Abstract
Integral regularities in the growth of 7YSZ thermal barrier coatings during MO CVD (Metal–Organic Chemical Vapor Deposition) are proposed. Within the framework of the model of the reacting boundary layer, the coating deposition process is considered as a process of independent global reactions of diffusion combustion of Zr(dpm)4 and Y(dpm)3 under convection conditions on a permeable surface. The rate of coating growth and the efficiency of using a precursor are analytically evaluated. The correctness of the proposed approach is confirmed by comparison with known experimental data. The considered model can be used to analyze the deposition of coatings from various mixtures of precursors, such as Nd(dpm)3, Hf(dpm)4, and Sm(dpm)3.
1. Introduction
Thermal barrier coatings (TBCs) are crucial in satisfying technical requirements in aerospace and energy industries []. Currently, the traditional system ZrO2-Y2O3 (7-8YSZ) remains the most commonly used TBC system, even though many efforts have been devoted to the development of ceramic layers that possess low thermal conductivity and are stable at high temperatures (above 1200 °C) [,]. An equally important task concerns lowering the cost of TBC deposition processes []. Chemical vapor deposition (CVD) is one of the promising, but poorly studied, methods of TBS deposition [].
This technique allows one to obtain a wide range of various functional layers to control coating thickness by varying it widely and to control parameters of deposited layers directly during their formation. This method is widely used when depositing coatings on geometrically complex objects []. The method is based on the process of decomposition of vapors of a chemical compound (precursor), often supplied by inert gas flow, on a heated substrate and in the presence of oxygen in the decomposition zone. The mechanism of coating formation is a result of a number of chemical processes. Several hypotheses have been developed to model the MO CVD (Metal–Organic CVD) process []. The composite process is as follows: precursors beta-diketonate complexes, namely, Zr and Y dipivaloylmethanates (Zr(dpm)4 and Y(dpm)3, respectively; dpm or thd is 2,2,6,6-tetramethylheptane-3,5-dionate), are commonly used for ZrO2 or Y2O3 MO CVD; argon is used as carrier gas; and oxygen is used as gas reactant. However, as noted in [,], a sufficiently high deposition rate occurs even without supplying O2 to the reactor. Coating deposition is governed by a competition of different physical and chemical processes. Thermal decomposition is a key process influenced by many important parameters (e.g., the growth rate and composition of the deposited film). Thermal stability and thermolysis of Y(dpm)3 in vacuum and in oxygen were investigated in []. It is noted that thermolysis of Y(dpm)3 vapors in oxygen begins at 395 °C, while in vacuum, this value is 95 °C higher. This temperature indicates the stability threshold of the precursor. For Zr(dpm)4 in the presence of oxygen, this temperature is 320 °C, according to data in []. It should be noted that the volatility of the above-mentioned Zr and Y precursors differs significantly []. Such a big difference in volatility may affect precursors feeding to the deposition zone.
Deposition of a binary system (mixed oxides) can be complicated not only by different thermal properties of the used precursors but also by the occurrence of possible mutual influence. For example, thermal stability and vaporization kinetics are different for the individual precursors Zr(dpm)4 and Nd(dpm)3 and for their mixture []. In particular, thermal stability for the Zr precursor as a component of the mixture is increased as compared with the individual substance. As shown in [], for Zr(dpm)4 and Y(dpm)3 mixtures of different ratios, the formation of low melting solid solution occurs. Heating these mixtures above their melting point affects the composition of the vapor due to partial removal of a more volatile Y component. This is critical for maintaining the component ratio in the deposited film. In contrast to a Zr–Nd system, the temperatures of decomposition onset for the studied Zr–Y mixtures are not changed as compared to individual precursors. The study of evaporation of Zr(dpm)4 and Y(dpm)3, both during separate heating of individual compounds in the presence of oxygen and during the heating of mixtures, shows that the composition of the gas phase does not change. This indicates that there is no mutual influence of precursor vapors on the surface at temperatures of up to 500 °C.
