Quaternary Holey Carbon Nanohorns/SnO2/ZnO/PVP Nano-Hybrid as Sensing Element for Resistive-Type Humidity Sensor
Abstract
:1. Introduction
2. Materials and Methods
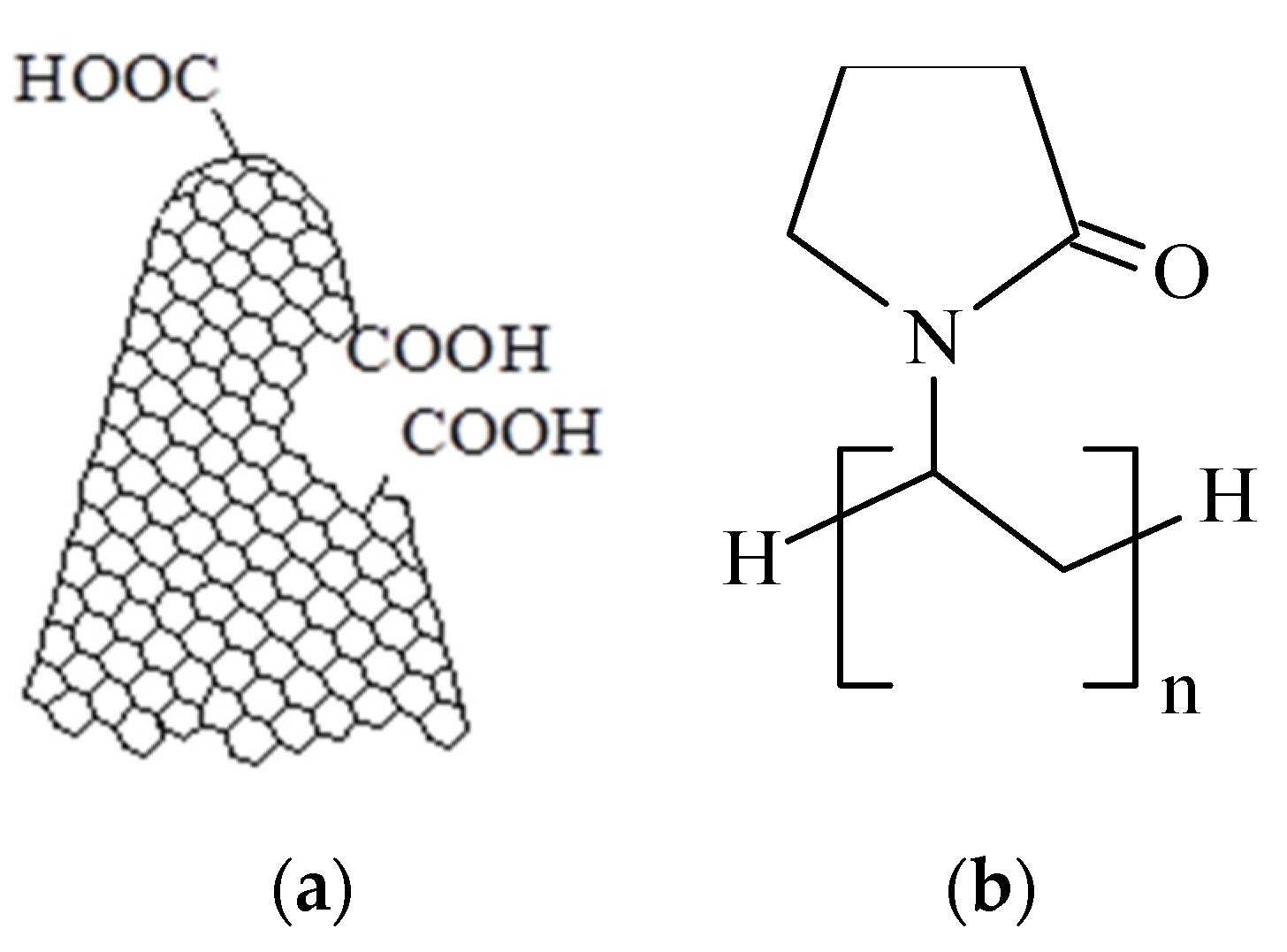
Materials
3. Results and Discussions
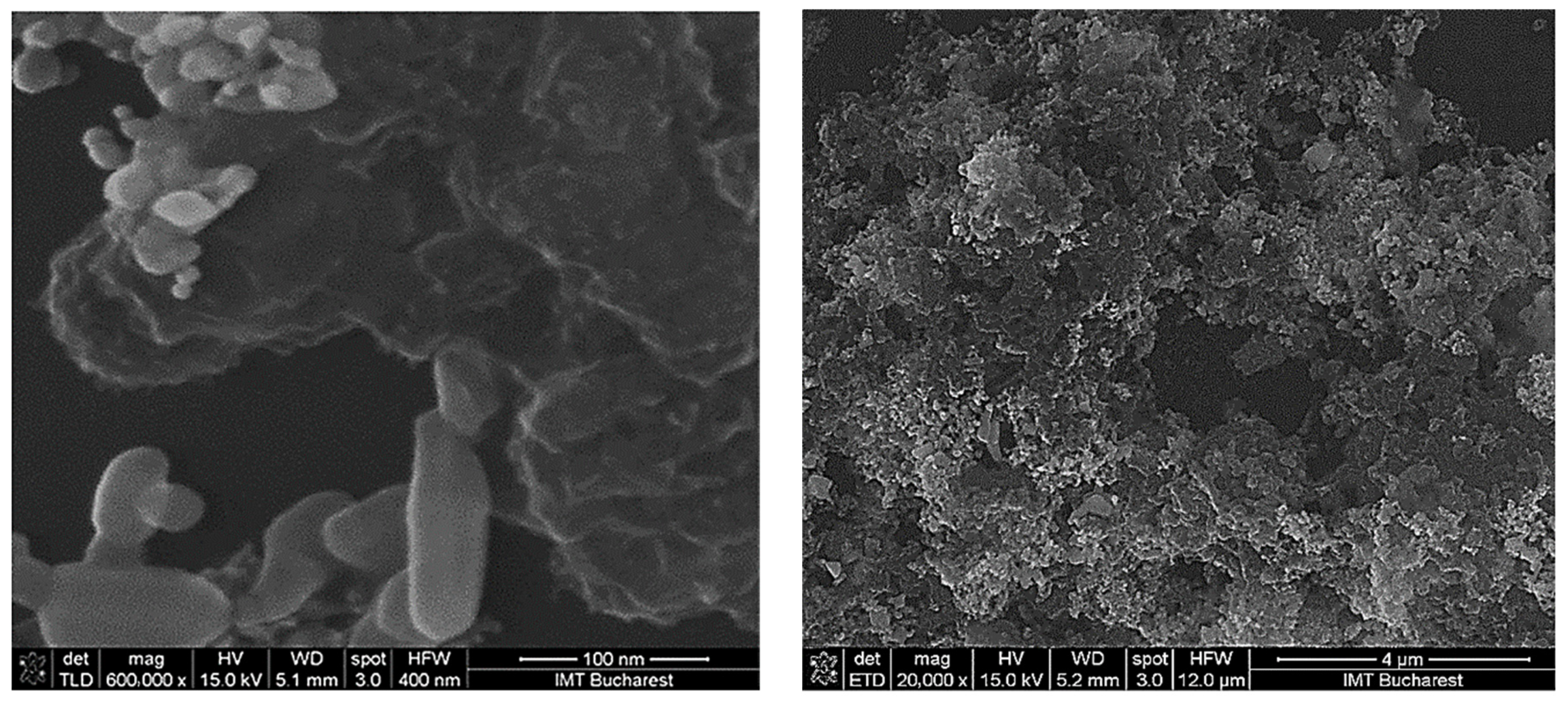
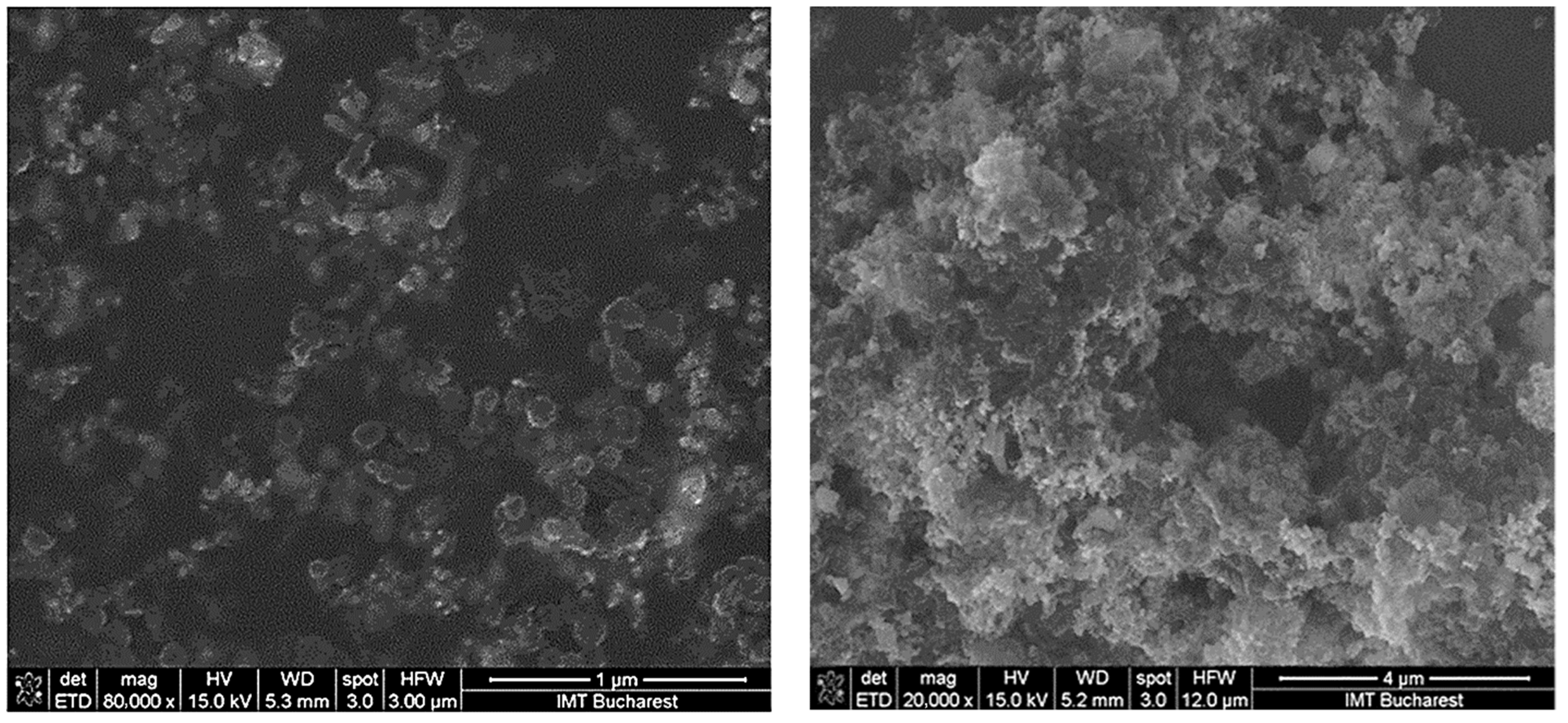
3.1. Surface Topography
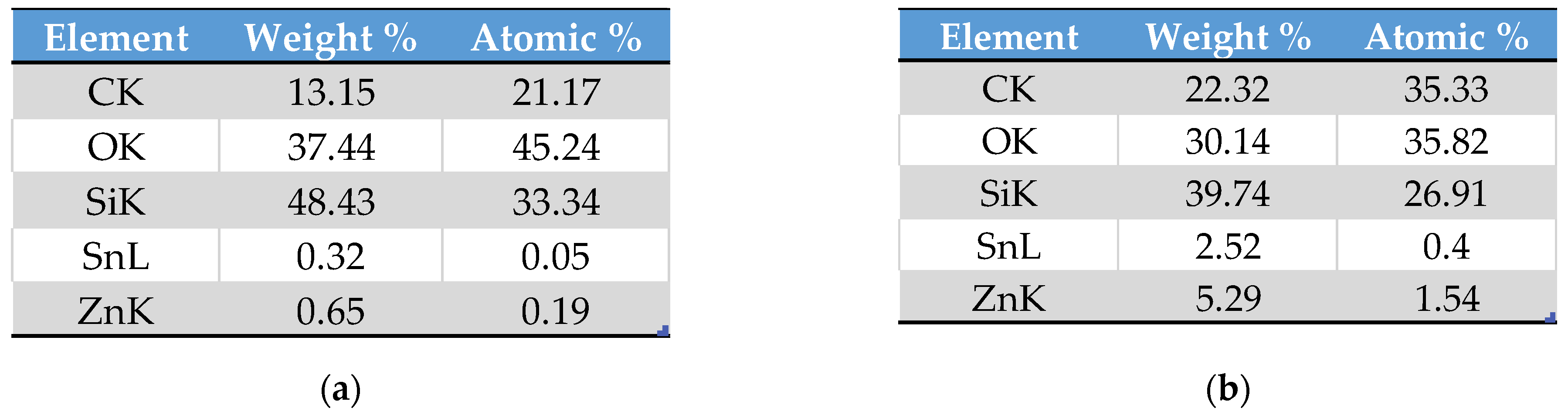
3.2. Surface Composition
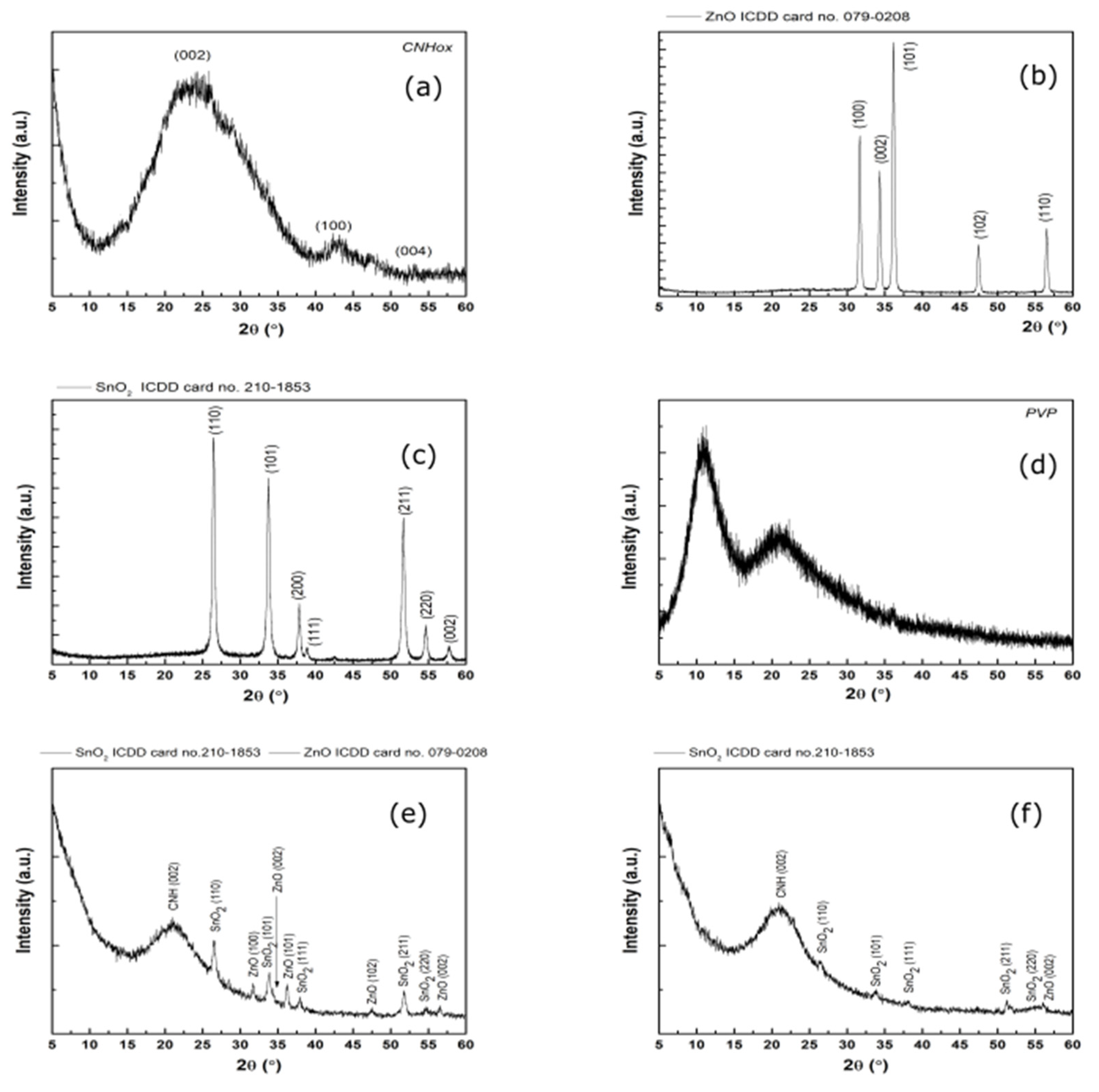
3.3. X-ray Diffraction Results
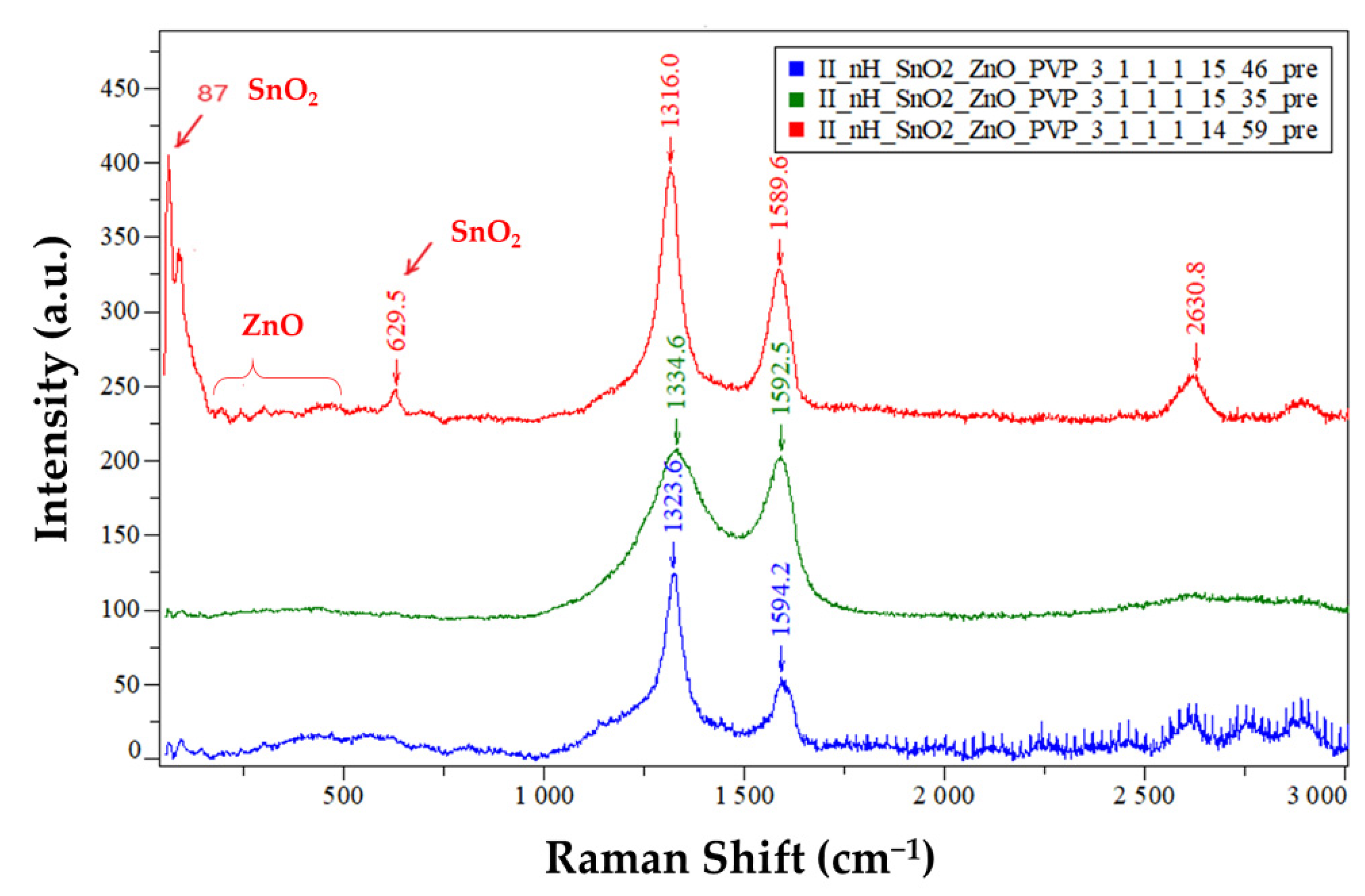
3.4. Raman Spectroscopy
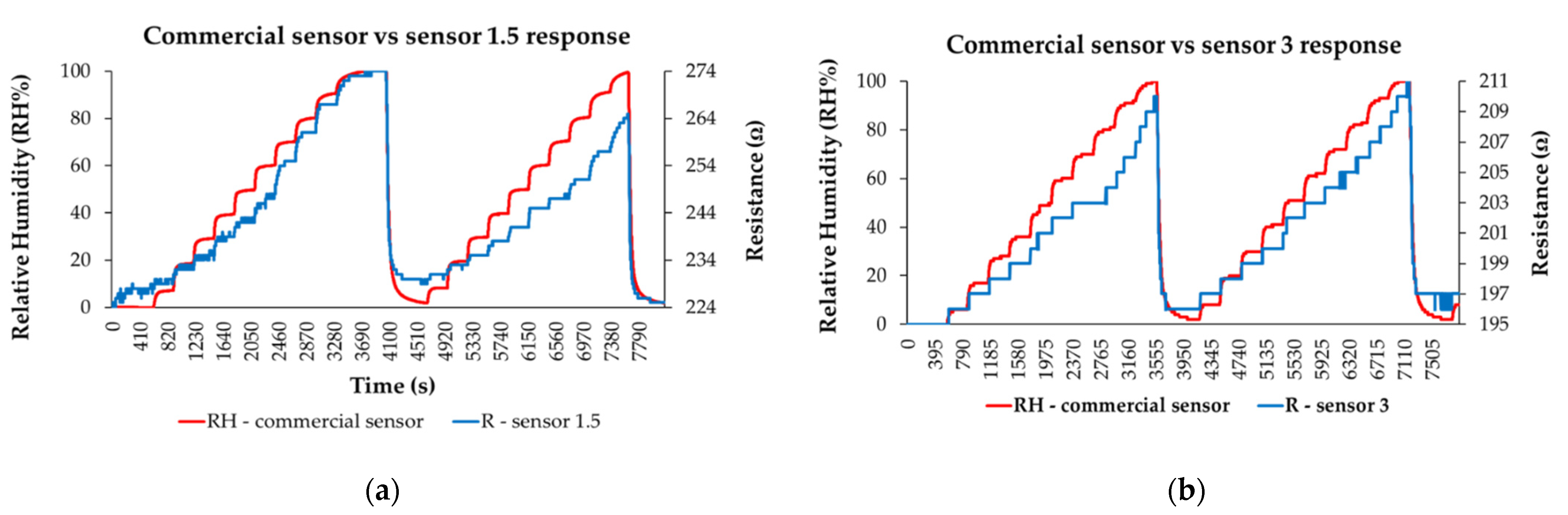
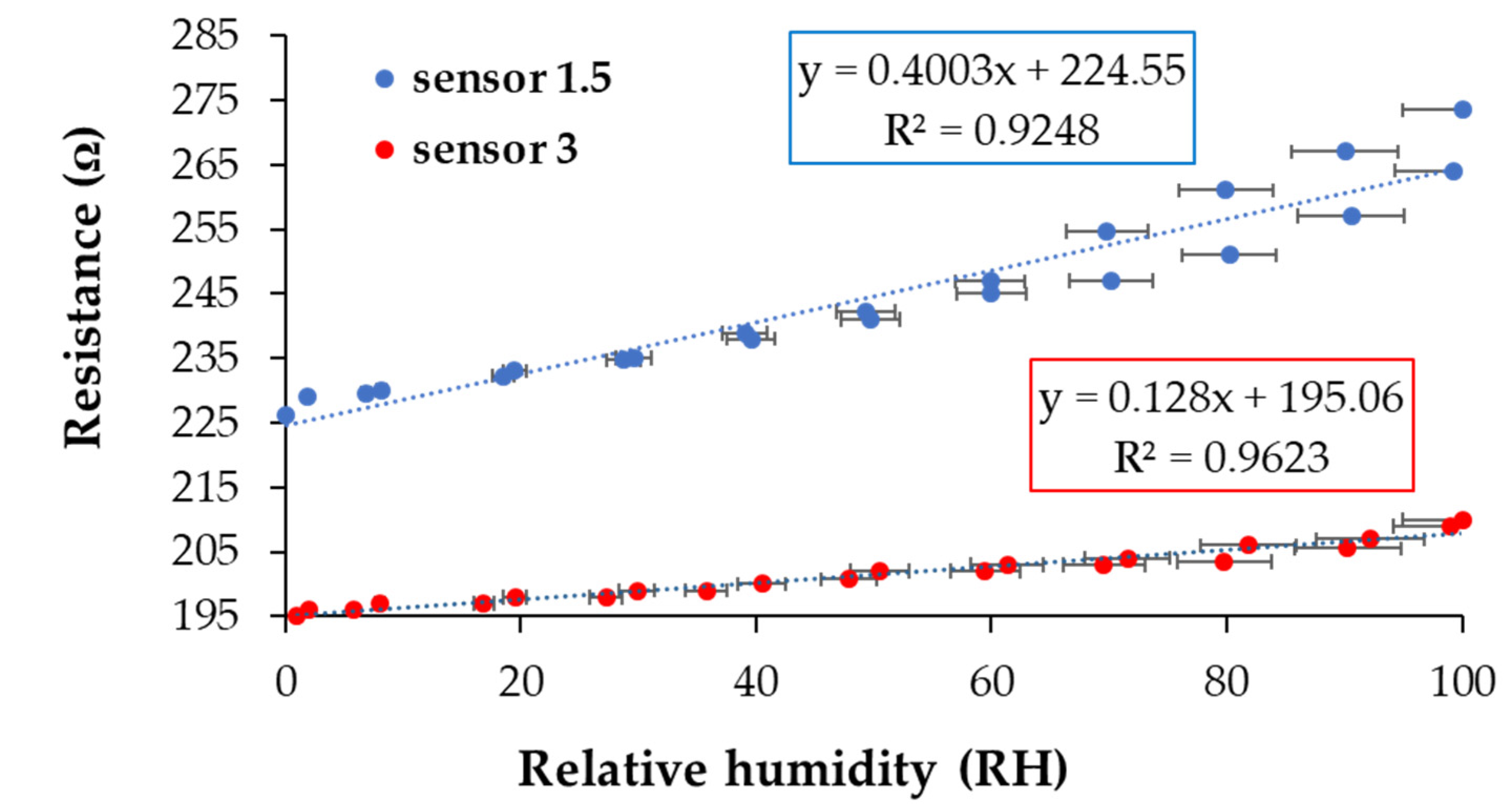
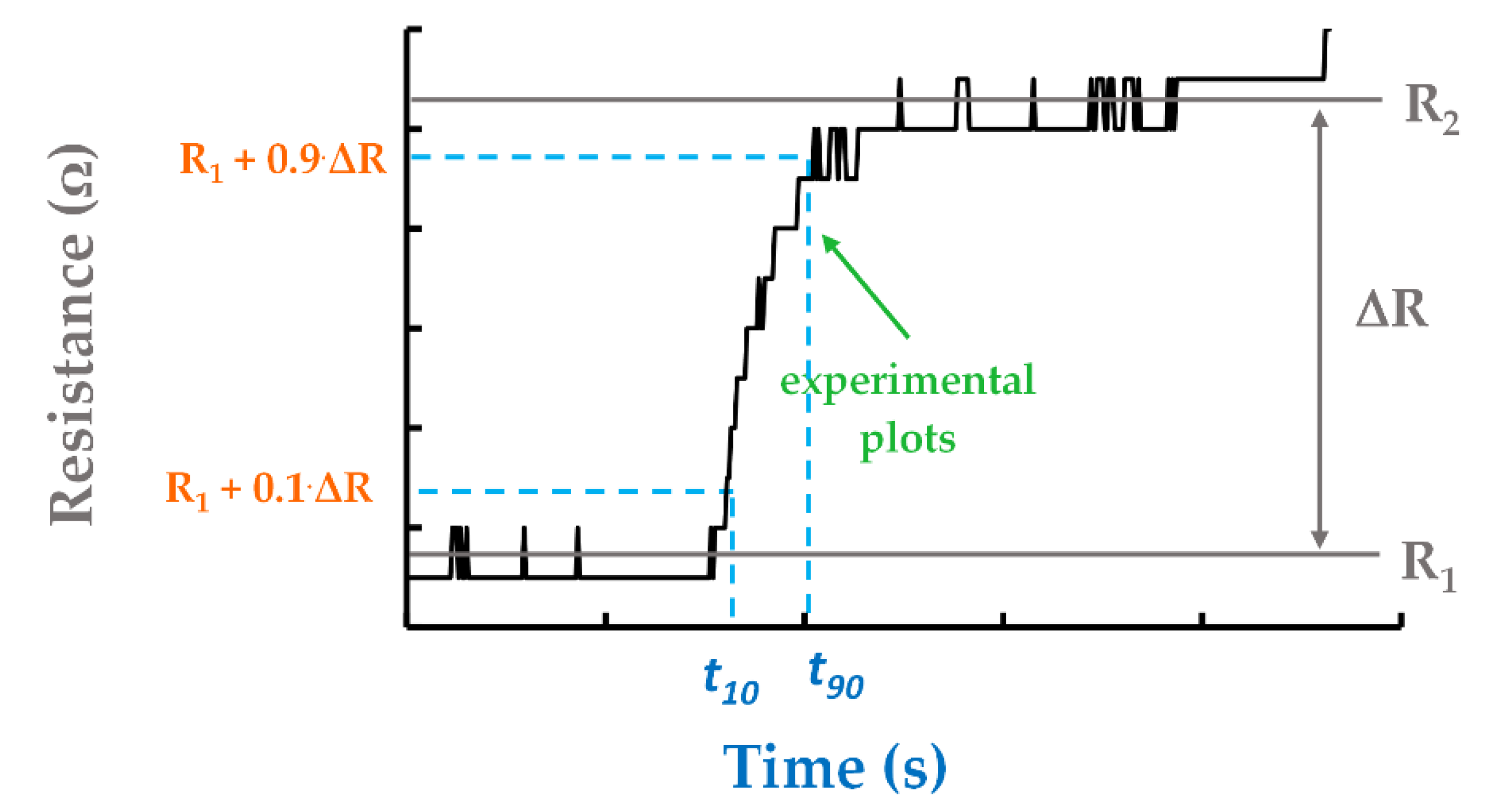
3.5. RH Monitoring Capability of the Quaternary Nanocomposite
3.6. Analysis of Sensing Mechanism
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farahani, H.; Wagiran, R.; Hamidon, M.N. Humidity sensors principle, mechanism, and fabrication technologies: A comprehensive review. Sensors 2014, 14, 7881–7939. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.Y.; Lee, G.B. Humidity sensors: A review. Sens. Lett. 2005, 3, 1–15. [Google Scholar] [CrossRef]
- Sikarwar, S.; Yadav, B.C. Opto-electronic humidity sensor: A review. Sens. Actuators A Phys. 2015, 233, 54–70. [Google Scholar] [CrossRef]
- Rittersma, Z.M. Recent achievements in miniaturised humidity sensors—A review of transduction techniques. Sens. Actuators A Phys. 2002, 96, 196–210. [Google Scholar] [CrossRef]
- Alwis, L.; Sun, T.; Grattan, K.T.V. Optical fibre-based sensor technology for humidity and moisture measurement: Review of recent progress. Measurement 2013, 46, 4052–4074. [Google Scholar] [CrossRef]
- Blank, T.A.; Eksperiandova, L.P.; Belikov, K.N. Recent trends of ceramic humidity sensors development: A review. Sens. Actuators B Chem. 2016, 228, 416–442. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, C. Humidity sensors: A review of materials and mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef] [Green Version]
- Moseley, P.T. Progress in the development of semiconducting metal oxide gas sensors: A review. Meas. Sci. Technol. 2017, 28, 082001. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Q.; Ren, Y.; Gao, Q.; Chen, J.; Wang, N.; Deng, J.; Xing, X. A new insight into cross-sensitivity to humidity of SnO2 sensor. Small 2018, 14, 1703974. [Google Scholar] [CrossRef]
- Qu, X.; Wang, M.H.; Chen, Y.; Sun, W.J.; Yang, R.; Zhang, H.P. Facile synthesis of hierarchical SnO2 twig-like microstructures and their applications in humidity sensors. Mater. Lett. 2017, 186, 182–185. [Google Scholar] [CrossRef]
- Feng, H.; Li, C.; Li, T.; Diao, F.; Xin, T.; Liu, B.; Wang, Y. Three-dimensional hierarchical SnO2 dodecahedral nanocrystals with enhanced humidity sensing properties. Sens. Actuators B Chem. 2017, 243, 704–714. [Google Scholar] [CrossRef]
