Formation of Aligned α-Si3N4 Microfibers by Plasma Nitridation of Si (110) Substrate Coated with SiO2
Abstract
:1. Introduction
2. Materials and Methods
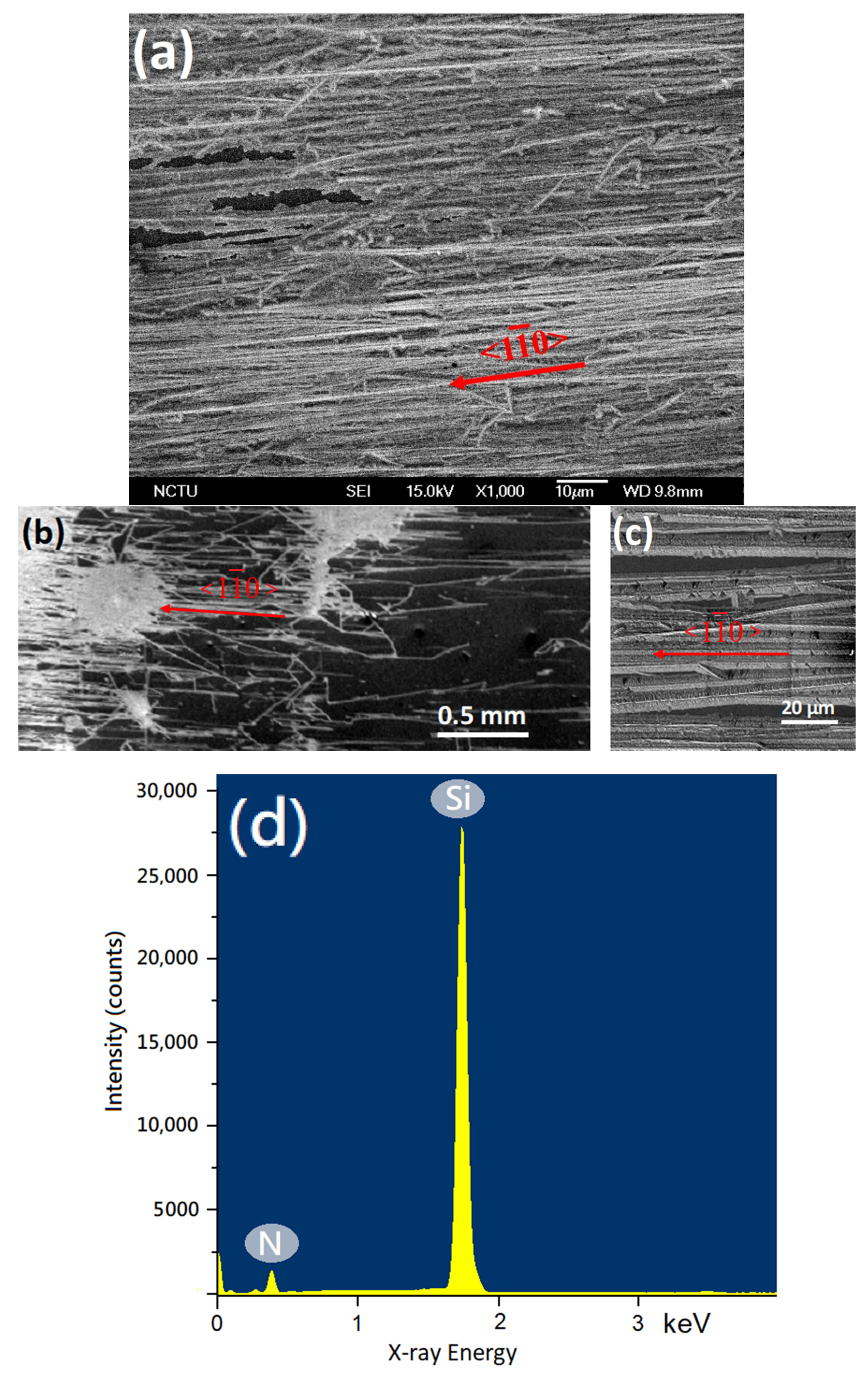
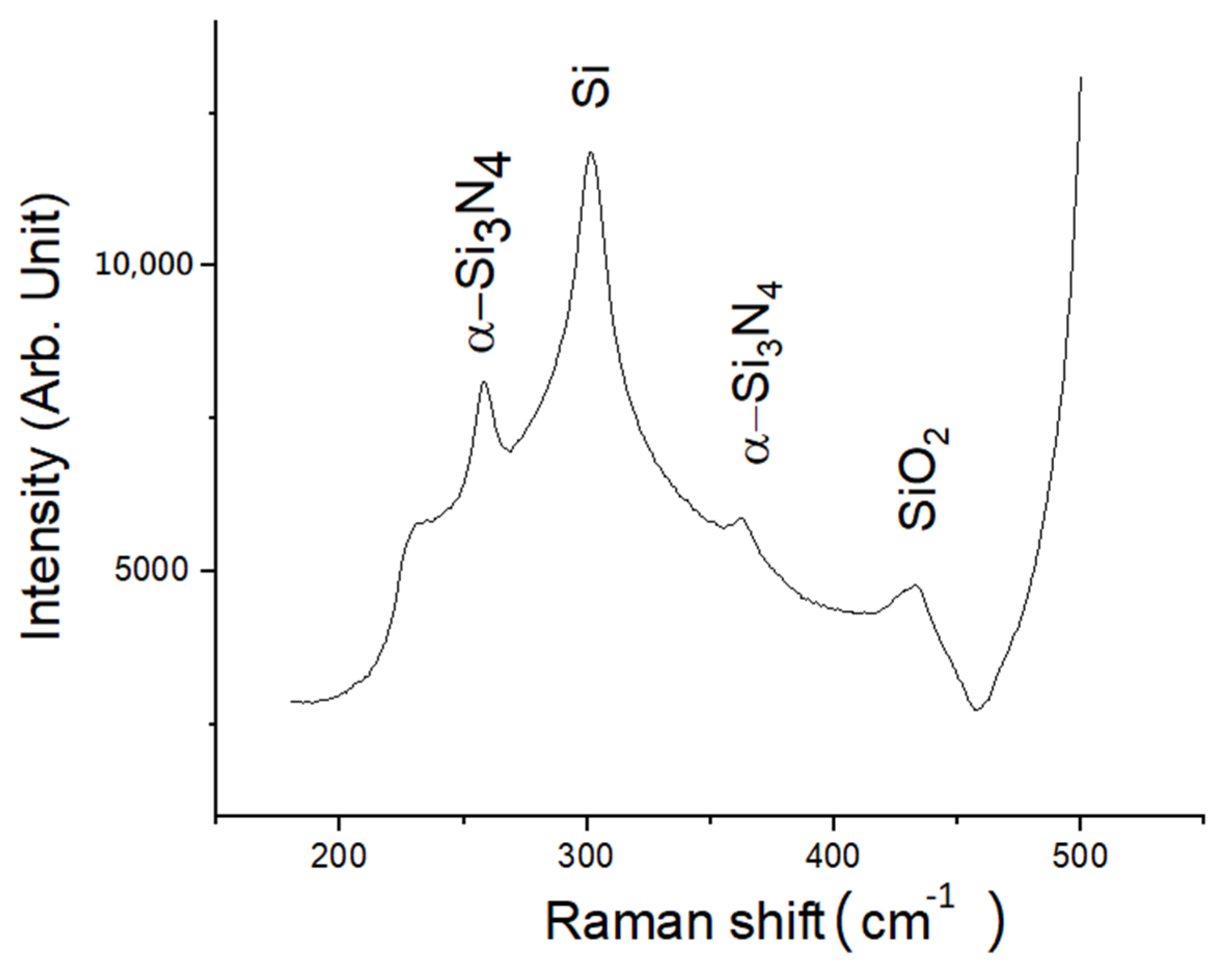
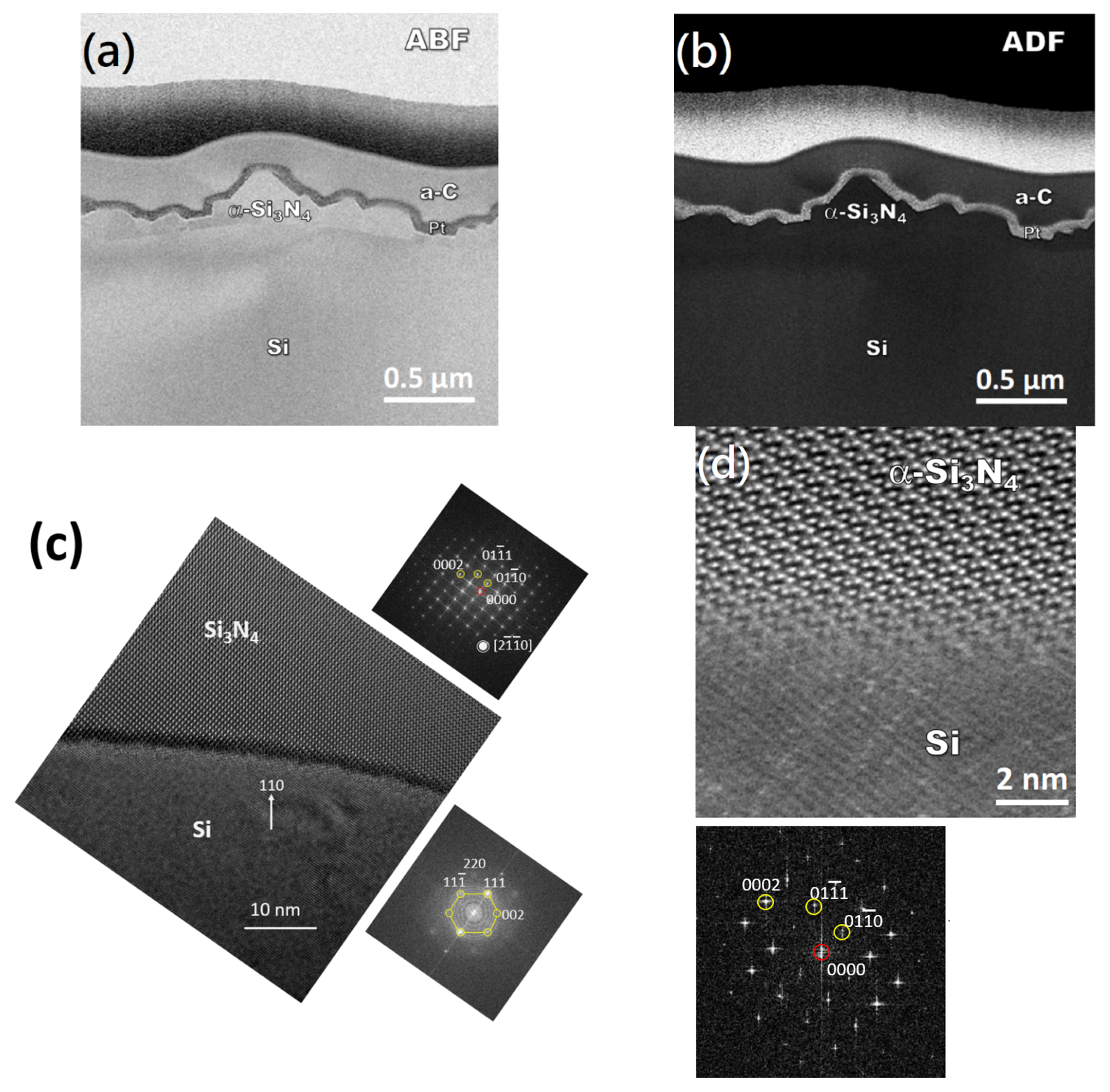
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Unal, O.; Petrovic, J.; Mitchell, T. CVD Si3N4 on single crystal SiC: Part I. Characterization and orientation relationship at the interface. J. Mater. Res. 1992, 7, 136–147. [Google Scholar] [CrossRef]
- Kim, J.W.; Yeom, H.W. Surface and interface structures of epitaxial silicon nitride on Si(111). Phys. Rev. B 2003, 67, 035304. [Google Scholar] [CrossRef]
- Riley, F.L. Silicon Nitride and Related Materials. J. Am. Ceram. Soc. 2004, 83, 245–265. [Google Scholar] [CrossRef]
- Murakawa, S.; Ishizuka, S.-I.; Nakanishi, T.; Suwa, T.; Teramoto, A.; Sugawa, S.; Hattori, T.; Ohmi, T. Depth Profile of Nitrogen Atoms in Silicon Oxynitride Films Formed by Low-Electron-Temperature Microwave Plasma Nitridation. Jpn. J. Appl. Phys. 2010, 49, 091301. [Google Scholar] [CrossRef]
- Higuchi, M.; Aratani, T.; Hamada, T.; Shinagawa, S.; Nohira, H.; Ikenaga, E.; Teramoto, A.; Hattori, T.; Sugawa, S.; Ohmi, T. Electric Characteristics of Si3N4Films Formed by Directly Radical Nitridation on Si(110) and Si(100) Surfaces. Jpn. J. Appl. Phys. 2007, 46, 1895–1898. [Google Scholar] [CrossRef]
- Seino, T.; Matsuura, T.; Murota, J. Atomic-order nitridation of SiO2 by nitrogen plasma. Surf. Interface Anal. 2002, 34, 451–455. [Google Scholar] [CrossRef]
- Sahu, B.B.; Yin, Y.; Han, J.G. Effect of plasma parameters on characteristics of silicon nitride film deposited by single and dual frequency plasma enhanced chemical vapor deposition. Phys. Plasmas 2016, 23, 033512. [Google Scholar] [CrossRef]
