Eco-Friendly Protective Coating to Extend the Life of Art-Works and Structures Made in Porous Stone Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials: Protective Product and Stone Substrate
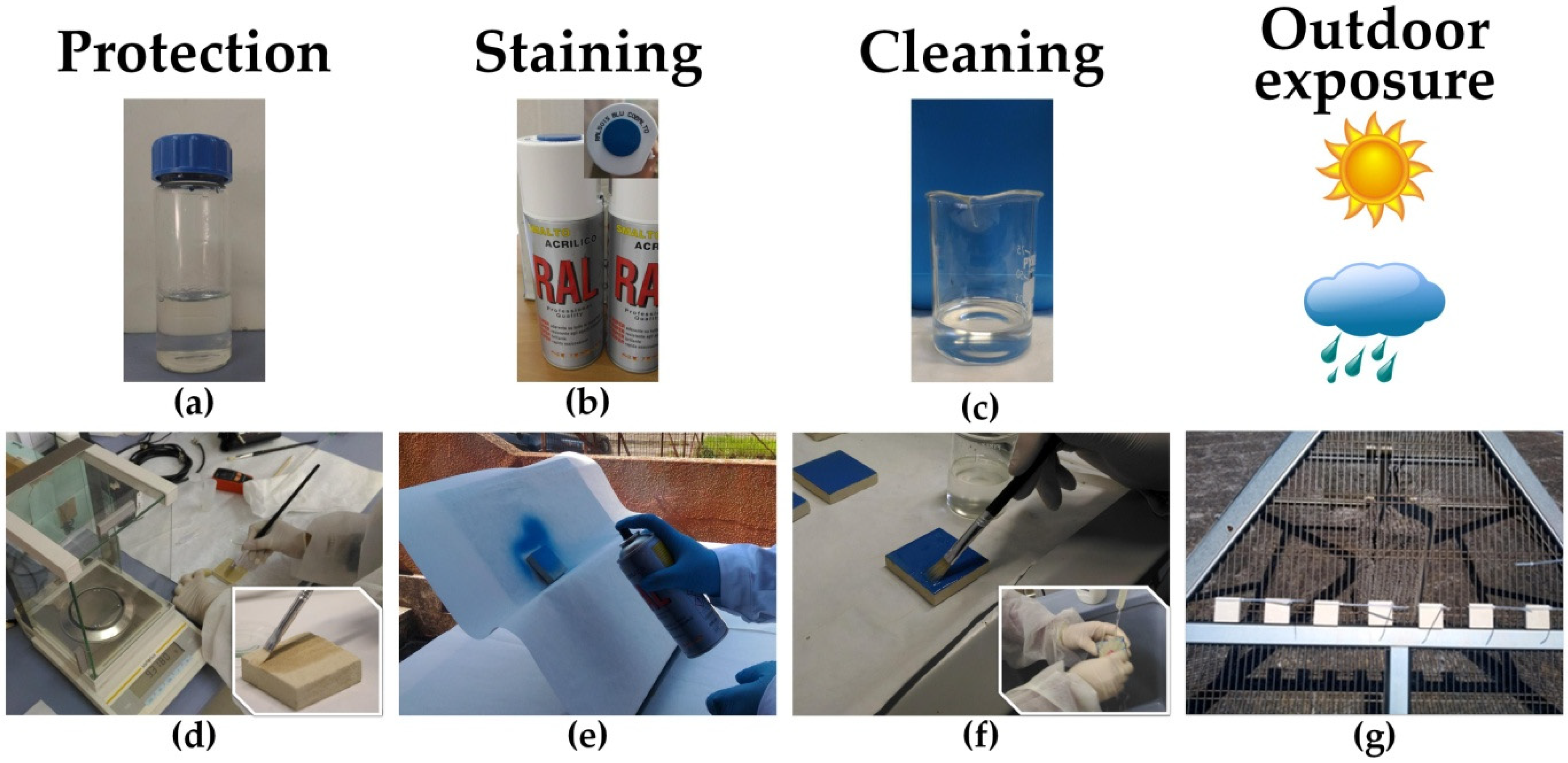
2.2. Application of the Protective Product and Experimental Investigations
2.2.1. Surface Protection
2.2.2. Anti-Graffiti Evaluation
2.2.3. Outdoor Exposure
2.3. Experimental Investigations
3. Results and Discussion
3.1. Evaluation of the Optimal Amount of Product
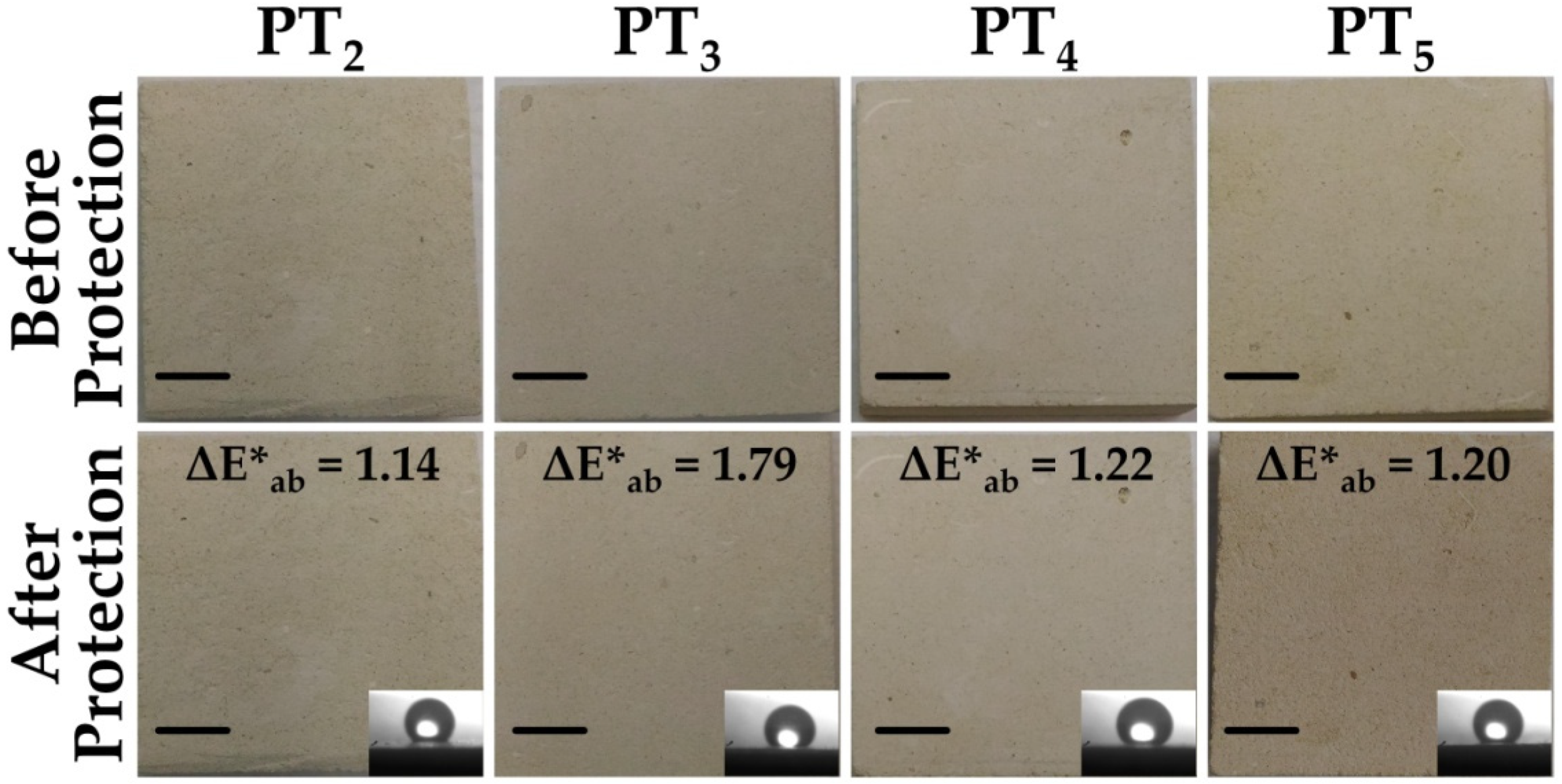
3.2. Harmlessness of the Treatment
3.3. Protective Efficacy
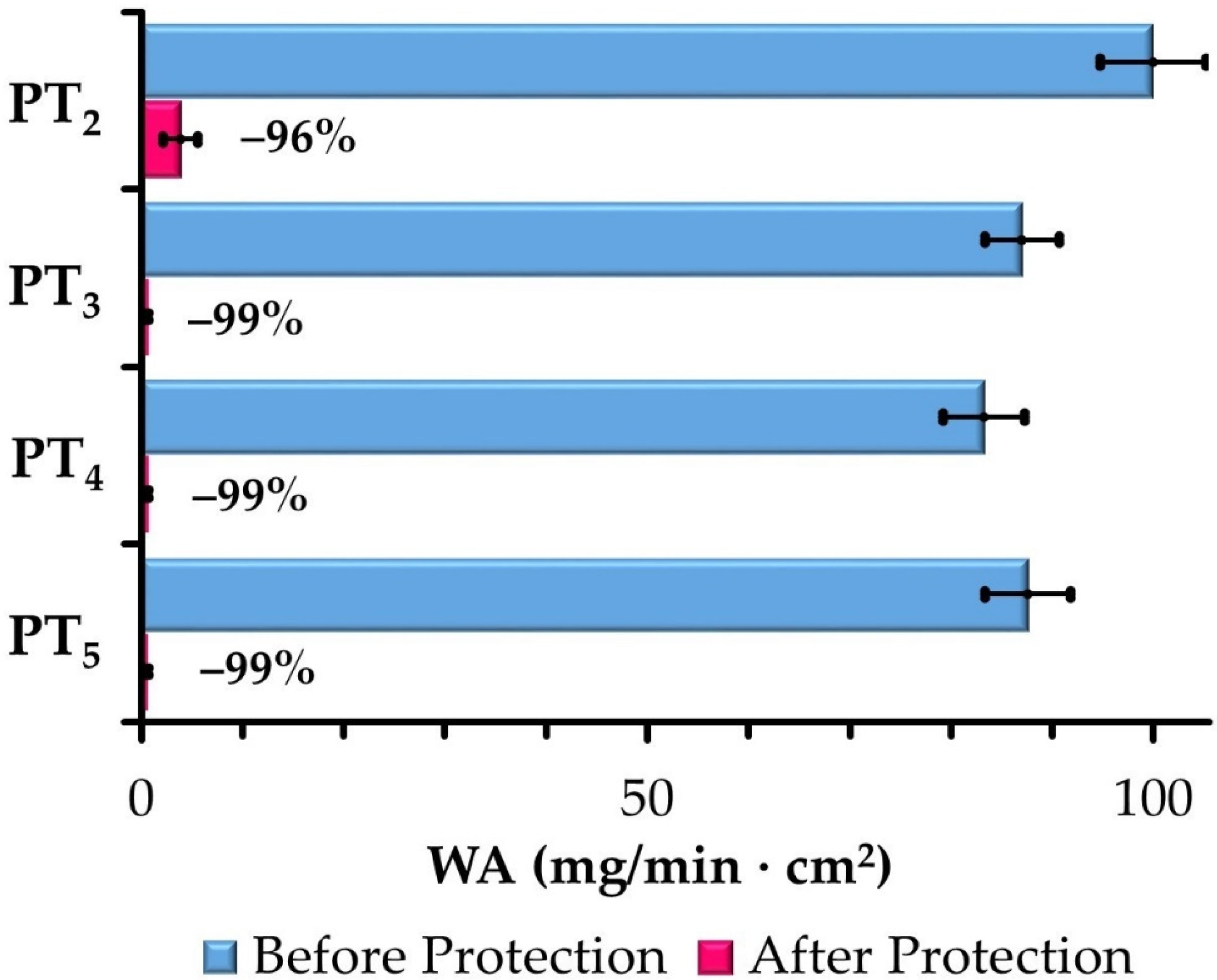
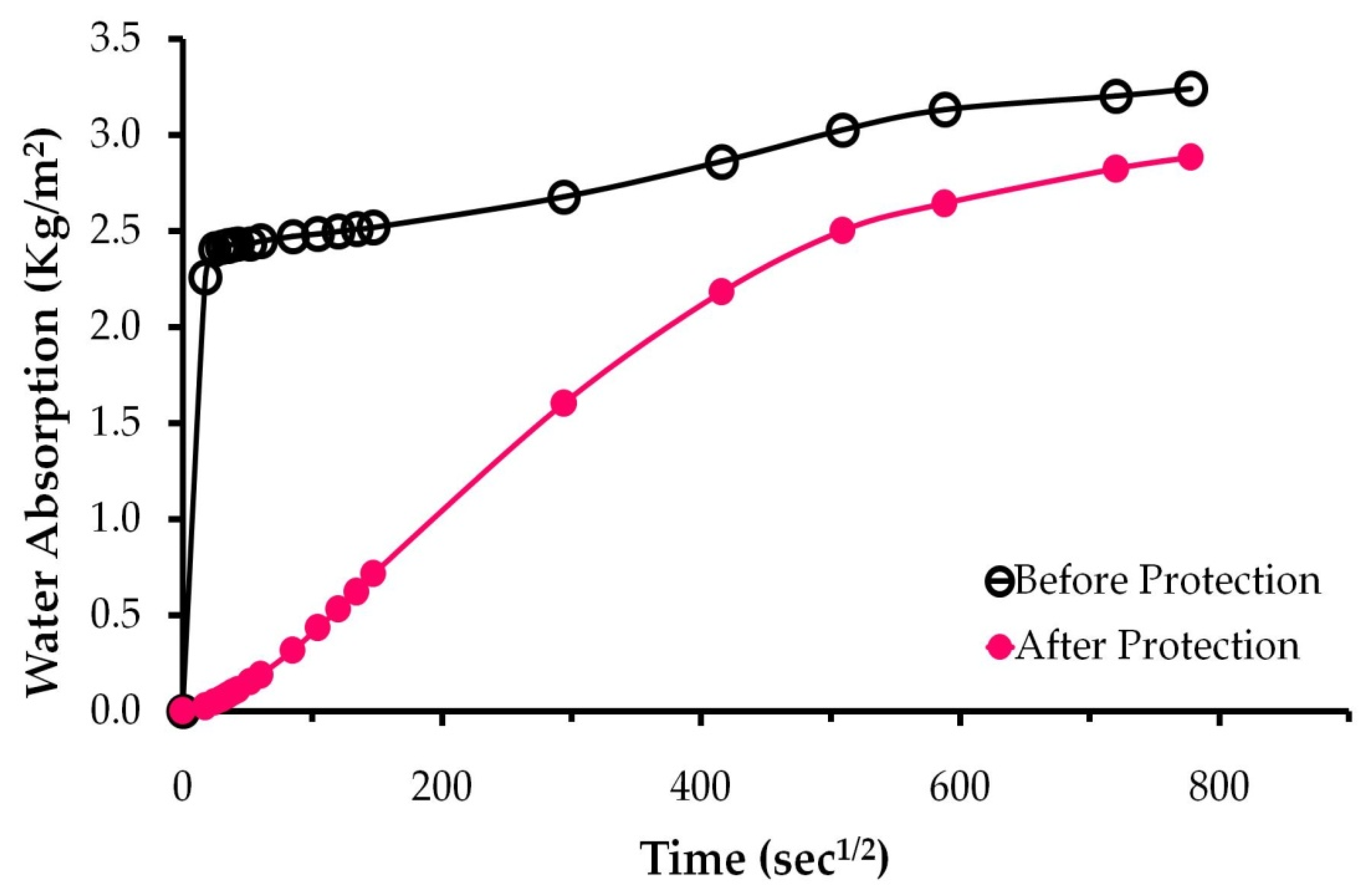
3.3.1. Barrier against Water Ingress
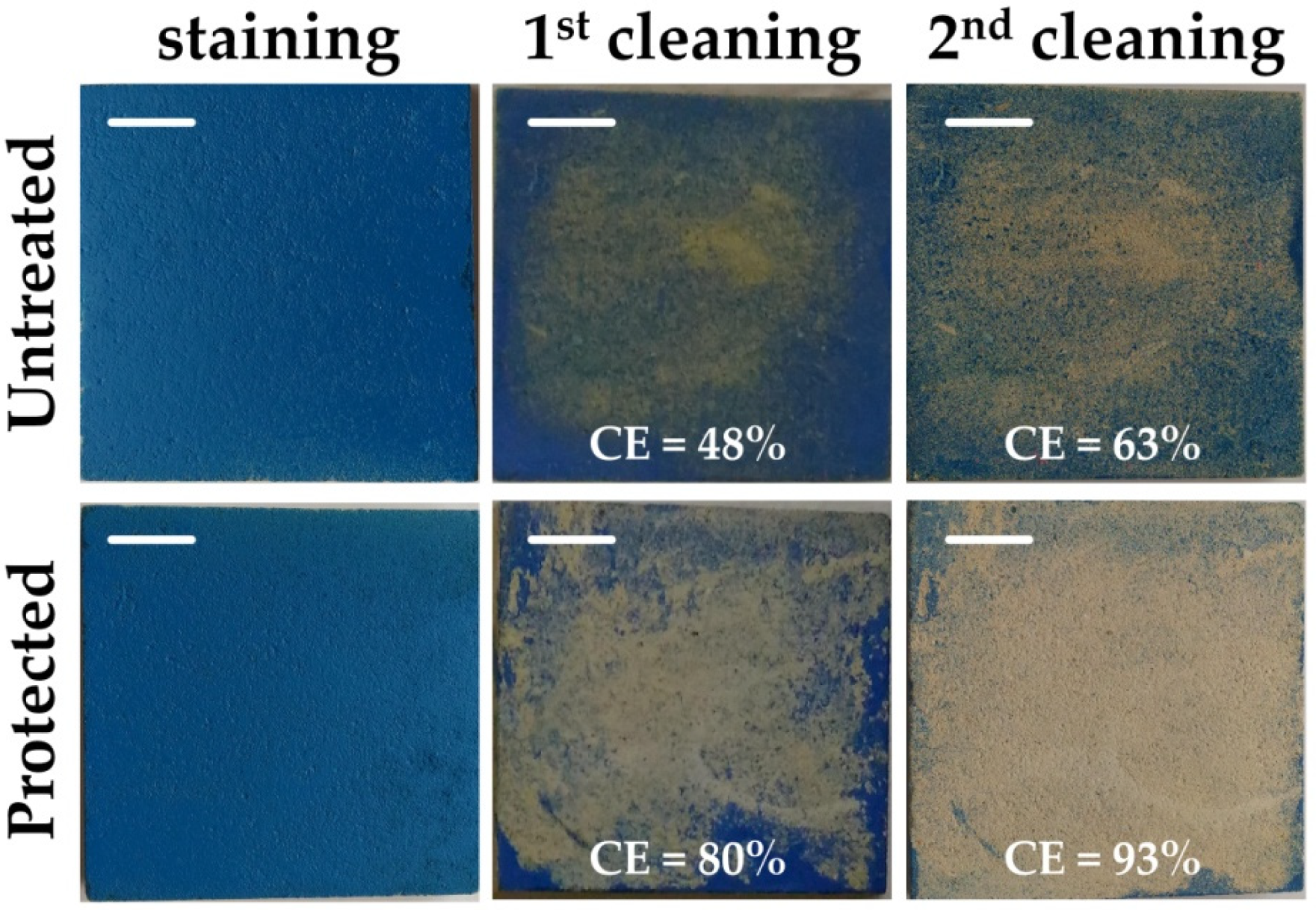
3.3.2. Anti-Graffiti Action
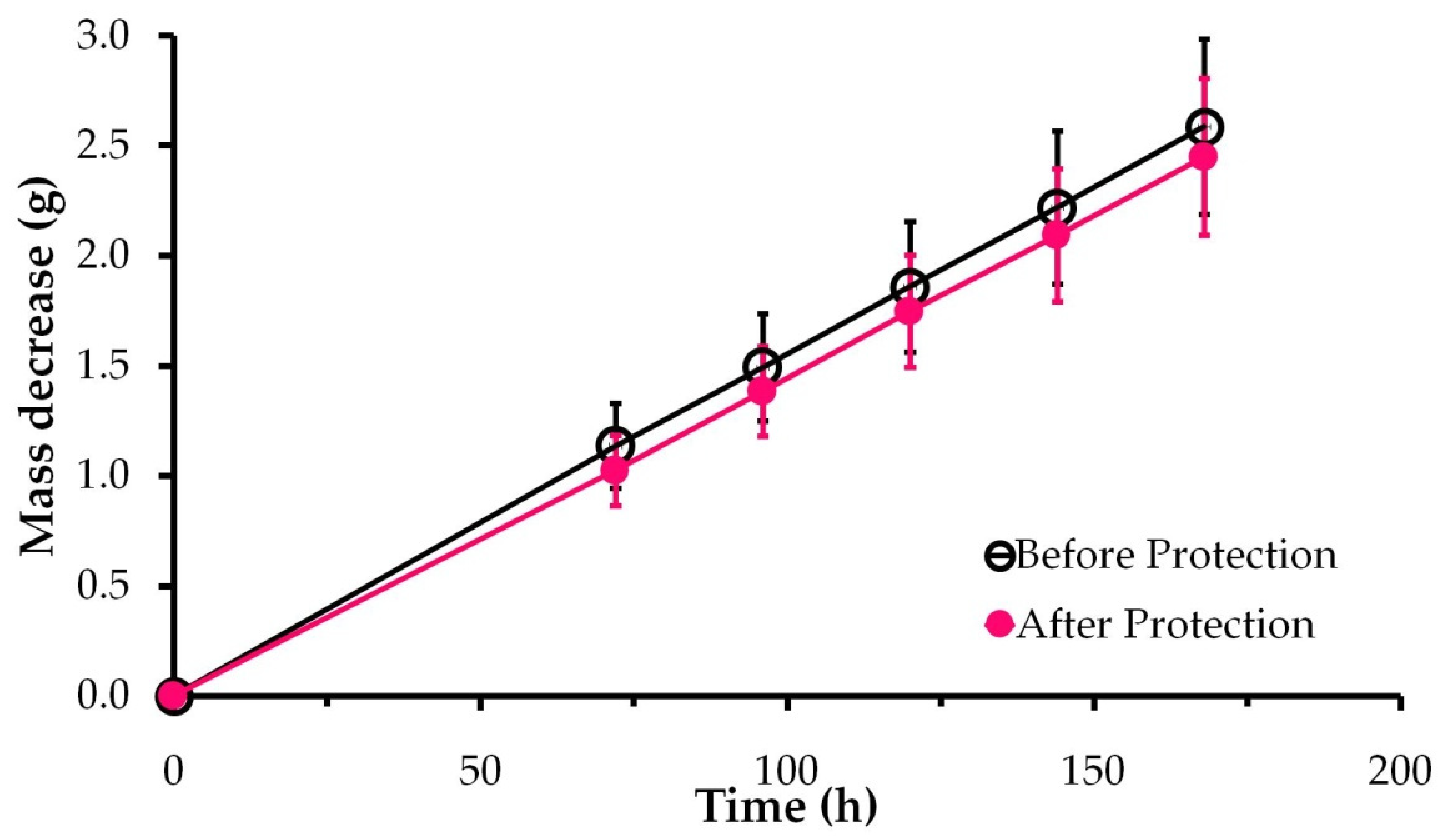
3.4. Durability to Short-Term Natural Aging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catarino, L.; Gil, F.P.S.C.; Quinta-Ferreira, M.; Marques, F. Characterization and rehabilitation of the “porta férrea” stone materials, university of coimbra, portugal. Environ. Earth Sci. 2018, 77, 416. [Google Scholar] [CrossRef]