- Gao, N.; Li, H.Y.; Zhang, W.; Zhang, Y.; Zeng, Y.; Zhixiang, H.; Liu, J.; Jiang, J.; Miao, L.; Yi, F.; et al. QCM-based humidity sensor and sensing properties employing colloidal SnO2 nanowires. Sens. Actuators B Chem. 2019, 293, 129–135. [Google Scholar] [CrossRef]
- Zhuo, M.; Chen, Y.; Sun, J.; Zhang, H.; Guo, D.; Zhang, H.; Li, Q.; Wang, T.; Wan, Q. Humidity sensing properties of a single Sb doped SnO2 nanowire field effect transistor. Sens. Actuators B Chem. 2013, 186, 78–83. [Google Scholar] [CrossRef]
- Kuang, Q.; Lao, C.; Wang, Z.L.; Xie, Z.; Zheng, L. High-sensitivity humidity sensor based on a single SnO2 nanowire. J. Am. Chem. Soc. 2007, 129, 6070–6071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parthibavarman, M.; Hariharan, V.; Sekar, C.J.M.S. High-sensitivity humidity sensor based on SnO2 nanoparticles synthesized by microwave irradiation method. Mater. Sci. Eng. C 2011, 31, 840–844. [Google Scholar] [CrossRef]
- Song, X.; Qi, Q.; Zhang, T.; Wang, C. A humidity sensor based on KCl-doped SnO2 nanofibers. Sens. Actuators B Chem. 2009, 138, 368–373. [Google Scholar] [CrossRef]
- Ates, T.; Tatar, C.; Yakuphanoglu, F. Preparation of semiconductor ZnO powders by sol–gel method: Humidity sensors. Sens. Actuators A Phys. 2013, 190, 153–160. [Google Scholar] [CrossRef]
- Narimani, K.; Nayeri, F.D.; Kolahdouz, M.; Ebrahimi, P. Fabrication, modeling and simulation of high sensitivity capacitive humidity sensors based on ZnO nanorods. Sens. Actuators B Chem. 2016, 224, 338–343. [Google Scholar] [CrossRef]
- Hong, H.S.; Phan, D.T.; Chung, G.S. High-sensitivity humidity sensors with ZnO nanorods based two-port surface acoustic wave delay line. Sens. Actuators B Chem. 2012, 171, 1283–1287. [Google Scholar] [CrossRef]
- Erol, A.; Okur, S.; Yağmurcukardeş, N.; Arıkan, M.Ç. Humidity-sensing properties of a ZnO nanowire film as measured with a QCM. Sens. Actuators B Chem. 2011, 152, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Harith, Z.; Irawati, N.; Batumalay, M.; Rafaie, H.A.; Harun, S.W.; Nor, R.M.; Ahmad, H. Relative humidity sensor employing optical fibers coated with ZnO nanostructures. Indian J. Sci. Technol. 2015, 8, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Yang, S. ZnO nanotetrapods: Controlled vapor-phase synthesis and application for humidity sensing. Adv. Funct. Mater. 2007, 17, 1345–1352. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, T.; Yu, Q.; Wang, R.; Zeng, Y.; Liu, L.; Yang, H. Properties of humidity sensing ZnO nanorods-base sensor fabricated by screen-printing. Sens. Actuators B Chem. 2008, 133, 638–643. [Google Scholar] [CrossRef]