- Lee, E.L.; Wachs, I.E. In Situ Raman Spectroscopy of SiO2-Supported Transition Metal Oxide Catalysts: An Isotopic 18O−16O Exchange Study. J. Phys. Chem. C 2008, 112, 6487–6498. [Google Scholar] [CrossRef]
- Unal, O.; Mitchell, T. CVD Si3N4 on single crystal SiC: Part II. High resolution electron microscopy and atomic models of the interface. J. Mater. Res. 1992, 7, 1445–1454. [Google Scholar] [CrossRef]
- Malvos, H.; Michel, H.; Ricard, A. Correlations between active species density and iron nitride layer growth in Ar-N2-H2microwave post-discharges. J. Phys. D Appl. Phys. 1994, 27, 1328–1332. [Google Scholar] [CrossRef]
- Tatarova, E.; Dias, F.; Gordiets, B.; Ferreira, C. Molecular dissociation in N2–H2 microwave discharges. Plasma Sources Sci. Technol. 2004, 14, 19–31. [Google Scholar] [CrossRef]
- Ma, Z.-C.; Chiu, K.-A.; Wei, L.-L.; Chang, L. Formation of m-plane AlN on plasma-nitrided m-plane sapphire. Jpn. J. Appl. Phys. 2019, 58, SC1033. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chang, L. Epitaxial AlN on c-plane sapphire by plasma nitriding. Jpn. J. Appl. Phys. 2019, 58, SC1012. [Google Scholar] [CrossRef]
- Do, T.H.; Chang, C.; Wei, L.-L.; Chiu, K.-A.; Chang, L. Epitaxial TiN formation on rutile titanium dioxide (0 0 1) single crystal by nitridation. Appl. Surf. Sci. 2019, 506, 144614. [Google Scholar] [CrossRef]
- Lin, L.W.; He, Y.H. Synthesis and optical property of ultra-long alpha-Si3N4 nanowires under superatmospheric pressure conditions. CrystEngComm 2012, 14, 3250–3256. [Google Scholar] [CrossRef]
- Gao, F.; Yang, W.; Fan, Y.; An, L. Aligned ultra-long single-crystalline α-Si3N4nanowires. Nanotechnology 2008, 19, 105602. [Google Scholar] [CrossRef]
