Clinical Application of Silver Nanoparticles Coated by Benzalkonium Chloride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Green Synthesis of AgNPs
2.3. Physico-Chemical Characterization of the Synthesized AgNPs
2.4. Antibacterial Assays
3. Results and Discussion
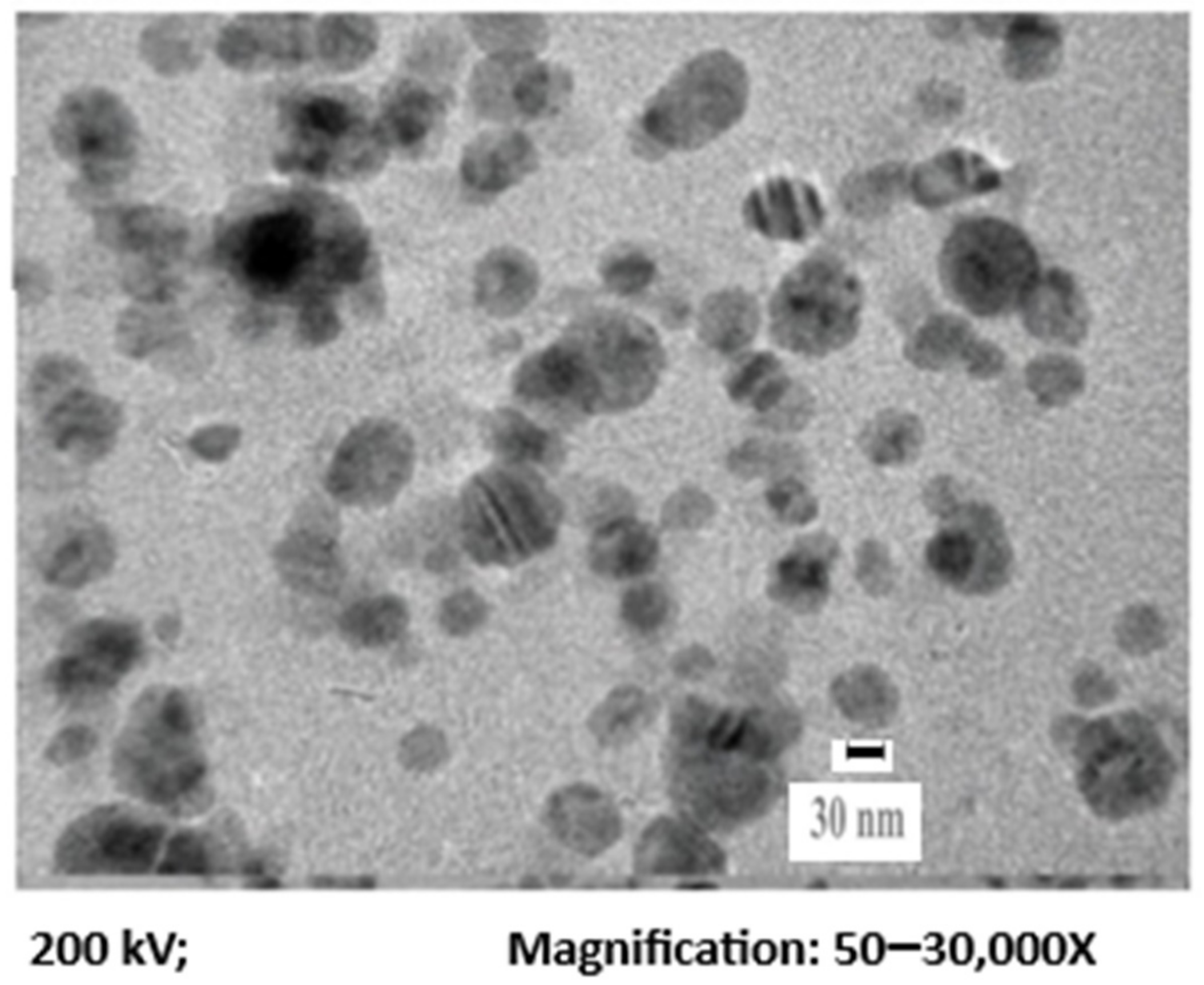
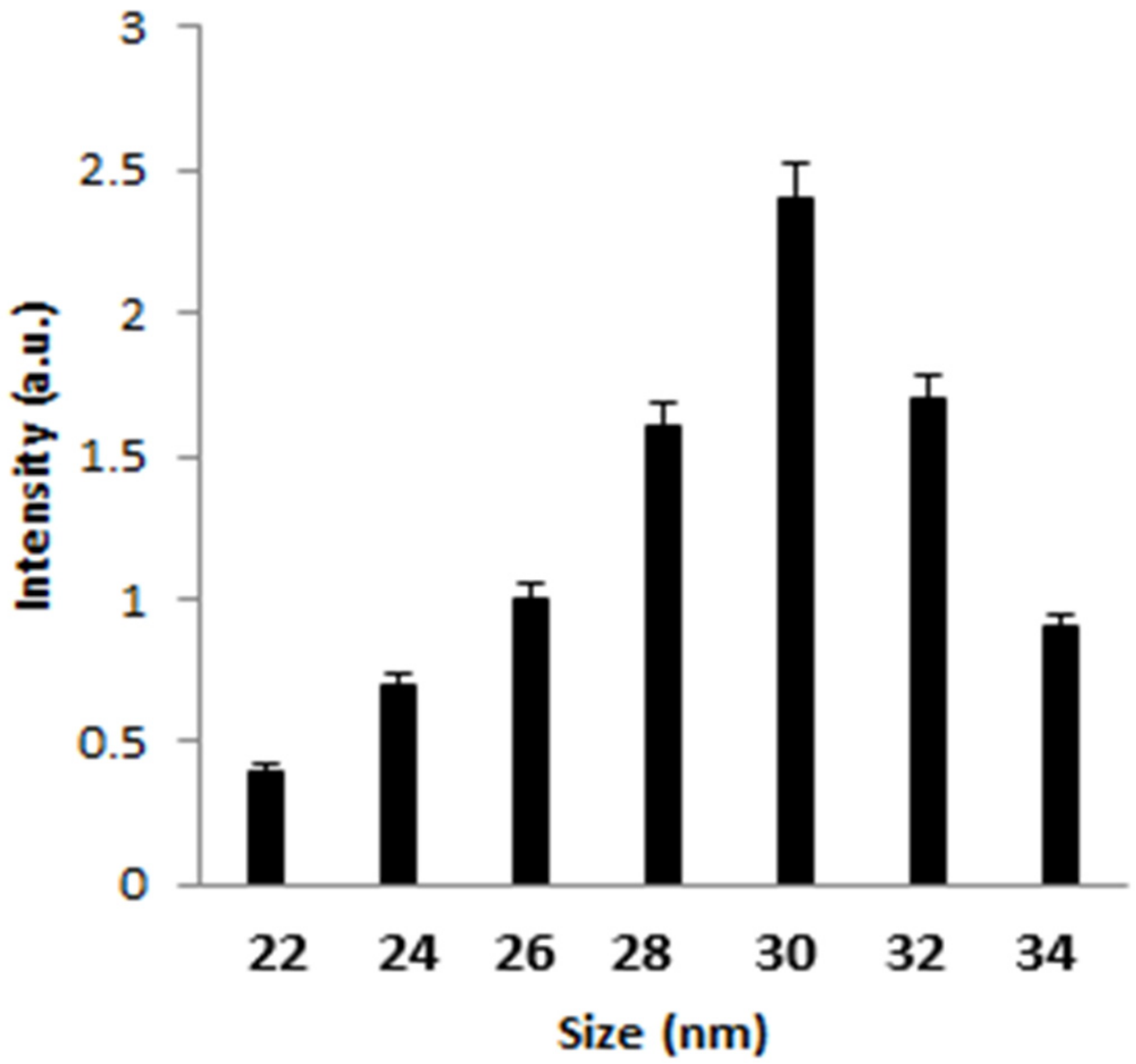
3.1. Physico-Chemical Characterization of the Synthesized AgNPs
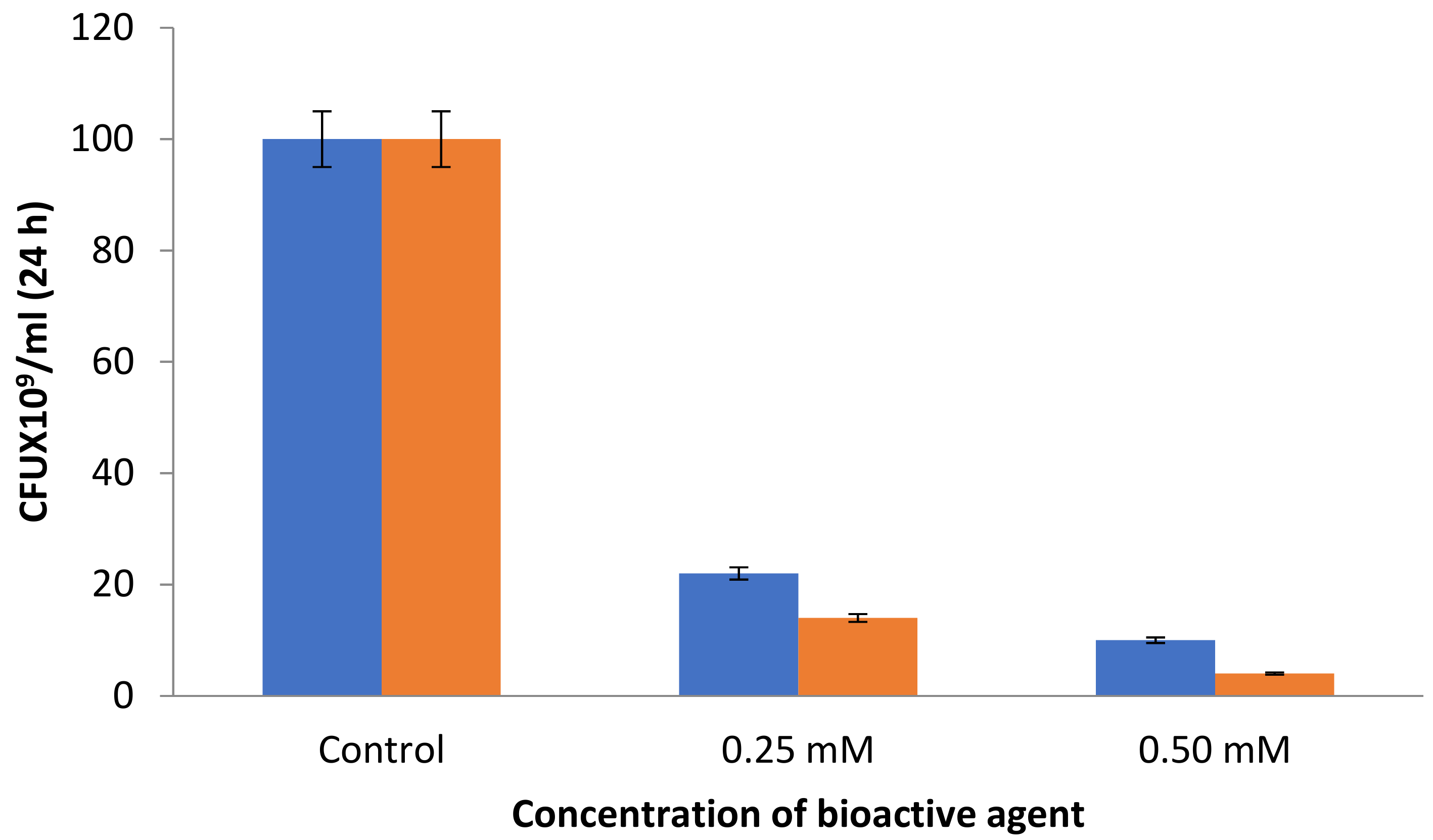
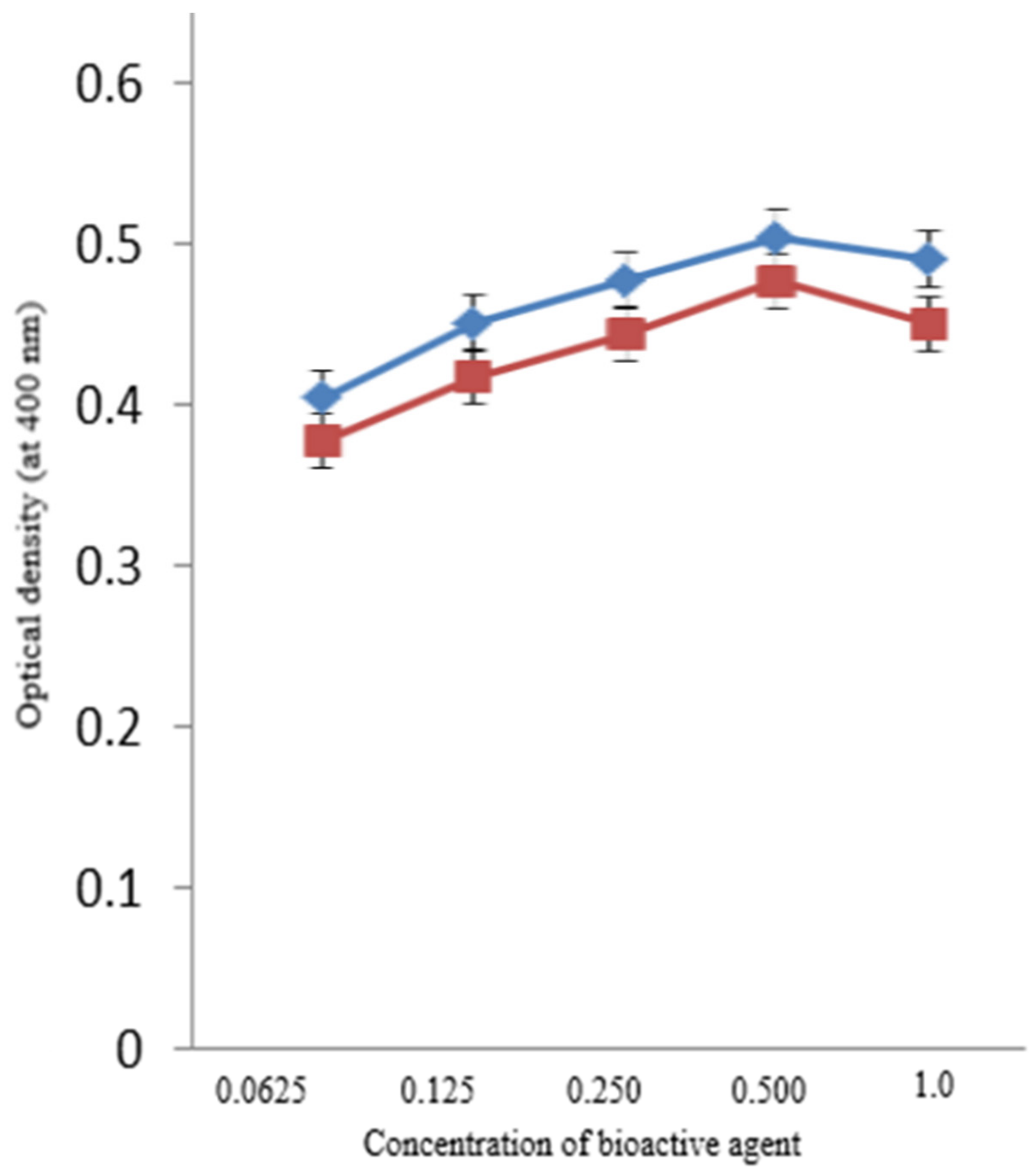
3.2. Antibacterial Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BAC | benzalkonium chloride |
| CFU | colony-forming units |
| ZOI | zone of inhibition |
References
- Nikaido, H. Multidrug resistance in bacteria. Ann. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef] [Green Version]
- Talebian, S.; Wallace, G.G.; Schroeder, A.; Stellacci, F.; Conde, J. Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat. Nanotechnol. 2020, 15, 618–621. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Varier, K.M.; Gudeppu, M.; Chinnasamy, A.; Thangarajan, S.; Balasubramanian, J. Nanoparticles: Antimicrobial applications and its prospects. In Advanced Nanostructured Materials for Environmental Remediation; Environmental Chemistry for a Sustainable World; Naushad, M., Rajendran, S., Gracia, F., Eds.; Springer: Cham, Switzerland, 2019; Volume 25, pp. 321–355. [Google Scholar]