- Tsai, F.S.; Wang, S.J. Enhanced sensing performance of relative humidity sensors using laterally grown ZnO nanosheets. Sens. Actuators B Chem. 2014, 193, 280–287. [Google Scholar] [CrossRef]
- Sin, N.M.; Ahmad, S.; Malek, M.F.; Mamat, M.H.; Rusop, M. Improvement sensitivity humidity sensor based on ZnO/SnO2 cubic structure. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2013; Volume 46, p. 012005. [Google Scholar]
- Wang, Z.; Shi, L.; Wu, F.; Yuan, S.; Zhao, Y.; Zhang, M. The sol–gel template synthesis of porous TiO2 for a high performance humidity sensor. Nanotechnology 2011, 22, 275502. [Google Scholar] [CrossRef]
- Chow, L.L.; Yuen, M.M.; Chan, P.C.; Cheung, A.T. Reactive sputtered TiO2 thin film humidity sensor with negative substrate bias. Sens. Actuators B Chem. 2001, 76, 310–315. [Google Scholar] [CrossRef]
- Fu, X.Q.; Wang, C.; Yu, H.C.; Wang, Y.G.; Wang, T.H. Fast humidity sensors based on CeO2 nanowires. Nanotechnology 2007, 18, 145503. [Google Scholar] [CrossRef]
- Xie, W.; Liu, B.; Xiao, S.; Li, H.; Wang, Y.; Cai, D.; Wang, D.; Wang, L.; Liu, Y.; Li, Q.; et al. High performance humidity sensors based on CeO2 nanoparticles. Sens. Actuators B Chem. 2015, 215, 125–132. [Google Scholar] [CrossRef]
- Wang, S.B.; Hsiao, C.H.; Chang, S.J.; Lam, K.T.; Wen, K.H.; Young, S.J.; Hung, S.C.; Huang, B.R. CuO nanowire-based humidity sensor. IEEE Sens. J. 2011, 12, 1884–1888. [Google Scholar] [CrossRef]
- Hsueh, H.T.; Hsueh, T.J.; Chang, S.J.; Hung, F.Y.; Tsai, T.Y.; Weng, W.Y.; Hsu, C.L.; Dai, B.T. CuO nanowire-based humidity sensors prepared on glass substrate. Sens. Actuators B Chem. 2011, 156, 906–911. [Google Scholar] [CrossRef]
- Rauti, R.; Musto, M.; Bosi, S.; Prato, M.; Ballerini, L. Properties and behavior of carbon nanomaterials when interfacing neuronal cells: How far have we come? Carbon 2019, 143, 430–446. [Google Scholar] [CrossRef]
- Chu, J.; Peng, X.; Feng, P.; Sheng, Y.; Zhang, J. Study of humidity sensors based on nanostructured carbon films produced by physical vapor deposition. Sens. Actuators B Chem. 2013, 178, 508–513. [Google Scholar] [CrossRef]
- Tulliani, J.M.; Inserra, B.; Ziegler, D. Carbon-based materials for humidity sensing: A short review. Micromachines 2019, 10, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Shen, C.; Lou, W.; Shentu, F.; Zhong, C.; Dong, X.; Tong, L. Fiber optic relative humidity sensor based on the tilted fiber Bragg grating coated with graphene oxide. Appl. Phys. Lett. 2016, 109, 031107. [Google Scholar] [CrossRef]
