Increase in Oxidation Resistance of MAR M-509 via LA-CVD Aluminizing
Abstract
:1. Introduction
2. Materials and Methods
3. Results
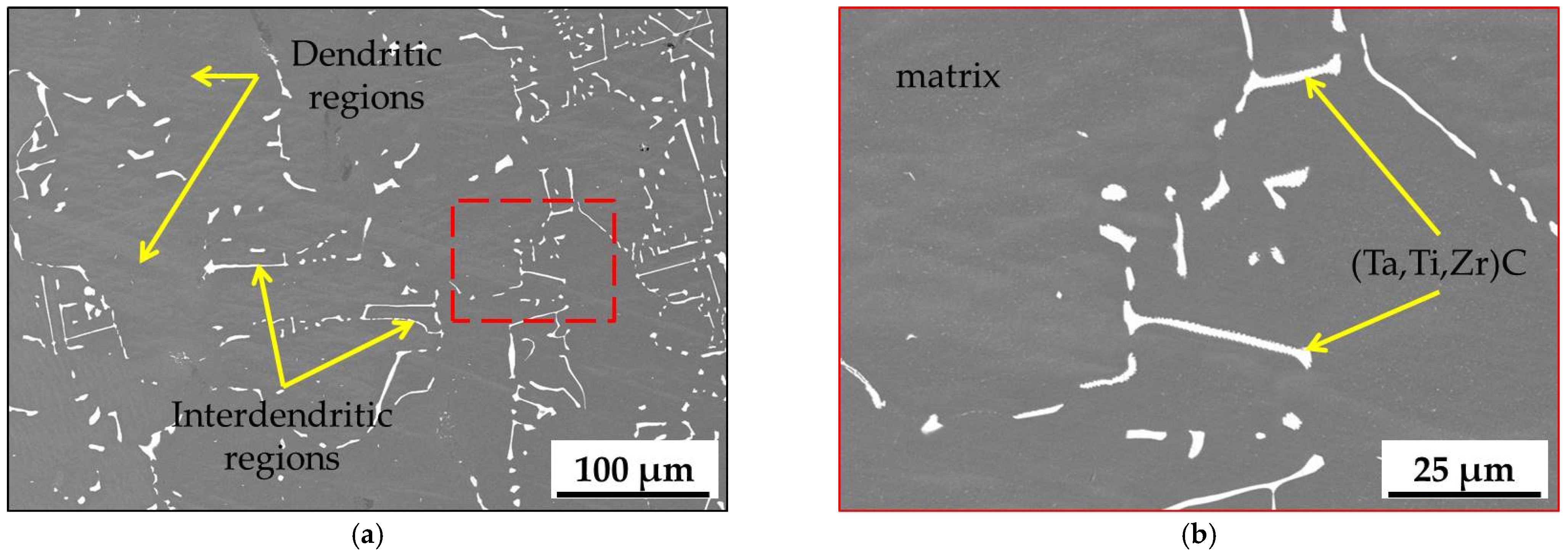
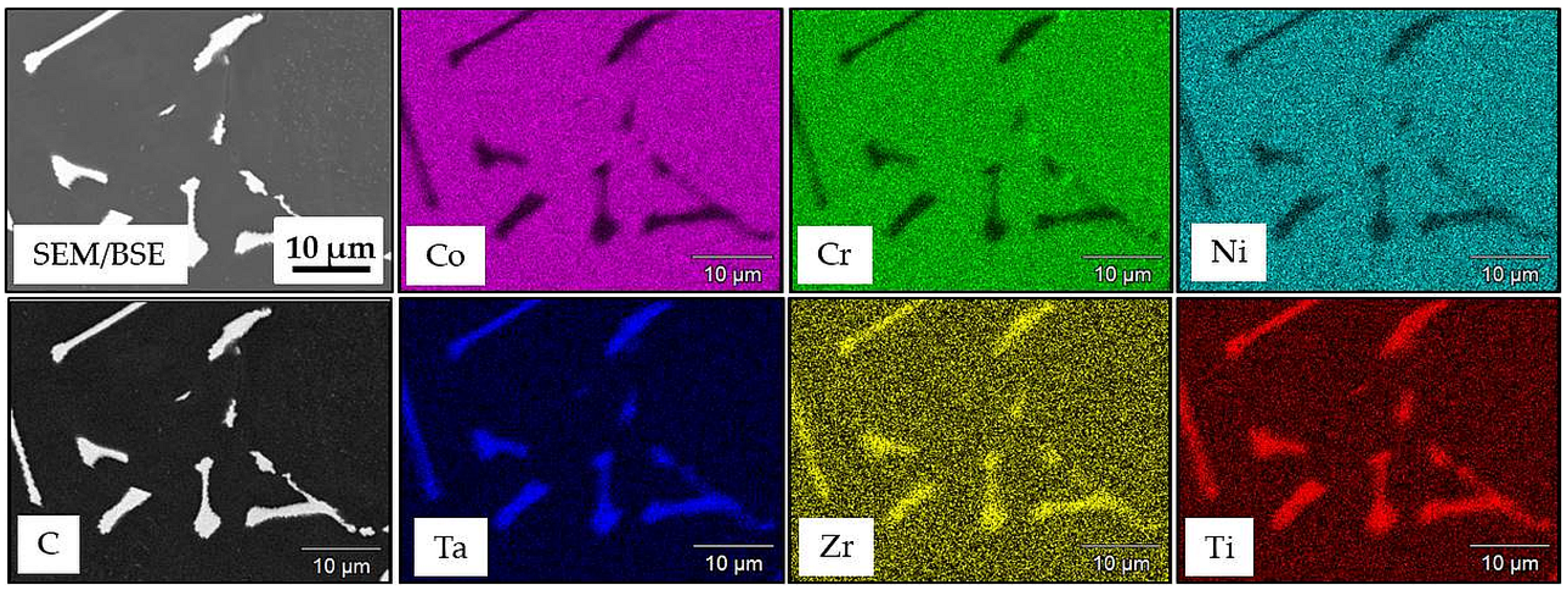
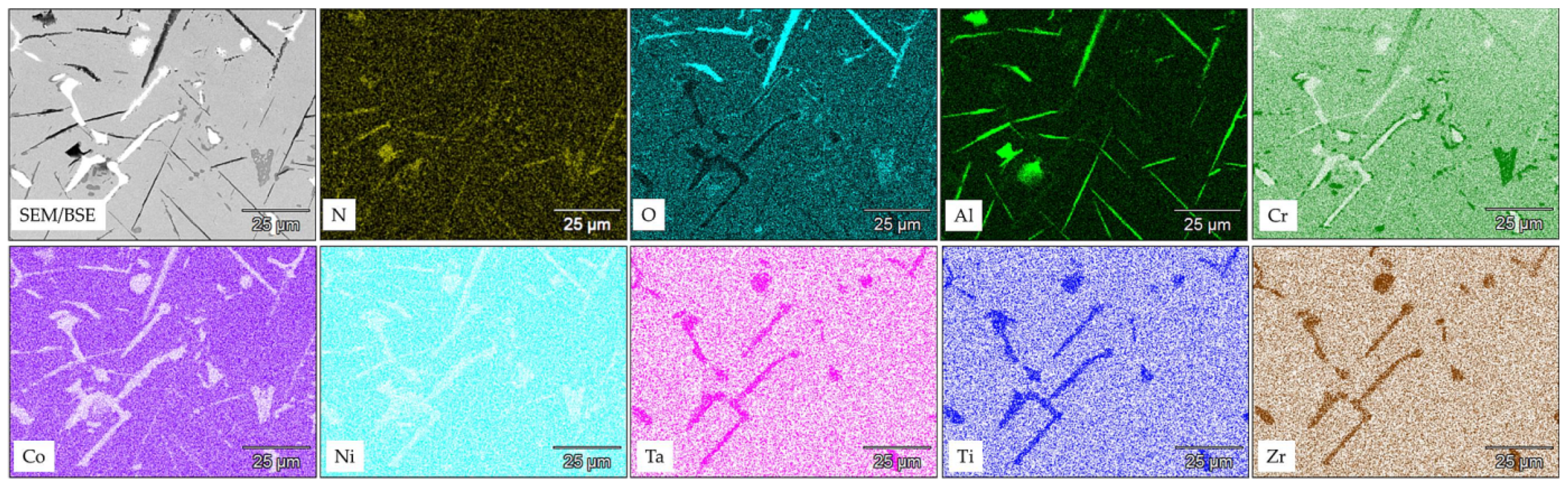
3.1. Microstructure of MAR M-509 in the As-Cast Condition
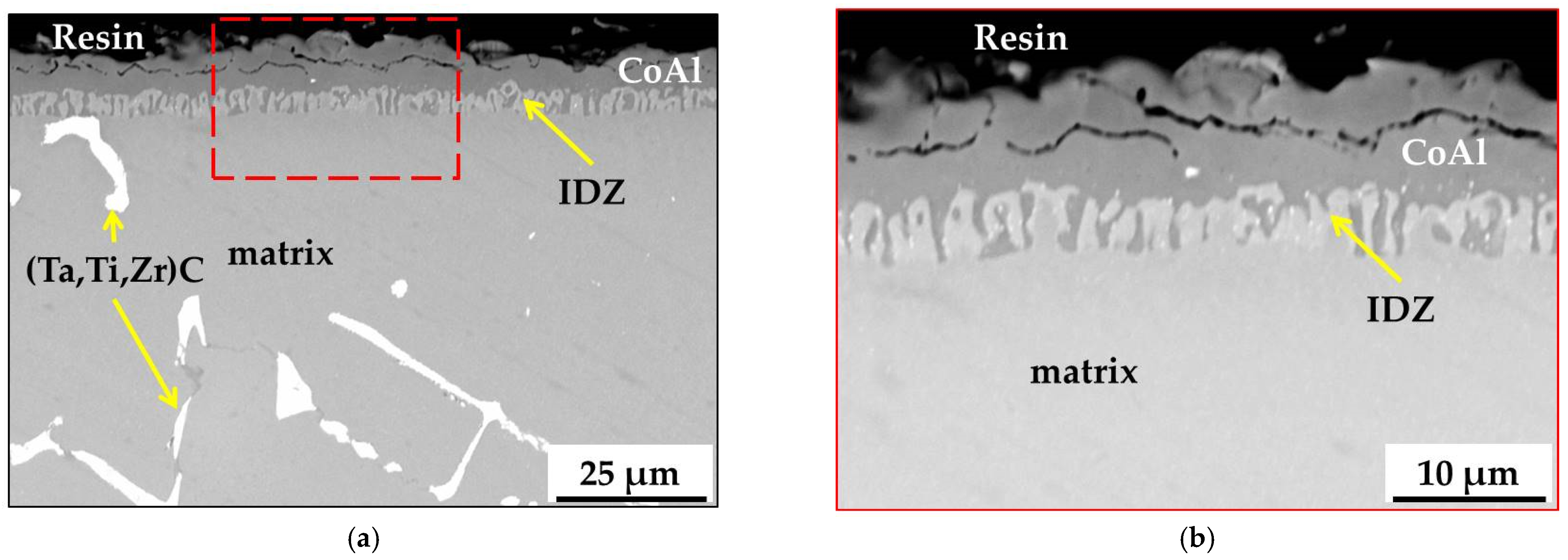
