New Approaches to Increasing the Superhydrophobicity of Coatings Based on ZnO and TiO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of ZnO/Au, ZnO/Fe2O3 Samples
2.2. Preparation of TiO2(N) Samples
2.3. Characterization Methods
3. Results
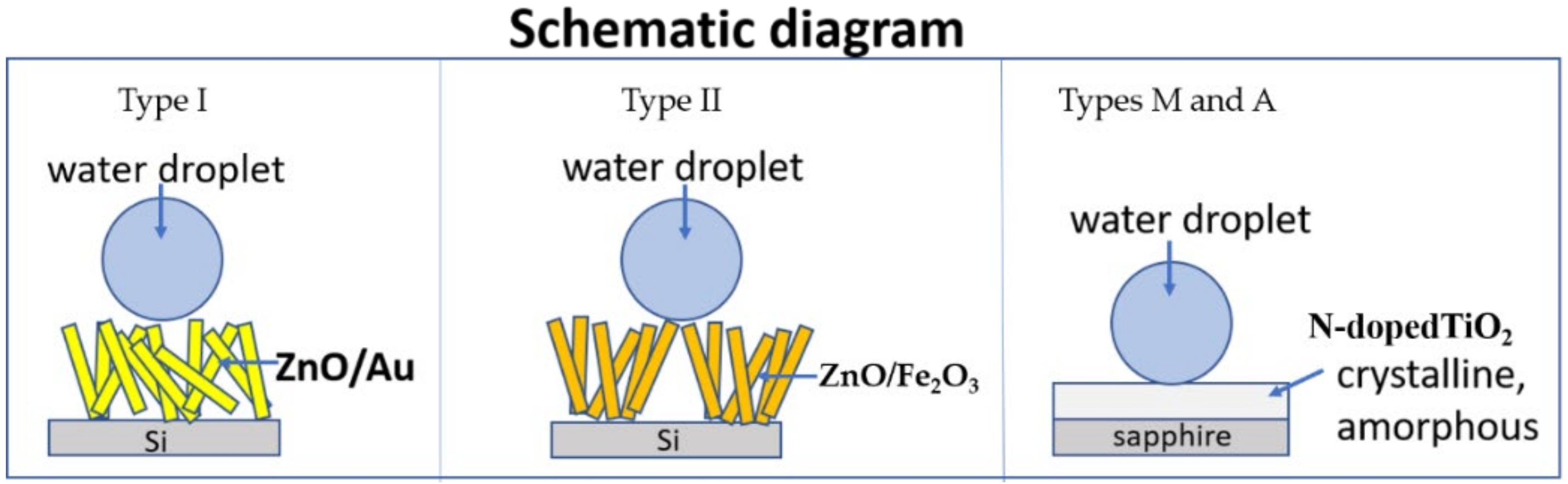
3.1. Superhydrophobicity of ZnO
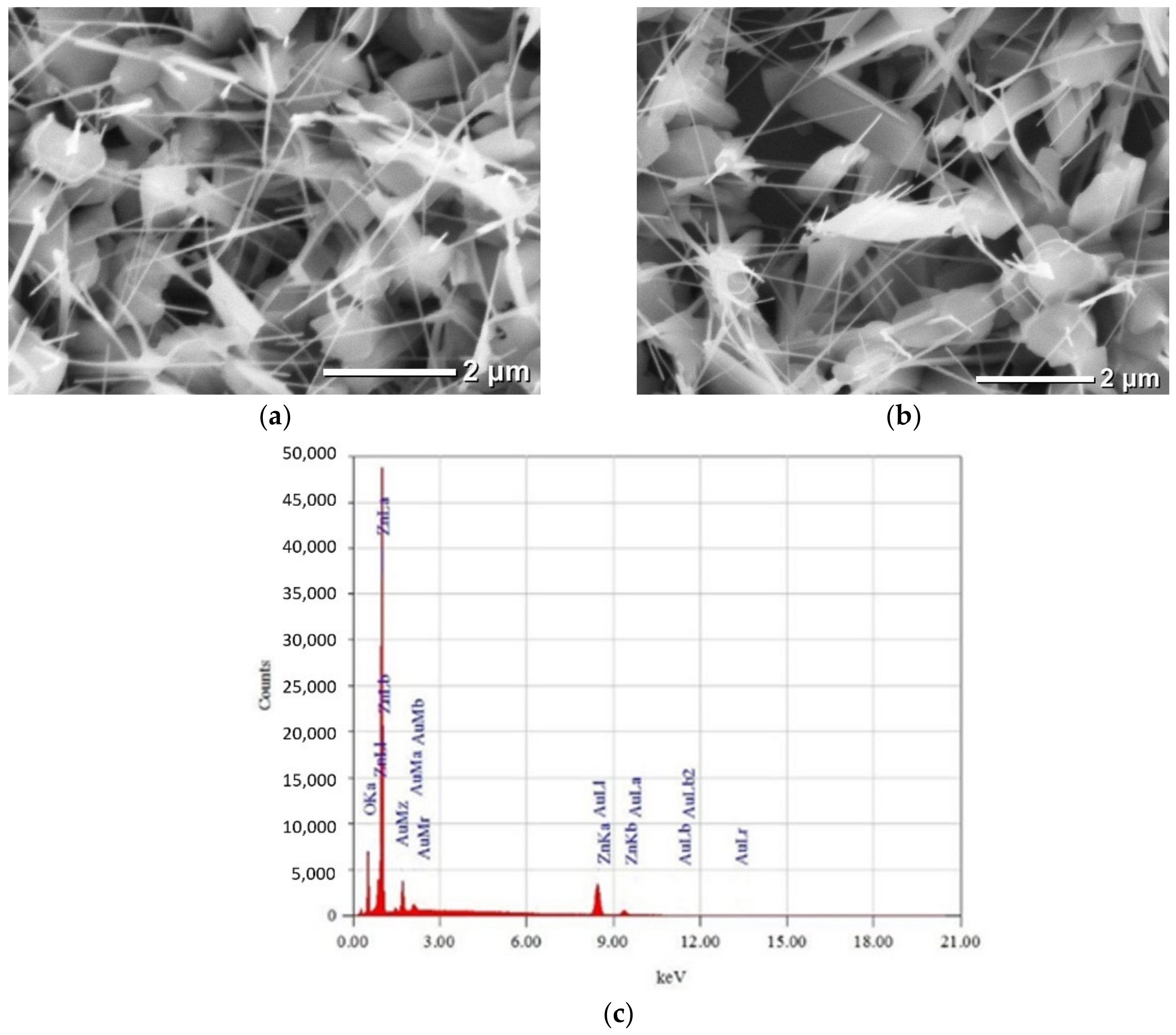
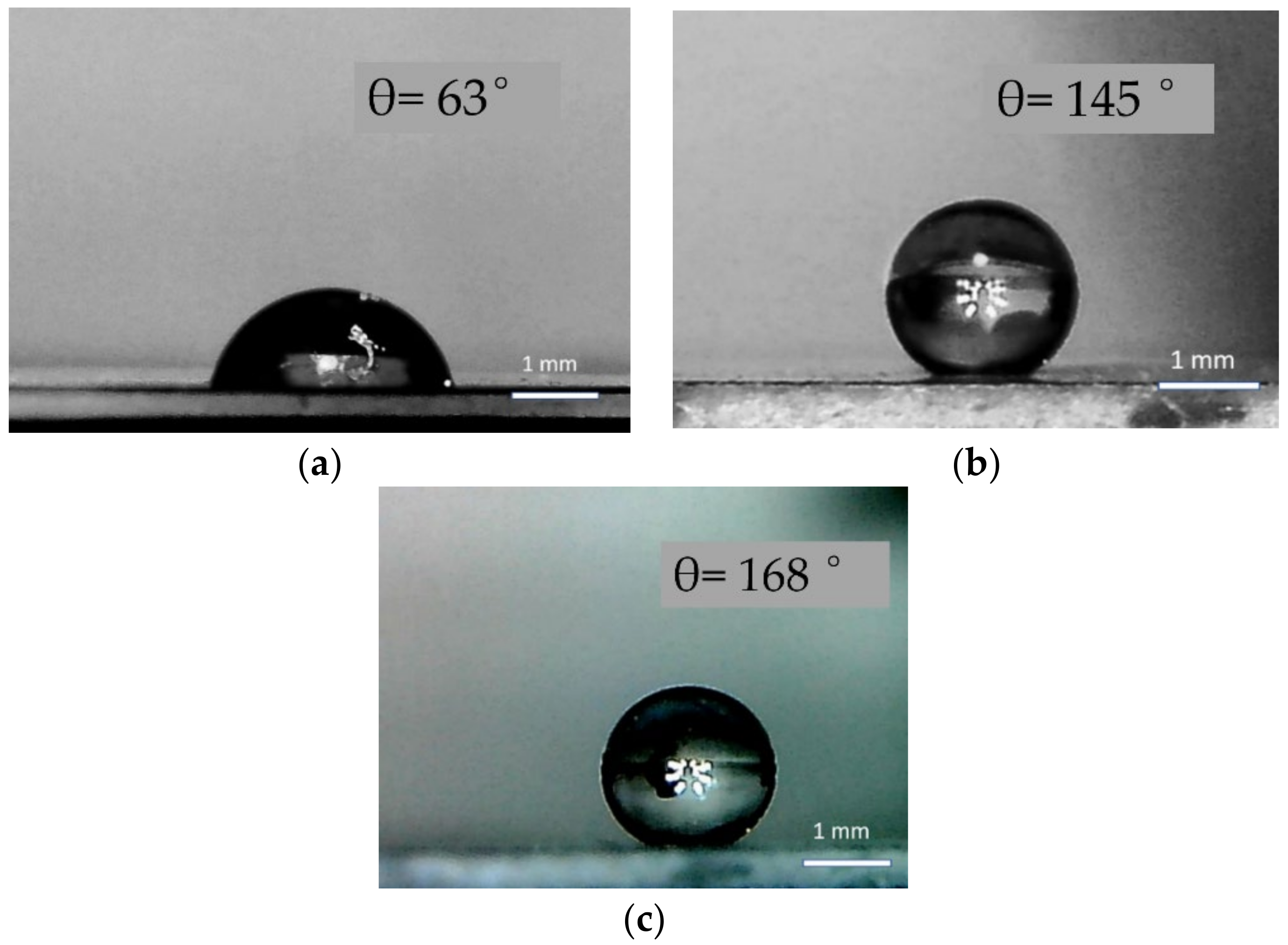
3.1.1. Samples of Type I: Au Plating
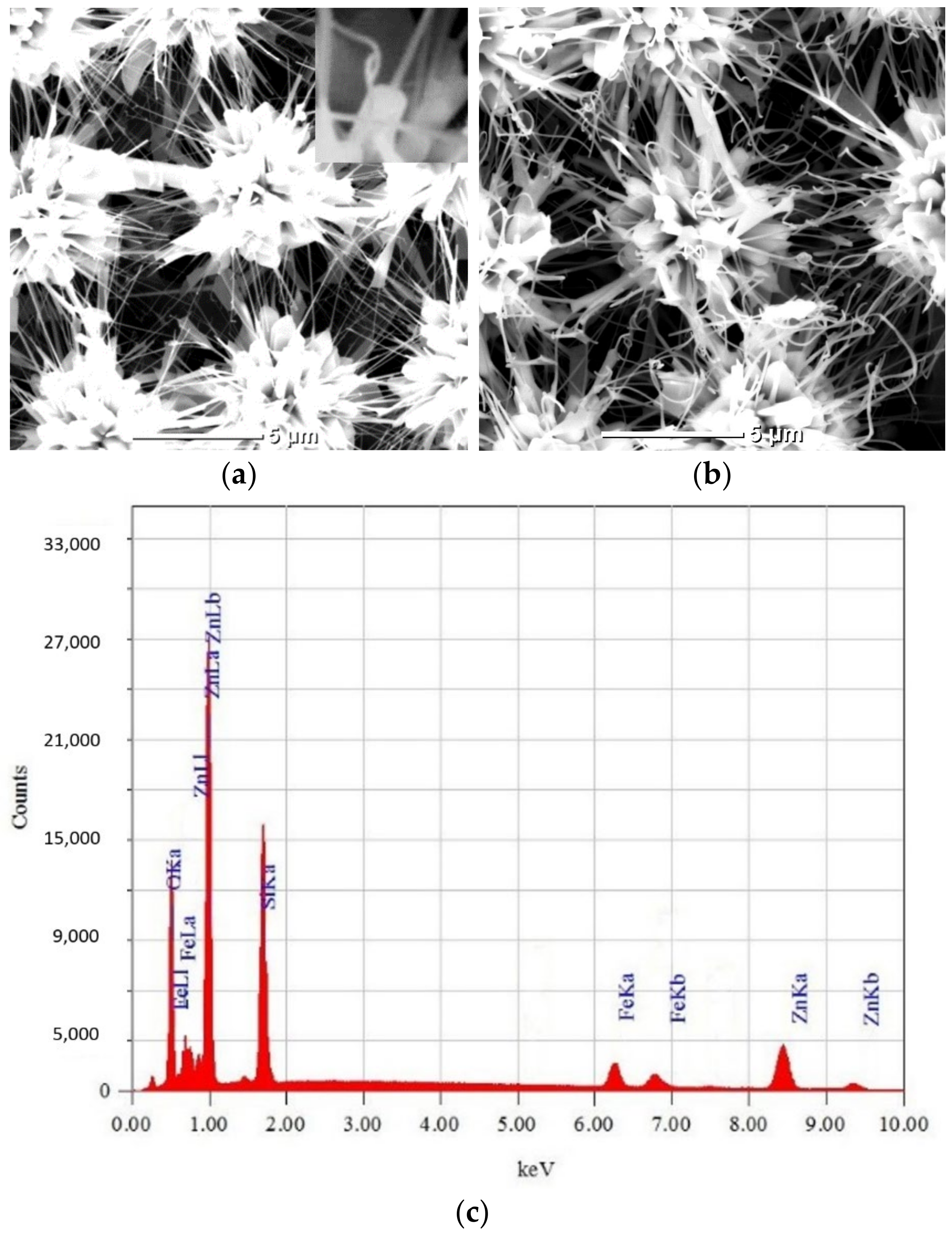
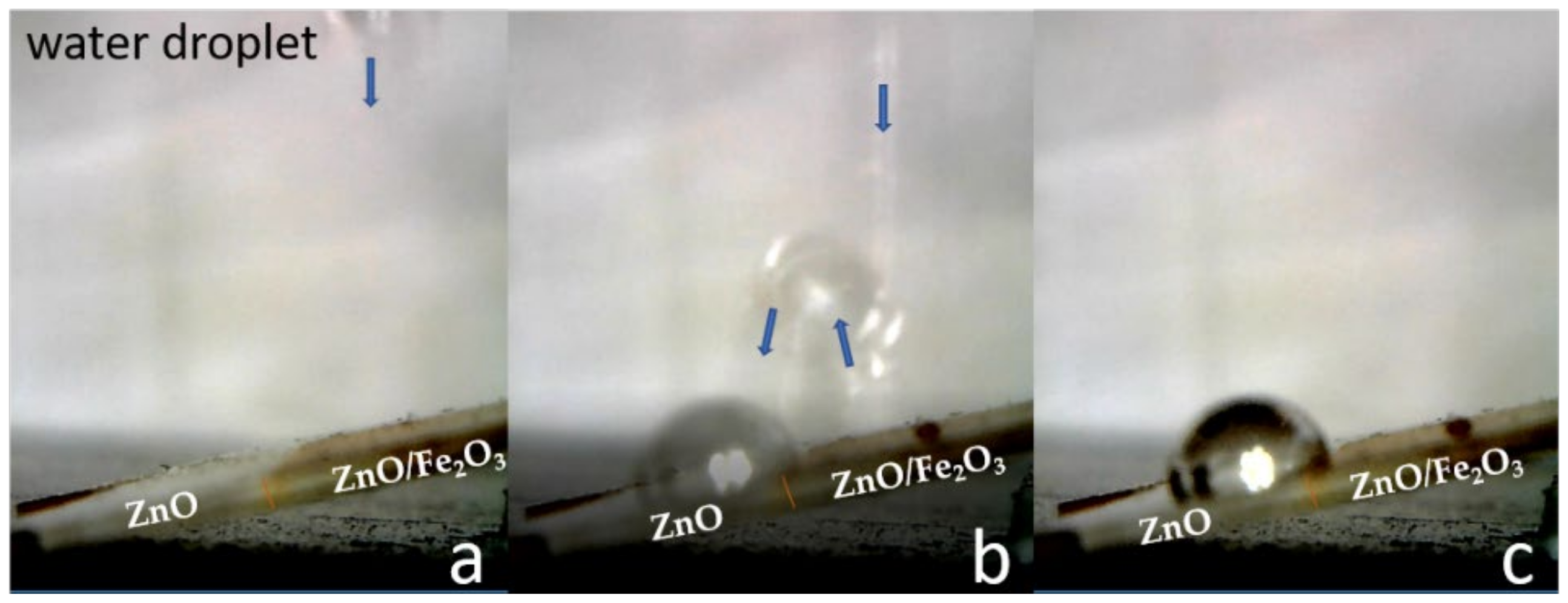
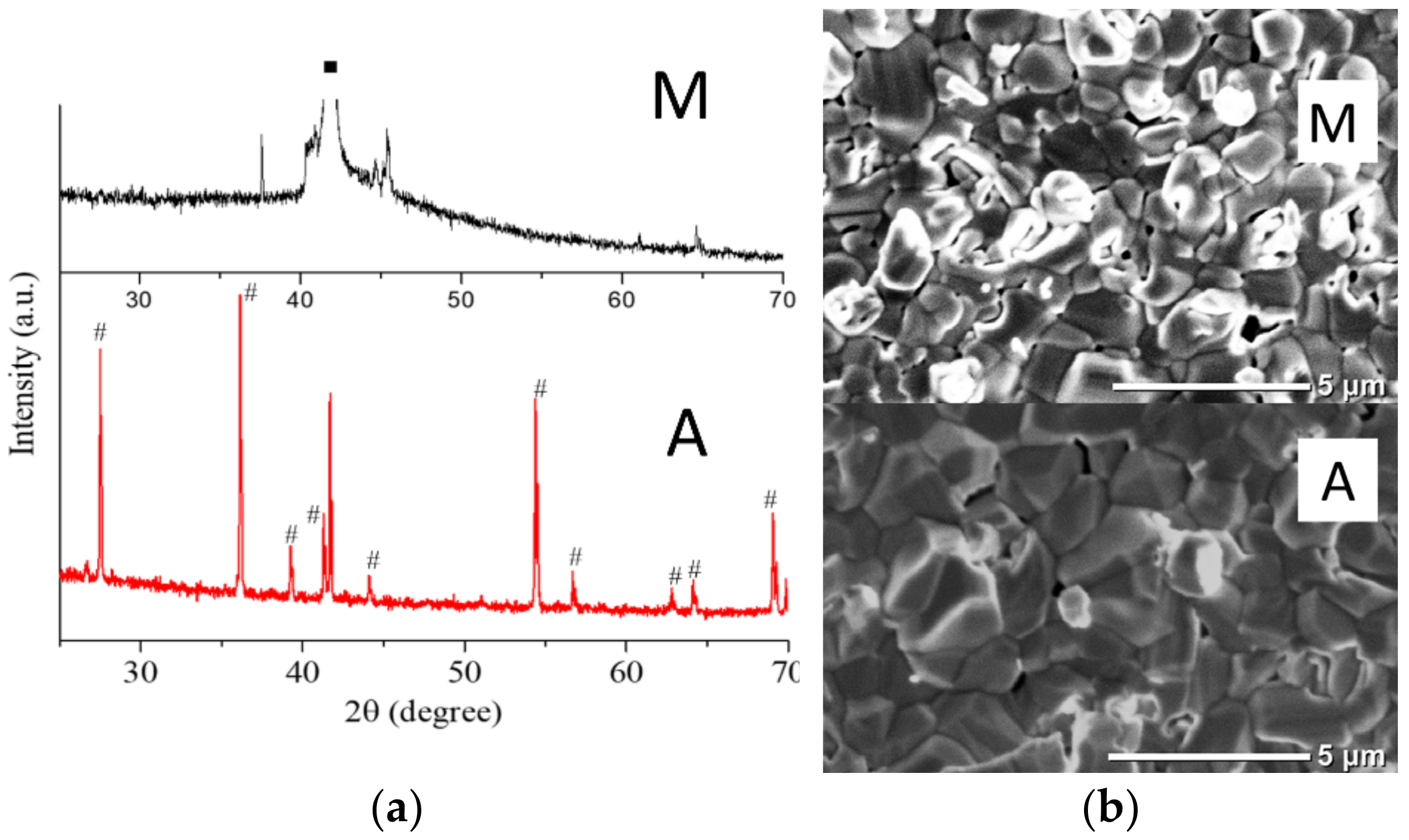
3.1.2. Samples of Type II: Fe2O3 Coating
3.2. Hydrophobicity of TiO2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srivastava, M.; Basu, B.B.J.; Rajam, K.S. Improving the hydrophobicity of ZnO by PTFE incorporation. J. Nanotechnol. 2011, 2011, 392754. [Google Scholar] [CrossRef] [Green Version]
- Kenmoe, S.; Biedermann, P.U. Water aggregation and dissociation on the ZnO(100) surface. Phys. Chem. Chem. Phys. 2017, 19, 1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, N.S.; Mahdjoub, N.; Vishnyakov, V.; Kelly, P.J.; Kriek, R.J. The