- De Luca, A.; Santra, S.; Ghosh, R.; Ali, S.Z.; Gardner, J.W.; Guha, P.K.; Udrea, F. Temperature-modulated graphene oxide resistive humidity sensor for indoor air quality monitoring. Nanoscale 2016, 8, 4565–4572. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghosh, R.; Guha, P.K.; Bhattacharyya, T.K. Humidity sensor based on high proton conductivity of graphene oxide. IEEE Trans. Nanotechnol. 2015, 14, 931–937. [Google Scholar] [CrossRef]
- Zhang, X.; Maddipatla, D.; Bose, A.K.; Hajian, S.; Narakathu, B.B.; Williams, J.D.; Mitchell, M.F.; Atashbar, M.Z. Printed Carbon Nanotubes-Based Flexible Resistive Humidity Sensor. IEEE Sens. J. 2020, 20, 12592–12601. [Google Scholar] [CrossRef]
- Zhang, X.; Turkani, V.S.; Hajian, S.; Bose, A.K.; Maddipatla, D.; Hanson, A.J.; Narakathu, B.B.; Atashbar, M.Z. Novel printed carbon nanotubes based resistive humidity sensors. In Proceedings of the 2019 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Glasgow, UK, 8–10 July 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1–3. [Google Scholar]
- Han, J.W.; Kim, B.; Li, J.; Meyyappan, M. Carbon nanotube based humidity sensor on cellulose paper. J. Phys. Chem. C 2012, 116, 22094–22097. [Google Scholar] [CrossRef]
- Chen, H.J.; Xue, Q.Z.; Ma, M.; Zhou, X.Y. Capacitive humidity sensor based on amorphous carbon film/n-Si heterojunctions. Sens. Actuators B Chem. 2010, 150, 487–489. [Google Scholar] [CrossRef]
- Yu, X.; Chen, X.; Yu, X.; Chen, X.; Ding, X.; Zhao, X. Flexible Wearable Humidity Sensor Based on Nanodiamond with Fast Response. IEEE Trans. Electron Devices 2019, 66, 1911–1916. [Google Scholar] [CrossRef]
- Yao, Y.; Xue, Y. Impedance analysis of quartz crystal microbalance humidity sensors based on nanodiamond/graphene oxide nanocomposite film. Sens. Actuators B Chem. 2015, 211, 52–58. [Google Scholar] [CrossRef]
- Svåsand, E.; Häberle, P. Humidity Sensing by Carbon Nanocones. J. Nanosci. Nanotechnol. 2014, 14, 3575–3580. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Chen, X.; Chen, X.; Zhao, X.; Li, N. A QCM humidity sensor based on fullerene/graphene oxide nanocomposites with high quality factor. Sens. Actuators B Chem. 2018, 266, 534–542. [Google Scholar] [CrossRef]
- Radeva, E.; Georgiev, V.; Spassov, L.; Koprinarov, N.; Kanev, S. Humidity adsorptive properties of thin fullerene layers studied by means of quartz micro-balance. Sens. Actuators B Chem. 1997, 42, 11–13. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Y.M.; Wu, Z.; Li, X.; Wang, N.; Tao, K.; Wang, G.P. Carbon nanocoil-based fast-response and flexible humidity sensor for multifunctional applications. ACS Appl. Mater. Interfaces 2019, 11, 4242–4251. [Google Scholar] [CrossRef]
- Serban, B.C.; Buiu, O.; Dumbravescu, N.; Cobianu, C.; Avramescu, V.; Brezeanu, M.; Nicolescu, C.M. Oxidized Carbon Nanohorns as Novel Sensing Layer for Resistive Humidity Sensor. Acta Chim. Slov. 2020, 67, 1–7. [Google Scholar] [CrossRef]