- Leplat, J.; Bousta, F.; François, A.; Guiavarc’h, M.; Mertz, J.-D.; Brissaud, D. The pink staircase of sully-sur-loire castle: Even bacteria like historic stonework. Int. Biodeterior. Biodegrad. 2019, 145, 104805. [Google Scholar] [CrossRef]
- Calia, A.; Laurenzi Tabasso, M.; Maria Mecchi, A.; Quarta, G. The study of stone for conservation purposes: Lecce Stone (Southern Italy). Geol. Soc. Lond. Spec. Publ. 2014, 391, 139. [Google Scholar] [CrossRef]
- Punturo, R.; Russo, L.G.; Giudice, A.L.; Mazzoleni, P.; Pezzino, A. building stone employed in the historical monuments of Eastern Sicily (Italy). An example: The ancient city centre of Catania. Environ. Geol. 2006, 50, 156–169. [Google Scholar] [CrossRef]
- Cassar, J. The use of limestone in a historic context—The experience of malta. Geol. Soc. Lond. Spec. Publ. 2010, 331, 13. [Google Scholar] [CrossRef]
- Artesani, A.; Di Turo, F.; Zucchelli, M.; Traviglia, A. Recent advances in protective coatings for cultural heritage–An overview. Coatings 2020, 10, 217. [Google Scholar] [CrossRef] [Green Version]
