Sol–Gel Encapsulation of ZnAl Alloy Powder with Alumina Shell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Powder Synthesis and Characterization
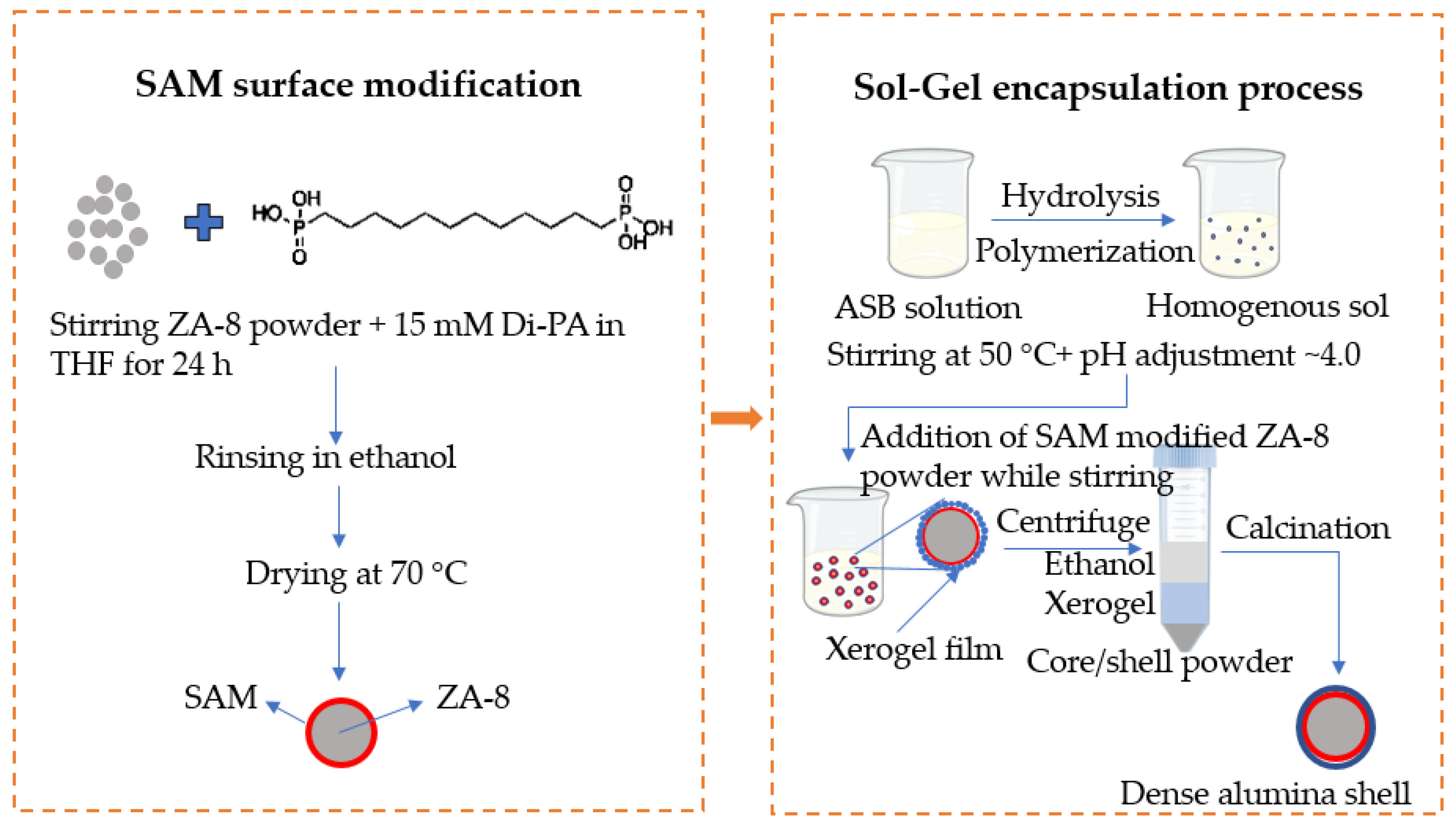
2.2. Application of a SAM on the Surface of the ZA-8 Powder Prior to Deposition of Alumina
2.3. Sol–Gel Processing of the Alumina Outer Shell
- (1)
- Hydrolysis of the alcohol groups at 50 °C:
- (2)
- Condensation and polymerization of the resulting hydroxyl groups:
- (3)
- Finally, a calcination reaction at 400–500 °C in air is conducted to convert the boehmite (aluminum oxide monohydrate, Al2O3·H2O, also known as γ-AlOOH) to alumina:
- ASB was added to DI water pre-heated to 50 °C at a molar ratio of 1:80 under vigorous mechanical stirring.
- A small amount of nitric acid was added to maintain the pH of the suspension at ~4.0.
- The suspension was stirred vigorously for 15 min, until a homogenous sol was formed.
- The SAM-modified powder was added to the sol, continue vigorous stirring for 15 min.
- The product was washed employing centrifugation in ethanol.
- The product was then dried at 100 °C for 12 h in air and calcined at 350–500 °C for 1 h. Both heating and cooling rates were 1 °C/min.