- Serban, B.C.; Buiu, O.; Dumbravescu, N.; Cobianu, C.; Avramescu, V.; Brezeanu, M.; Bumbac, M.; Pachiu, C.; Nicolescu, C.M. Oxidized Carbon Nanohorn-Hydrophilic Polymer Nanocomposite as the Resistive Sensing Layer for Relative Humidity. Anal. Lett. 2020, 54, 527–540. [Google Scholar] [CrossRef]
- Santra, S.; Hu, G.; Howe, R.C.; De Luca, A.; Ali, S.Z.; Udrea, F.; Gardner, J.W.; Ray, S.K.; Guha, P.K.; Hasan, T. CMOS integration of inkjet-printed graphene for humidity sensing. Sci. Rep. 2015, 5, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Serban, B.C.; Cobianu, C.; Buiu, O.; Bumbac, M.; Dumbravescu, N.; Avramescu, V.; Nicolescu, C.M.; Brezeanu, M.; Pachiu, C.; Craciun, G.; et al. Ternary Nanocomposites Based on Oxidized Carbon Nanohorns as Sensing Layers for Room Temperature Resistive Humidity Sensing. Materials 2021, 14, 2705. [Google Scholar] [CrossRef]
- Serban, B.C.; Cobianu, C.; Dumbravescu, N.; Buiu, O.; Avramescu, V.; Bumbac, M.; Nicolescu, C.M.; Cobianu, C.; Brezeanu, M. Electrical Percolation Threshold in Oxidized Single Wall Carbon Nanohorn-Polyvinylpyrrolidone Nanocomposite: A Possible Application for High Sensitivity Resistive Humidity Sensor. In Proceedings of the International Semiconductor Conference (CAS), Sinaia, Romania, 7–9 October 2020; pp. 239–242. [Google Scholar]
- Șerban, B.C.; Cobianu, C.; Buiu, O.; Dumbrăvescu, N.; Avramescu, V.; Brezeanu, M.; Marinescu, M.R. Ternary oxidized carbon nanohorn-based nanohybrid as sensing layer for resistive humidity sensor. In Proceedings of the 3RD International Conference on Emerging Technologies in Materials Engineering, Bucharest, Romania, 6–8 November 2019; In Book of Abstracts. p. 83. [Google Scholar]
- Șerban, B.C.; Cobianu, C.; Buiu, O.; Dumbrăvescu, N.; Avramescu, V.; Brezeanu, M.; Marinescu, M.R. Ternary hydrophilic carbon nanohorn/ZnO/PVP nanohybrid structure for room temperature resistive humidity sensing. In Proceedings of the 3RD International Conference on Emerging Technologies in Materials Engineering, Bucharest, Romania, 6–8 November 2019; In Book of Abstracts. p. 84. [Google Scholar]
- Serban, B.C.; Cobianu, C.; Dumbravescu, N.; Buiu, O.; Bumbac, M.; Nicolescu, C.M.; Cobianu, C.; Brezeanu, M.; Pachiu, C.; Serbanescu, M. Electrical Percolation Threshold and Size Effects in Polyvinylpyrrolidone-Oxidized Single-Wall Carbon Nanohorn Nanocomposite: The Impact for Relative Humidity Resistive Sensors Design. Sensors 2021, 21, 1435. [Google Scholar] [CrossRef]
- Epeloa, J.; Repetto, C.E.; Gómez, B.J.; Nachez, L.; Dobry, A. Resistivity humidity sensors based on hydrogenated amorphous carbon films. Mater. Res. Express 2018, 6, 025604. [Google Scholar] [CrossRef]
