Corrosion Law of Metal Pipeline in Tahe Oilfield and Application of New Materials
Abstract
:1. Introduction
2. Analysis of Main Corrosion Factors
2.1. Experimental Conditions
2.2. Experimental Results
2.2.1. The Effect of CO2 on the Material of 20# Steel
2.2.2. The Effect of H2S on the Material of 20# Steel
2.2.3. The Effect of Cl− on the Material of 20# Steel
3. Optimization of Corrosion-Resistant Metal Pipe Material
3.1. Chemical Composition Optimization
3.2. Mechanical Properties of Optimized Materials
3.3. Corrosion Resistance Comparison
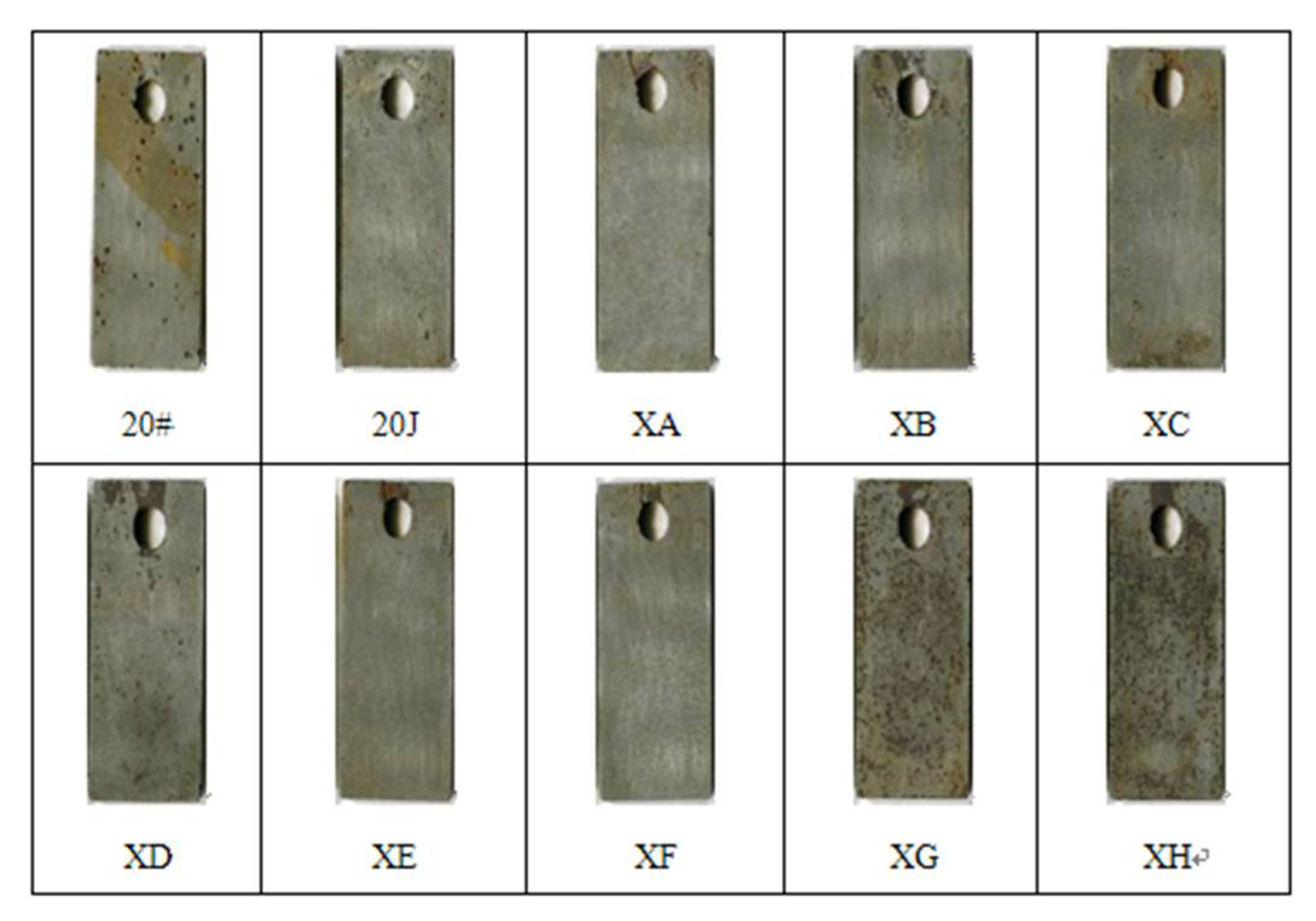
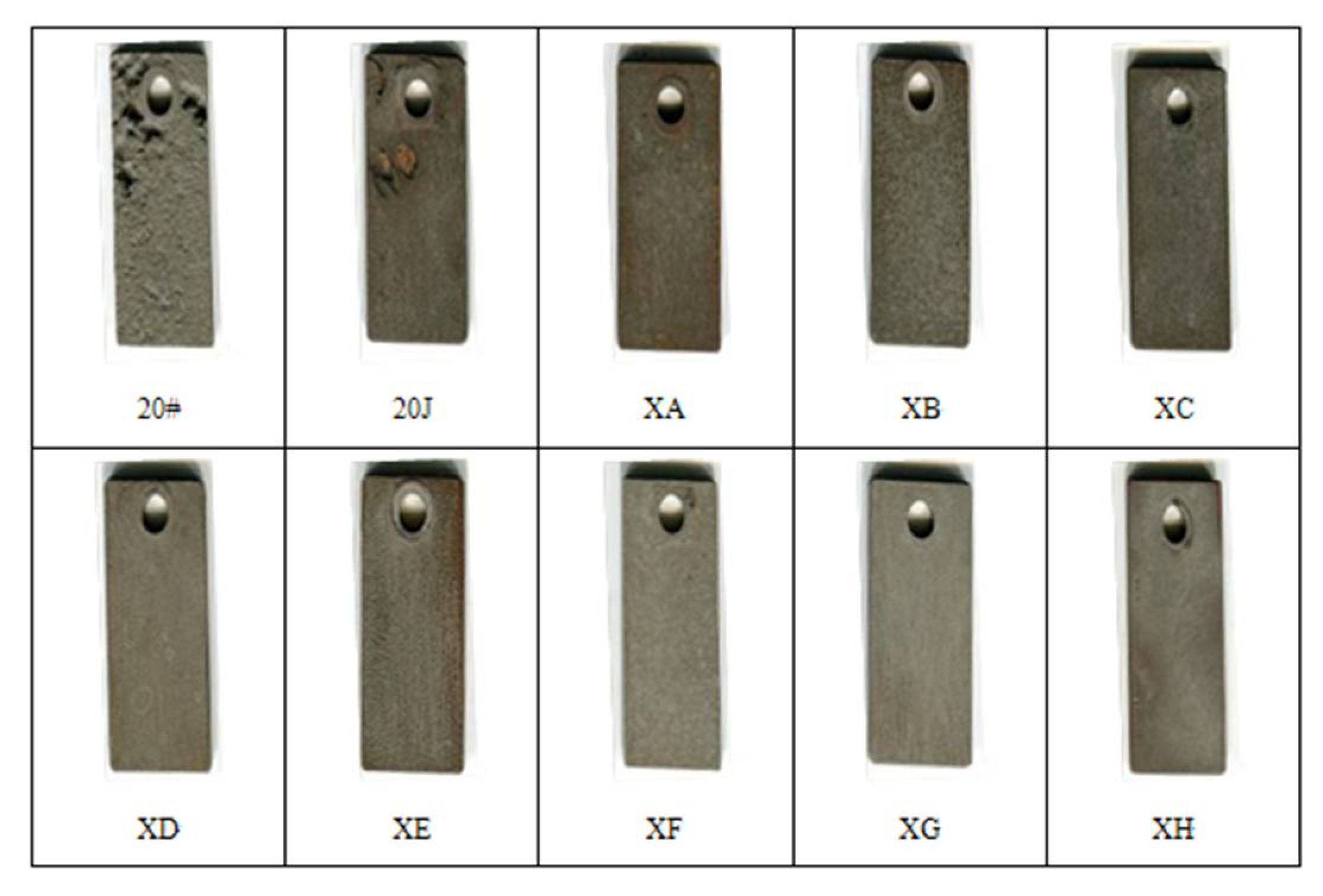
3.3.1. Immersion Experiment
Metal Coupons Were Immersed in a Solution of CO2 Partial Pressure of 0.1 MPa
Metal Coupons Were Immersed in a Solution of pH Value of 1.5
3.3.2. Medium Pressure Kettle Experiment
3.3.3. High-Pressure Kettle Experiment
3.3.4. Experimental Results
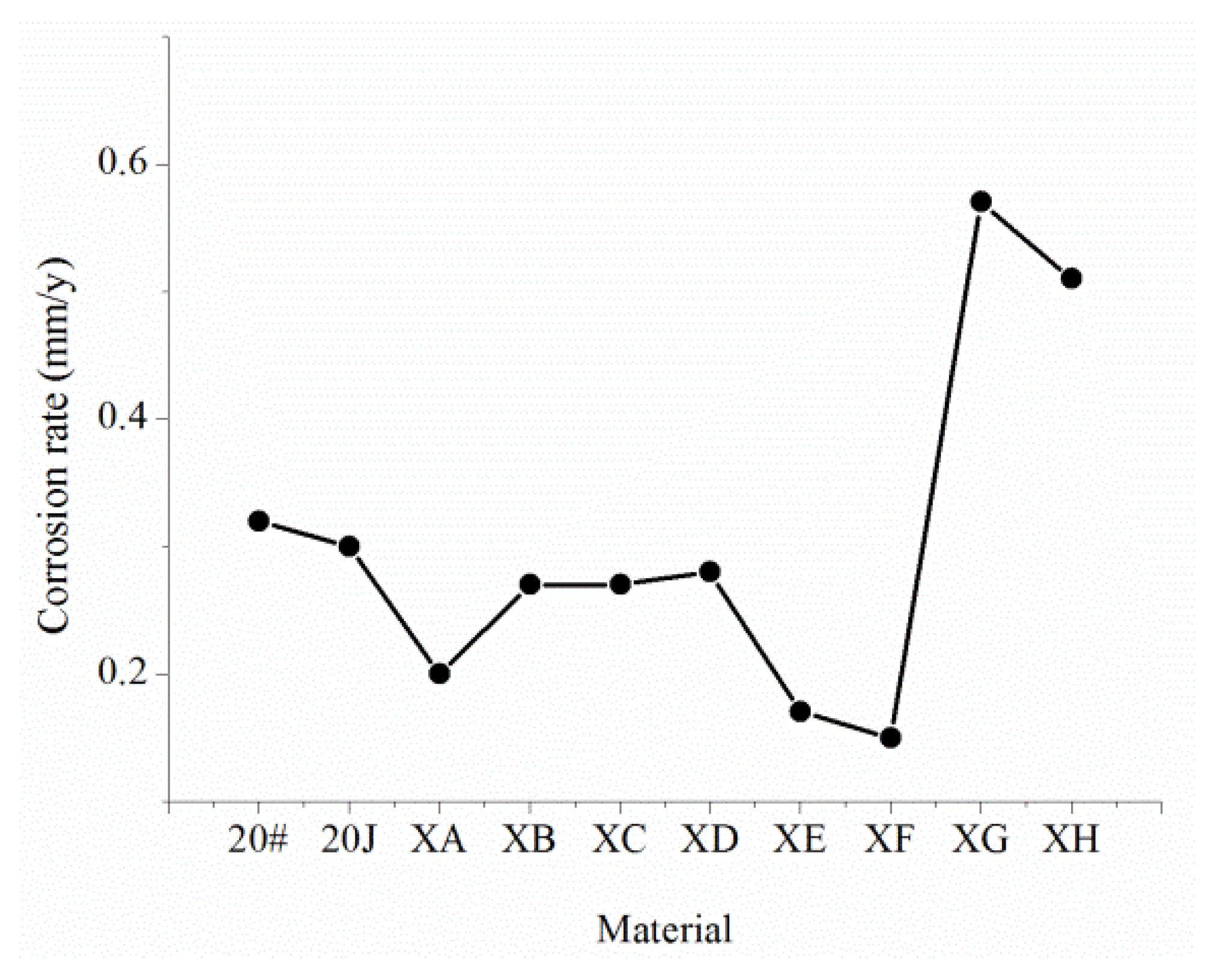
- The corrosion resistance of 20J steel is higher than that of ordinary 20# steel, but the pitting tendency is still high, and the maximum pitting rate is up to 25 mm/year.
- The XE and XF steel with a Cr content of 2.1% have better corrosion resistance, but the carbon equivalent of the two steels reaches 0.58. During the welding process, preheating or subsequent heat treatment is necessary, which will increase the difficulty and cost of construction.
- Mo can effectively improve the hardenability of materials. The XH steel containing Mo exhibits an unbalanced bainitic structure and martensite structure. Although it does not affect the corrosion resistance of CO2, it is very sensitive to the corrosion of dissolved oxygen, and the tendency of pitting corrosion is pronounced.