3. Results and Discussion
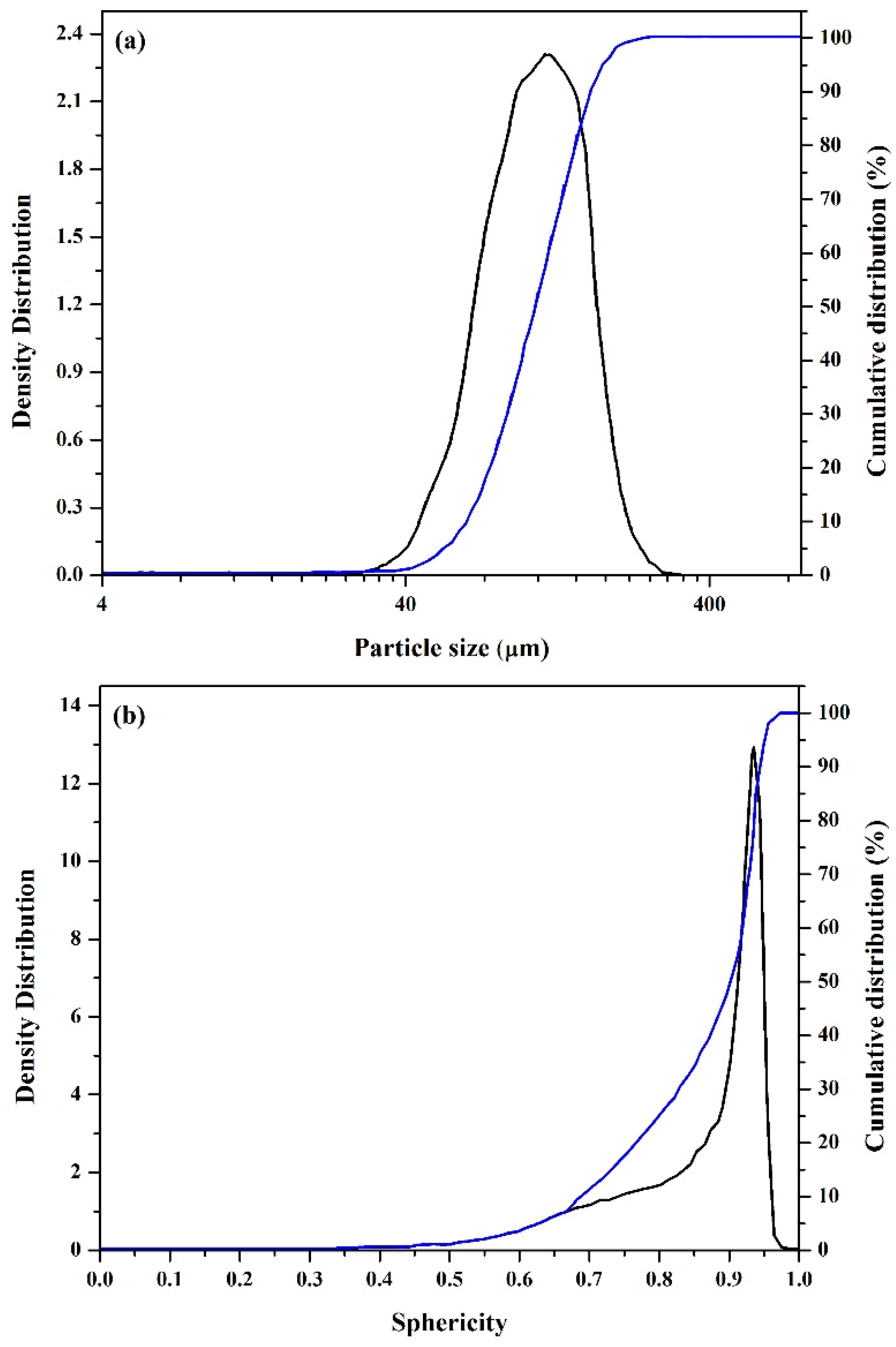
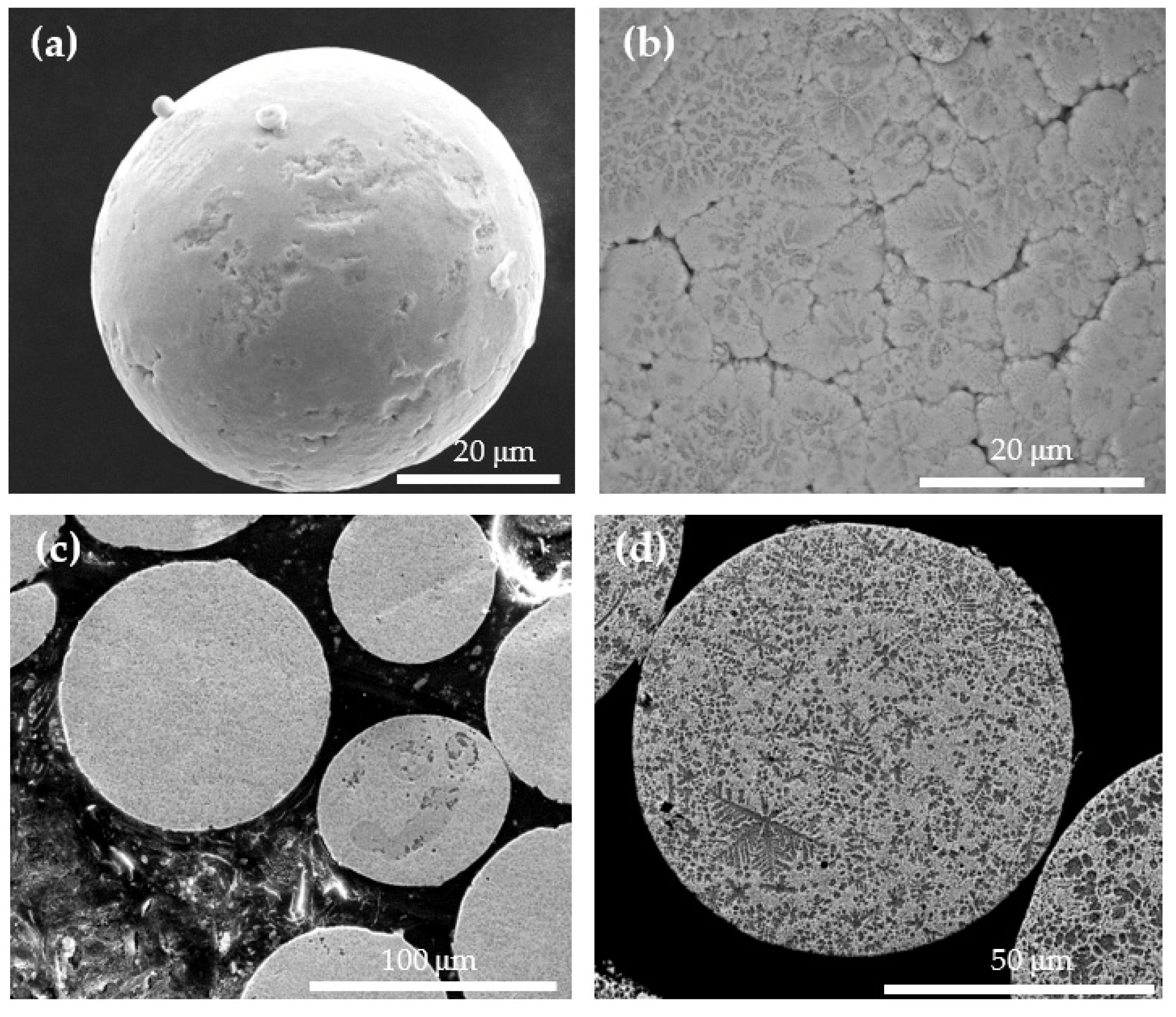
3.1. Characterization of the As-Atomized ZA-8 Powder
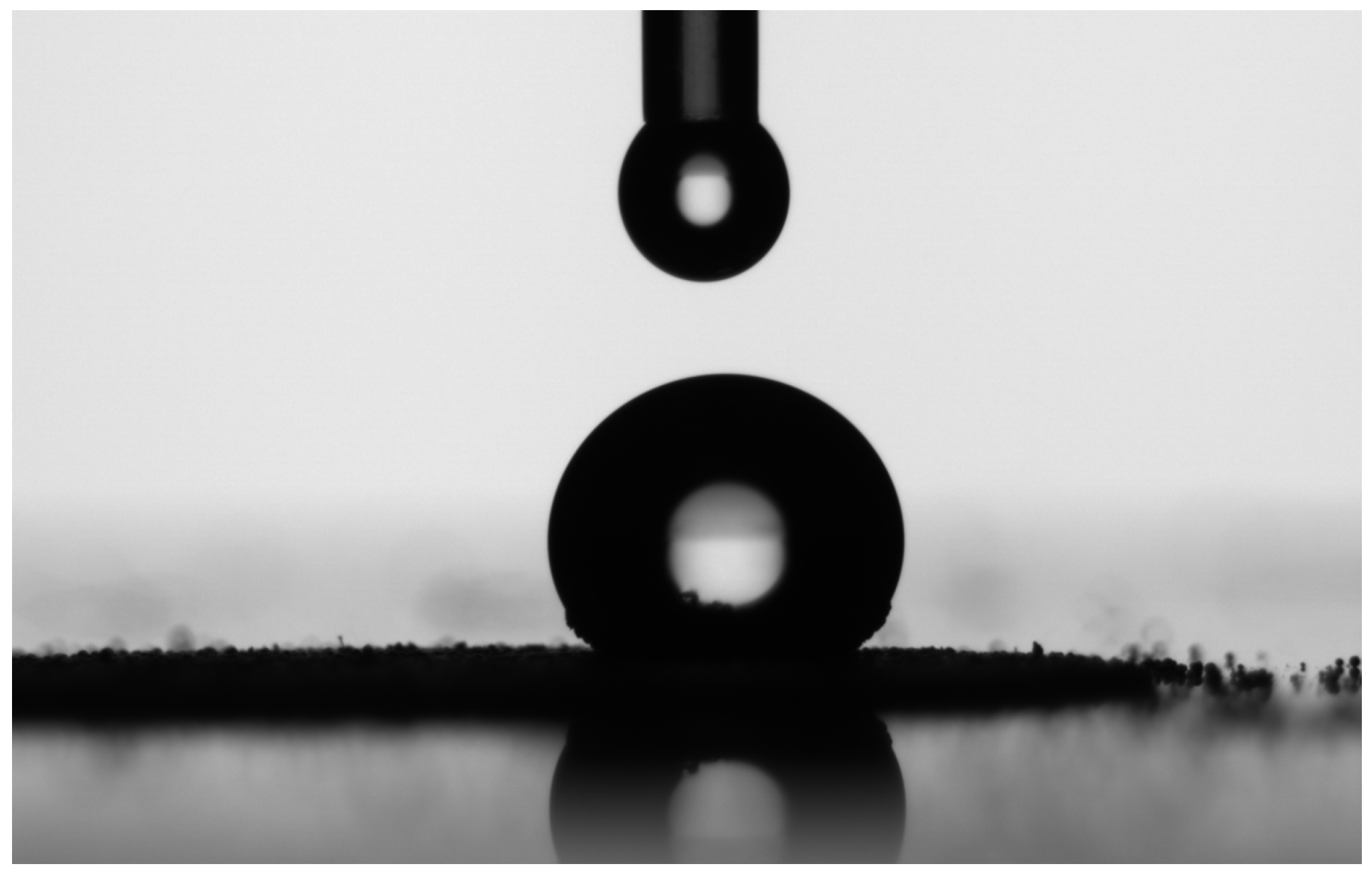
3.2. The SAM-Modified Powder—Contact Angle Measurements
3.3. The Core/Shell ZA-8/Alumina Powder
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grabowski, B.; Tasan, C.C. Self-healing metals. In Self-Healing Materials. Advances in Polymer Science; Hager, M.D., van der Zwaag, S., Schubert, U.S., Eds.; Springer: Basel, Switzerland, 2016; Volume 273, pp. 387–407. [Google Scholar]
- Manuel, M.V. Design of a Biomimetic Self-Healing Alloy Composite. Ph.D. Thesis, Northwestern University, Evanston, IL, USA, 2007. [Google Scholar]
- Misra, S.K. Shape Memory Alloy Reinforced Self-Healing Metal Matrix Composites. Master’s Thesis, The University of Wisconsin-Milwaukee, Milwaukee, WI, USA, 2013. [Google Scholar]