In regard to the mechanism of thermal decomposition of metal dipivaloylmethanates, the analysis of limited works dealing with data on gaseous decomposition products shows that metal oxides are formed due to a series of intramolecular rearrangements. For example, lead oxide is formed from Pb(dpm)2 by two parallel routes, with diketone Hdpm and different ketones evolving into the gas phase []. It is noted that decomposition occurs on the surface of a thermal reactor but not in the gas phase, since the flow regime of particles is molecular under the experimental conditions. In general, it is the heterogeneous decomposition in the vicinity of a hot substrate that leads to deposition of a smooth uniform film. This forces us to pay special attention to different types of interaction of gas reaction mixture with a substrate.
The authors of [] mention that the interaction of a substrate with gas flow significantly affects the deposition process. It is important that coatings grow uniformly over the entire substrate area. One of the well-proven ways of solving this problem is to use a rotating substrate that is streamlined by a reagent impinging jet. The morphology of the 7YSZ crystallite can be controlled by varying the rate of substrate rotation during deposition []. In this case, the content of phases in the columnar structure of 7YSZ remains almost constant. This approach can be used for flows propagating near a flat wall as well as for a vortex reactor with a rotating fluidized bed of solid particles []. A numerical solution is obtained for a steady thermal flow associated with multi-species and chemical reactions in an atmospheric pressure reactor with an axisymmetrically rotating disk []. It is reported that the final product is formed as a result of heterogeneous reaction, while the reaction scheme includes intermediate reactions of reactants in the gas phase.
There are quite reasonable approaches implying the kinetic representation of heterogeneous oxidation of precursor vapors on the substrate surface in the composite reaction approximation []. In this case, the growth rate of coating thickness can be described by the simplest Arrhenius model, determined by vapor concentration and wall temperature. In the approximation of a one-dimensional model description of a laminar reagent flow around the substrate, the kinetic rate constants of the global reaction of ZrO2 formation were estimated from the experimental growth rates of the films. In [], the coating growth in the MO CVD process was described as taking into account the kinetics of chemical reactions and diffusion transfer of substances in the reacting boundary layer. The mechanisms that control the growth of a coating within the conceptual framework of a reacting boundary layer are analyzed in []. In particular, it is stated that the layer synthesis process is controlled by mass diffusion to the substrate. This work echoes the conclusions of [] that, according to kinetics studies, at high substrate temperatures, the mass transport is the controlling factor.
The authors of [] consider a 2D boundary layer under conditions where hydrogen fuel mixtures diluted by an inert gas are injected into the air flow through a porous plate upon diffusion combustion. The distance between the reaction front and the wall in the reacting boundary layer is uniquely determined by the composition of the chemical system. By means of the approaches describing diffusion combustion in the boundary layer on a permeable surface [], heterogeneous combustion in the MO CVD process can be represented by a condition where the reaction front is located “on the wall”, and the resulting coating is considered as a substance withdrawn from the reaction surface. The purpose of this work is to investigate the applicability of such a model to the processes of thermal barrier coating deposition.
2. Methods
When 7YSZ coatings are deposited on a substrate with a temperature higher than 600 °C, the rate of coating growth stops depending on temperature [,,], indicating a diffusion regime of combustion. The coating growth in the MO CVD process can be viewed as a combustion of the precursor vapor in the oxygen–argon flow. This process proceeds efficiently under conditions of homogeneous reaction without forming metal oxides in the gas phase. This fact can be used as an additional condition when constructing the analytical model. We consider a boundary layer formed by reagent flow around a flat wall (Figure 1).
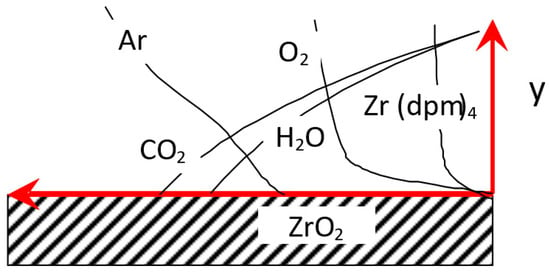
Figure 1.
Flow diagram.