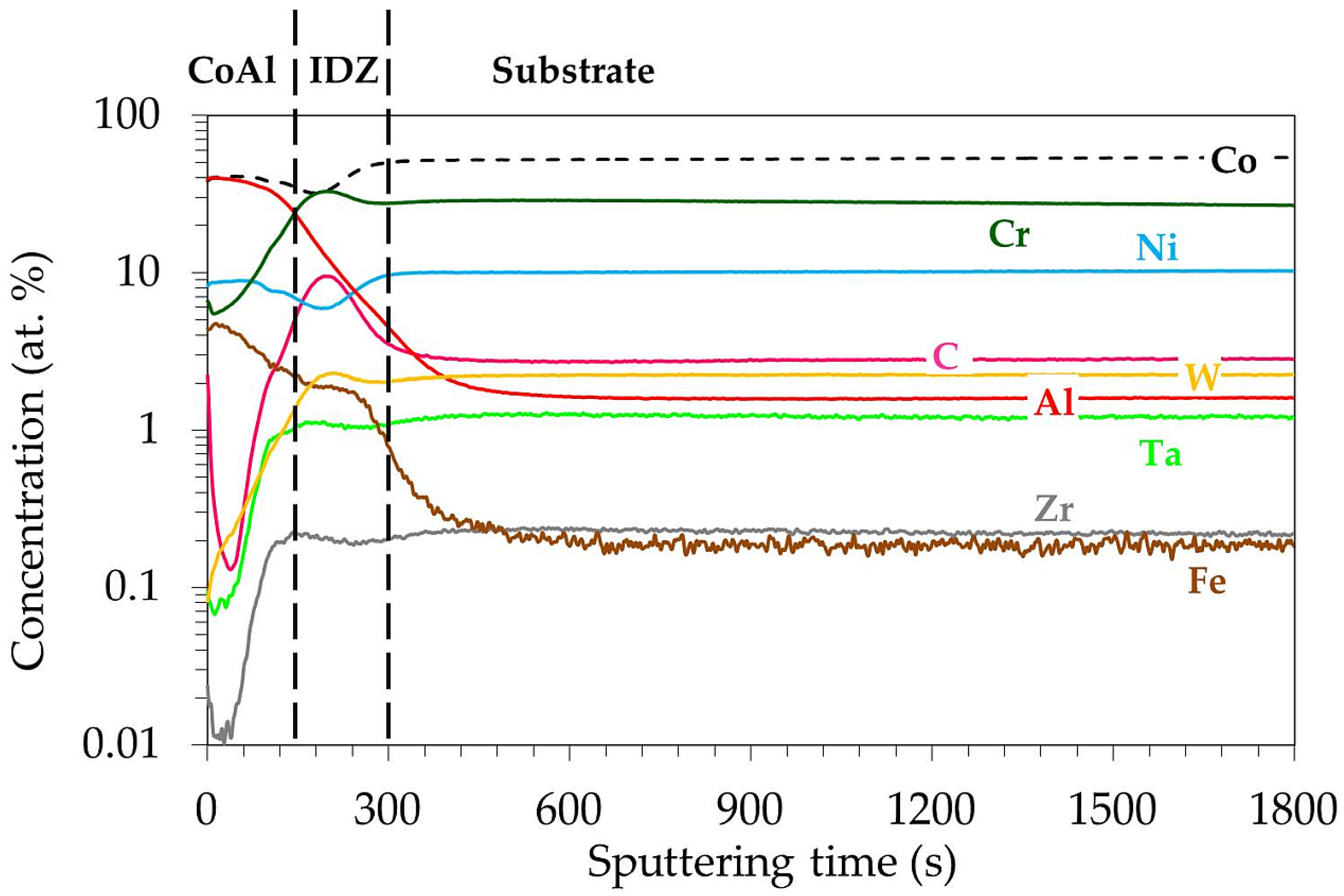
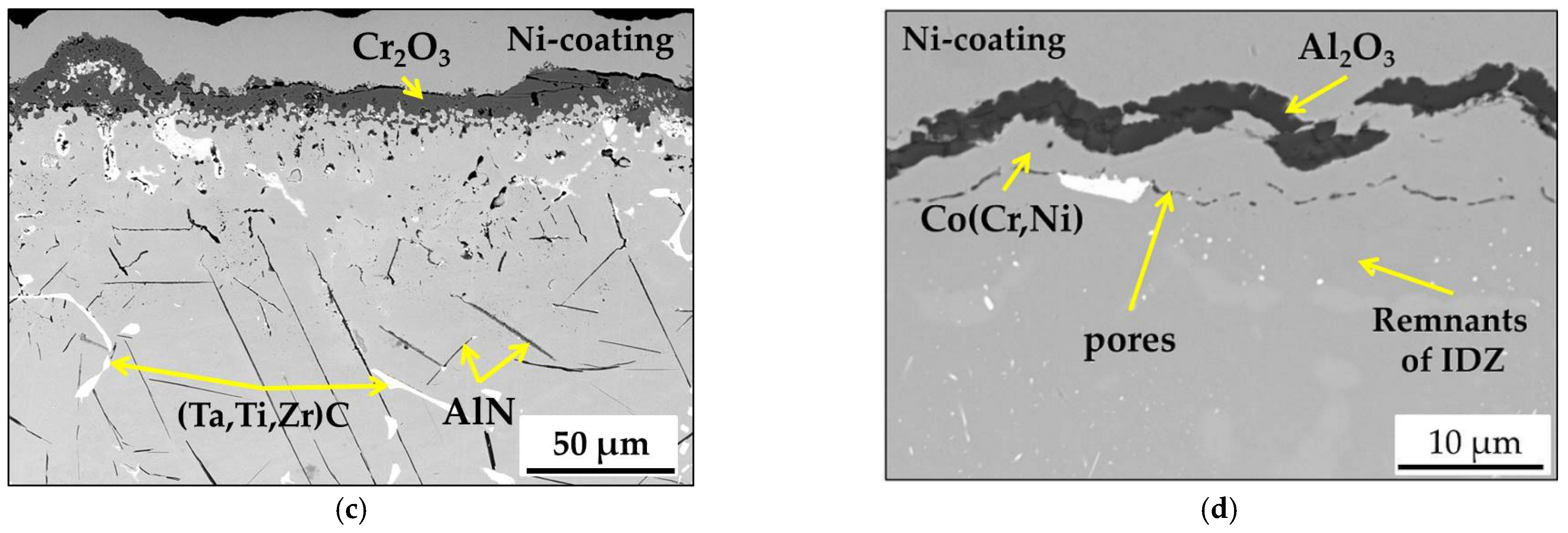
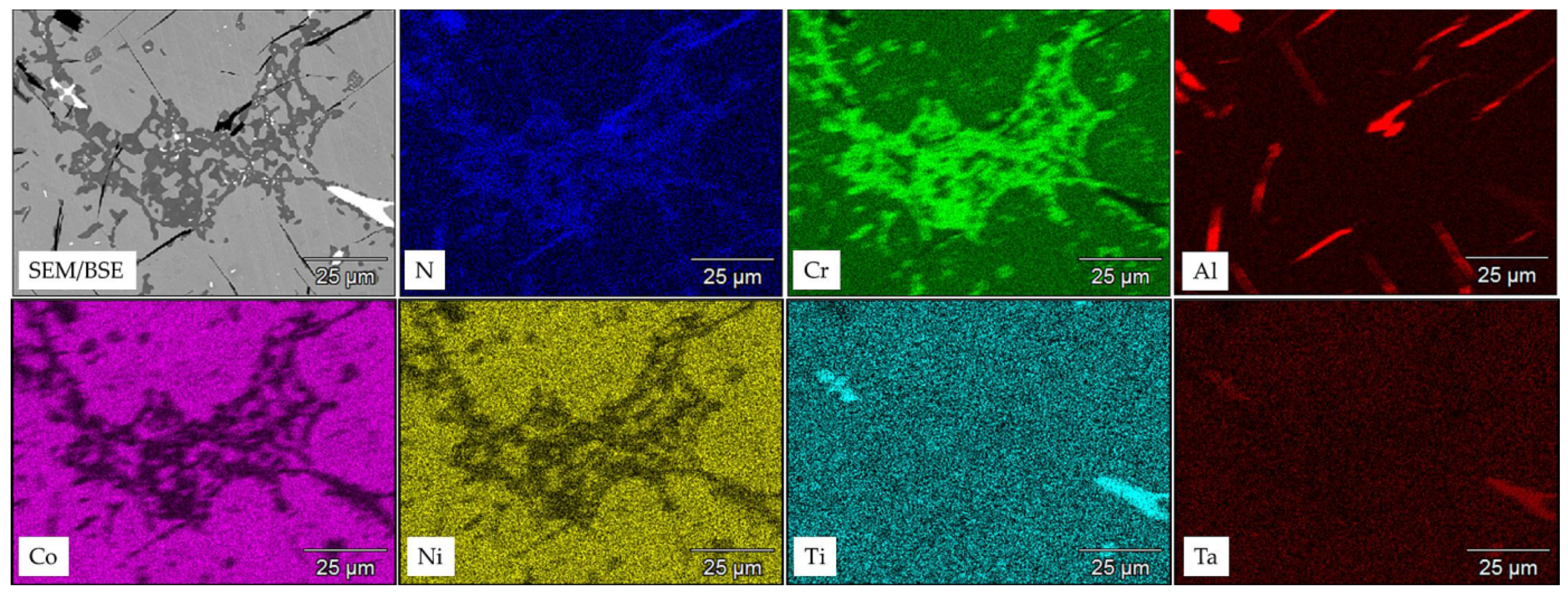
3.2. Microstructure of MAR M-509 after CVD Aluminizing
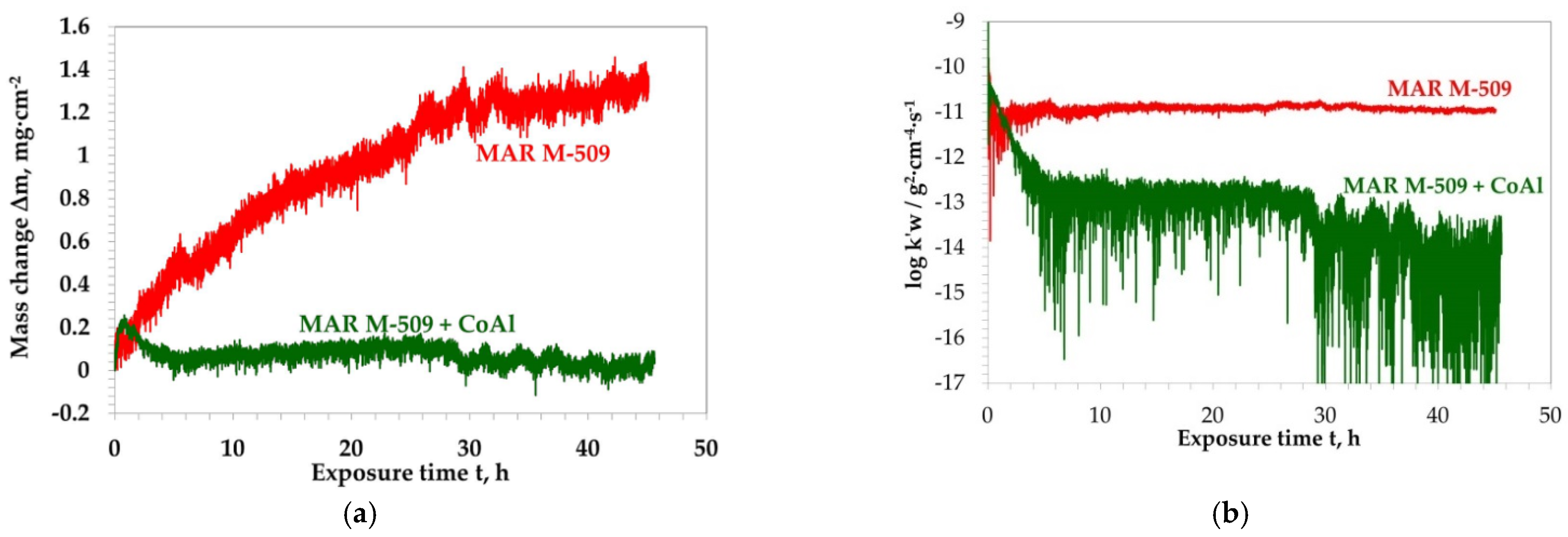
3.3. Air Oxidation Tests at 1000 °C
3.3.1. Isothermal Oxidation Test
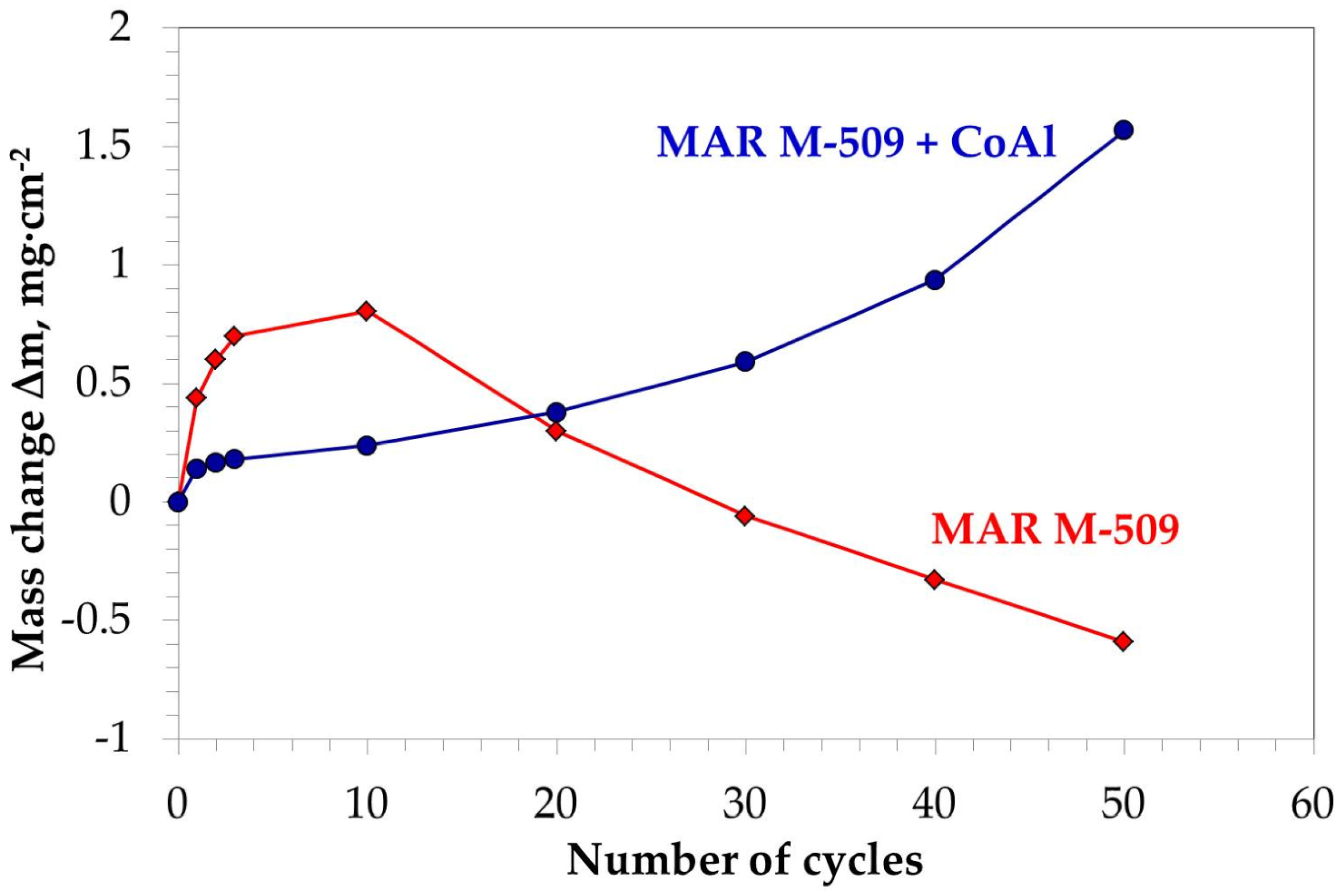
3.3.2. Cyclic Oxidation Test