- Ferguson, J.B.; Schultz, B.F.; Rohatgi, P.K. Self-healing metals and metal matrix composites. JOM 2014, 66, 866–871. [Google Scholar] [CrossRef]
- Svetlizky, D.; Zheng, B.; Buta, T.; Zhou, Y.; Golan, O.; Breiman, U.; Haj-Ali, R.; Schoenung, J.M.; Lavernia, E.J.; Eliaz, N. Directed energy deposition of Al 5xxx alloy using Laser Engineered Net Shaping (LENS®). Mater. Des. 2020, 192, 108763. [Google Scholar] [CrossRef]
- Eliaz, N.; Svetlizky, D.; Schoenung, J.; Lavernia, E.; Zhou, Y.; Zheng, B. Deposition of Al 5xxx Alloy Using Laser Engineered Net Shaping. International Patent Application PCT/US2020/065373, 16 December 2020. [Google Scholar]
- Eliaz, N.; Fuks, N.; Geva, D.; Oren, S.; Shriki, N.; Vaknin, D.; Fishman, D.; Levi, O. Comparative quality control of titanium alloy Ti–6Al–4V, 17–4 pH stainless steel, and aluminum alloy 4047 either manufactured or repaired by Laser Engineered Net Shaping (LENS). Materials 2020, 13, 4171. [Google Scholar] [CrossRef]
- Svetlizky, D.; Kazimierczak, H.; Ovadia, B.; Sharoni, A.; Eliaz, N. Electrochemical two-step encapsulation of ZnAl alloy microparticles with a metallic Ni-based shell. Materials 2021, 14, 834. [Google Scholar] [CrossRef]
- Eliaz, N.; Svetlizky, D.; Kazimierczak, H. Method for Forming Functional Coatings. U.S. Provisional Patent 63/093,535, 19 October 2020. [Google Scholar]
- Kawaguchi, T.; Sakai, H.; Sheng, N.; Kurniawan, A.; Nomura, T. Microencapsulation of Zn-Al alloy as a new phase change material for middle-high-temperature thermal energy storage applications. Appl. Energy 2020, 276, 115487. [Google Scholar] [CrossRef]
- Ferguson, J.B.; Schultz, B.F.; Rohatgi, P.K. Zinc alloy ZA-8/shape memory alloy self-healing metal matrix composite. Mater. Sci. Eng. A 2015, 620, 85–88. [Google Scholar] [CrossRef]
- Granta EduPack 2021; Granta Design: Cambridge, UK, 2021.