- Șerban, B.C.; Buiu, O.; Cobianu, C.; Marinescu, M.R. New Sensitive Layer for Relative Humidity Sensor and Itsmanufacturing Method. RO 134519 A2, 30 October 2020. Official Bulletin of Industrial Property—Patents Section. [Google Scholar]
- Șerban, B.C.; Buiu, O.; Cobianu, C.; Avramescu, V.R.; Marinescu, M.R. Humidity Sensor. RO 134520 A2, 30 October 2020. Official Bulletin of Industrial Property—Patents Section. [Google Scholar]
- Șerban, B.C.; Buiu, O.; Cobianu, C.; Marinescu, M.R. New Chemiresistive Sensor for Humidity Detection. RO 134521 A2, 30 October 2020. Official Bulletin of Industrial Property—Patents Section. [Google Scholar]
- Șerban, B.C.; Buiu, O.; Cobianu, C.; Marinescu, M.R. Sensitive Layer for Humidity Sensor with Surface Acoustic Waves. RO 134499 A2, 30 October 2020. Official Bulletin of Industrial Property—Patents Section. [Google Scholar]
- Zhang, X.; Ming, H.; Liu, R.; Han, X.; Kang, Z.; Liu, Y.; Zhang, Y. Highly sensitive humidity sensing properties of carbon quantum dots films. Mater. Res. Bull. 2013, 48, 790–794. [Google Scholar] [CrossRef]
- Zhang, D.; Chang, H.; Li, P.; Liu, R.; Xue, Q. Fabrication and characterization of an ultrasensitive humidity sensor based on metal oxide/graphene hybrid nanocomposite. Sens. Actuators B Chem. 2016, 225, 233–240. [Google Scholar] [CrossRef]
- Leghrib, R.; Pavelko, R.; Felten, A.; Vasiliev, A.; Cané, C.; Gràcia, I.; Pireaux, J.J.; Llobet, E. Gas sensors based on multiwall carbon nanotubes decorated with tin oxide nanoclusters. Sens. Actuators B Chem. 2010, 145, 411–416. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Xia, B. Layer-by-layer self-assembly of zinc oxide/graphene oxide hybrid toward ultrasensitive humidity sensing. IEEE Electron Device Lett. 2016, 37, 916–919. [Google Scholar] [CrossRef]
- Yuan, Z.; Tai, H.; Bao, X.; Liu, C.; Ye, Z.; Jiang, Y. Enhanced humidity-sensing properties of novel graphene oxide/zinc oxide nanoparticles layered thin film QCM sensor. Mater. Lett. 2016, 174, 28–31. [Google Scholar] [CrossRef]
- Kalim, B.; Ansar, M.T.; Ullah, Z.; Abbas, S.K.; Riaz, S.; Siddiqi, S.A.; Atiq, S. CNTs/ZnO and CNTs/ZnO/Ag multilayers spray coated on cellulose fiber for use as an efficient humidity sensor. Ceram. Int. 2020, 46, 25593–25597. [Google Scholar] [CrossRef]
- Zhang, D.; Yin, N.; Xia, B.; Sun, Y.; Liao, Y.; He, Z.; Hao, S. Humidity-sensing properties of hierarchical ZnO/MWCNTs/ZnO nanocomposite film sensor based on electrostatic layer-by-layer self-assembly. J. Mater. Sci. Mater. Electron. 2016, 27, 2481–2487. [Google Scholar] [CrossRef]