- Baglioni, P.; Chelazzi, D. How science can contribute to the remedial conservation of cultural heritage. Chem. A Eur. J. 2021, 27, 10798–10806. [Google Scholar] [CrossRef]
- Sbardella, F.; Pronti, L.; Santarelli, M.L.; Asua Gonzàlez, J.; Bracciale, M. Waterborne acrylate-based hybrid coatings with enhanced resistance properties on stone surfaces. Coatings 2018, 8, 283. [Google Scholar] [CrossRef] [Green Version]
- Sbardella, F.; Bracciale, M.P.; Santarelli, M.L.; Asua, J.M. Waterborne modified-silica/acrylates hybrid nanocomposites as surface protective coatings for stone monuments. Prog. Org. Coat. 2020, 149, 105897. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Striani, R.; Frigione, M. Novel hydrophobic free-solvent UV-cured hybrid organic–inorganic methacrylic-based coatings for porous stones. Prog. Org. Coat. 2014, 77, 803–812. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Striani, R.; Frigione, M. Organic–inorganic UV-cured methacrylic-based hybrids as protective coatings for different substrates. Prog. Org. Coat. 2014, 77, 1117–1125. [Google Scholar] [CrossRef]
- Ocak, Y.; Sofuoglu, A.; Tihminlioglu, F.; Böke, H. Sustainable bio-nano composite coatings for the protection of marble surfaces. J. Cult. Herit. 2015, 16, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Zucchelli, M.; Mazzon, G.; Bertolacci, L.; Carzino, R.; Zendri, E.; Athanassiou, A. Stone sustainable protection and preservation using a zein-based hydrophobic coating. Prog. Org. Coat. 2021, 159, 106434. [Google Scholar] [CrossRef]
- Geurts, J.; Bouman, J.; Overbeek, A. New waterborne acrylic binders for zero VOC paints. J. Coat. Technol. Res. 2008, 5, 57–63. [Google Scholar] [CrossRef]
- Ho, J.; Mudraboyina, B.; Spence-Elder, C.; Resendes, R.; Cunningham, M.F.; Jessop, P.G. Water-Borne coatings that share the mechanism of action of oil-based coatings. Green Chem. 2018, 20, 1899–1905. [Google Scholar] [CrossRef]
- Hunek, D.B.-; Fic, S.; Styczen, J. Roughness and surface free energy of silicate brick hydrophobised with emulsions of low VOC content. IOP Conf. Ser. Mater. Sci. Eng. 2019, 471, 032012. [Google Scholar] [CrossRef]
- Lettieri, M.; Masieri, M.; Morelli, A.; Pipoli, M.; Frigione, M. Oleo/hydrophobic coatings containing nano-particles for the protection of stone materials having different porosity. Coatings 2018, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Lettieri, M.; Masieri, M.; Frigione, M. Novel nano-filled coatings for the protection of built heritage stone surfaces. Nanomaterials 2021, 11, 301. [Google Scholar] [CrossRef]
- Lettieri, M.; Masieri, M.; Pipoli, M.; Morelli, A.; Frigione, M. Anti-graffiti behavior of oleo/hydrophobic nano-filled coatings applied on natural stone materials. Coatings 2019, 9, 740. [Google Scholar] [CrossRef] [Green Version]
- Lettieri, M.; Masieri, M.; Frigione, M. Durability to simulated bird guano of nano-filled oleo/hydrophobic coatings for the protection of stone materials. Prog. Org. Coat. 2020, 148, 105900. [Google Scholar] [CrossRef]
- IBIX Biocare Website. Available online: https://www.ibixbiocare.it/images/documenti/schede-tecniche/scheda-tecnica-protect-itr.pdf (accessed on 4 October 2021).