- Li, D.; Zhou, B.; Lv, B. Antibacterial therapeutic agents composed of functional biological molecules. J. Chem. 2020, 2020, 6578579. [Google Scholar] [CrossRef] [Green Version]
- Hooper, D.C. Mechanisms of action of antimicrobials: Focus on fluoroquinolones. Clin. Infect. Dis. 2001, 32, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Bazaka, K.; Jacob, M.V.; Chrzanowski, W.; Ostrikov, K. Anti-bacterial surfaces: Natural agents, mechanisms of action, and plasma surface modification. RSC Adv. 2015, 5, 48739–48759. [Google Scholar] [CrossRef] [Green Version]
- Herman, A.; Herman, A.P. Nanoparticles as antimicrobial agents: Their toxicity and mechanisms of action. J. Nanosci. Nanotechnol. 2014, 14, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Almatar, M.; Makky, E.A.; Var, I.; Koksal, F.; Var, I. The role of nanoparticles in the inhibition of multidrug-resistant bacteria and biofilms. Curr. Drug Deliv. 2018, 15, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Gudikandula, K.; Maringanti, S.C. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. 2016, 11, 714–721. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [Green Version]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14–22. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications–a review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Islam, A.; Jacob, M.V.; Antunes, E. A critical review on silver nanoparticles: From synthesis and applications to its mitigation through low-cost adsorption by biochar. J. Environ. Manag. 2021, 281, 111918–111932. [Google Scholar] [CrossRef] [PubMed]
