Abstract
Film-coating is widely applied in pharmaceutics to enhance aspect/taste and mechanical properties of dosage forms, to protect them from the environment and to modify their release performance. In this respect, a film-coating process was recently involved in the development of 4D printed prolonged-release systems intended for organ retention. During coating processes, liquid formulations are sprayed onto moving cores, whose shape, weight and surface characteristics are essential to attain a homogeneous film. Devices of complex shapes, composed of smart materials and fabricated by hot-processing techniques, such as extrusion and fused deposition modeling 3D printing, might be poorly compatible with the requirements of traditional coating methods, e.g., need for spherical substrates with smooth surface and stable under process temperatures. This work was aimed at evaluating, at a small scale level, the feasibility of a versatile equipment for film-coating of rod-shaped extruded and printed prototypes with different section. Equipment design and set up of process parameters were performed starting from polymeric solutions and suspensions and selecting as cores 50 mm-long rod-shaped samples based on shape memory poly(vinyl alcohol). Integrity and thickness of the applied layer and its impact on shape memory and release performance of prototypes were investigated.
1. Introduction
Accessibility of manufacturing techniques based on hot-processing and availability of smart polymers (e.g., materials changing their shape/characteristics in response to an external stimulus) has opened new possibilities in pharmaceutics, the most interesting ones regarding 4D printing [1,2,3,4,5,6]. At the same time, the accomplishment of challenging therapeutic goals hitherto considered unattainable and the development of personalized drug products through small-scale production processes might become a viable reality [7].
In previous works, self-expanding systems based on pharmaceutical-grade poly(vinyl alcohol) (PVA) and fabricated by hot melt extrusion (HME) and fused deposition modeling (FDM), thus involving 4D printing, were proposed for intravesical and intragastric applications [8,9,10]. Prototypes were conceived in an original shape (e.g., U-, S- and helix-like) with such a spatial encumbrance that their rapid emptying from the target organ could be avoided, thus ensuring long-lasting residence and release. Moreover, being composed of shape memory polymers, they were able to (i) take on a temporary shape suitable for administration inside either a catheter or a capsule and (ii) recover the original one upon contact with aqueous fluids at body temperature. In the development of these organ-retentive PVA-based systems, film-coating was taken into account in order to improve their mechanical properties and prolong the release duration, without affecting the shape memory effect.
In the drug delivery field, film-coating of solid dosage forms is often performed to improve aspect, mechanical properties, swallowability as well as taste of the final product, to protect it from the outer environment (e.g., humidity, light) and to control the release of the drug conveyed [11,12,13]. The process generally consists in spraying polymeric solutions/suspensions onto tumbling or fluidized substrates (e.g., granules/pellets, tablets) depending on the equipment employed (i.e., rotating pans or fluid beds). In order to achieve a continuous film of uniform thickness onto the cores, formulation and process parameters need to be adjusted. In particular, drying conditions defined by the air temperature and relevant flow rate, and the ability to expose continuously or alternately the whole surface of the substrates may turn out critical. In this respect, the proper exposure of the cores during the process mainly depends on their shape, weight as well as surface characteristics, and the best results are obtained with spherical smooth ones. Substrates characterized by complex shape (e.g., with edges and folds) as well as by peculiar thermal and mechanical properties, for instance, those involved in thermal programming of the desired temporary shape, could be difficult to coat and will require the development of dedicated processes.
In the present manuscript, the feasibility of a lab-scale, versatile and easy to set up film-coating process for self-expanding rod-shaped PVA-based devices fabricated by HME and FDM was demonstrated. A dedicated equipment was designed, and a new coating process was developed. In view of the desired performance and based on preliminary trials performed by casting, two coating formulations based on methacrylic acid copolymers were selected. Data on equipment and process set up, relevant robustness and reproducibility as well as impact of the applied coating on shape memory and release performance of the prototypes were collected.