- Wu, J.; Yin, C.; Zhou, J.; Li, H.; Liu, Y.; Shen, Y.; Garner, S.; Fu, Y.; Duan, H. Ultrathin Glass-Based Flexible, Transparent, and Ultrasensitive Surface Acoustic Wave Humidity Sensor with ZnO Nanowires and Graphene Quantum Dots. ACS Appl. Mater. Interfaces 2020, 12, 39817–39825. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Dong, X.; Chan, C.C.; Ni, K.; Zhang, S.; Shum, P.P. Humidity sensor with a PVA-coated photonic crystal fiber interferometer. IEEE Sens. J. 2013, 13, 2214–2216. [Google Scholar] [CrossRef]
- Buvailo, A.; Xing, Y.; Hines, J.; Borguet, E. Thin polymer film based rapid surface acoustic wave humidity sensors. Sens. Actuators B Chem. 2011, 156, 444–449. [Google Scholar] [CrossRef]
- Zeng, F.W.; Liu, X.X.; Diamond, D.; Lau, K.T. Humidity sensors based on polyaniline nanofibres. Sens. Actuators B Chem. 2010, 143, 530–534. [Google Scholar] [CrossRef]
- Tan, X.; Wu, X.; Hu, Z.; Ma, D.; Shi, Z. Synthesis and catalytic activity of palladium supported on heteroatom doped single-wall carbon nanohorns. RSC Adv. 2017, 7, 19985–29991. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-G.; Kresse, I.; Springer, J.; Nissen, J.; Yang, Y.-L. Morphology and gas perm selectivity of blend membranes of polyvinylpyridine with ethylcellulose. Polymer 2001, 42, 6859–6869. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Šćepanović, M.G.B.M.; Grujić-Brojčin, M.; Vojisavljević, K.; Bernik, S.; Srećković, T. Raman study of structural disorder in ZnO nanopowders. J. Raman Spectrosc. 2010, 41, 914–921. [Google Scholar] [CrossRef]
- Serban, B.C.; Cobianu, C.; Buiu, O.; Bumbac, M.; Dumbravescu, N.; Avramescu, V.; Nicolescu, C.M.; Brezeanu, M.; Radulescu, C.; Craciun, G.; et al. Quaternary Oxidized Carbon Nanohorns—Based Nanohybrid as Sensing Coating for Room Temperature Resistive Humidity Monitoring. Coatings 2021, 11, 530. [Google Scholar] [CrossRef]
- Cao, C.L.; Hu, C.G.; Fang, L.; Wang, S.X.; Tian, Y.S.; Pan, C.Y. Humidity sensor based on multi-walled carbon nanotube thin films. J. Nanomater. 2011, 2011, 707303. [Google Scholar] [CrossRef] [Green Version]
- Zahab, A.; Spina, L.; Poncharal, P.; Marliere, C. Water-vapor effect on the electrical conductivity of a single-walled carbon nanotube mat. Phys. Rev. B 2000, 62, 10000. [Google Scholar] [CrossRef]
- Bi, H.; Yin, K.; Xie, X.; Ji, J.; Wan, S.; Sun, L.; Dresselhaus, M.S. Ultrahigh humidity sensitivity of graphene oxide. Sci. Rep. 2013, 3, 2714. [Google Scholar] [CrossRef] [Green Version]
- Heiland, G.; Kohl, D. Chemical Sensor Technology; Seiyama, T., Ed.; Kodansha: Tokyo, Japan, 1988; Volume 1, Chapter 2; pp. 15–38. [Google Scholar]
- Serban, B.C.; Buiu, O.; Dumbravescu, N.; Cobianu, C.; Avramescu, V.; Brezeanu, M.; Bumbac, M.; Nicolescu, C.M. Optimization of Sensing Layers Selection Process for Relative Humidity Sensors. Sci. Technol. 2020, 23, 93–104. [Google Scholar]
- Henrich, V.A.; Cox, P.A. The Surface Science of Metal Oxides; Cambridge University Press: Cambridge, UK, 1994; p. 312. [Google Scholar]