- Torrisi, A. Evaluation of five fluorinated compounds as calcarenite protectives. J. Cult. Herit. 2008, 9, 135–145. [Google Scholar] [CrossRef]
- Lettieri, M.; Masieri, M. Surface characterization and effectiveness evaluation of anti-graffiti coatings on highly porous stone materials. Appl. Surf. Sci. 2014, 288, 466–477. [Google Scholar] [CrossRef]
- Camaiti, M.; Bortolotti, V.; Cao, Y.; Papacchini, A.; Salvini, A.; Brizi, L. High efficiency fluorinated oligo(ethylenesuccinamide) coating for stone. Coatings 2021, 11, 452. [Google Scholar] [CrossRef]
- Gemelli, G.M.C.; Zarzuela, R.; Fernandez, F.; Mosquera, M.J. Compatibility, effectiveness and susceptibility to degradation of alkoxysilane-based consolidation treatments on a carbonate stone. J. Build. Eng. 2021, 42, 102840. [Google Scholar] [CrossRef]
- Bianco, L. Geochemistry, mineralogy and textural properties of the lower globigerina limestone used in the built heritage. Minerals 2021, 11, 740. [Google Scholar] [CrossRef]
- Beck, K.; Al-Mukhtar, M.; Rozenbaum, O.; Rautureau, M. Characterization, water transfer properties and deterioration in tuffeau: Building material in the Loire Valley—France. Build. Environ. 2003, 38, 1151–1162. [Google Scholar] [CrossRef] [Green Version]
- Ševčík, R.; Viani, A.; Mancini, L.; Appavou, M.-S.; Machová, D. Investigation of nano-microstructural changes in maastricht limestone after treatment with nanolime suspension. Appl. Phys. A 2020, 126, 367. [Google Scholar] [CrossRef]
- Sena da Fonseca, B.; Ferreira Pinto, A.P.; Rodrigues, A.; Piçarra, S.; Montemor, M.F. The role of properties on the decay susceptibility and conservation issues of soft limestones: Contribution of Ançã Stone (Portugal). J. Build. Eng. 2021, 44, 102997. [Google Scholar] [CrossRef]
- Bugani, S.; Camaiti, M.; Morselli, L.; Van de Casteele, E.; Koen, J. Investigation on porosity changes of lecce stone due to conservation treatments by means of X-Ray nano- and improved micro-computed tomography: Preliminary results. X-ray Spectrom. 2007, 36, 316–320. [Google Scholar] [CrossRef]
- Föllmi, K.B.; Hofmann, H.; Chiaradia, M.; de Kaenel, E.; Frijia, G.; Parente, M. Miocene phosphate-rich sediments in Salento (Southern Italy). Sediment. Geol. 2015, 327, 55–71. [Google Scholar] [CrossRef]
- Tiano, P.; Accolla, P.; Tomaselli, L. Phototrophic biodeteriogens on lithoid surfaces: An ecological study. Microb. Ecol. 1995, 29, 299–309. [Google Scholar] [CrossRef] [PubMed]
- UNI 10921: Beni Culturali Materiali Lapidei Naturali Ed Artificiali—Prodotti Idrorepellenti—Applicazione Su Provini E Determinazione in Laboratorio Delle Loro Caratteristiche; Ente Italiano di normazione: Milan, Italy, 2001.
- Sacchi, B.; Vettori, S.; Andreotti, A.; Rampazzi, L.; Colombini, M.P.; Tiano, P. Assessment of water repellent treatments for the stone of the matera Cathedral Facade (Italy). Int. J. Archit. Herit. 2020, 1–9. [Google Scholar] [CrossRef]
- Occhipinti, R.; Stroscio, A.; Maria Belfiore, C.; Barone, G.; Mazzoleni, P. Chemical and colorimetric analysis for the characterization of degradation forms and surface colour modification of building stone materials. Constr. Build. Mater. 2021, 302, 124356. [Google Scholar] [CrossRef]
- EN 15886. Conservation of Cultural Property—Test Methods—Colour Measurement of Surfaces; CEN (European Committee for Standardization): Brussels, Belgium, 2010. [Google Scholar]
- UNI 11432: Beni culturali Materiali Lapidei Naturali Ed Artificiali—Misura Della Capacità Di Assorbimento Di Acqua Mediante Spugna Di Contatto; Ente Italiano di normazione: Milan, Italy, 2011.
- Vandevoorde, D.; Pamplona, M.; Schalm, O.; Vanhellemont, Y.; Cnudde, V.; Verhaeven, E. Contact sponge method: Performance of a promising tool for measuring the initial water absorption. J. Cult. Herit. 2009, 10, 41–47. [Google Scholar] [CrossRef]
- EN 15802. Conservation of Cultural Property—Test Methods—Determination of Static Contact Angle; CEN (European Committee for Standardization): Brussels, Belgium, 2010. [Google Scholar]
- Lettieri, M.; Masieri, M. Performances and coating morphology of a siloxane-based hydrophobic product applied in different concentrations on a highly porous stone. Coatings 2016, 6, 60. [Google Scholar] [CrossRef]
- NORMAL Rec. 21/85 Permeabilità al Vapor D’acqua; CNR/ICR: Rome, Italy, 1985.