- The XC and XB steel with a Cr content of 0.6% and the XC and XD steel with a Cr content of 1.1% all have uniform corrosion characteristics. The uniform corrosion resistance of XA and XB relative to 20# is improved by 50%, and the uniform corrosion resistance of XC and XD relative to 20# is improved by 70%. XA and XB steel have obvious pitting corrosion problems and a maximum pitting rate of 15 mm/year. Moreover, XC and XD steel do not have a pitting corrosion tendency, so they can be used as potential pipe materials for corrosion resistance.
- The corrosion resistance of these steel materials is: XE, XF, XC, XD > XA, XB, 20J > 20# > XG, XH.
3.4. Comparison of Welding Performance
3.5. Determination of New Pipe Material
4. Laboratory and Field Evaluation of the New Pipe Material
4.1. Laboratory Evaluation
4.2. Field Evaluation
5. Conclusions
- In the H2S–CO2–Cl− corrosion environment, H2O is the carrier of corrosion, Cl− is the corrosion catalyst, H2S is a strong hydrogen permeation medium, CO2 dissolves in water to cause electrochemical corrosion, and O2 is a kind of depolarization agent.
- The content of CO2 has a significant influence on the corrosion form of 20# steel; the content of H2S has a certain influence on the corrosion morphology and corrosion tendency of 20# steel, and the concentration of Cl− has a great influence on the local corrosion rate but little effect on the surface film and the uniform corrosion rate.