Here, y is the distance normal to the surface. The direction of the flow of reagents will be taken parallel to the surface. The curves qualitatively describe the distributions of basic substances. In the diffusion approximation, the formation of ZrO2 (Y2O3) from a Zr(dpm)4, Y(dpm)3, Ar, and O2 mixture proceeds in the reaction front, according to the following equation of global reaction:
If another inert gas is used instead of argon, it is necessary to set the appropriate value instead of the molar mass of argon. In the approximation of the kinetic regime of combustion on the “wall”, a similar approach is used in []. As shown in [], these two chemical processes proceed independently of each other. By writing the balance of diffusion flows on the wall using the atomic fractions of elements, we obtain [,]
where i corresponds to atomic fraction, corresponds to atomic fractions of elements in solid film, represents the atomic fractions of the elements on the “wall”and is the mass flux of solid film during the MO CVD process. In the Shvab–Zeldovich approximation, all molecular diffusion coefficients D are the same for any pair of substances. The parameter of permeability characterizing convective mass transfer in the reacting boundary layer is
As can be seen from (2), the flow of ZrO2 to the wall is determined by three groups of conditions:
- −
- Composition of the mixture at the reactor inlet;
- −
- Intensity of heat and mass transfer. during convective flow around the substrate surface ;
- −
- Composition of the gas mixture on the “wall”, i.e., for 0.
According to our estimates, the magnitude of under the considered conditions is of the order of unity. For laminar boundary layer on the plate, the Stanton heat transfer number is . ReX is the Reynolds number for a plate. For turbulent flow in the boundary layer, there is the known expression . The vicinity of the critical point of laminar impact flow is described in [] by the expression for Sherwood mass transfer number . Sc is the Schmidt number. The coefficient values (l, m and n) depend on the distance between the nozzle and the substrate surface.
The quantity describing the concentration conditions for the flame front can be estimated from the stoichiometric ratio from global reaction. Within the considered approach, we assume that the resulting solid substance is continuously removed from the gas phase; thus, we assume that a heterogeneous reaction proceeds on the surface. In the boundary layer theory, such problem statement is referred to as the task of heat and mass transfer on an impinged permeable wall with suction. The expression for can be written as
where are corresponding molecular weights; is the oxidizer excess ratio, determined from the composition of the mixture at the inlet to the reactor; and . The ratios obtained above can be extended to other groups of various precursor mixtures, such as Nd(dpm)3, Hf(dpm)4, and Sm(dpm)3. It is only necessary to take into account the corresponding values of the molecular weights of corresponding substances. Similarly, the transition from argon to another inert gas can be taken into account by considering the corresponding values of the molecular weight.
3. Results and Discussion
As noted in [], the deposition of the coating occurs at a sufficiently high rate of . This conclusion is consistent with studies on the mechanism of thermal decomposition of precursors. From the analysis of our studies on the temperature dependence of the gas phase composition of the considered precursors Zr and Y, it follows that in the absence of oxygen, thermal decomposition of adsorbed precursor molecules with the formation of oxides occurs through intramolecular rearrangements, such as migration of the Y-atom from one dpm ligand to another and the C–O bond breaking with release of C(CH3)3C≡CC(O)C(CH3)3 into the gas phase. The main pathway can be represented as follows:
{Zr(dpm)4}ads = ZrO2 + 2H(dpm)↑ + 2(dpm − OH) ↑ and
{Y(dpm)3}ads = Y2O3 + 3H(dpm)↑ + 3(dpm − OH) ↑
where (dpm − OH) is C(CH3)3C≡CC(O)C(CH3)3
Moreover, in the presence of oxygen, the intramolecular decomposition of the precursor also occurs in the reactor, and the oxygen oxidizes the formed primary organic decomposition products; as a result, carbon oxides and water are released. Thus, for metals with high affinity for oxygen, the formation of oxide during thermolysis occurs without participation of oxygen, but its role in the oxidation of organic by-products is obvious. In addition, we can assume its influence on the competition of surface processes (adsorption, nucleation, and film growth) as well as its possible participation in gas-phase reactions.