- Karapanagiotis, I.; Hosseini, M. Superhydrophobic coatings for the protection of natural stone. In Advanced Materials for the Conservation of Stone; Hosseini, M., Karapanagiotis, I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–25. ISBN 978-3-319-72260-3. [Google Scholar]
- EN 15801. Conservation of Cultural Property—Test Methods—Determination of Water Absorption by Capillarity; CEN (European Committee for Standardization): Brussels, Belgium, 2009. [Google Scholar]
- Chatzigrigoriou, A.; Manoudis, P.N.; Karapanagiotis, I. Fabrication of water repellent coatings using waterborne resins for the protection of the cultural heritage. Macromol. Symp. 2013, 331–332, 158–165. [Google Scholar] [CrossRef]
- Pargoletti, E.; Motta, L.; Comite, V.; Fermo, P.; Cappelletti, G. The hydrophobicity modulation of glass and marble materials by different si-based coatings. Prog. Org. Coat. 2019, 136, 105260. [Google Scholar] [CrossRef] [Green Version]
- Esposito Corcione, C.; De Simone, N.; Santarelli, M.L.; Frigione, M. Protective properties and durability characteristics of experimental and commercial organic coatings for the preservation of porous stone. Prog. Org. Coat. 2017, 103, 193–203. [Google Scholar] [CrossRef]
- Calia, A.; Lettieri, M.; Quarta, G.; Laurenzi Tabasso, M.; Mecchi, A. Documentation and assessment of the most important conservation treatments carried out on lecce stone monuments in the last two decades. In Proceedings of the 10th International Congress on Deterioration and Conservation of Stone, Stockholm, Sweden, 27 June–2 July 2004; Kwiatkowski, D., Lofvendahl, R., Eds.; ICOMOS: Stockholm, Sweden, 2004; Volume 1, pp. 431–438. [Google Scholar]
- Mosquera, M.J.; Pozo, J.; Esquivias, L. Stress during drying of two stone consolidants applied in monumental conservation. J. Sol Gel Sci. Technol. 2003, 26, 1227–1231. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Panayiotou, C. Tuning the wetting properties of siloxane-nanoparticle coatings to induce superhydrophobicity and superoleophobicity for stone protection. Mater. Des. 2016, 108, 736–744. [Google Scholar] [CrossRef]
- Krzemień, L.; Łukomski, M.; Bratasz, Ł.; Kozłowski, R.; Mecklenburg, M.F. Mechanism of craquelure pattern formation on panel paintings. Stud. Conserv. 2016, 61, 324–330. [Google Scholar] [CrossRef]
- Atkinson, A.; Guppy, R.M. Mechanical stability of sol-gel films. J. Mater. Sci. 1991, 26, 3869–3873. [Google Scholar] [CrossRef]
- Doehne, E.; Price, C.A. Stone Conservation: An Overview of Current Research; Research in Conservation, 2nd ed.; Getty Conservation Institute: Los Angeles, CA, USA, 2010; ISBN 978-1-60606-046-9. [Google Scholar]
- Snethlage, R.; Sterflinger, K. Stone conservation. In Stone in Architecture; Springer: Berlin/Heidelberg, Germany, 2011; pp. 411–544. [Google Scholar]
- Bergamonti, L.; Alfieri, I.; Franzò, M.; Lorenzi, A.; Montenero, A.; Predieri, G.; Raganato, M.; Calia, A.; Lazzarini, L.; Bersani, D.; et al. Synthesis and characterization of nanocrystalline TiO2 with application as photoactive coating on stones. Environ. Sci. Pollut. Res. 2014, 21, 13264–13277. [Google Scholar] [CrossRef] [PubMed]
- Vasanelli, E.; Calia, A.; Masieri, M.; Baldi, G. Stone consolidation with SiO2 nanoparticles: Effects on a high porosity limestone. Constr. Build. Mater. 2019, 219, 154–163. [Google Scholar] [CrossRef]
- Di Tullio, V.; Proietti, N.; Capitani, D.; Nicolini, I.; Mecchi, A.M. NMR depth profiles as a non-invasive analytical tool to probe the penetration depth of hydrophobic treatments and inhomogeneities in treated porous stones. Anal. Bioanal. Chem. 2011, 400, 3151–3164. [Google Scholar] [CrossRef]
- Scrivano, S.; Gaggero, L. Non-Invasive analytical technique to address water uptake on stone surfaces: The implemented contact sponge method (i-CSM). J. Cult. Herit. 2017, 28, 9–15. [Google Scholar] [CrossRef]
- Rodrigues, J.D.; Grossi, A. Indicators and ratings for the compatibility assessment of conservation actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]
- Becherini, F.; Pastorelli, G.; Valotto, G.; Gambirasi, A.; Bianchin, S.; Favaro, M. Effects of protective treatments on particle deposition and colour variation in stone surfaces exposed to an urban environment. Prog. Org. Coat. 2017, 112, 75–85. [Google Scholar] [CrossRef]
