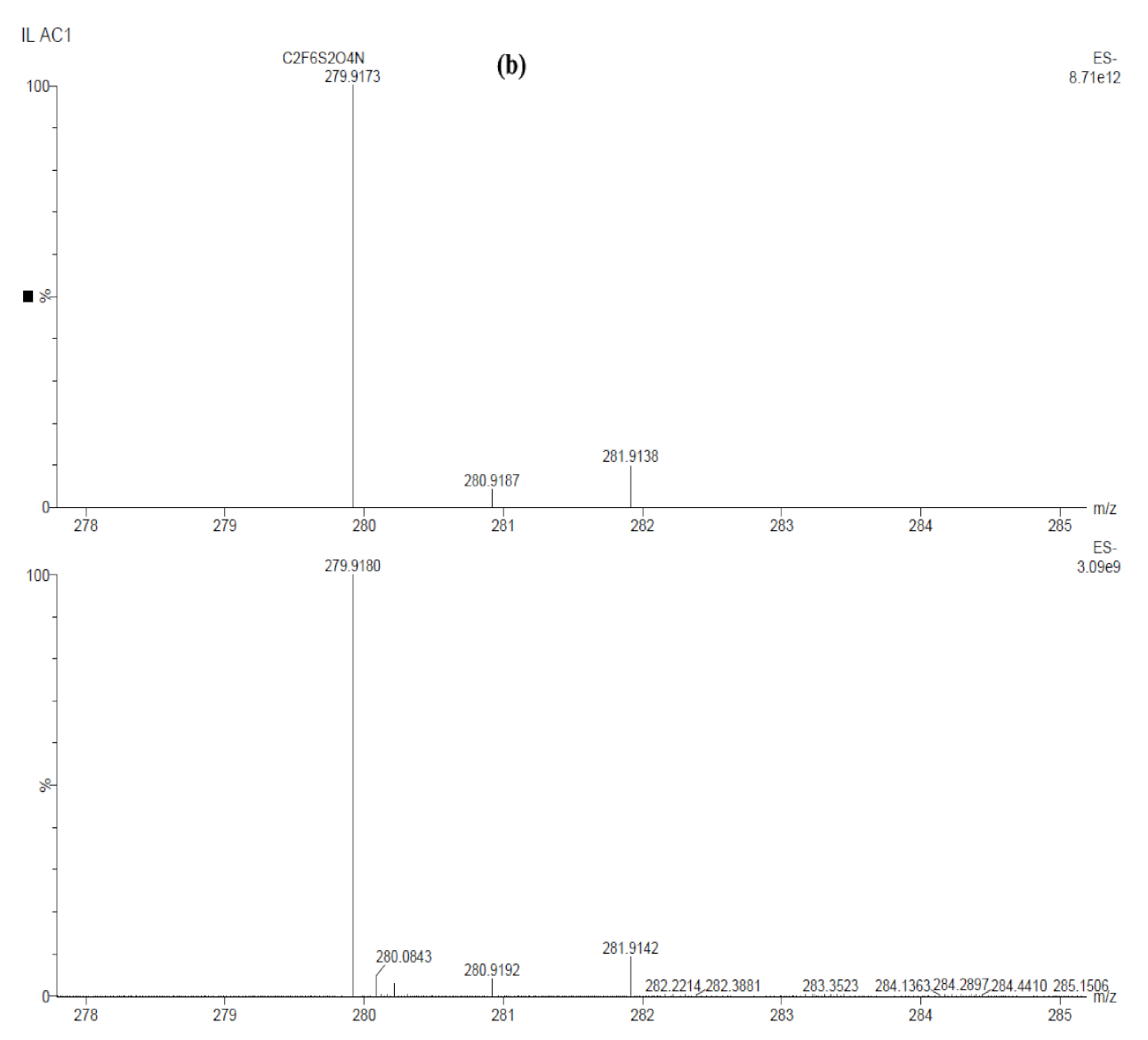
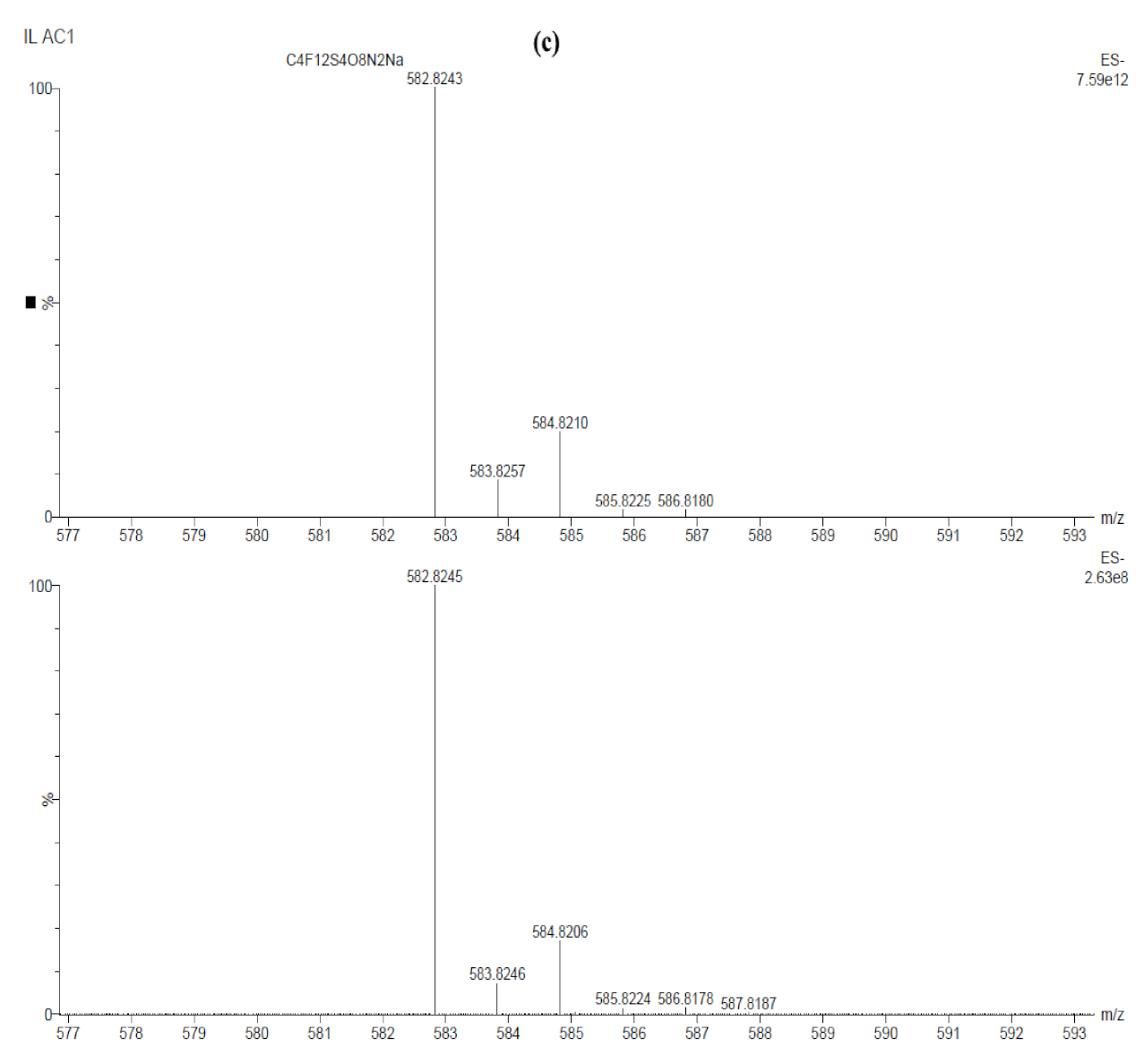
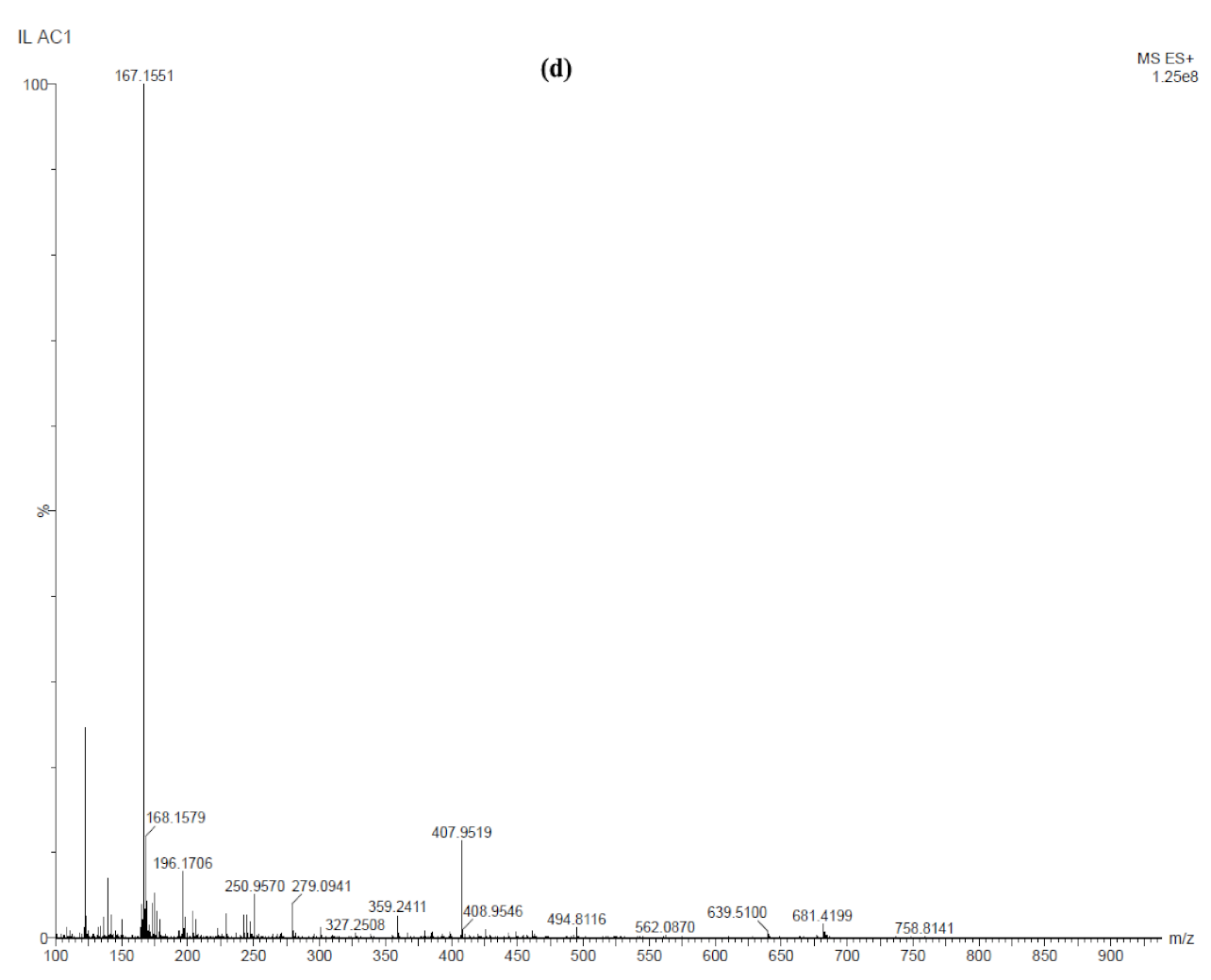


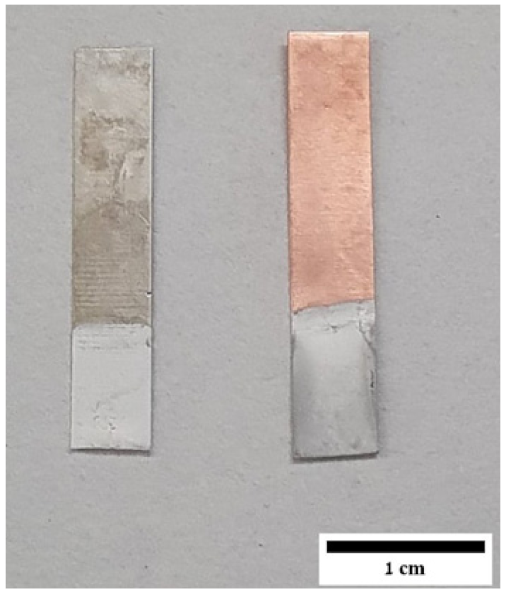
| Sample ID | Potential (V) | Time (min) | Al | O | Cl |
|---|---|---|---|---|---|
| E52 | −1.3 | 120 | 97.4 | 2.6 | - |
| E50 | −1.4 | 20 | 100.0 | - | - |
| E46 | −1.45 | 20 | 100.0 | - | - |
| E40 | −1.5 | 20 | 93.5 | 3.0 | 3.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melamed, Y.; Maity, N.; Meshi, L.; Eliaz, N. Electroplating of Pure Aluminum from [HMIm][TFSI]–AlCl3 Room-Temperature Ionic Liquid. Coatings 2021, 11, 1414. https://doi.org/10.3390/coatings11111414
Melamed Y, Maity N, Meshi L, Eliaz N. Electroplating of Pure Aluminum from [HMIm][TFSI]–AlCl3 Room-Temperature Ionic Liquid. Coatings. 2021; 11(11):1414. https://doi.org/10.3390/coatings11111414
Chicago/Turabian StyleMelamed, Yarden, Nabasmita Maity, Louisa Meshi, and Noam Eliaz. 2021. "Electroplating of Pure Aluminum from [HMIm][TFSI]–AlCl3 Room-Temperature Ionic Liquid" Coatings 11, no. 11: 1414. https://doi.org/10.3390/coatings11111414
APA StyleMelamed, Y., Maity, N., Meshi, L., & Eliaz, N. (2021). Electroplating of Pure Aluminum from [HMIm][TFSI]–AlCl3 Room-Temperature Ionic Liquid. Coatings, 11(11), 1414. https://doi.org/10.3390/coatings11111414