From Expressions (1)–(3), it follows that the mass flow in the case of CVD process for Zr(dpm)4 and Y(dpm)3 may be written as
For the purpose of accurate comparison of Estimations (4) and (5) with experimental data, it is convenient to neglect the convective interaction of flows with the substrate. For this, we can consider a dimensionless flow, where the scale factor is chosen as the value of the same quantity at the maximum precursor concentration in the considered experiments. Figure 2 shows the dependence of the normalized mass flow of ZrO2 during the deposition of the coating, depending on the molar fraction of precursor vapor at the reactor inlet. The maximum value of Jw at was chosen for normalization. Under experimental conditions, the substrate temperature was quite high; i.e., the growth rate of the coating did not change with increasing substrate temperature. A jet of reagents flowed from above onto the substrate. As can be seen from the data shown in Figure 2, the experimental points from [] are quite accurately described by relation (4), although in the region of low values of the precursor concentration, there is a noticeable discrepancy with the analytical assessment.
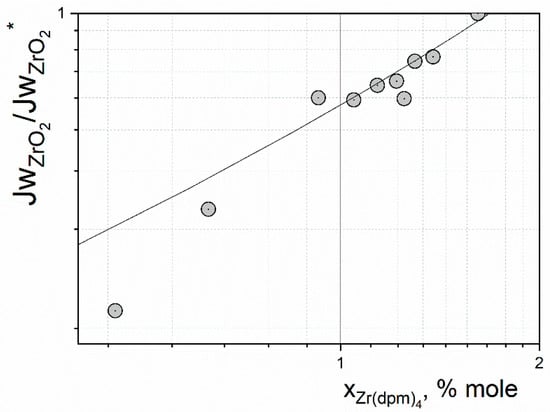
Figure 2.
Comparison of analytical estimation with experimental data of [].
The authors of experimental works [,] noted that the coating growth rate begins to increase as O2 is introduced. It then stabilizes at the oxygen mole fraction in the initial mixture 0.1 … 0.2 and no longer depends on the oxygen content in the initial mixture. Apparently, this is due to the competition of two factors. On the one hand, adding oxygen increases the total gas mixture consumption, thereby increasing the intensity of convective heat and mass transfer. On the other hand, Equation (4) shows that the intensity of ZrO2 flow to the wall decreases with increasing oxygen content.
Using the proposed approach, some theoretical estimates can be made. The mass flow of Zr atoms supplied to the reactor is
where V0 = 22.4 L/mol.
The mass flow of Zr atoms deposited on the coating is
The surface area of substrate S and conditions of convective heat and mass transfer St are very individual characteristics and are not always correctly described in the literature. Therefore, we consider the following normalized consumption factor:
Then
The optimal concentration of Zr(dpm)4 increases together with the oxygen content in the reagent mixture (Figure 3a). The saturation degree of precursor vapors does not affect the coating growth rate (Figure 3b).

Figure 3.
Optimum conditions for coating deposition depending on the precursor mole fraction. (a)–the effect of oxygen content; (b)–the effect of the degree of saturation of the precursor vapor.
For each precursor, the optimal concentration occurs when the maximum possible amount of zirconium (or yttrium) remains in the coating material. In the plots, it corresponds to η* = 1.
The highest growth rate of 108 µm/h in the experiments reported in [] was achieved for a precursor mole fraction of 0.018% and an oxygen content of 25% in the initial mixture, i.e., for the excess oxidant factor . As can be seen, this result also agrees well with the estimates shown in Figure 4. Equation (2) shows that the molecular weight of the initial mixture depends on the type of inert carrier gas. As can be seen from the data presented, the characteristics of vapor deposition of ZrO2 (Figure 4b) and Y2O3 (Figure 4a) are different. It can be expected that with an intensive growth of the thermal barrier coating, the Y/Zr ratio in the initial mixture may differ from the composition of the solid film.
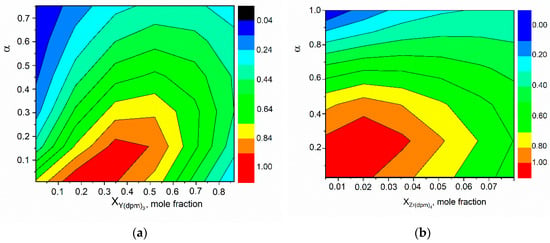
Figure 4.
Optimum conditions for coating deposition depending on the mole fraction of precursor and the factor of oxygen excess. (a)–Y(dpm)3 vapor deposition, (b)–Zr(dpm)4 vapor deposition.