3.4. Air Oxidation Tests at 1100 °C
3.4.1. Isothermal Oxidation Test
3.4.2. Cyclic Oxidation Test
4. Discussion
5. Conclusions
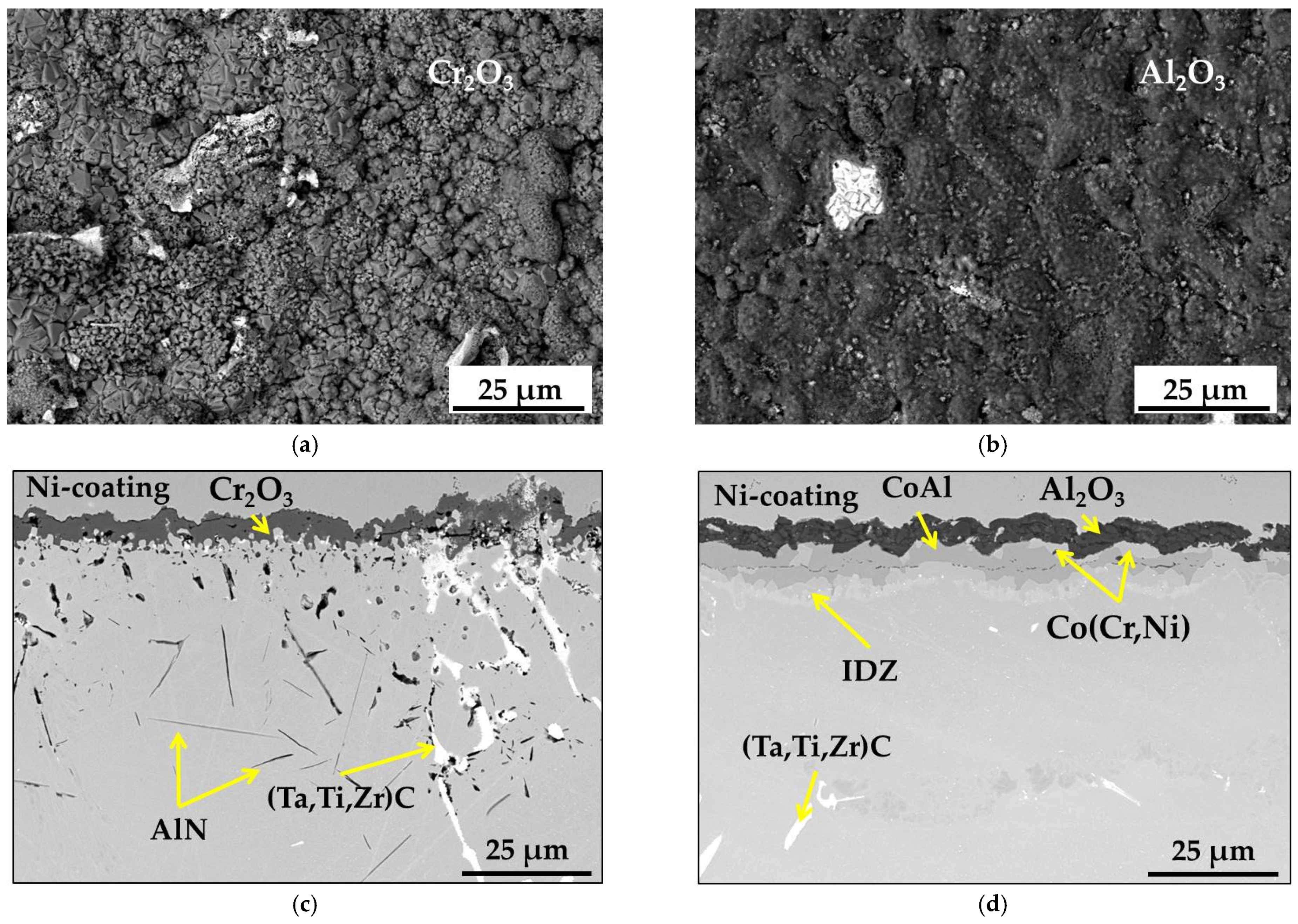
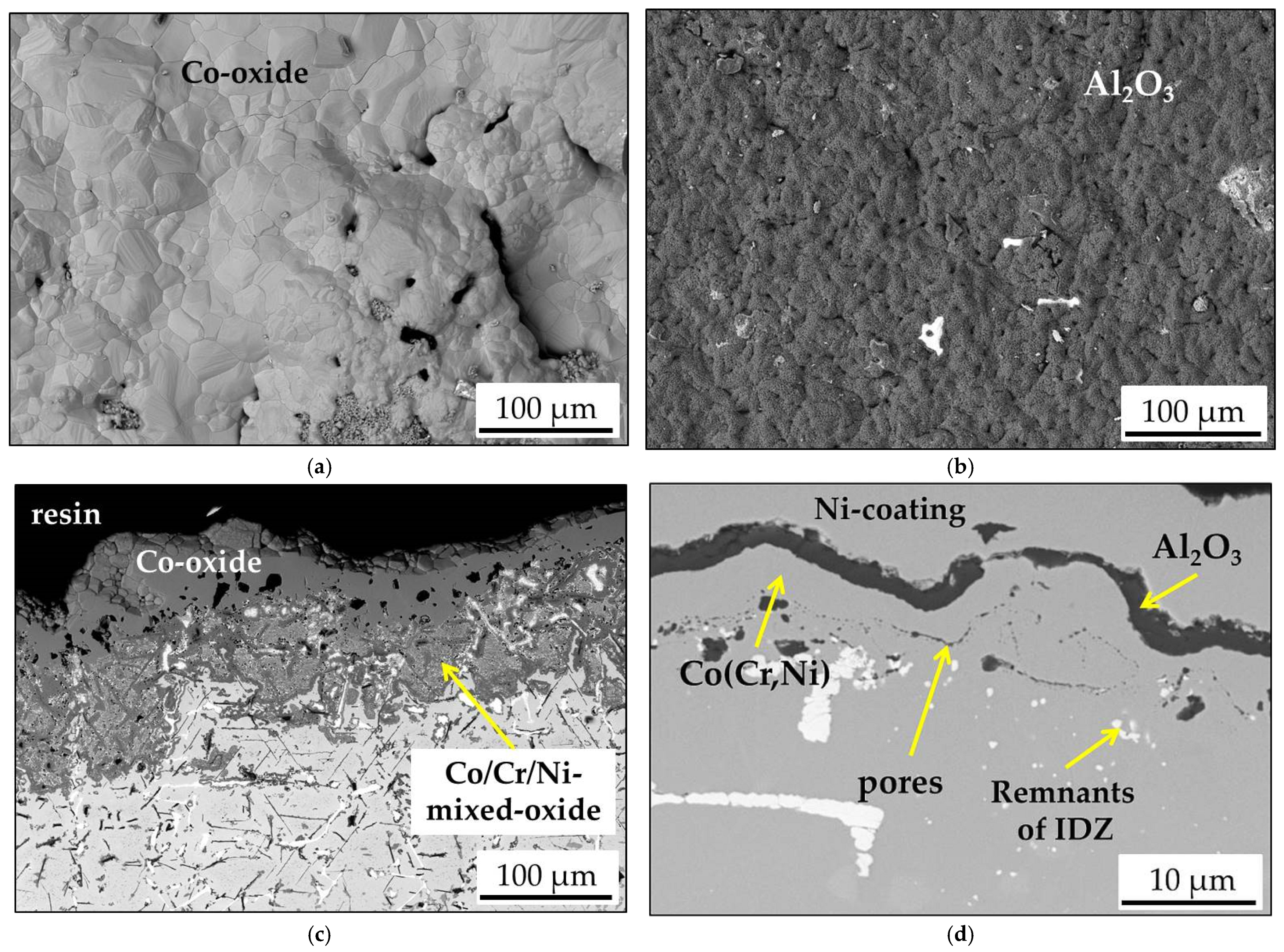
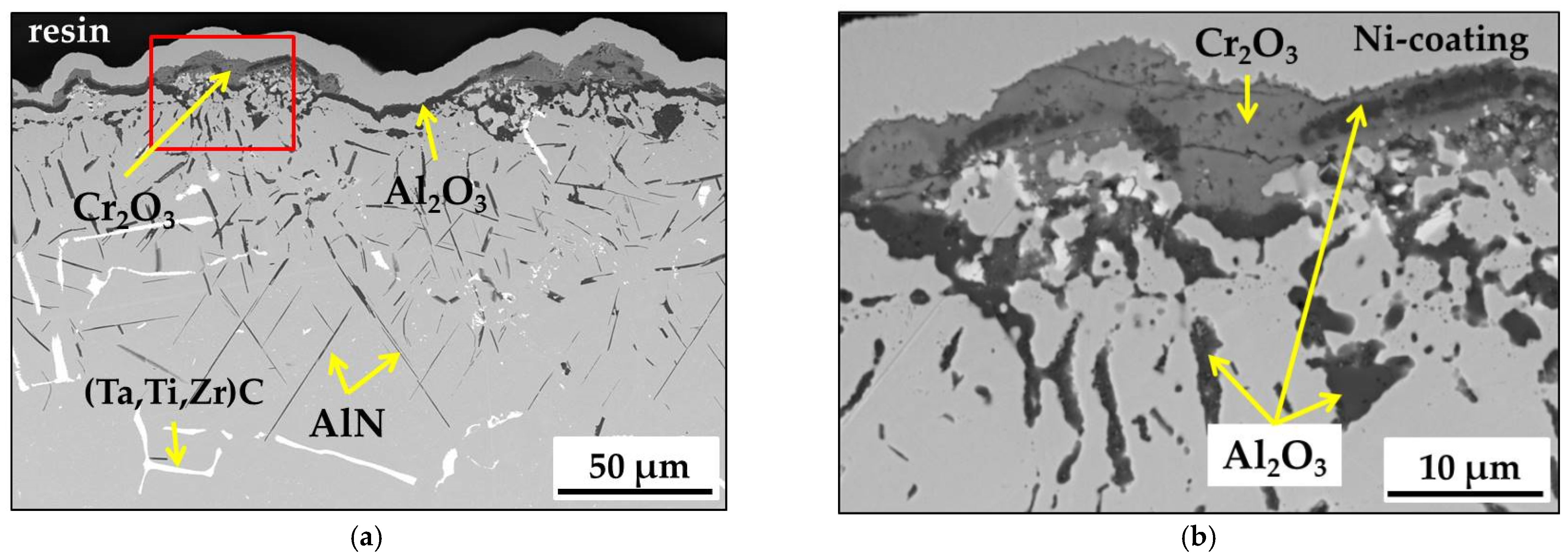
- During the short-term isothermal oxidation test, uncoated MAR M-509 forms an external oxide scale based on Cr2O3 accompanied by intensive formation of nitrides below the outer oxide scale. The formed nitrides were mainly identified as AlN.
- It was observed that internal nitridation plays an important role in the material degradation mechanism due to significant participation in the destabilization of strengthening precipitates, which are TaC via the formation of CrN at the edge of carbides, which becomes oxidized at a later stage.
- Aluminizing of MAR M-509 increases the oxidation resistance of MAR M-509 by decreasing the oxidation rate by a factor of 2.5 at 1000 °C and 1.5 at 1100 °C.
- The aluminide CoAl layer on MAR M-509 suppressed the observed degradation mechanism, including nitridation until the occurrence of breakaway oxidation.