- Dong, S.; Hu, P.; Zhang, X.; Cheng, Y.; Fang, C.; Xu, J.; Chen, G. Facile synthesis of silicon nitride nanowires with flexible mechanical properties and with diameters controlled by flow rate. Sci. Rep. 2017, 7, 45538. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Jian, K. Synthesis and formation mechanism of α-Si3N4 single-crystalline nanowires via direct nitridation of H2-treated SiC fibres. Ceram. Int. 2018, 44, 12847–12852. [Google Scholar] [CrossRef]
- Yu, C.-H.; Chiu, K.-A.; Do, T.-H.; Chang, L. Oriented Si3N4 crystallites formed by plasma nitriding of SiO2/Si (111) substrate. Surf. Coat. Technol. 2020, 395, 125877. [Google Scholar] [CrossRef]
- Truscott, B.S.; Kelly, M.W.; Potter, K.J.; Johnson, M.; Ashfold, M.N.R.; Mankelevich, Y.A. Microwave Plasma-Activated Chemical Vapor Deposition of Nitrogen-Doped Diamond. I. N2/H2 and NH3/H2 Plasmas. J. Phys. Chem. A 2015, 119, 12962–12976. [Google Scholar] [CrossRef] [Green Version]
- Loureiro, J.; Ricard, A. Electron and vibrational kinetics in an N2-H2 glow discharge with application to surface processes. J. Phys. D Appl. Phys. 1993, 26, 163–176. [Google Scholar] [CrossRef]
- Kuzuba, T.; Kijima, K.; Bando, Y. Raman-active modes of alpha silicon nitride. J. Chem. Phys. 1978, 69, 40. [Google Scholar] [CrossRef]
- Faraci, G.; Mannino, G.; Pennisi, A.R.; Ruggeri, R.; Sberna, P.; Privitera, V. Raman and photoluminescence spectroscopy of Si nanocrystals: Evidence of a form factor. J. Appl. Phys. 2013, 113, 63518. [Google Scholar] [CrossRef]
- Spizzirri, P.G.; Fang, J.-H.; Rubanov, S.; Gauja, E.; Prawer, S. Nano-Raman spectroscopy of silicon surfaces. arXiv 2010, arXiv:1002.2692. [Google Scholar]
- Swift, G.A.; Ustundag, E.; Clausen, B.; Bourke, M.A.M.; Lin, H.-T. High-temperature elastic properties of in situ-reinforced Si3N4. Appl. Phys. Lett. 2003, 82, 1039. [Google Scholar] [CrossRef]