4. Conclusions
The proposed estimates are obtained using rather serious assumptions: the diffusion approximation of the global reaction and probably the most simplified model of diffusion transfer processes based on similarity. However, they adequately describe the known experimental facts and, importantly, do not depend on the specifics of a particular precursor. The growth of the thermal barrier coating in the MO CVD process in the presence of oxygen is determined by two factors: the intensity of convective heat and mass transfer on the substrate surface, and the composition of the gas mixture on the “wall”, which can be estimated using the condition of the global combustion reaction of the precursor on the wall. In the absence of oxygen, it appears to be the defining mechanism of thermal decomposition of precursors. The most efficient precursor concentration depends on both the type of precursor and the type of carrier gas. The coating growth rates and concentration ranges, corresponding to optimal deposition conditions, are different for precursors Me1(dpm)4 and Me2(dpm)3. In particular, this can lead to the ratio Me1/Me2 in the coating differing from the ratio of precursors in the initial mixture of reagents.
Author Contributions
Conceptualization, V.V.L. and I.K.I.; methodology, V.V.L. and A.E.T.; validation, V.V.L. and I.K.I.; investigation, resources, data curation, writing—original draft preparation, A.E.T., V.V.L. and I.K.I.; writing—review and editing, A.E.T., V.V.L. and I.K.I. All authors have read and agreed to the published version of the manuscript.
Funding
The analysis of chemical mechanisms of coating formation as a result of thermal decomposition was funded by the Ministry of Science and Higher Education of the Russian Federation, No. 121031700314-5. The integral estimations of coating growth rates and the efficiency of precursor consumption were supported by the Ministry of Science and Higher Education of the Russian Federation (mega-grant 075-15-2021-575).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morumpalle, S.S.; Giridharar, G.; Kumar, S. Development and analysis of thermal barrier coatings on gas turbine blades—A Review. Mater. Today Proc. 2018, 5, 2746–2751. [Google Scholar]
- Lee, K. Protective coatings for gas turbines. In The Gas Turbine Handbook; National Energy Technology Laboratory: Pittsburgh, PA, USA, 2005; pp. 419–437. [Google Scholar]
- Zhang, X.; Deng, Z.; Li, H.; Mao, J.; Deng, C.; Deng, C.; Niu, S.; Chen, W.; Song, J.; Fan, J.; et al. Al2O3-modified PS-PVD 7YSZ thermal barrier coatings for advanced gas-turbine engines. Mater. Degrad. 2020, 4, 31. [Google Scholar] [CrossRef]
- Feuerstein, A. Technical and Economical Aspects of Current Thermal Barrier Coating Systems for Gas Turbine Engines by Thermal Spray and EBPVD: A Review. J. Therm. Spray Technol. 2008, 17, 199–213. [Google Scholar] [CrossRef]
- Hitchman, M.L.; Jensen, K.F. (Eds.) Chemical Vapor Deposition: Principles and Applications; Academic Press: London, UK, 1993. [Google Scholar]
- Demir, H. The Effects on Thermal Efficiency of Yttria-Stabilized Zirconia and Lanthanum Zirconate-Based Thermal Barrier Coatings on Aluminum Heating Block for 3D Printer. Coatings 2021, 11, 792. [Google Scholar] [CrossRef]
- Kleijn, C.R.; Dorsman, R.; Kuijlaars, K.J.; Okkerse, M.; Santen, H. Multi-scale modeling of chemical vapor deposition processes for thin film technology. J. Cryst. Growth 2007, 303, 362–380. [Google Scholar] [CrossRef]