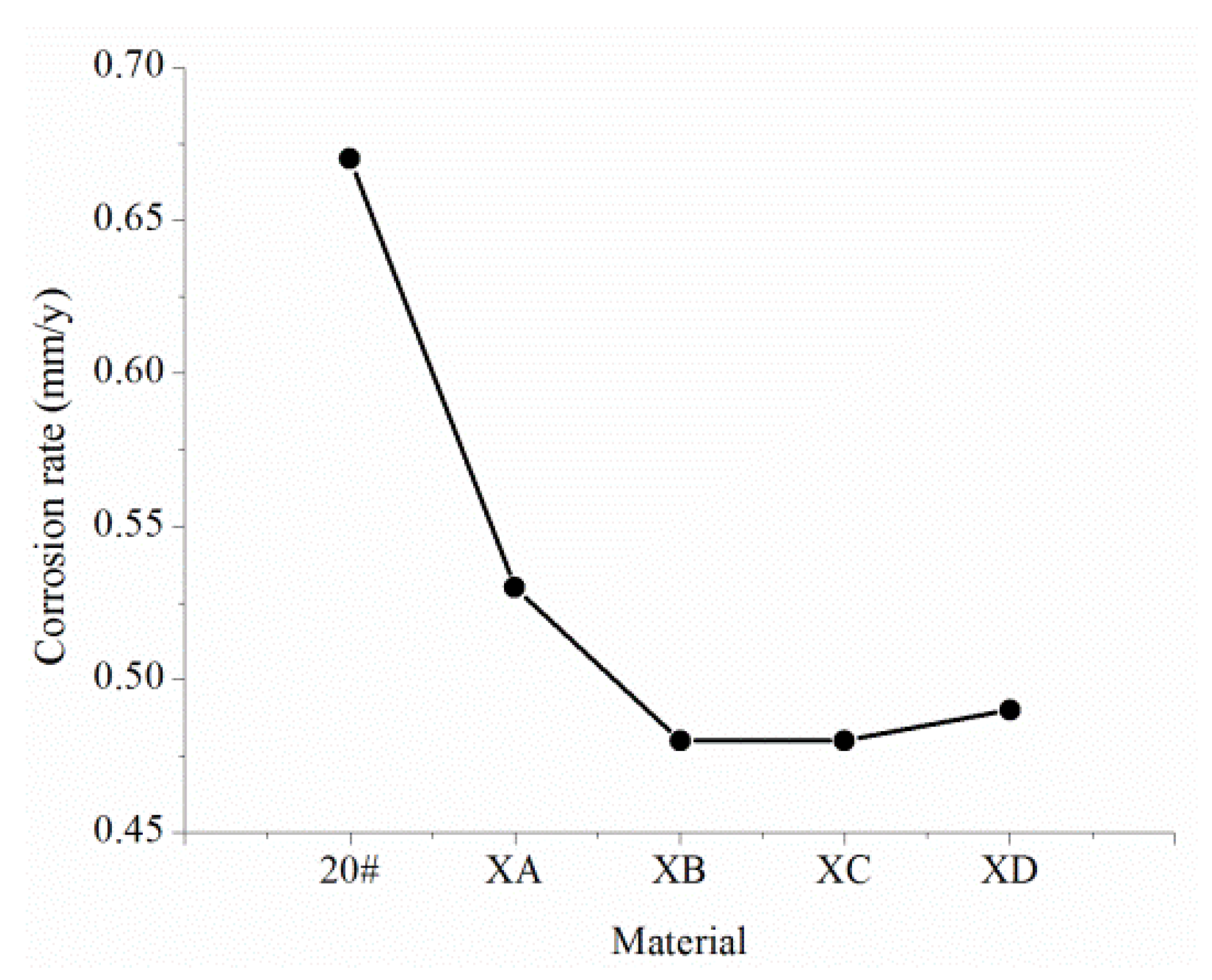
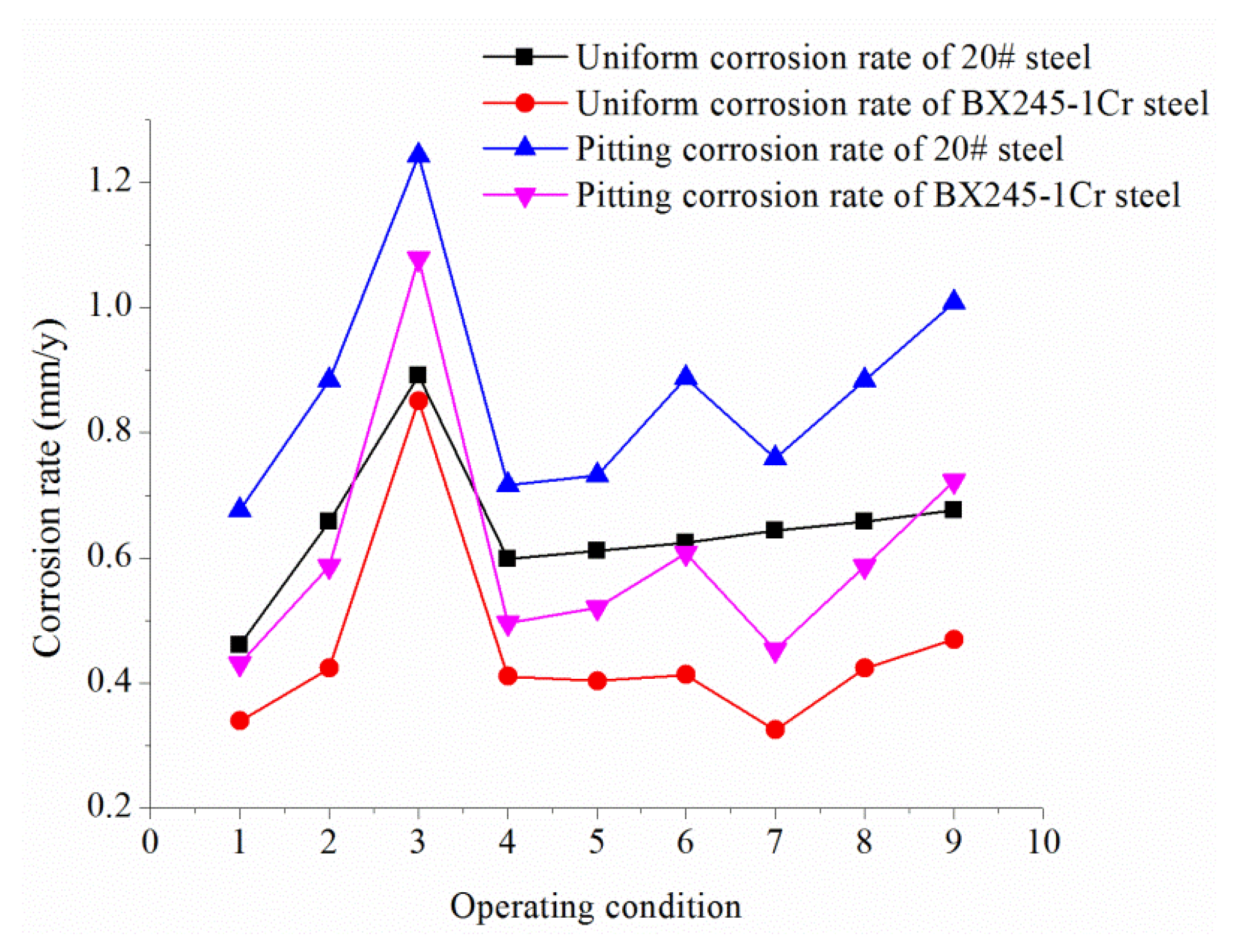
- Under the simulated experimental conditions, the uniform corrosion resistance and the pitting corrosion resistance of BX245-1Cr steel was 30.25% and 29.66% higher than that of 20# steel, which means BX245-1Cr steel has better resistance to pitting corrosion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Material | Surface Area of Coupon (m2) | Weighing before the Experiment (g) | Weighing after the Experiment (g) | Weight Loss (g) | Corrosion Rate (mm/year) | Average Corrosion Rate (mm/year) |
|---|---|---|---|---|---|---|
| 20# | 0.002884 | 27.9962 | 27.8860 | 0.1102 | 0.089 | 0.085 |
| 0.002884 | 27.4859 | 27.3854 | 0.1005 | 0.081 | ||
| 20J | 0.002884 | 27.4367 | 27.3523 | 0.0844 | 0.068 | 0.074 |
| 0.002884 | 26.3870 | 26.2875 | 0.0995 | 0.080 | ||
| XA | 0.002884 | 27.8179 | 27.7474 | 0.0705 | 0.057 | 0.059 |
| 0.002884 | 27.8340 | 27.7589 | 0.0751 | 0.060 | ||
| XB | 0.002884 | 28.0804 | 28.0291 | 0.0513 | 0.041 | 0.055 |
| 0.002884 | 28.2411 | 28.1554 | 0.0857 | 0.069 | ||
| XC | 0.002884 | 27.8875 | 27.7990 | 0.0885 | 0.071 | 0.062 |
| 0.002884 | 27.6426 | 27.5780 | 0.0646 | 0.052 | ||
| XD | 0.002884 | 27.8113 | 27.7386 | 0.0727 | 0.059 | 0.061 |
| 0.002884 | 27.8542 | 27.7748 | 0.0794 | 0.064 | ||
| XE | 0.002884 | 27.6187 | 27.5488 | 0.0699 | 0.056 | 0.056 |
| 0.002884 | 27.3169 | 27.2465 | 0.0704 | 0.057 | ||
| XF | 0.002884 | 27.8958 | 27.8469 | 0.0489 | 0.039 | 0.055 |
| 0.002884 | 28.0224 | 27.9359 | 0.0865 | 0.070 | ||
| XG | 0.002884 | 27.6899 | 27.6075 | 0.0824 | 0.066 | 0.063 |
| 0.002884 | 27.8840 | 27.8088 | 0.0752 | 0.061 | ||
| XH | 0.002884 | 27.3599 | 27.2879 | 0.072 | 0.058 | 0.062 |
| 0.002884 | 27.6775 | 27.5943 | 0.0832 | 0.067 |
| Material | Surface Area of Coupon (m2) | Weighing before the Experiment (g) | Weighing after the Experiment (g) | Weight Loss (g) | Corrosion Rate (mm/year) |
|---|---|---|---|---|---|
| 20# | 0.002884 | 28.6523 | 28.1380 | 0.5143 | 0.32 |
| 20J | 0.002884 | 27.5736 | 27.0887 | 0.4849 | 0.30 |
| XA | 0.002884 | 28.2801 | 27.9700 | 0.3101 | 0.20 |
| XB | 0.002884 | 28.4495 | 28.0112 | 0.4383 | 0.27 |
| XC | 0.002884 | 28.2940 | 27.8512 | 0.4428 | 0.27 |
| XD | 0.002884 | 28.4559 | 27.9913 | 0.4646 | 0.28 |
| XE | 0.002884 | 28.1906 | 27.9080 | 0.2826 | 0.17 |
| XF | 0.002884 | 28.2113 | 27.9654 | 0.2459 | 0.15 |
| XG | 0.002884 | 28.2116 | 27.2803 | 0.9313 | 0.57 |
| XH | 0.002884 | 28.1653 | 27.3393 | 0.826 | 0.51 |
| Material | Surface Area of Coupon (m2) | Weighing before the Experiment (g) | Weighing after the Experiment (g) | Weight Loss (g) | Corrosion Rate (mm/year) |
|---|---|---|---|---|---|
| 20# | 0.002884 | 28.2034 | 22.1055 | 6.0979 | 4.91 |
| 20J | 0.002884 | 26.8695 | 22.7886 | 4.0809 | 3.29 |
| XA | 0.002884 | 27.9966 | 24.8759 | 3.1207 | 2.51 |
| XB | 0.002884 | 28.2109 | 25.3080 | 2.9029 | 2.34 |
| XC | 0.002884 | 27.7977 | 25.9100 | 1.8877 | 1.52 |
| XD | 0.002884 | 28.0624 | 26.1737 | 1.8887 | 1.52 |
| XE | 0.002884 | 27.8477 | 26.1219 | 1.7258 | 1.39 |
| XF | 0.002884 | 27.9163 | 26.2380 | 1.6783 | 1.35 |
| XG | 0.002884 | 27.9493 | 26.3295 | 1.6198 | 1.30 |
| XH | 0.002884 | 27.7710 | 27.5841 | 0.1869 | 0.15 |
References
- Lu, H.; Iseley, T.; Matthews, J.; Liao, W. Hybrid machine learning for pullback force forecasting during horizontal directional drilling. Autom. Constr. 2021, 129, 103810. [Google Scholar] [CrossRef]