- Syafiuddin, A.; Salmiati; Salim, M.R.; Kueh, A.B.H.; Hadibarata, T.; Nur, H. A review of silver nanoparticles: Research trends, global consumption, synthesis, properties, and future challenges. J. Chin. Chem. Soc. 2017, 64, 732–756. [Google Scholar] [CrossRef]
- Verma, A.; Gautam, S.P.; Bansal, K.K.; Prabhakar, N.; Rosenholm, J.M. Green Nanotechnology: Advancement in phytoformulation research. Medicines 2019, 6, 39–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, J.K.; Baek, K.-H. Green nanobiotechnology: Factors affecting synthesis and characterization techniques. J. Nanomater. 2014, 2014, 417305. [Google Scholar] [CrossRef] [Green Version]
- Ansari, S.A.; Satar, R.; Alam, F.; Alqahtani, M.H.; Chaudhary, A.G.; Naseer, M.I.; Karim, S.; Sheikh, I.A. Cost effective surface functionalization of silver nanoparticles for high yield immobilization of Aspergillus oryzae β-galactosidase and its application in lactose hydrolysis. Proc. Biochem. 2012, 47, 2427–2433. [Google Scholar] [CrossRef]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S.M.; et al. Green nanotechnology: A review on green synthesis of silver nanoparticles—An ecofriendly approach. Int. J. Nanomed. 2019, 14, 5087–5107. [Google Scholar] [CrossRef] [Green Version]