- Kuwabara, A.; Matsunaga, K.; Tanaka, I. Lattice dynamics and thermodynamical properties of silicon nitride polymorphs. Phys. Rev. B 2008, 78, 064104. [Google Scholar] [CrossRef] [Green Version]
- Messmer, C.; Bilello, J.C. The surface energy of Si, GaAs, and GaP. J. Appl. Phys. 1981, 52, 4623–4629. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, J.; Yang, H. Synthesis of silicon nitride nanowires directly from the silicon substrates. Chem. Phys. Lett. 2003, 372, 269–274. [Google Scholar] [CrossRef]
- Hayafuji, Y.; Kajiwara, K. Nitridation of Silicon and Oxidized-Silicon. J. Electrochem. Soc. 1982, 129, 2102–2108. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, N.; He, R.; Liu, J.; Zhang, X.; Zhu, J. A simple method to synthesize Si3N4 and SiO2 nanowires from Si or Si/SiO2 mixture. J. Cryst. Growth 2001, 233, 803–808. [Google Scholar] [CrossRef]
- Kobayashi, H.; Mizokuro, T.; Nakato, Y.; Yoneda, K.; Todokoro, Y. Nitridation of silicon oxide layers by nitrogen plasma generated by low energy electron impact. Appl. Phys. Lett. 1997, 71, 1978–1980. [Google Scholar] [CrossRef]
- Manjunatha, K.N.; Paul, S. Stability study: Transparent conducting oxides in chemically reactive plasmas. Appl. Surf. Sci. 2017, 424, 316–323. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Cheng, B.; Yu, J.; Wang, Q. Metal catalysis-free, direction-controlled planar growth of single-crystalline α-Si3N4 nanowires on Si(100) substrate. Nanotechnology 2006, 17, 3989–3993. [Google Scholar] [CrossRef]
- Dervišbegović, H.; Riley, F.L. The role of hydrogen in the nitridation of silicon powder compacts. J. Mater. Sci. 1981, 16, 1945–1955. [Google Scholar] [CrossRef]