2. Materials and Methods
2.1. Materials
PVA05 and PVA48 (Gohsenol™ EG 05P and 48P, Mitsubishi Chemical, Tokio, Japan) were selected as the main polymeric components for the manufacturing via HME and FDM of shape-memory prototypes; glycerol (GLY; Pharmagel, Milan, Italy) was the plasticizer employed to favor hot-processability of PVAs; allopurinol (ALP; FarmaQuimica Sur S.L., Malaga, Spain) was added as a model drug; methacrylic acid copolymers, Eudragit® RS 100 and RL 100 (Evonik, Essen, Germany); ready-to-use dispersion of methacrylic acid copolymers, Eudragit® NE (Evonik, Essen, Germany) were the main components of the liquid formulations to be sprayed during the coating process; triethyl citrate (TEC; Sigma Aldrich, Darmstadt, Germany) was the plasticizer chosen for the preparation of the Eudragit® RS 100 and RL 100 solution, and ethanol (Sigma Aldrich, Darmstadt, Germany) represented the solvent for the latter; PLA filament (TreeD Filaments, Seregno, Italy; glass transition temperature = 55–60 °C; melting temperature = 144 °C, density = 1.24 g/cm3) was used as received to print the different parts of the coating equipment.
2.2. Methods
2.2.1. Fabrication of Prototypes and Equipment Parts
PVA05 and PVA48 were kept in an oven at 40 °C for 24 h prior to use. Plasticized PVA formulations were prepared by mixing PVAs with 15% GLY in a mortar. The amount of plasticizer was expressed as percentage by weight on the dry polymer. 10% of ALP, calculated as percentage by weight on the plasticized polymeric formulation, was added as a model drug by mixing in a mortar. Starting from the PVA48-based formulation rod-shape prototypes with circular cross-section were prepared by HME. A twin-screw extruder (Haake™ MiniLab II, Thermo Scientific, Milwaukee, WI, USA) equipped with counter-rotating screws and a circular die of 1.50 mm in diameter was employed and the extruded rods were cut into 50 mm-long samples. Starting from the PVA05-based formulation, filaments with circular cross section of nominal 1.75 ± 0.05 mm in diameter were prepared by HME for feeding the FDM printer. In this respect, a custom-made aluminum circular die of 1.80 mm in diameter was employed for the HME process. Extruded rods were manually pulled and forced to pass through a caliper connected with the extruder and set at 1.80 mm as previously described [14]. After cooling, filament diameter was verified every 5 cm in length, and portions out of specifications were discarded. In Table 1, HME parameters for the PVA-based formulations are reported.

Table 1.
HME process parameters.
FDM was performed by a dual arm 3D printer (Kloner3D 240® Twin, Kloner3D, Florence, Italy) equipped with 0.5 mm nozzles. This was employed to fabricate both rod-shaped prototypes with squared cross-section (side = 1.5 mm) and parts of the rotating mechanism of the coating equipment. They were designed by means of Autodesk® Autocad® 2016 (Autodesk Inc., software version 14.0, San Rafael, CA, USA), and the CAD files were saved in .stl format and imported to the equipment software (Simplify 3D, I, software version 4.1, Milan, Italy). Rod-shaped samples were fabricated starting from the in-house prepared filaments based on PVA05 (printing conditions: nozzle temperature = 180 °C, build plate temperature = 70 °C, infill = 100%, layer height = 0.10 mm, printing speed = 23 mm/s). On the other hand, the equipment parts were printed from commercial poly (lactic acid) (PLA) filament used as received (printing default conditions provided by the software for high-resolution PLA-based prints: nozzle temperature = 220 °C, infill = 100%, layer height = 0.10 mm, printing speed = 50 mm/s).
Extruded and printed rod-shaped prototypes were coated with (i) an ethanolic solution (final concentration 30% weight/volume) containing Eudragit® RS and RL (mixed in a 3:1 ratio by weight) and TEC as the plasticizer (15% by weight on the dry polymeric blend) and (ii) a 30% ready-to-use aqueous suspension of Eudragit® NE. Description of the equipment and coating process developed is the main topic of the manuscript. Coating processes were carried out at ambient conditions (21 ± 0.5 °C and 55% RH).
2.2.2. Characterization of Prototypes
Uncoated and coated prototypes were characterized for weight (n = 6; Sartorius, Varedo, Italy) and dimensions. The diameter of the extruded specimens was checked using a caliper in 3 different positions to rule out any possible ovalization phenomena. The caliper was also used to measure width and height of FDM prototypes in 3 different points along their length. To evaluate thickness of the coated prototypes, samples (n = 6) were cut, and photographs of their cross section were acquired using a digital microscope (Digital Microscope AM-413T, Dino-Lite, Milan, Italy; resolution = 1.3 Megapixel − 1280 × 1024). The latter were processed (ImageJ, software version 1.8.0_172, Milan, Italy) to evaluate the coating thickness in at least 6 different positions along the circumference of the samples, as highlighted in Figure 1.