- Colangiuli, D.; Lettieri, M.; Masieri, M.; Calia, A. Field study in an urban environment of simultaneous self-cleaning and hydrophobic nanosized TiO2-based coatings on stone for the protection of building surface. Sci. Total Environ. 2019, 650, 2919–2930. [Google Scholar] [CrossRef] [PubMed]
- Licchelli, M.; Marzolla, S.; Carò, F.; Moggi, G. Stone surface protection by fluoropolymers from decay caused by mural writings. Struct. Anal. Hist. Constr. Ed. Paulo B. Lourenco Pere Roca Claudio Modena Shailesh Agrawal MacMillan India Ltd Delhi 2006, 1405–1412. [Google Scholar]
- Sabatini, V.; Pargoletti, E.; Comite, V.; Ortenzi, M.A.; Fermo, P.; Gulotta, D.; Cappelletti, G. Towards novel fluorinated methacrylic coatings for cultural heritage: A combined polymers and surfaces chemistry study. Polymers 2019, 11, 1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Samples | Suggested Coverage (m2/L) | Actual Amount of Product (mL/specimen) | Consumption (L/m2) |
|---|---|---|---|
| PT2 | 2 | 1.25 | 0.200 |
| PT3 | 3 | 0.83 | 0.248 |
| PT4 | 4 | 0.62 | 0.332 |
| PT5 | 5 | 0.50 | 0.500 |
| Daily Average | Maximum | Minimum | Cumulative | |
|---|---|---|---|---|
| T (°C) | 22.2 | 34.1 | 12.4 | --- |
| R.H. (%) | 67.2 | 99.7 | 26.3 | --- |
| Rain (mm) | --- | 0.8 | 0.0 | 5.2 |
| Solar light dose (MJ/m2) | 0.04 | 0.08 | 0.00 | 30.02 |
| Wind speed (m/s) | 2.031 | 6.971 | 0.017 | --- |
| Samples | Before Protection | After Protection | |
|---|---|---|---|
| L* | PT2 | 82.54 ± 0.50 | 82.04 ± 0.44 |
| PT3 | 82.64 ± 0.53 | 81.64 ± 0.44 | |
| PT4 | 82.52 ± 0.60 | 81.81 ± 0.24 | |
| PT5 | 83.04 ± 0.36 | 82.25 ± 0.58 | |
| a* | PT2 | 2.02 ± 0.18 | 2.10 ± 0.14 |
| PT3 | 1.94 ± 0.13 | 2.15 ± 0.10 | |
| PT4 | 2.00 ± 0.13 | 2.13 ± 0.09 | |
| PT5 | 2.12 ± 0.15 | 2.26 ± 0.14 | |
| b* | PT2 | 14.05 ± 0.46 | 15.08 ± 0.42 |
| PT3 | 13.91 ± 0.51 | 15.38 ± 0.41 | |
| PT4 | 13.80 ± 0.45 | 14.79 ± 0.42 | |
| PT5 | 14.73 ± 0.44 | 15.62 ± 0.50 | |
| WA (mg/min × cm2) | PT2 | 99.95 ± 5.24 | 3.83 ± 1.70 |
| PT3 | 87.00 ± 3.68 | 0.66 ± 0.10 | |
| PT4 | 83.24 ± 4.03 | 0.65 ± 0.08 | |
| PT5 | 87.60 ± 4.23 | 0.62 ± 0.11 | |
| A (°) | PT2 | Not determinable | 141 ± 6 |
| PT3 | Not determinable | 141 ± 4 | |
| PT4 | Not determinable | 132 ± 4 | |
| PT5 | Not determinable | 140 ± 4 |
| Untreated | Protected | |
|---|---|---|
| G ((g/h) × 10−3) | 15.4 ± 2.4 | 14.6 ± 2.1 |
| WVTR (g/m2 × 24 h) | 212 ± 30 | 196 ± 26 |
| RVP (%) | --- | 8 ± 4 |
| Samples | Before Exposure | After Exposure | Variation | |
|---|---|---|---|---|
| L* | Untreated | 82.27 ± 0.36 | 84.72 ± 0.42 | 2.45 ± 0.12 |
| Protected | 82.12 ± 0.82 | 84.95 ± 0.74 | 2.83 ± 0.34 | |
| a* | Untreated | 2.17 ± 0.08 | 1.55 ± 0.13 | −0.62 ± 0.01 |
| Protected | 2.10 ± 0.26 | 1.43 ± 0.28 | −0.67 ± 0.07 | |
| b* | Untreated | 15.36 ± 0.62 | 13.77 ± 0.74 | −1.58 ± 0.07 |
| Protected | 15.08 ± 0.82 | 12.52 ± 0.92 | −2.56 ± 0.28 | |
| ΔE*ab | Untreated | --- | 2.98 ± 0.07 | --- |
| Protected | --- | 3.88 ± 0.41 | --- | |
| WA (mg/min × cm2) | Untreated | 102.76 ± 9.28 | 100.36 ± 7.36 | −2.40 ± 1.92 |
| Protected | 0.82 ± 0.15 | 1.01 ± 0.25 | 0.19 ± 0.13 | |
| A (°) | Untreated | N.D. | N.D. | --- |
| Protected | 144 ± 2 | 134 ± 5 | −10 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lettieri, M.; Masieri, M.; Aquaro, M.; Dilorenzo, D.; Frigione, M. Eco-Friendly Protective Coating to Extend the Life of Art-Works and Structures Made in Porous Stone Materials. Coatings 2021, 11, 1270. https://doi.org/10.3390/coatings11111270
Lettieri M, Masieri M, Aquaro M, Dilorenzo D, Frigione M. Eco-Friendly Protective Coating to Extend the Life of Art-Works and Structures Made in Porous Stone Materials. Coatings. 2021; 11(11):1270. https://doi.org/10.3390/coatings11111270
Chicago/Turabian StyleLettieri, Mariateresa, Maurizio Masieri, Marika Aquaro, Debora Dilorenzo, and Mariaenrica Frigione. 2021. "Eco-Friendly Protective Coating to Extend the Life of Art-Works and Structures Made in Porous Stone Materials" Coatings 11, no. 11: 1270. https://doi.org/10.3390/coatings11111270