- Xu, Z.D.; Zhu, C.; Shao, L.W. Damage identification of pipeline based on ultrasonic guided wave and wavelet denoising. J. Pipeline Syst. Eng. Pract. 2021, 12, 04021051. [Google Scholar] [CrossRef]
- Xu, Z.D.; Yang, Y.; Miao, A.N. Dynamic analysis and parameter optimization of pipelines with multidimensional vibration isolation and mitigation device. J. Pipeline Syst. Eng. Pract. 2021, 12, 04020058. [Google Scholar] [CrossRef]
- Lu, H.; Xu, Z.D.; Iseley, T.; Matthews, J.C. Novel data-driven framework for predicting residual strength of corroded pipelines. J. Pipeline Syst. Eng. Pract. 2021, 12, 04021045. [Google Scholar] [CrossRef]
- Lu, H.; Iseley, T.; Matthews, J.; Liao, W.; Azimi, M. An ensemble model based on relevance vector machine and multi-objective salp swarm algorithm for predicting burst pressure of corroded pipelines. J. Pet. Sci. Eng. 2021, 203, 108585. [Google Scholar] [CrossRef]
- Yang, D.; Ge, P.; Zhu, Y. Corrosion prevention and control technology application of gathering and transportation pipelines in Tahe oilfield. Surf. Technol. 2016, 45, 57–64. [Google Scholar]
- Rogers, W.F.; Rowe, A., Jr. 3. Corrosion effects of hydrogen sulphide and carbon dioxide in oil production. In Proceedings of the 4th World Petroleum Congress, Rome, Italy, 6–15 June 1955. [Google Scholar]
- Kermani, B.; Dougan, M.; Gonzalez, J.C.; Linne, C.; Cochrane, R. Development of low carbon Cr–Mo steels with exceptional corrosion resistance for oilfield applications. In Proceedings of the CORROSION, Houston, TX, USA, 11–16 March 2001. [Google Scholar]
- Kermani, B.; Gonzales, J.C.; Turconi, G.L.; Pigliacampo, L.; Perez, T.; Morales, C. Window of application and operational track record of low carbon 3Cr steel tubular. In Proceedings of the CORROSION, San Diego, CA, USA, 12–16 March 2006. [Google Scholar]
- Liu, H.X.; Zhang, G.L.; Zhao, J.M.; Liu, D.Y. Corrosion behaviors of four steels in CO2-saturated brine. Corros. Prot. 2007, 28, 202–204. [Google Scholar]
- Ding, J.; Zhang, L.; Li, D.; Lu, M.; Xue, J.; Zhong, W. Corrosion and stress corrosion cracking behavior of 316L austenitic stainless steel in high H2S–CO2–Cl− environment. J. Mater. Sci. 2013, 48, 3708–3715. [Google Scholar] [CrossRef]
- Li, D.P.; Zhang, L.; Yang, J.W.; Lu, M.X.; Ding, J.H.; Liu, M.L. Effect of H2S concentration on the corrosion behavior of pipeline steel under the coexistence of H2S and CO2. Int. J. Miner. Metall. Mater. 2014, 21, 388–394. [Google Scholar] [CrossRef]
- Guo, Z.J.; Chen, D.F.; Li, Y.J.; Li, X.J.; Xuan, P.C.; Mao, Z.Q.; Xie, S. research progress of oil field pressure vessel corrosion mechanisms in H2S-CO2-Cl− environment. J. Petro-Chem. Equip. 2008, 37, 53–58. [Google Scholar]
- Zhou, J.; Li, Y. Anodic electrochemical behavior of X80 pipeline steel in NaHCO3 solution. Acta Met. Sin 2010, 46, 251–256. [Google Scholar] [CrossRef]
- Xia, X. Stress corrosion-induced cracking of 20 steel in H2S solution. Mater. Prot. 2007, 40, 15–17. [Google Scholar]
- Lu, J.Z.; Wang, B.; Zhang, D.F.; Zhou, D.M. Corrosion mechanism research of 20G steel in high Cl− glycol solution. Press. Vessel. Technol. 2010, 27, 19–23. [Google Scholar]
- Pots, B.F.; Kapusta, S.D.; John, R.C.; Thomas, M.J.J.; Rippon, I.J.; Whitham, T.S.; Girgis, M. Improvements on de Waard-Milliams corrosion prediction and applications to corrosion management. In Proceedings of the CORROSION 2002, Denver, CO, USA, 7–11 April 2002. [Google Scholar]
- NACE RP0775, Preparation, Installation, Analysis, and Interpretation of Corrosion Coupons in Oilfield Operations; NACE International: Houston, TX, USA, 2005.