- Aluminide layer with thickness of 10 µm provided increased oxidation resistance of MAR M-509 up to 730 cycles, i.e., 1460 hot hours at 1000 °C. At this stage, breakaway oxidation was observed.
- It is proposed that a further increase in oxidation resistance of MAR M-509 can be achieved by increasing the thickness of the produced CoAl layer.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schütze, M.; Quadakkers, W.J. Future Directions in the Field of High-Temperature Corrosion Research. Oxid. Met. 2017, 87, 681–704. [Google Scholar] [CrossRef]
- Leng, W.; Pillai, R.; Naumenko, D.; Galiullin, T.; Quadakkers, W.J. Effect of substrate alloy composition on the oxidation behaviour and degradation of aluminide coatings on two Ni base superalloys. Corros. Sci. 2020, 167, 108494. [Google Scholar] [CrossRef]
- Nowak, W.J.; Ochał, K.; Wierzba, P.; Gancarczyk, K.; Wierzba, B. Effect of Substrate Roughness on Oxidation Resistance of an Aluminized Ni-Base Superalloy. Metals 2019, 9, 782. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, S.; Jiang, C.Y.; Yu, C.T.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. Preparation and oxidation performance of a low-diffusion Pt-modified aluminide coating with Re-base diffusion barrier. Corros. Sci. 2020, 168, 108582. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Hadavi, S.M.M.; Palizdar, Y. Characterization, growth kinetics and formation mechanism of aluminide coating by plasma paste aluminizing on IN738. Vacuum 2021, 184, 109968. [Google Scholar] [CrossRef]
- Ghadami, F.; Aghdam, A.S.R.; Zakeri, A.; Saeedi, B.; Tahvili, P. Synergistic effect of CeO2 and Al2O3 nanoparticle dispersion on the oxidation behavior of MCrAlY coatings deposited by HVOF. Ceram. Int. 2020, 46, 4556–4567. [Google Scholar] [CrossRef]
- Zhang, P.; Yuan, K.; Peng, R.L.; Li, X.; Johansson, S. Long-term oxidation of MCrAlY coatings at 1000 °C and an Al-activity based coating life criterion. Surf. Coat. Technol. 2017, 332, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Kilic, M.; Ozkan, D.; Gok, M.S.; Karaoglanli, A.C. Room- and high temperature Wear Resistance of MCrAlY Coatings Deposited by Detonation Gun (D-gun) and Supersonic Plasma Spraying (SSPS) Techniques. Coatings 2020, 10, 1107. [Google Scholar] [CrossRef]
- Guo, D.; Zhao, L.; Jodoin, B. Cold spray for production of in-situ nanocrystalline MCrAlY coatings—Part II: Isothermal oxidation performance. Surf. Coat. Technol. 2021, 409, 126828. [Google Scholar] [CrossRef]
- Nowak, W.J.; Kubaszek, T.; Góral, M.; Wierzba, B. Durability of underaluminized thermal barrier coatings during exposure at high temperature. Surf. Coat. Technol. 2020, 382, 125236. [Google Scholar] [CrossRef]
- Ozgurluk, Y.; Doleker, K.M.; Ozkan, D.; Ahlatci, H.; Karaoglanli, A.C. Cyclic Hot Corrosion Failure Behaviors of EB-PVD TBC Systems in the Presence of Sulfate and Vanadate Molten Salts. Coatings 2019, 9, 166. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimzade, V.; Uchtmann, H.; Singheiser, L.; Küger, M.; Malzbender, J. Microstructure and cyclic oxidation behavior of APS TBC systems drilled with various laser methods. Surf. Coat. Technol. 2019, 378, 125018. [Google Scholar] [CrossRef]
- Moskal, G.; Mikuśkiewicz, M.; Jasik, A. Thermal diffusivity measurement of ceramic materials used in spraying of TBC systems. J. Therm. Anal. Calorim. 2019, 138, 4261–4269. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Tang, C.; Jianu, A.; Fetzer, R.; Weisenburger, A.; Steinbrueck, M.; Grosse, M.; Stieglitz, R.; Müller, G. Oxidation behavior and microstructure evolution of alumina-forming austenitic & high entropy alloys in steam environment at 1200 °C. Corros. Sci. 2020, 170, 108654. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Zhang, H.; Ni, N.; Li, L.; He, L.; Mu, R.; Zhao, X.; Guo, F. Y/Hf-doped AlCoCrFeNi high-entropy alloy with ultra oxidation and spallation resistance. Corros. Sci. 2020, 166, 108426. [Google Scholar] [CrossRef]
- Osei-Agyemang, E.; Balasubramanian, G. Surface oxidation mechanism of a refractory high-entropy alloy. NPJ Mater. Degrad. 2019, 3, 20. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Kong, X.; Chen, M.; Wang, Q.; Wang, F. High-entropy FeNiCoCr alloys with improved mechanical and tribological properties by tailoring composition and controlling oxidation. J. Mater. Sci. Technol. 2021, 82, 207–213. [Google Scholar] [CrossRef]