- Kulwicki, M. Humidity sensors. J. Am. Ceram. Soc. 1991, 74, 697–708. [Google Scholar] [CrossRef]
- Schaub, R.; Thostrup, P.; Lopez, N.; Lægsgaard, E.; Stensgaard, I.; Nørskov, J.K.; Besenbacher, F. Oxygen vacancies as active sites for water dissociation on rutile TiO2(110). Phys. Rev. Lett. 2001, 87, 266104/1–266104/4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasab, S.S.; Zahmatkesh, S. Preparation, structural characterization, and gas separation properties of functionalized zinc oxide particle filled poly(ether-amide) nanocomposite films. J. Plast. Film Sheet. 2017, 33, 92–113. [Google Scholar] [CrossRef]



| Sensing Layer | Relative Sensitivity |
|---|---|
| CNHox [48] | 0.013–0.021 |
| CNHox-PVP 1/1 [49] | 0.020–0.058 |
| CNHox-PVP 2/1 [49] | 0.017–0.025 |
| Graphene-PVP inkjet printed [50] | 0.21–0.30 |
| GO-CNHox–PVP 1/1/1 [51] | 0.150–0.200 |
| GO-CNHox–PVP 1/2/1 [51] | 0.063–0.070 |
| GO-CNHox–PVP 1/3/1 [51] | 0.043–0.051 |
| CNHox/GO/SnO2/PVP 0.75/0.75/1/1 [76] | 0.548–0.770 |
| CNHox/GO/SnO2/PVP 1/1/1/1 [76] | 0.798–0.980 |
| CNHox/SnO2/ZnO/PVP = 1.5/1/1/1-present study | 0.320–0.489 |
| CNHox/SnO2/ZnO/PVP = 3/1/1/1-present study | 0.105–0.242 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serban, B.-C.; Cobianu, C.; Buiu, O.; Bumbac, M.; Dumbravescu, N.; Avramescu, V.; Nicolescu, C.M.; Brezeanu, M.; Radulescu, C.; Craciun, G.; et al. Quaternary Holey Carbon Nanohorns/SnO2/ZnO/PVP Nano-Hybrid as Sensing Element for Resistive-Type Humidity Sensor. Coatings 2021, 11, 1307. https://doi.org/10.3390/coatings11111307
Serban B-C, Cobianu C, Buiu O, Bumbac M, Dumbravescu N, Avramescu V, Nicolescu CM, Brezeanu M, Radulescu C, Craciun G, et al. Quaternary Holey Carbon Nanohorns/SnO2/ZnO/PVP Nano-Hybrid as Sensing Element for Resistive-Type Humidity Sensor. Coatings. 2021; 11(11):1307. https://doi.org/10.3390/coatings11111307
Chicago/Turabian StyleSerban, Bogdan-Catalin, Cornel Cobianu, Octavian Buiu, Marius Bumbac, Niculae Dumbravescu, Viorel Avramescu, Cristina Mihaela Nicolescu, Mihai Brezeanu, Cristiana Radulescu, Gabriel Craciun, and et al. 2021. "Quaternary Holey Carbon Nanohorns/SnO2/ZnO/PVP Nano-Hybrid as Sensing Element for Resistive-Type Humidity Sensor" Coatings 11, no. 11: 1307. https://doi.org/10.3390/coatings11111307
APA StyleSerban, B.-C., Cobianu, C., Buiu, O., Bumbac, M., Dumbravescu, N., Avramescu, V., Nicolescu, C. M., Brezeanu, M., Radulescu, C., Craciun, G., Romanitan, C., & Comanescu, F. C. (2021). Quaternary Holey Carbon Nanohorns/SnO2/ZnO/PVP Nano-Hybrid as Sensing Element for Resistive-Type Humidity Sensor. Coatings, 11(11), 1307. https://doi.org/10.3390/coatings11111307