- Rahman, I.A.; Riley, F.L. The control of morphology in silicon nitride powder prepared from rice husk. J. Eur. Ceram. Soc. 1989, 5, 11–22. [Google Scholar] [CrossRef]
- Saranin, A.; Tarasova, O.; Kotljar, V.; Khramtsova, E.; Lifshits, V. Thermal nitridation of the Si(110) by NH3: LEED and AES study. Surf. Sci. 1995, 331–333, 458–463. [Google Scholar] [CrossRef]
- Atanassova, E.D.; Popova, L.I. Plasma nitridation of thin SiO2 films: AES, ELS and IR study. J. Nucl. Mater. 1993, 200, 421–425. [Google Scholar] [CrossRef]
- Ramesh, P.D.; Rao, K.J. Carbothermal reduction and nitridation reaction of SiOx and preoxidized SiOx: Formation of α-Si3N4 fibers. J. Mater. Res. 1994, 9, 2330–2340. [Google Scholar] [CrossRef]
- Wang, Q.; Cong, R.; Li, M.; Zhang, J.; Cui, Q. A simple method to synthesize α-Si3N4, β-SiC and SiO2 nanowires by carbothermal route. J. Cryst. Growth 2010, 312, 2133–2136. [Google Scholar] [CrossRef]
- Gedevanishvili, S.; Cherian, K.; Agrawal, D.; Roy, R. Synthesis of Silicon Nitride Whiskers by Microwave Heating. MRS Proc. 1998, 547, 13. [Google Scholar] [CrossRef]
- Chen, F.; Li, Y.; Liu, W.; Shen, Q.; Zhang, L.; Jiang, Q.; Lavernia, E.J.; Schoenung, J.M. Synthesis of α silicon nitride single-crystalline nanowires by nitriding cryomilled nanocrystalline silicon powder. Scr. Mater. 2009, 60, 737–740. [Google Scholar] [CrossRef]
- Farjas, J.; Pinyol, A.; Rath, C.; Roura, P.; Bertran, E. Kinetic study of the oxide-assisted catalyst-free synthesis of silicon nitride nanowires. Phys. Status Solidi 2006, 203, 1307–1312. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Qin, X.; Yang, L.; Meng, Y.; Sun, L. Synthesis and photoluminescence of Si3N4 nanowires from La/SiO2 composites and Si powders. Ceram. Int. 2015, 41, 1505–1510. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, K.; Sun, S.; Zhang, J.; Cui, W.; Xie, Z.; Liu, G. Synthesis of Si3N4 nanowires by catalyst-free nitridation of (Si + SiO2) mixture. Micro Nano Lett. 2019, 14, 919–921. [Google Scholar] [CrossRef]