- Pan, Y.; Lu, X.; Hayat, M.D.; Yang, F.; Liu, C.C.; Li, Y.; Li, X.Y.; Xu, W.; Qu, H.H.; Cao, P. Effect of Sn addition on the high-temperature oxidation behavior of high Nb-containing TiAl alloys. Corros. Sci. 2020, 166, 108449. [Google Scholar] [CrossRef]
- Li, D.; Zhang, G.; Lu, G.; Wang, J.; Liu, C. Optimizing high-temperature oxidation behaviors of high-Nb-containing TiAl alloys by addition of boron. Corros. Sci. 2020, 177, 108971. [Google Scholar] [CrossRef]
- Pan, Y.; Lu, X.; Liu, C.; Hui, T.; Zhang, C.; Qu, X. Sintering densification, microstructure and mechanical properties of Sn-doped high Nb-containing TiAl alloys fabricated by pressureless sintering. Intermetallics 2020, 125, 106891. [Google Scholar] [CrossRef]
- Lohfeld, S.; Schütze, M.; Böhm, A.; Güther, V.; Rix, R.; Scholl, R. Oxidation behaviour of particle reinforced MoSi2 composites at temperatures up to 1700 °C. Mater. Corros. 2015, 56, 250–258. [Google Scholar] [CrossRef] [Green Version]
- Perepezko, J.H.; Harris, C. Oxidation of Mo-Si-B Alloys and Coatings in a Water Vapor Environment. Oxid. Met. 2021, 96, 323–332. [Google Scholar] [CrossRef]
- Su, R.; Zhang, H.; Ouyang, G.; Liu, L.; Nachlas, W.; Cui, J.; Johnson, D.D.; Perepezko, J.H. Enhanced oxidation resistance of (Mo95W5)85Ta10(TiZr)5 refractory multi-principal element alloy up to 1300 °C. Acta Mater. 2021, 215, 117114. [Google Scholar] [CrossRef]
- Makineni, S.K.; Singh, M.P.; Chattopadhyay, K. Low-Density, High-Temperature Co Base Superalloys. Annu. Rev. Mater. Res. 2021, 51, 187–208. [Google Scholar] [CrossRef]
- Ruan, J.; Xu, W.; Yang, T.; Yu, Y.; Yang, S.; Luan, J.; Omori, T.; Wang, C.; Kainuma, R.; Ishida, K.; et al. Accelerated design of novel W-free high-strength Co-base superalloys with extremely wide γ/γ’ region by machine learning and CALPHAD methods. Acta Mater. 2020, 186, 425–433. [Google Scholar] [CrossRef]
- Feng, L.; Lv, D.; Rhein, R.K.; Goiri, J.G.; Titus, M.S.; Van der Ven, A.; Pollock, T.M.; Wang, Y. Shearing of γ’ particles in Co-base and Co-Ni-base superalloys. Acta Mater. 2018, 161, 99–109. [Google Scholar] [CrossRef]
- Weiser, M.; Galetz, M.C.; Zschau, H.E.; Zenk, C.H.; Neumeier, S.; Göken, M.; Virtanen, S. Influence of Co to Ni ratio in γ’-strengthened model alloys on oxidation resistance and the efficacy of the halogen effect at 900 °C. Corros. Sci. 2019, 156, 84–95. [Google Scholar] [CrossRef]
- Pollock, T.M.; Dibbern, J.; Tsunekane, M.; Zhu, J.; Suzuki, A. New Co-based γ-γ′ high-temperature alloys. JOM 2010, 62, 58–63. [Google Scholar] [CrossRef]
- Bond, A.P.; Uhlig, H.H. Corrosion Behavior and Passivity of Nickel-Chromium and Cobalt-Chromium Alloys. J. Electrochem. Soc. 1960, 107, 488. [Google Scholar] [CrossRef]
- Kocijan, A.; Milošev, I.; Pihlar, B. Cobalt-based alloys for orthopaedic applications studied by electrochemical and XPS analysis. J. Mater. Sci. Mater. Med. 2004, 15, 643–650. [Google Scholar] [CrossRef]
- Migas, D.; Moskal, G.; Myalska, H.; Mikuszewski, T. The effect of alloying elements on oxide scale spallation of multicomponent Co-based superalloys. Corros. Sci. 2021, 192, 109787. [Google Scholar] [CrossRef]
- Weiser, M.; Chater, R.J.; Shollock, B.A.; Virtanen, S. Transport mechanisms during the high-temperature oxidation of ternary γ/γ′ Co-base model alloys. NPJ Mater. Degrad. 2019, 3, 33. [Google Scholar] [CrossRef]
- Kubacka, D.; Weiser, M.; Spiecker, E. Early stages of high-temperature oxidation of Ni- and Co-base model superalloys: A comparative study using rapid thermal annealing and advanced electron microscopy. Corros. Sci. 2021, 191, 109744. [Google Scholar] [CrossRef]
- Stewart, C.A.; Murray, S.P.; Suzuki, A.; Pollock, T.M.; Levi, C.G. Accelerated discovery of oxidation resistant CoNi-base γ/γ’ alloys with high L12 solvus and low density. Mater. Des. 2020, 189, 108445. [Google Scholar] [CrossRef]
- Rosas, A.O.P.; Epler, M.E.; Kernion, A.J.; Forsik, S.A.J.; Colombo, G.A.; Wang, T.; Zhou, N. High-Temperature Oxidation Behavior of a Novel Co-Base Superalloy. Metall. Mater. Trans. A 2018, 49, 4058–4069. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Sayre, G. Synthesis of simple and platinum-modified aluminide coatings on cobalt (Co)-base superalloys via a vapor phase aluminizing process. Surf. Coat. Technol. 2008, 203, 256–263. [Google Scholar] [CrossRef]
- Lee, J.W.; Kuo, Y.C. Cyclic oxidation behavior of a cobalt aluminide coating on Co-base superalloy AMS 5608. Surf. Coat. Technol. 2005, 200, 1225–1230. [Google Scholar] [CrossRef]