- Javed, R.; Zia, M.; Naz, S.; Asida, S.O.; Ain, N.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 172–185. [Google Scholar] [CrossRef]
- Verma, A.; Mehata, M.S. Controllable synthesis of silver nanoparticles using Neem leaves and their antimicrobial activity. J. Rad. Res. Appl. Sci. 2016, 9, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, P.; Satapathy, M.; Mukhopahayay, A.; Das, P. Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: Synthesis, characterization, antimicrobial property and toxicity analysis. Biores. Bioproc. 2014, 1, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Saware, K.; Venkataraman, A. Biosynthesis and Characterization of Stable Silver Nanoparticles Using Ficus religiosa Leaf Extract: A Mechanism Perspective. J. Clust. Sci. 2014, 25, 1157–1171. [Google Scholar] [CrossRef]
- Goudarzi, M.; Mir, N.; Mehdi, M.K.; Bagheri, S.; Mosaoud, S.N. Biosynthesis and characterization of silver nanoparticles prepared from two novel natural precursors by facile thermal decomposition methods. Sci. Rep. 2016, 6, 32539–32549. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Kim, M.; Weigand, M.R.; Oh, S.; Hatt, J.K.; Krishnan, R.; Tezel, U.; Pavlostathis, S.G.; Konstantinidis, K.T. Widely used benzalkonium chloride disinfectants can promote antibiotic resistance. Appl. Environ. Microbiol. 