- Wright, M.C.; Manuel, M.; Wallace, T.; Newman, A.; Brinson, C. Self-Repairing Fatigue Damage in Metallic Structures for Aerospace Vehicles using Shape Memory Alloy Self-Healing (SMASH) Technology. In Proceedings of the LEARN/Seeding Technical Seminar, Webinar, 18–19 March 2015. [Google Scholar]
- Wright, M.C.; Manuel, M.; Wallace, T. Fatigue Resistance of Liquid-Assisted Self-Repairing Aluminum Alloys Reinforced with Shape Memory Alloys; NASA/TM-2013-216629; NASA: Hanover, MD, USA, 2013.
- Eliaz, N.; Gileadi, E. Physical Electrochemistry: Fundamentals, Techniques, and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2019. [Google Scholar]
- Quiñones, R.; Garretson, S.; Behnke, G.; Fagan, J.W.; Mueller, K.T.; Agarwal, S.; Gupta, R.K. Fabrication of phosphonic acid films on nitinol nanoparticles by dynamic covalent assembly. Thin Solid Film. 2017, 642, 195–206. [Google Scholar] [CrossRef]
- Quiñones, R.; Rodriguez, K.; Iuliucci, R.J. Investigation of phosphonic acid surface modifications on zinc oxide nanoparticles under ambient conditions. Thin Solid Film. 2014, 565, 155–164. [Google Scholar] [CrossRef]
- Guerrero, G.; Alauzun, J.G.; Granier, M.; Laurencin, D.; Mutin, P.H. Phosphonate coupling molecules for the control of surface/interface properties and the synthesis of nanomaterials. Dalton Trans. 2013, 42, 12569–12585. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Zhao, X.; Zhang, Y. Sol–gel fabrication of compact, crack-free alumina film. Mater. Res. Bull. 2007, 42, 600–608. [Google Scholar] [CrossRef]
- He, F.; Chao, S.; He, X.; Li, M. Inorganic microencapsulated core/shell structure of Al–Si alloy micro-particles with silane coupling agent. Ceram. Int. 2014, 40, 6865–6874. [Google Scholar] [CrossRef]
- He, F.; Chao, S.; He, X.; Li, M. Comparison of structure and phase change characteristic of microencapsulated core/shell Al–Si alloy microparticles synthesized by two methods. J. Sol-Gel Sci. Technol. 2015, 76, 1–10. [Google Scholar] [CrossRef]
- Levin, I.; Brandon, D. Metastable alumina polymorphs: Crystal structures and transition sequences. J. Am. Ceram. Soc. 1998, 81, 1995–2012. [Google Scholar] [CrossRef]
- Amrute, A.P.; Łodziana, Z.; Schreyer, H.; Weidenthaler, C.; Schüth, F. High-surface-area corundum by mechanochemically induced phase transformation of boehmite. Science 2019, 366, 485–489. [Google Scholar] [CrossRef]
- Carstens, S.; Meyer, R.; Enke, D. Towards macroporous α-Al2O3—Routes, possibilities and limitations. Materials 2020, 13, 1787. [Google Scholar] [CrossRef] [Green Version]
- Peintinger, M.F.; Kratz, M.J.; Bredow, T. Quantum-chemical study of stable, meta-stable and high-pressure alumina polymorphs and aluminum hydroxides. J. Mater. Chem. A 2014, 2, 13143–13158. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Dewil, R.; Appels, L.; Zhang, H.; Li, S.; Baeyens, J. The need to accurately define and measure the properties of particles. Standards 2021, 1, 19–38. [Google Scholar] [CrossRef]
- Li, L.; Iskander, M. Comparison of 2D and 3D dynamic image analysis for characterization of natural sands. Eng. Geol. 2021, 290, 106052. [Google Scholar] [CrossRef]
- ISO 9276-1:1998: Representation of Results of Particle Size Analysis—Part 1: Graphical Representation; ISO: Geneva, Switzerland, 1998.
- Türk, A.; Kurnaz, C.; Şevik, H. Comparison of the wear properties of modified ZA-8 alloys and conventional bearing bronze. Mater. Des. 2007, 28, 1889–1897. [Google Scholar] [CrossRef]
- Purcek, G.; Karaman, I.; Yapici, G.G.; Al-Maharbi, M.; Kuçukomeroglu, T.; Saray, O. Enhancement in mechanical behavior and wear resistance of severe plastically deformed two-phase Zn–Al alloys. Int. J. Mater. Res. 2007, 98, 332–338. [Google Scholar] [CrossRef]
- Zhu, Y.H. General rule of phase decomposition in Zn-Al based alloys (II)—On effects of external stresses on phase transformation. Mater. Trans. 2004, 45, 3083–3097. [Google Scholar] [CrossRef] [Green Version]
- Degtyareva, V.F.; Afonikova, N.S. Simple metal and binary alloy phases based on the fcc structure: Electronic origin of distortions, superlattices and vacancies. Crystals 2017, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Pola, A.; Tocci, M.; Goodwin, F.E. Review of microstructures and properties of Zinc alloys. Metals 2020, 10, 253. [Google Scholar] [CrossRef] [Green Version]
- Nomura, T.; Zhu, C.; Sheng, N.; Saito, G.; Akiyama, T. Microencapsulation of metal-based phase change material for high-temperature thermal energy storage. Sci. Rep. 2015, 5, 9117. [Google Scholar] [CrossRef] [Green Version]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-Ray Photoelectron Spectroscopy Database, Version 4.1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012.