effect of crystalline phase (anatase, brookite and rutile) and size on the photocatalytic activity of calcined polymorphic titanium dioxide (TiO2). Polym. Degrad. Stab. 2018, 150, 31. [Google Scholar] [CrossRef]
- Bahramian, A. Molecular interactions insights underlying temperature-dependent structure of water molecules on TiO2 nanostructured film: A computational study using reactive and non-reactive force fields. Fluid Phase Equilibria 2017, 438, 53–66. [Google Scholar] [CrossRef]
- Shirolkar, M.M.; Phase, D.; Sathe, V.; Rodríguez-Carvajal, J.; Choudhary, R.J.; Kulkarni, S.K. Relation between crystallinity and chemical nature of surface on wettability: A study on pulsed laser deposited TiO2 thin films. J. Appl. Phys. 2011, 109, 123512. [Google Scholar] [CrossRef]
- Balajka, J.; Hines, M.A.; DeBenedetti, W.J.I.; Komora, M.; Pavelec, J.; Schmid, M.; Diebold, U. High-affinity adsorption leads to molecularly ordered interfaces on TiO2 in air and solution. Science 2018, 361, 786–789. [Google Scholar] [CrossRef] [Green Version]
- Drelich, J.W.; Boinovich, L.; Chibowski, E.; Volpe, C.D.; Hołysz, L.; Marmur, A.; Siboni, S. Contact angles: History of over 200 years of open questions. Surf. Innov. 2019, 8, 3–27. [Google Scholar] [CrossRef] [Green Version]
- Ennaceri, H.; Wang, L.; Erfurt, D.; Riedel, W.; Mangalgiri, G.; Khaldoun, A.; Ennaoui, A. Water-resistant surfaces using zinc oxide structured nanorod arrays with switchable wetting property. Surf. Coat. Technol. 2016, 299, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Xia, J.; Lei, W.; Wang, B. Superhydrophobic surface based on a coral-like hierarchical structure of ZnO. PLoS ONE 2010, 5, e14475. [Google Scholar] [CrossRef]
- Bouhjar, F.; Marí, B.; Bessaïs, B. Hydrothermal fabrication and characterization of ZnO/Fe2O3 heterojunction devices for hydrogen production. J. Anal. Pharm. Res. 2018, 7, 315–321. [Google Scholar] [CrossRef] [Green Version]
- Nawrocki, G.; Cieplak, M. Aqueous amino acids and proteins near the surface of gold in hydrophilic and hydrophobic force fields. J. Phys. Chem. C 2014, 118, 12929–12943. [Google Scholar] [CrossRef] [Green Version]