- Lu, H.; Behbahani, S.; Ma, X.; Iseley, T. A multi-objective optimizer-based model for predicting composite material properties. Constr. Build. Mater. 2021, 284, 122746. [Google Scholar] [CrossRef]

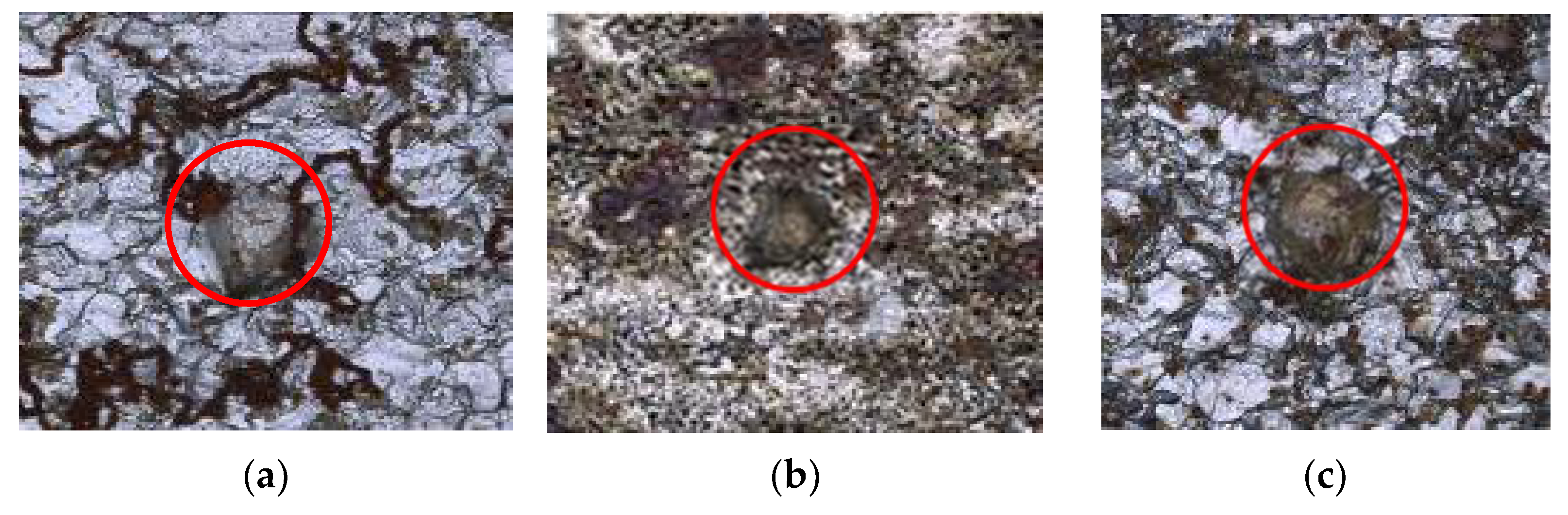
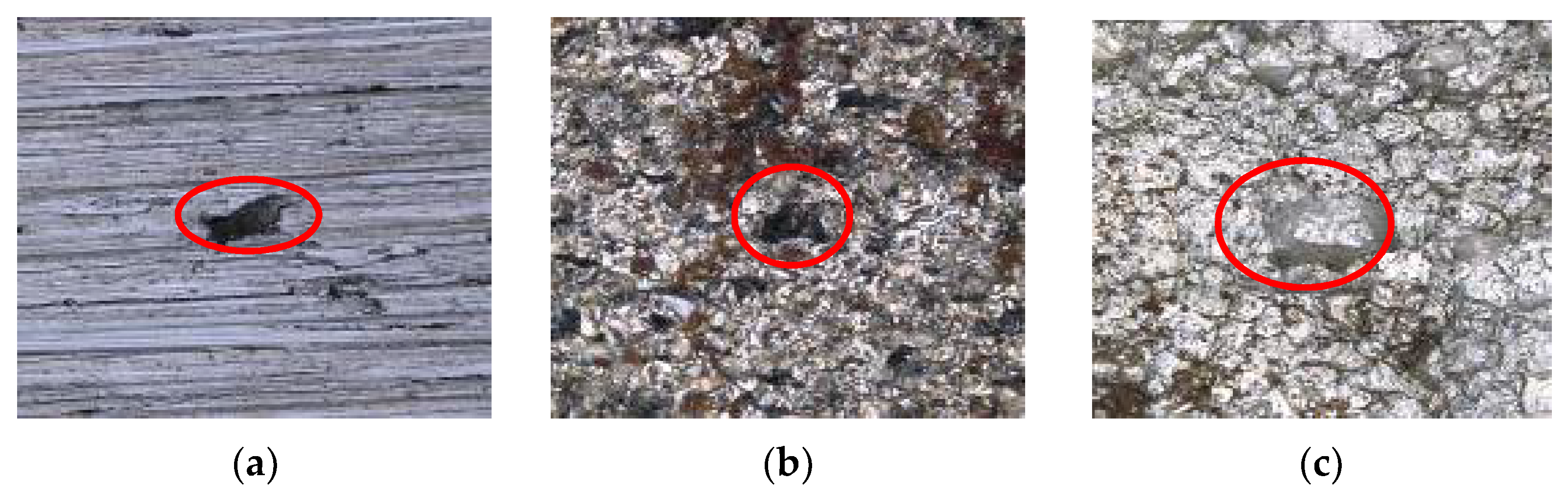


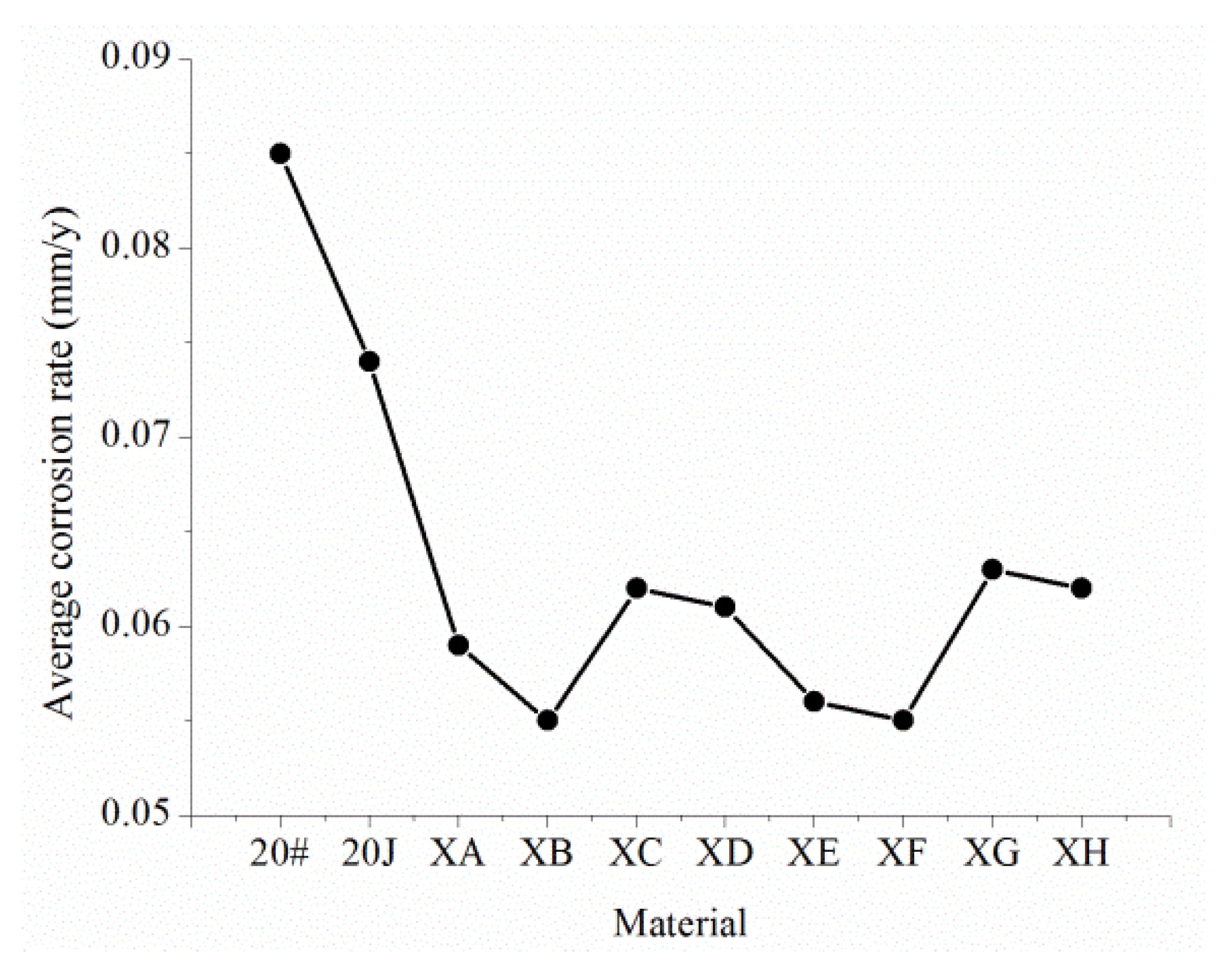
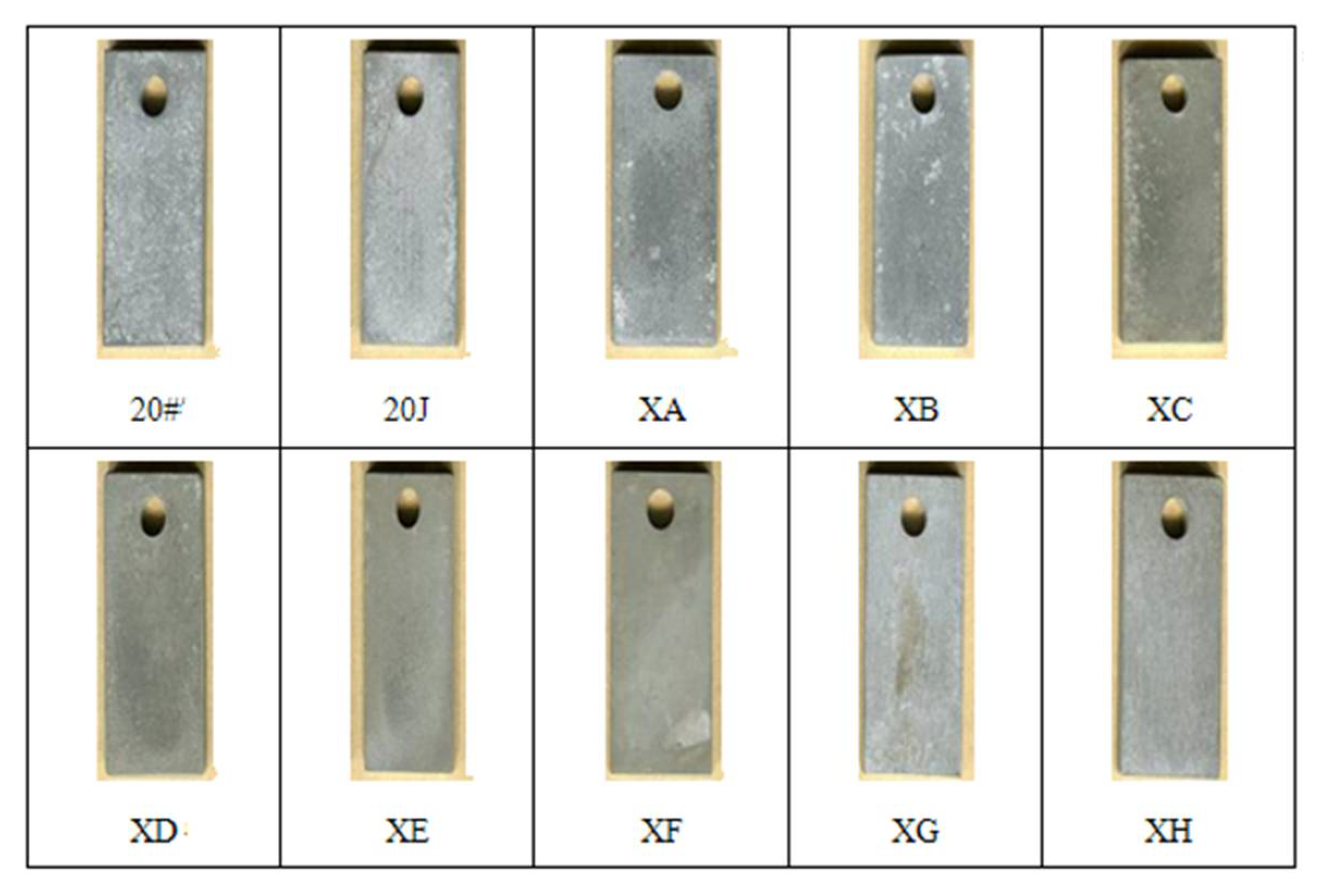


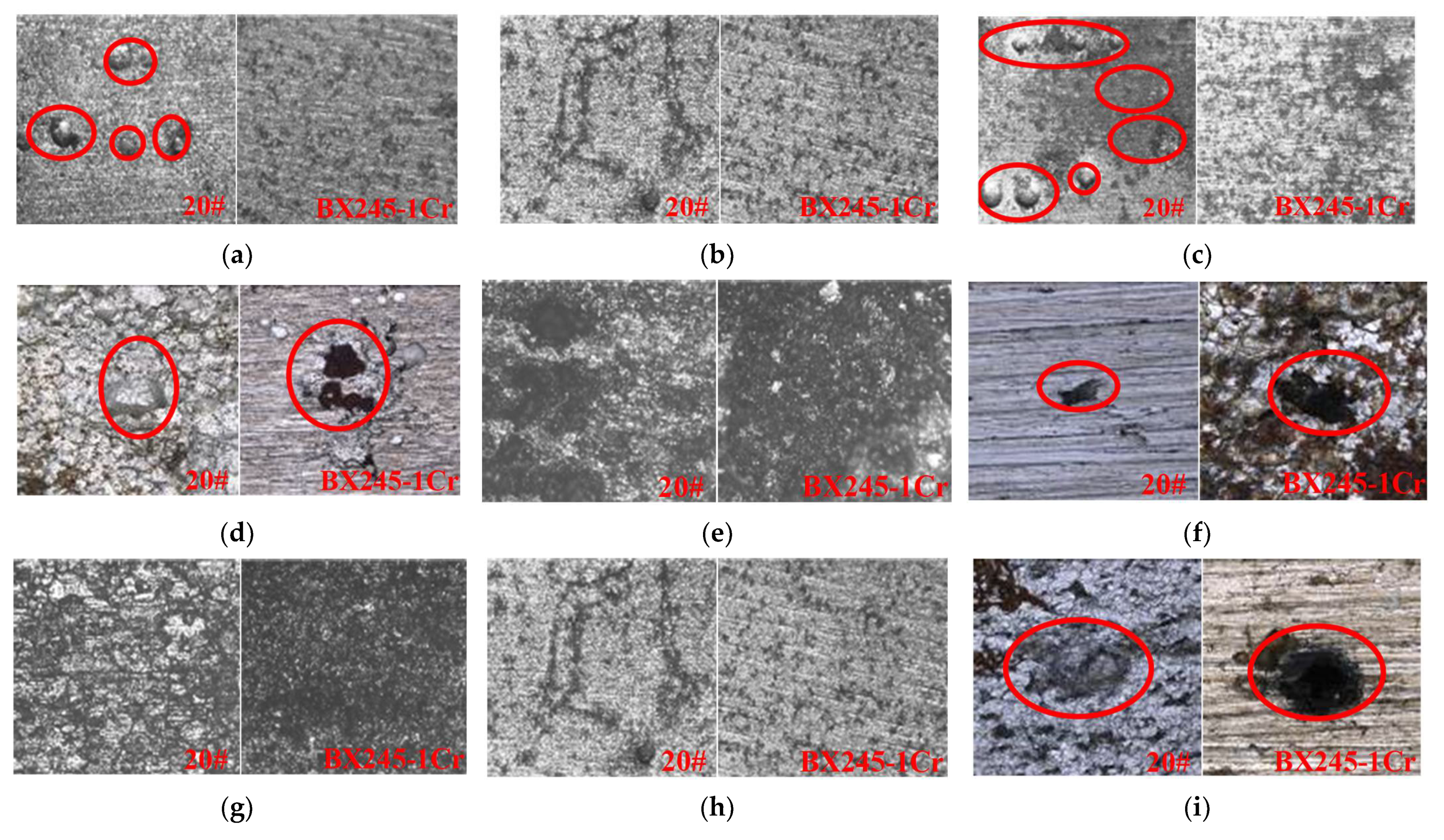
| Medium | Minimum Value | Maximum Value | Average Value |
|---|---|---|---|
| H2S | 2.4 mg/m3 | 170,571 mg/m3 | 38,962 mg/m3 |
| CO2 | 4.88% | 27.2% | 8.07% |
| Cl− | 60,929 mg/L | 150,325 mg/L | 113,316 mg/L |
| Cl− (mg/L) | SO42− (mg/L) | Ca2+ (mg/L) | Mg2+ (mg/L) | HCO3− (mg/L) | Total Mineralization | PH Value |
|---|---|---|---|---|---|---|
| 113,316 | 302.7 | 16,460.3 | 955.4 | 620.4 | 189,287 | 6.0 |
| Partial Pressure of CO2 (MPa) | Partial Pressure of H2S (MPa) | PCO2/PH2S | Concentration of Cl− (mg/L) | Temperature (°C) | Total Pressure (MPa) | Test Cycle (days) |
|---|---|---|---|---|---|---|
| 0.01 | 0.04 | 0.25 | 110,000 | 70 | 1.6 | 30 |
| 0.15 | 3.75 | |||||
| 0.45 | 11.25 |
| Partial Pressure of CO2 (MPa) | Partial Pressure of H2S (MPa) | PCO2/PH2S | Concentration of Cl− (mg/L) | Temperature (°C) | Total Pressure (MPa) | Test Cycle (days) |
|---|---|---|---|---|---|---|
| 0.15 | 0.003 | 50 | 110,000 | 70 | 1.6 | 30 |
| 0.0008 | 200 | |||||
| 0.0003 | 450 |
| Partial Pressure of CO2 (MPa) | Partial Pressure of H2S (MPa) | PCO2/PH2S | Concentration of Cl− (mg/L) | Temperature (°C) | Total Pressure (MPa) | Test Cycle (days) |
|---|---|---|---|---|---|---|
| 0.15 | 0.04 | 3.75 | 60,000 | 70 | 1.6 | 30 |
| 110,000 | ||||||
| 150,000 |
| Grade | Uniform Corrosion Rate (mm/year) | Maximum Pitting Rate (mm/year) |
|---|---|---|
| Low | <0.025 | <0.13 |
| Moderate | 0.025–0.12 | 0.13–0.20 |
| High | 0.13–0.25 | 0.21–0.38 |
| Severe | >0.25 | >0.38 |
| Partial Pressure of CO2 (MPa) | Partial Pressure of H2S (MPa) | Uniform Corrosion Rate | Pitting Rate | ||