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Duong, L.V.; Wood, B.J.; Frost, R.L. XPS study of the major minerals in bauxite: Gibbsite, bayerite and (pseudo-)boehmite. J. Colloid Interface Sci. 2006, 296, 572–576. [Google Scholar] [CrossRef] [Green Version]
- King, D.E.; Swartz, W.E. Variable-angle X-ray photoelectron spectroscopic determination of the thickness of the oxide layer on aluminum metal: An advanced undergraduate laboratory experiment. J. Chem. Educ. 1987, 64, 981. [Google Scholar] [CrossRef]
- Di Castro, V.; Polzonetti, G.; Contini, G.; Cozza, C.; Paponetti, B. XPS study of MnO2 minerals treated by bioleaching. Surf. Interface Anal. 1990, 16, 571–574. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corp.: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Nguefack, M.; Popa, A.F.; Rossignol, S.; Kappenstein, C. Preparation of alumina through a sol–gel process. Synthesis, characterization, thermal evolution and model of intermediate boehmite. Phys. Chem. Chem. Phys. 2003, 5, 4279–4289. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Kaushik, S.C.; Tyagi, S.K.; Akiyama, T. Development of phase change materials based microencapsulated technology for buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1373–1391. [Google Scholar] [CrossRef]
- Gil, A.; Medrano, M.; Martorell, I.; Lazaro, A.; Dolado, P.; Zalba, B.; Cabeza, L.F. State of the art on high temperature thermal energy storage for power generation. Part 1—Concepts, materials and modellization. Renew. Sustain. Energy Rev. 2010, 14, 31–55. [Google Scholar] [CrossRef]
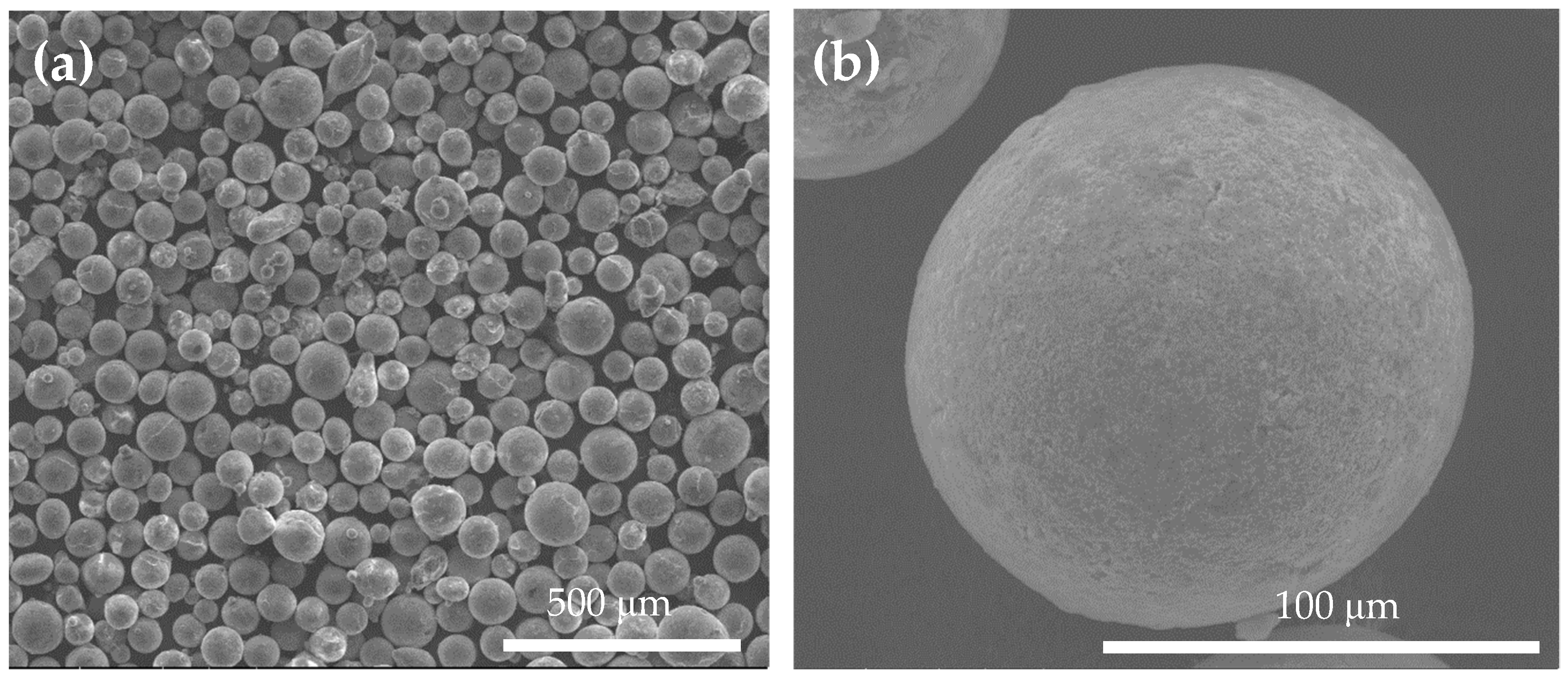

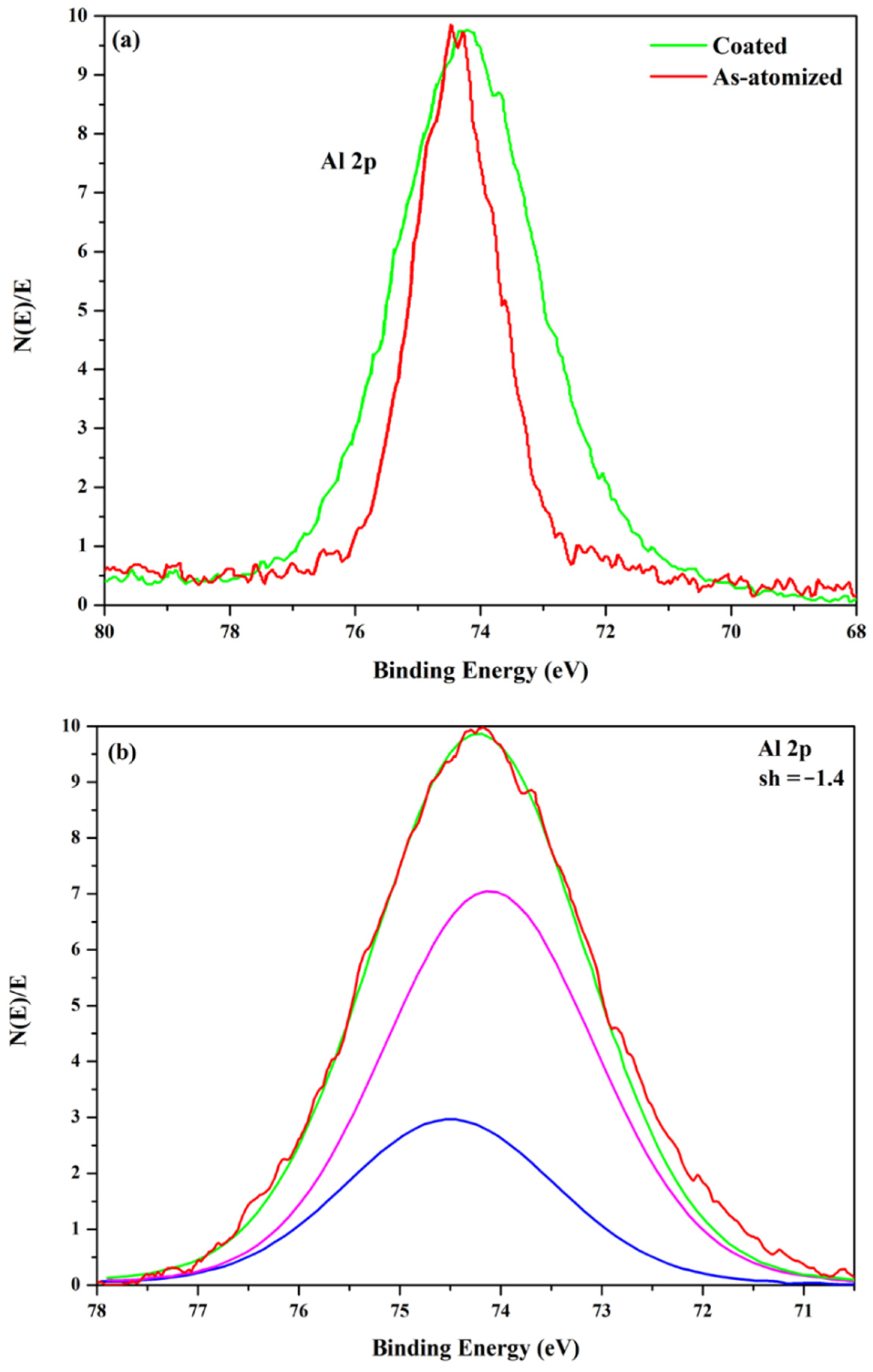
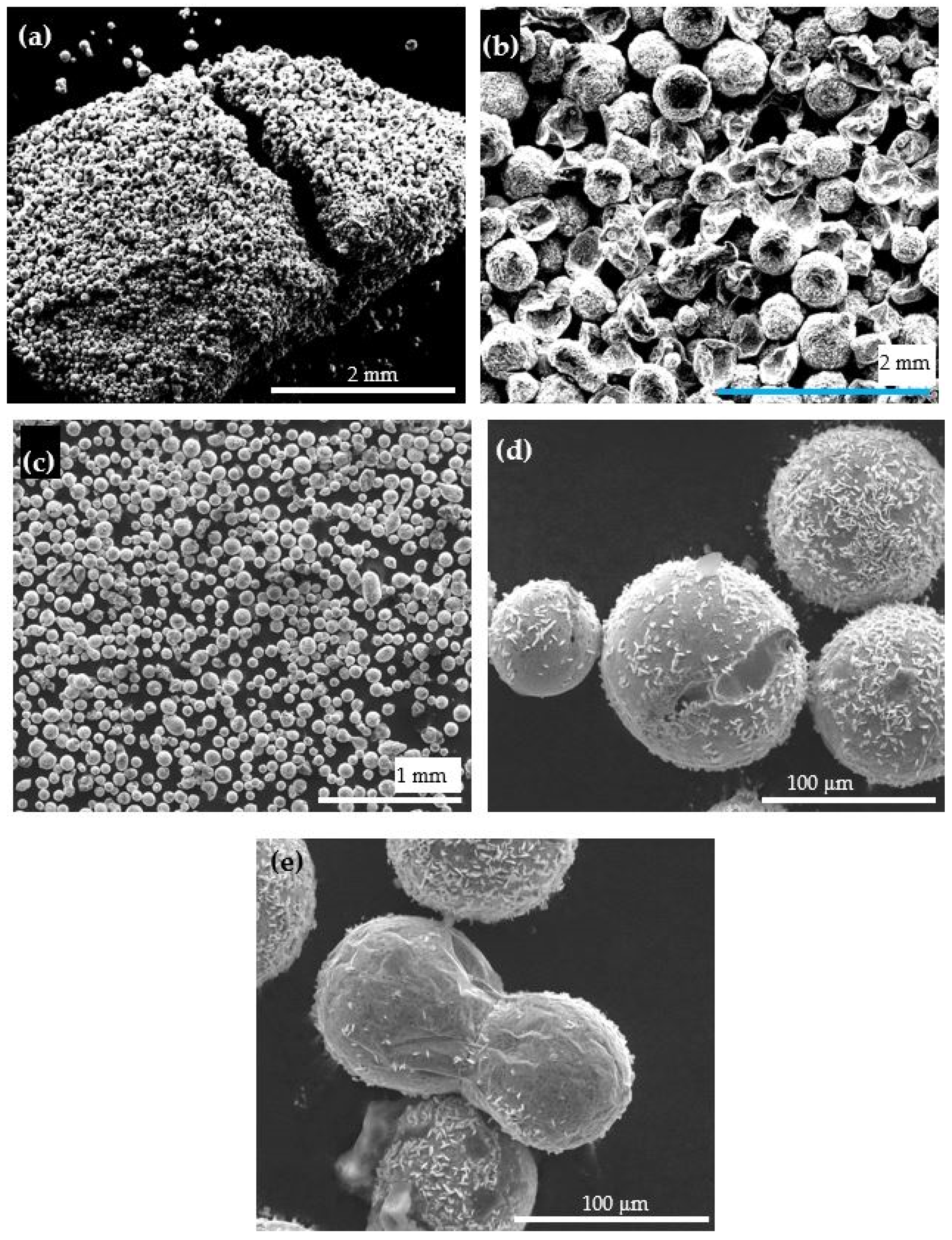
| Property | ZA-8 | Al 5083-O |
|---|---|---|
| Melting point (°C) | 375–404 | 571–637 |
| Tensile stress (MPa) | 220–425 | 248–283 |
| Yield strength (MPa) | 200–290 | 110–131 |
| Fatigue strength (MPa) | 50–105 | 64–67 |
| Young’s modulus (GPa) | 84–88 | 70–73.6 |