2018, 84, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ansari, S.A.; Oves, M.; Satar, R.; Khan, A.; Ahmad, S.I.; Jafri, M.A.; Zaidi, S.K.; Alqahtani, M.H. Antibacterial activity of iron oxide nanoparticles synthesized by co-precipitation technology against Bacillus cereus and Klebsiella pneumoniae. Pol. J. Chem. Technol. 2017, 19, 110–115. [Google Scholar] [CrossRef] [Green Version]
- Khajav, R.; Sattar, M.; Ashjaran, A. The antimicrobial effect of benzalkonium chloride on some pathogenic microbes observed on fibers of acrylic carpet. Pak. J. Biol. Sci. 2007, 10, 598–601. [Google Scholar] [CrossRef]
- Mazur, P.; Skiba-Kurek, I.; Mrowiec, P.; Karczewska, E.; Drozdz, R. Synergistic ROS-associated antimicrobial activity of silver nanoparticles and gentamicin against staphylococcus epidermidis. Int. J. Nanomed. 2020, 15, 3551–3562. [Google Scholar] [CrossRef] [PubMed]
- Krce, L.; Sprung, M.; Roncevic, T.; Maravic, A.; Culic, V.C.; Blazeka, D.; Krstulovic, N.; Aviani, I. Probing the mode of antibacterial action of silver nanoparticles synthesized by laser ablation in water: What fluorescence and AFM data tell us. Nanomaterials 2020, 10, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Koschevic, M.T.; de Araujo, R.P.; dos Santos Garcia, V.A.; Fakhouri, F.M.; de Oliveira, K.M.P.; Arruda, A.J.; Dufresne, A.; Martelli, S.M. Antimicrobial activity of bleached cattail fibers (Typha domingensis) impregnated with silver nanoparticles and benzalkonium chloride. J. Appl. Polym. Sci. 2021, 138, 55–67. [Google Scholar] [CrossRef]