|---|---|---|---|---|---|
| Value (mm/year) | Grade of Corrosion | Value (mm/year) | Grade of Corrosion | ||
| 0.01 | 0.04 | 0.4605 | Severe | 0.6765 | Severe |
| 0.15 | 0.6572 | Severe | 0.8835 | Severe | |
| 0.45 | 0.8913 | Severe | 1.2435 | Severe | |
| Partial Pressure of CO2 (MPa) | Partial Pressure of H2S (MPa) | Uniform Corrosion Rate | Pitting Rate | ||
|---|---|---|---|---|---|
| Value (mm/year) | Grade of Corrosion | Value (mm/year) | Grade of Corrosion | ||
| 0.15 | 0.0003 | 0.5984 | Severe | 0.7166 | Severe |
| 0.008 | 0.6105 | Severe | 0.7324 | Severe | |
| 0.003 | 0.6235 | Severe | 0.8877 | Severe | |
| Partial Pressure of CO2 (MPa) | Partial Pressure of H2S (MPa) | Content of Cl− (mg/L) | Uniform Corrosion Rate | Pitting Rate | ||
|---|---|---|---|---|---|---|
| Value (mm/year) | Grade of Corrosion | Value (mm/year) | Grade of Corrosion | |||
| 0.15 | 0.04 | 60,000 | 0.6438 | Severe | 0.7585 | Severe |
| 110,000 | 0.6572 | Severe | 0.8835 | Severe | ||
| 150,000 | 0.6760 | Severe | 1.0081 | Severe | ||
| Material | C (%) | Si (%) | Mn (%) | P (%) | S (%) | Cr (%) | Cu (%) | Nb (%) | Mo (%) | Carbon Equivalent (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 20# | 0.23 | 0.26 | 0.49 | 0.014 | 0.009 | - | - | - | - | 0.31 |
| 20J | 0.20 | 0.30 | 0.51 | 0.009 | 0.001 | - | - | - | - | 0.29 |
| XA | 0.07 | 0.29 | 0.48 | 0.007 | 0.002 | 0.65 | - | - | - | 0.25 |
| XB | 0.09 | 0.34 | 0.52 | 0.009 | 0.002 | 0.63 | 0.18 | - | - | 0.31 |
| XC | 0.07 | 0.33 | 0.51 | 0.006 | 0.002 | 1.09 | - | - | - | 0.37 |
| XD | 0.09 | 0.31 | 0.51 | 0.009 | 0.003 | 1.11 | 0.23 | - | - | 0.41 |
| XE | 0.08 | 0.34 | 0.52 | 0.010 | 0.002 | 2.08 | - | - | - | 0.58 |
| XF | 0.08 | 0.33 | 0.53 | 0.009 | 0.003 | 2.1 | - | 0.04 | - | 0.59 |
| XG | 0.08 | 0.35 | 0.50 | 0.009 | 0.002 | 1.09 | 0.21 | - | 0.23 | 0.44 |
| XH | 0.09 | 0.33 | 0.51 | 0.008 | 0.002 | 2.11 | 0.21 | - | 0.24 | 0.65 |