- Bakar, M.; Sugiuchi, M.; Iwasaki, M. Hydrogen bonds to Au atoms in coordinated gold clusters. Nat. Commun. 2017, 8, 576. [Google Scholar] [CrossRef] [PubMed]
- Iveson, S.M.; Holt, S.; Biggs, S. Advancing contact angle of iron ores as a function of their hematite and goethite content: Implications for pelletising and sintering. Int. J. Miner. Process. 2004, 74, 281–287. [Google Scholar] [CrossRef]
- Shrimali, K.; Jin, J.; Hassas, B.V.; Wang, X.; Miller, J.D. The surface state of hematite and its wetting characteristics. J. Colloid Interface Sci. 2016, 477, 16–24. [Google Scholar] [CrossRef]
- Kaminski, J.; Witkowska, J.; Plocinski, T.; Tarnowski, M.; Wierzchon, T. Structure and corrosion resistance of titanium oxide layers produced on NiTi alloy in low-temperature plasma. Int. J. Mater. Res. 2018, 109, 443–450. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Mynuddin, M. Kinetic modelling of atmospheric pressure nitrogen plasma. Am. J. Mod. Phys. 2018, 7, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Kongsong, P.; Taleb, A.; Masae, M.; Jeenarong, A.; Hansud, P.; Khumruean, S. Effect of nitrogen doping on the photocatalytic activity and hydrophobic property of rutile TiO2 nanorods array. Surf. Interface Anal. 2018, 50, 1271–1277. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Surface Science Techniques; Bracco, G., Holst, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 51, pp. 3–34. [Google Scholar]
- Shih, P.-H.; Sheng, Y.W. Growth mechanism studies of ZnO nanowires: Experimental observations and short-circuit diffusion analysis. Nanomaterials 2017, 7, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Wan, Y.; Wu, W.; Yang, C.; He, J.-H.; Cheng, J.; Chen, Y. A lotus effect-inspired flexible and breathable membrane with hierarchical electrospinning micro/nanofibers and ZnO nanowires. Mater. Des. 2019, 162, 246–248. [Google Scholar] [CrossRef]
- Latthe, S.; Terashima, C.; Nakata, K.; Fujishima, A. Superhydrophobic surfaces developed by mimicking hierarchical surface morphology of lotus leaf. Molecules 2014, 19, 4256–4283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter. 2004, 16, R829. [Google Scholar] [CrossRef]
- Mardosaitė, R.; Jurkevičiutė, A.; Račkauskas, S. Superhydrophobic ZnO Nanowires: Wettability Mechanisms and Functional Applications. Cryst. Growth Des. 2021, 21, 4765–4779. [Google Scholar] [CrossRef]
- Ketteler, G.; Weiss, W.; Ranke, W.; Schlögl, R. Bulk and surface phases of iron oxides in an oxygen and water atmosphere at low pressure. Phys. Chem. Chem. Phys. 2001, 3, 1114–1122. [Google Scholar] [CrossRef]
- Özdemir, Ö.; Banerjee, S.K. High temperature stability of maghemite (γ-Fe2O3). Geophys. Res. Lett. 1984, 11, 161–164. [Google Scholar] [CrossRef]
- Butashin, A.V.; Muslimov, A.E.; Asvarov, A.S. Formation of nanoporous films during solid-phase reactions. Nanotechnol. Russ. 2020, 15, 747–752. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Bernstein, J.L. Remeasurement of the structure of hexagonal ZnO. Acta Crystallogr. Sect. B Struct. Crystallogr. Crystal Chem. 1969, 25, 1233–1236. [Google Scholar] [CrossRef]
- Kraushofer, F.; Jakub, Z.; Bichler, M.; Hulva, J.; Drmota, P.; Weinold, M.; Parkinson, G.S. Atomic-scale structure of the hematite α-Fe2O3(102) “R-Cut” surface. J. Phys. Chem. C 2018, 122, 1657–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Chen, L.; Liao, X.; Jiang, W. Effect of heat treatment temperature on super-hydrophilic property of enamels with titanium dioxide thin film. J. Sol-Gel Sci. Technol. 2016, 80, 606–611. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muslimov, A.E.; Gadzhiev, M.K.; Kanevsky, V.M. New Approaches to Increasing the Superhydrophobicity of Coatings Based on ZnO and TiO2. Coatings 2021, 11, 1369. https://doi.org/10.3390/coatings11111369
Muslimov AE, Gadzhiev MK, Kanevsky VM. New Approaches to Increasing the Superhydrophobicity of Coatings Based on ZnO and TiO2. Coatings. 2021; 11(11):1369. https://doi.org/10.3390/coatings11111369
Chicago/Turabian StyleMuslimov, Arsen E., Makhach Kh. Gadzhiev, and Vladimir M. Kanevsky. 2021. "New Approaches to Increasing the Superhydrophobicity of Coatings Based on ZnO and TiO2" Coatings 11, no. 11: 1369. https://doi.org/10.3390/coatings11111369
APA StyleMuslimov, A. E., Gadzhiev, M. K., & Kanevsky, V. M. (2021). New Approaches to Increasing the Superhydrophobicity of Coatings Based on ZnO and TiO2. Coatings, 11(11), 1369. https://doi.org/10.3390/coatings11111369