- Ahmad, M.; Zhao, J.; Zhang, F.; Pan, C.; Zhu, J. One-step synthesis route of the aligned and non-aligned single crystalline α-Si3N4 nanowires. Sci. China Ser. E Technol. Sci. 2009, 52, 1–5. [Google Scholar] [CrossRef]
- Ahmad, M.; Zhao, J.; Pan, C.; Zhu, J. Ordered arrays of high-quality single-crystalline α-Si3N4 nanowires: Synthesis, properties and applications. J. Cryst. Growth 2009, 311, 4486–4490. [Google Scholar] [CrossRef]
- Cui, J.; Li, B.; Zou, C.; Zhang, C.; Wang, S. Direct Synthesis of α-Silicon Nitride Nanowires from Silicon Monoxide on Alumina. Nanomater. Nanotechnol. 2015, 5, 32. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.-H.; Chiu, K.-A.; Do, T.-H.; Chang, L.; Chen, W.-C. Formation of Aligned α-Si3N4 Microfibers by Plasma Nitridation of Si (110) Substrate Coated with SiO2. Coatings 2021, 11, 1251. https://doi.org/10.3390/coatings11101251
Yu C-H, Chiu K-A, Do T-H, Chang L, Chen W-C. Formation of Aligned α-Si3N4 Microfibers by Plasma Nitridation of Si (110) Substrate Coated with SiO2. Coatings. 2021; 11(10):1251. https://doi.org/10.3390/coatings11101251
Chicago/Turabian StyleYu, Chang-Hua, Kun-An Chiu, Thi-Hien Do, Li Chang, and Wei-Chun Chen. 2021. "Formation of Aligned α-Si3N4 Microfibers by Plasma Nitridation of Si (110) Substrate Coated with SiO2" Coatings 11, no. 10: 1251. https://doi.org/10.3390/coatings11101251
APA StyleYu, C.-H., Chiu, K.-A., Do, T.-H., Chang, L., & Chen, W.-C. (2021). Formation of Aligned α-Si3N4 Microfibers by Plasma Nitridation of Si (110) Substrate Coated with SiO2. Coatings, 11(10), 1251. https://doi.org/10.3390/coatings11101251