| Material | Yield Strength (MPa) | Tensile Strength (MPa) | Extensibility (%) | Impact Energy (J) (0 °C) | Yield Ratio | Metallographic Structure |
|---|---|---|---|---|---|---|
| 20# | 308 | 469 | 28.5 | 83, 90, 87 | 0.67 | F 1 + P 1 |
| 20J | 288 | 454 | 36.5 | 153, 177, 139 | 0.63 | F + P |
| XA | 251 | 390 | 43.5 | 233, 240, 236 | 0.64 | F + P |
| XB | 286 | 446 | 36.5 | 109, 109, 94 | 0.64 | F + P |
| XC | 266 | 404 | 43.0 | 281, 274, 274 | 0.66 | F + P |
| XD | 294 | 491 | 36 | 135, 120, 116 | 0.60 | F + P |
| XE | 248 | 501 | 36.0 | 41, 34, 26 | 0.50 | F + B 1 |
| XF | 367 | 607 | 25.0 | 28, 16, 23 | 0.60 | F + B |
| XG | 366 | 606 | 25.5 | 15, 15, 16 | 0.60 | F + B + M 1 |
| XH | 565 | 851 | 22 | 11, 9, 10 | 0.66 | B + M |
| Icon Content (mg/L) | CO2 Partial Pressure (MPa) | Experimental Duration (h) | |||||
|---|---|---|---|---|---|---|---|
| K+, Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | 0.1 | 480 |
| 71,993 | 15,000 | 1298 | 134,345 | 300 | 113 | ||
| Composition | NaCl | KCl | MgCl2 | CaCl2 | Na2SO4 | NaHCO3 | PH Value |
|---|---|---|---|---|---|---|---|
| Content (g/L) | 154.93 | 20.97 | 5.14 | 41.63 | 0.44 | 0.156 | 4.5 |
| Material | Maximum Pitting Depth (mm) | Average Pitting Depth (mm) | Maximum Pitting Rate (mm/year) | Average Pitting Rate (mm/year) |
|---|---|---|---|---|
| 20# | 2.990 | 2.914 | 109.135 | 106.361 |
| 20J | 0.690 | 0.578 | 25.185 | 21.097 |
| XA | 0.440 | 0.152 | 16.060 | 5.548 |
| XB | 0.430 | 0.302 | 15.695 | 11.023 |
| XC | No pitting phenomenon | |||
| XD | ||||
| XE | ||||
| XF | ||||
| XG | ||||
| XH | ||||
| Composition | C | Si | Mn | P | S | Cr |
|---|---|---|---|---|---|---|
| Mass fraction (%) | 0.06–0.09 | 0.25–0.35 | 0.4–0.6 | ≤0.015 | ≤0.003 | 0.01–0.012 |
| Steel | C | Si | Mn | P | S | Cr | Ni | Cu |
|---|---|---|---|---|---|---|---|---|
| 20# | 0.21 | 0.32 | 0.52 | 0.032 | 0.035 | 0.21 | 0.23 | 0.22 |
| BX245-1Cr | 0.07 | 0.30 | 0.5 | 0.007 | 0.0011 | 0.011 | - | - |