- Liu, P.S.; Liang, K.M.; Zhou, H.Y.; Gu, S.R.; Sun, X.F.; Guan, H.R.; Jin, T.; Yang, K.N. Cyclic oxidation behavior of aluminide coatings on the Co-base superalloy DZ40M. Surf. Coat. Technol. 2001, 145, 75–79. [Google Scholar] [CrossRef]
- Mróz, M.; Orłowicz, W.; Tupaj, M. Evaluation of fractures in MAR-M509 alloy samples after fatigue strength tests. Arch. Foundry Eng. 2010, 10, 115–118. [Google Scholar]
- Franclois, M.; Rémy, L. thermal-mechanical fatigue of mar-m 509 superalloy. comparison with low-cycle fatigue behaviour. Fatigue Fract. Eng. Mater. Struct. 1991, 14, 115–129. [Google Scholar] [CrossRef]
- Baufeld, B.; Tzimas, E.; Müllejans, H.; Peteves, S.; Bressers, J.; Stamm, W. Thermal-mechanical fatigue of MAR-M 509 with a thermal barrier coating. Mater. Sci. Eng. A 2001, 315, 231–239. [Google Scholar] [CrossRef]
- Galiullin, T.; Gobereit, B.; Naumenko, D.; Buck, R.; Amsbeck, L. Neises-von Puttkamer, M.; Quadakkers, W.J. High temperature oxidation and erosion of candidate materials for particle receivers of concentrated solar power tower systems. Sol. Energy 2019, 188, 883–889. [Google Scholar] [CrossRef]
- Berthod, P.; Gomis, J.P.; Medjahdi, G.; Panteix, P.J.; Aranda, L. A study of the dependence on the Co and Ni proportions of the oxidation at elevated temperature of TaC-strengthened {Ni and Co}-based cast superalloys. Mater. Chem. Phys. 2020, 251, 123088. [Google Scholar] [CrossRef]
- Pfeifer, J.P.; Holzbrecher, H.; Quadakkers, W.J.; Speier, J. Quantitative analysis of oxide films on ODS-alloys using MCs+-SIMS and e-beam SNMS. J. Anal. Chem. 1993, 346, 186–191. [Google Scholar] [CrossRef]
- Quadakkers, W.J.; Elschner, A.; Speier, W.; Nickel, H. Composition and growth mechanisms of alumina scales on FeCrAl-based alloys determined by SNMS. Appl. Surf. Sci. 1991, 52, 271–287. [Google Scholar] [CrossRef]
- Nowak, W.J. Characterization of oxidized Ni-based superalloys by GD-OES. J. Anal. At. Spectrom. 2017, 32, 1730–1738. [Google Scholar] [CrossRef]
- Nowak, W.J.; Wierzba, B. Effect of Surface Treatment on High Temperature Oxidation Behavior of IN 713C. J. Mater. Eng. Perform. 2018, 27, 5280–5290. [Google Scholar] [CrossRef]
- Nowak, W.J.; Siemek, K.; Ochał, K.; Kościelniak, B.; Wierzba, B. Consequences of different mechanical surface preparation of Ni-base alloys during high temperature exposure. Materials 2020, 13, 3529. [Google Scholar] [CrossRef]
- Essuman, E.; Meier, G.H.; Zurek, J.; Hänsel, M.; Norby, T.; Singheiser, L.; Quadakkers, W.J. Protective and non-protective scale formation of NiCr alloys in water vapour containing high- and low-pO2 gases. Corr. Sci. 2008, 50, 1753–1760. [Google Scholar] [CrossRef]
- Zurek, J.; Young, D.J.; Essuman, E.; Hänsel, M.; Penkalla, H.J.; Niewolak, L.; Quadakkers, W.J. Growth and adherence of chromia based surface scales on Ni-base alloys in high- and low-pO2 gases. Mater. Sci. Eng. A 2008, 477, 259–270. [Google Scholar] [CrossRef]
- Giggins, C.S.; Pettit, F.S. Oxidation of Ni-Cr-Al alloys between 1100 °C and 1200 °C. Solid. State Sci. 1971, 118, 1782–1790. [Google Scholar]
- Young, D.J. High Temperature Oxidation and Corrosion of Metals, 2nd ed.; Elsevier: Cambridge, UK, 2008. [Google Scholar]
- Hou, P.Y.; Cannon, R.M. Spallation Behavior of Thermally Grown Nickel Oxide on Nickel. Oxid. Met. 2009, 71, 237–256. [Google Scholar] [CrossRef]
- Mrowec, S.; Werber, T. Korozja Gazowa; WYDAWNICTWO “ŚLĄSK”: Katowice, Poland, 1965. [Google Scholar]
- Nix, F.C.; Jaumot, F.E., Jr. Self-Diffusion in Cobalt. Phys. Rev. 1951, 82, 72. [Google Scholar] [CrossRef]
- Barin, I. Thermochemical Data of Pure Substances; VCH: Weinheim, Germany, 1995. [Google Scholar]





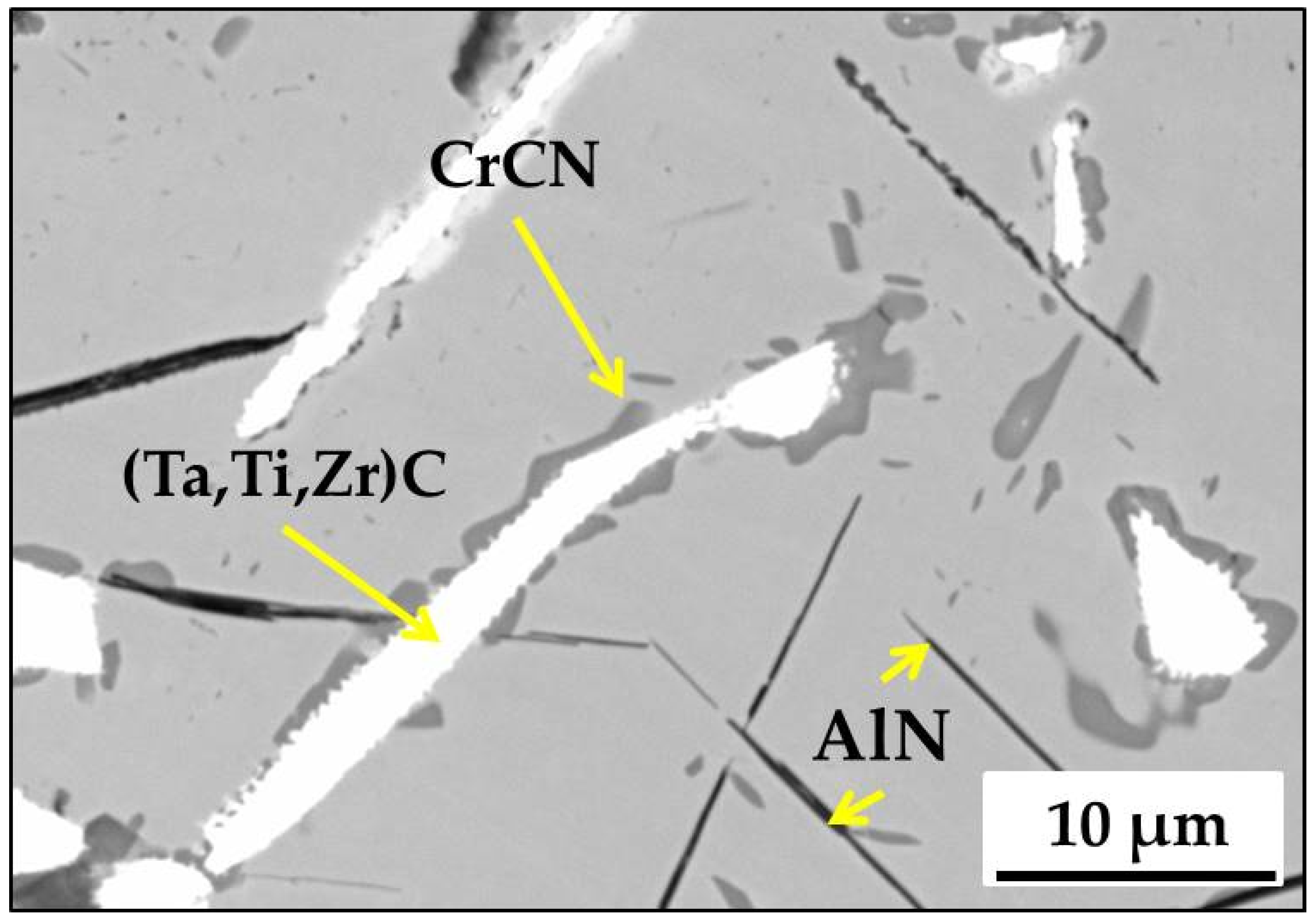
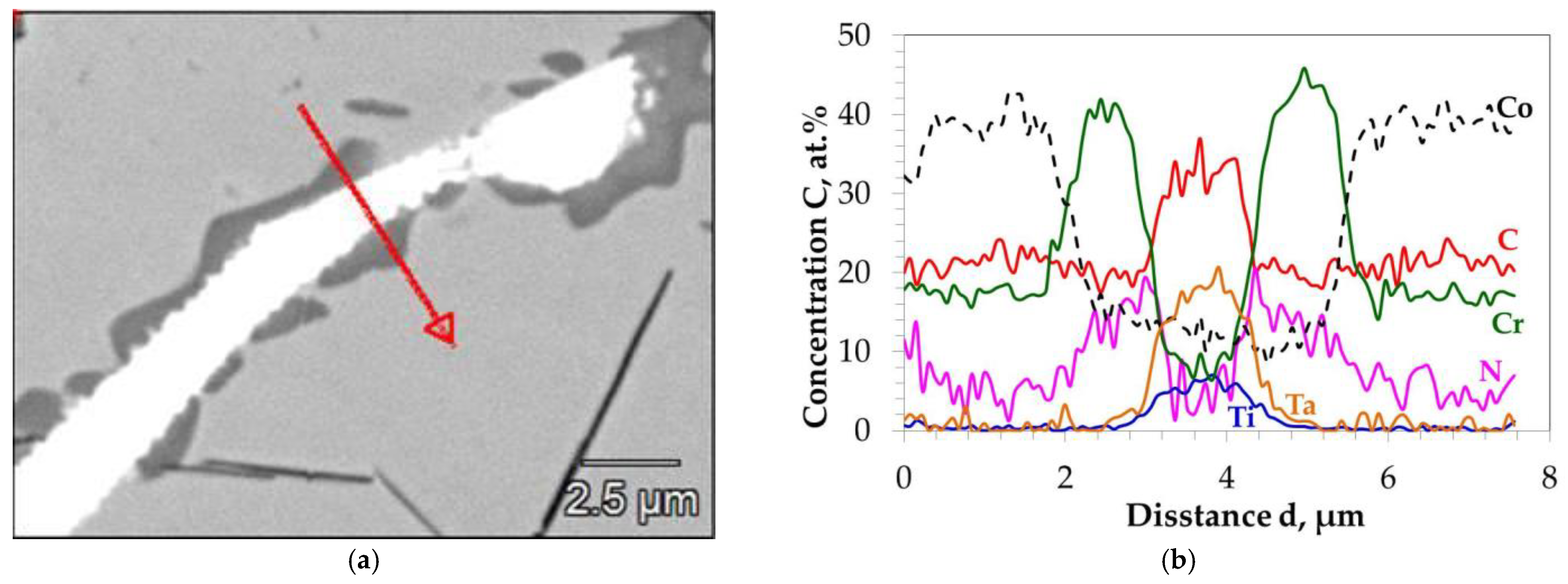
| Element | C | Ni | Cr | W | Ta | Zr | Ti | Fe | Al | Si | Co |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content in wt.% | 0.55 | 9.82 | 22.88 | 6.89 | 3.79 | 0.35 | 0.18 | 0.16 | 0.25 | 0.05 | BAL. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, W.J.; Hader, B.; Ochał, K.; Wierzba, B. Increase in Oxidation Resistance of MAR M-509 via LA-CVD Aluminizing. Coatings 2021, 11, 1306. https://doi.org/10.3390/coatings11111306
Nowak WJ, Hader B, Ochał K, Wierzba B. Increase in Oxidation Resistance of MAR M-509 via LA-CVD Aluminizing. Coatings. 2021; 11(11):1306. https://doi.org/10.3390/coatings11111306
Chicago/Turabian StyleNowak, Wojciech J., Bernadeta Hader, Kamil Ochał, and Bartek Wierzba. 2021. "Increase in Oxidation Resistance of MAR M-509 via LA-CVD Aluminizing" Coatings 11, no. 11: 1306. https://doi.org/10.3390/coatings11111306