| Distribution Type | PSD | Sphericity |
|---|---|---|
| d10 (µm) | 63.57 | 0.69 |
| d50 (µm) | 106.75 | 0.90 |
| d90 (µm) | 163.43 | 0.94 |
| Spectrum | Zn | Al | Cu | Mg | O | C |
|---|---|---|---|---|---|---|
| 1 | 62.19 | 9.20 | 0.90 | 0.90 | 20.18 | 6.64 |
| 2 | 52.63 | 11.64 | 1.95 | 1.01 | 26.55 | 6.21 |
| 3 | 68.43 | 7.76 | 0.86 | - | 18.52 | 4.43 |
| 4 | 85.43 | 2.64 | 1.23 | - | 5.51 | 5.19 |
| 5 | 72.23 | 15.81 | 1.08 | - | 4.93 | 5.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svetlizky, D.; Eliaz, N. Sol–Gel Encapsulation of ZnAl Alloy Powder with Alumina Shell. Coatings 2021, 11, 1389. https://doi.org/10.3390/coatings11111389
Svetlizky D, Eliaz N. Sol–Gel Encapsulation of ZnAl Alloy Powder with Alumina Shell. Coatings. 2021; 11(11):1389. https://doi.org/10.3390/coatings11111389
Chicago/Turabian StyleSvetlizky, David, and Noam Eliaz. 2021. "Sol–Gel Encapsulation of ZnAl Alloy Powder with Alumina Shell" Coatings 11, no. 11: 1389. https://doi.org/10.3390/coatings11111389