| Condition | Partial Pressure of CO2 (MPa) | Partial Pressure of H2S (MPa) | Concentration of Cl− (mg/L) | Total Pressure (MPa) | Temperature (°C) | Experimental Duration (days) | Flow Rate (m/s) |
|---|---|---|---|---|---|---|---|
| 1 | 0.01 | 0.04 | 110,000 | 70 | 1.6 | 30 | 1 |
| 2 | 0.15 | 0.04 | 110,000 | 70 | 1.6 | 30 | 1 |
| 3 | 0.45 | 0.04 | 110,000 | 70 | 1.6 | 30 | 1 |
| 4 | 0.15 | 0.0003 | 110,000 | 70 | 1.6 | 30 | 1 |
| 5 | 0.15 | 0.0008 | 110,000 | 70 | 1.6 | 30 | 1 |
| 6 | 0.15 | 0.003 | 110,000 | 70 | 1.6 | 30 | 1 |
| 7 | 0.15 | 0.04 | 60,000 | 70 | 1.6 | 30 | 1 |
| 8 | 0.15 | 0.04 | 110,000 | 70 | 1.6 | 30 | 1 |
| 9 | 0.15 | 0.04 | 150,000 | 70 | 1.6 | 30 | 1 |
| Phase | Uniform Corrosion Rate (mm/year) | Corrosion rate Decline (%) | Pitting Corrosion Rate (mm/year) | Corrosion Rate Decline (%) | ||
|---|---|---|---|---|---|---|
| BX245-1Cr | 20# | BX245-1Cr | 20# | |||
| I | 0.0270 | 0.0294 | 8.16 | 0.3224 | 0.5659 | 43.03 |
| II | 0.0091 | 0.0105 | 13.33 | 0.1024 | 0.1217 | 15.86 |
| III | 0.0617 | 0.0633 | 2.53 | 0.2585 | 0.4032 | 35.89 |
| Average | 0.0235 | 0.025 | 8.01 | 0.2463 | 0.3717 | 31.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Zhang, Z.; Wu, L.; Li, X.; Zhang, Z. Corrosion Law of Metal Pipeline in Tahe Oilfield and Application of New Materials. Coatings 2021, 11, 1269. https://doi.org/10.3390/coatings11111269
Shi X, Zhang Z, Wu L, Li X, Zhang Z. Corrosion Law of Metal Pipeline in Tahe Oilfield and Application of New Materials. Coatings. 2021; 11(11):1269. https://doi.org/10.3390/coatings11111269
Chicago/Turabian StyleShi, Xiaolong, Zhi Zhang, Lanjie Wu, Xincai Li, and Zhenwu Zhang. 2021. "Corrosion Law of Metal Pipeline in Tahe Oilfield and Application of New Materials" Coatings 11, no. 11: 1269. https://doi.org/10.3390/coatings11111269
APA StyleShi, X., Zhang, Z., Wu, L., Li, X., & Zhang, Z. (2021). Corrosion Law of Metal Pipeline in Tahe Oilfield and Application of New Materials. Coatings, 11(11), 1269. https://doi.org/10.3390/coatings11111269