| Concentration (mM) | E. coli | B. subtilis | P. aeruginosa | S. pneumonia | ||||
|---|---|---|---|---|---|---|---|---|
| AgNPs | BAC–AgNPs | AgNPs | BAC–AgNPs | AgNPs | BAC–AgNPs | AgNPs | BAC–AgNPs | |
| 0.25 | 3.45 ± 0.85 | 3.56 ± 0.54 | 4.28 ± 0.39 | 4.40 ± 0.58 | 3.36 ± 1.2 | 3.47 ± 1.34 | 3.44 ± 0.96 | 3.62 ± 0.68 |
| 0.50 | 4.28 ± 0.73 | 4.40 ± 0.68 | 4.36 ± 0.98 | 4.44 ± 0.77 | 4.28 ± 0.98 | 4.37 ± 1.55 | 3.78 ± 0.27 | 3.91 ± 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, S.A.; Alshanberi, A.M. Clinical Application of Silver Nanoparticles Coated by Benzalkonium Chloride. Coatings 2021, 11, 1382. https://doi.org/10.3390/coatings11111382
Ansari SA, Alshanberi AM. Clinical Application of Silver Nanoparticles Coated by Benzalkonium Chloride. Coatings. 2021; 11(11):1382. https://doi.org/10.3390/coatings11111382
Chicago/Turabian StyleAnsari, Shakeel Ahmed, and Asim Muhammed Alshanberi. 2021. "Clinical Application of Silver Nanoparticles Coated by Benzalkonium Chloride" Coatings 11, no. 11: 1382. https://doi.org/10.3390/coatings11111382
APA StyleAnsari, S. A., & Alshanberi, A. M. (2021). Clinical Application of Silver Nanoparticles Coated by Benzalkonium Chloride. Coatings, 11(11), 1382. https://doi.org/10.3390/coatings11111382