- Akiyama, Y.; Sato, T.; Imaishi, N. Reaction analysis for ZrO2 and Y2O3 film growth by low-pressure metal-organic chemical vapor deposition using b-diketonate complexes. J. Cryst. Growth 1995, 147, 130–146. [Google Scholar] [CrossRef]
- Tu, R.; Kimura, T.; Goto, T. High-speed deposition of yttria stabilized zirconia by MOCVD. Surf. Coat. Technol. 2004, 187, 238–244. [Google Scholar] [CrossRef]
- Bykov, A.F.; Semyannikov, P.P.; Igumenov, I.K. Mass spectrometric study of gas-phase thermal stability of yttrium(III) tris(dipivaloylmethanate). J. Thermal Anal. 1992, 38, 1447–1486. [Google Scholar] [CrossRef]
- Turgambaeva, A.; Zherikova, K.; Mosyagina, S.; Igumenov, I. A Study of the Thermal Behavior of the System of Zirconium and Neodymium Dipivaloylmethanates. Russ. J. Appl. Chem. 2017, 90, 1062–1067. [Google Scholar] [CrossRef]
- Zelenina, L.; Chusova, T.; Zherikova, K. Thermal study of CVD metal-organic precursors. J Anal. Calorim. 2017, 133, 1157–1165. [Google Scholar] [CrossRef]
- Turgambaeva, A.; Zherikova, K.; Mosyagina, S.; Krisyuk, V.; Lukashov, V.; Igumenov, I.K. Thermal Behavior of Mixtures of Zirconium(IV) and Yttrium(III) Dipivaloylmethanates. Rus. J. Appl. Chem. 2021, 94, 450–456. [Google Scholar] [CrossRef]
- Krisyuk, V.; Turgambaeva, A.; Igumenov, I. Volatile lead β-diketonates as CVD precursors. Chem. Vap. Depos. 1998, 4, 43–46. [Google Scholar] [CrossRef]
- Abedi, S.; Farhadi, F.; Boozarjomehry, R.B. Integration of CFD and Nelder—Mead algorithm for optimization of MOCVD process in an atmospheric pressure vertical rotating disk reactor. Int. Communic. Heat Mass Transf. 2013, 43, 138–145. [Google Scholar] [CrossRef]
- Park, C.; Choi, S.; Chae, J.; Kim, S.; Kim, H.; Oh, Y.-S. Effect of Substrate Rotation on the Phase Evolution and Microstructure of 8YSZ Coatings Fabricated by EB-PVD. J. Korean Ceram. Soc. 2016, 53, 81–86. [Google Scholar] [CrossRef]
- Liu, S.S.; Xiao, W.D. CFD-PBM coupled simulation of silicon CVD growth in a fluidized bed reactor: Effect of silane pyrolysis kinetic models. Chem. Eng. Sci. 2015, 127, 84–94. [Google Scholar] [CrossRef]
- Si, J.; Desu, S.B. Metal-organic chemical vapor deposition of ZrO2 films using Zr(thd)4 as precursors. J. Mater. Res. 1994, 9, 1208–1212. [Google Scholar] [CrossRef]
- de Croon, M.H.; Giling, L.J. Chemical boundary layers in (MO)CVD. Prog. Cryst. Growth Charact. 1989, 19, 125–136. [Google Scholar] [CrossRef]
- Sawka, A. ZrO2-Sm2O3 Layer Growth Using the MOCVD Method at Low Temperatures and Under Reduced Pressure. Coatings 2020, 10, 1126. [Google Scholar] [CrossRef]
- Aghajani, H.; Hoseini, N.; Mirzakhani, B. Deposition kinetics and boundary layer theory in the chemical vapor deposition of β-sic on the surface of c/c composite. Mater. Phys. Mech. 2020, 44, 34–47. [Google Scholar]
- Volchkov, E.P.; Lukashov, V.V.; Terekhov, V.V.; Hanjalic, K. Characterization of the flame blow-off conditions in a laminar boundary layer with hydrogen injection. Combust. Flame 2013, 160, 1999–2009. [Google Scholar] [CrossRef]
- Nemetz, W. Chemical Vapour Deposition of Thermal Barrier Coatings on Turbine Blades; Shaker Verlag: Aachen, Germany, 2005. [Google Scholar]
- Pulver, M.; Nemetz, W.; Wahl, G. CVD of ZrO2, Al2O3 and Y2O3 from metalorganic compounds in different reactors. Surf. Coat. Technol. 2000, 125, 400–406. [Google Scholar] [CrossRef]
- Law Chung, K. Combustion Physics; University Press: Cambridge, UK, 2006. [Google Scholar]
- Chin, D.-T.; Tsang, C.-H. Mass transfer at Impinging jet Electrode. J. Elecrochem. Soc. 1978, 152, 1461–1470. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).