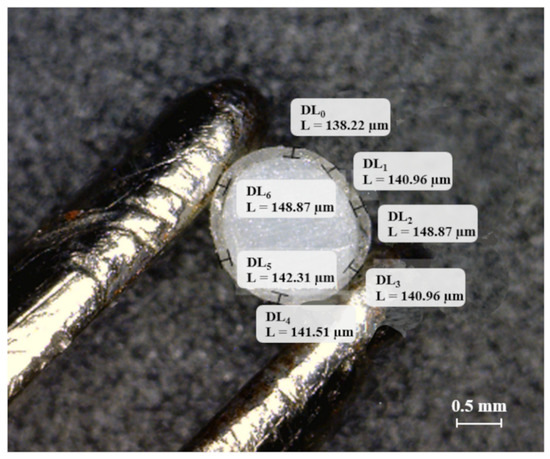
Figure 1.
Digital photograph of the cross-section of an extruded rod coated for 16 min with the Eudragit® NE aqueous suspension with thickness measurements taken in 7 different positions (DL0–DL6).
Uncoated and coated prototypes were tested for shape memory effect according to [8]. Samples were heated at 35 °C above their glass transition temperature (Tg) and forced to take on a temporary U-shape. Then, they were cooled below Tg in the deformed shape. Shape recovery was studied upon immersion of the deformed specimens into a crystallization vessel, filled with 250 mL of HCl 0.1N and kept at 37 ± 0.5 °C by means of a thermoregulated bath. The recovery of the original shape was monitored using a digital camera positioned above (distance 13 cm) the specimens (GoPro Hero Session, Milan, Italy; n = 3). The photographs acquired were processed using a specific software (ImageJ, software version 1.8.0_172, Milan, Italy) to calculate the recovery index (RI) as follows.
where αp is the angle obtained in the programming phase (angles in rad).
By linear interpolation of the recovery data immediately before and after the time point of interest, times to 50% (t50%RI) and 80% (t80%RI) recovery, respectively, were calculated.
Tg of the rod-shaped prototypes was determined by DSC (DSC Q100, TA Instruments, New Castle, DE, USA; n = 1) using nitrogen as a purge gas (70 mL/min). Indium was used as a calibration standard. Samples of about 10 mg were heated in aluminum crucibles from −50 to 240 °C, maintained at this temperature for 1 min, cooled down to −50 °C and reheated up to 240 °C. Both heating and cooling steps were run at 10 °C/min. In this respect, Tg of PVA05- and PVA48-based samples were 20 and 28 °C respectively.
Uncoated and coated samples in their original shape were tested for release using a USP38 dissolution apparatus 2 (50 rpm, 37 ± 0.5 °C, 900 mL HCl 0.1 N; Distek Inc., North Brunswick, NJ, USA; n = 6). Fluid samples were withdrawn at specific time points (0.5, 2 and 6 h) and assayed spectrophotometrically (λ = 251 nm) to calculate the percentage of ALP released.
3. Results and Discussion
3.1. Equipment Setup
A lab-scale equipment comprising two assemblies for spraying the coating formulations and implementing the rotation of rod-shaped samples was conceived and in-house assembled. An outline of the equipment developed is reported in Figure 2.
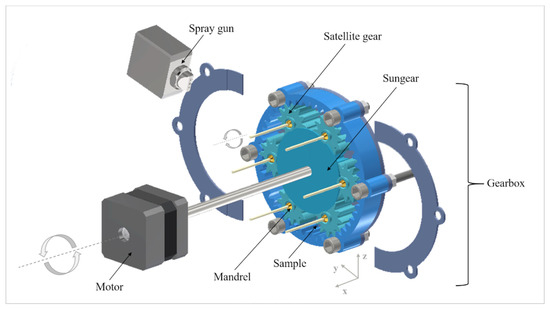
Figure 2.
Outline of the assembled coating equipment.
The spraying system was derived from a pan coating setup. It comprised a low-pressure spray gun entailing a 3-ways (i.e., control, atomizer and pattern) nozzle. This was maintained in a fixed position by means of a stand provided with different clamps connecting the nozzle to the chassis of the rotating assembly. The positioning of the spray gun (i.e., 20 cm, distance from the gearbox in the x axis; 30 cm, distance from the base of the gearbox in the z axis; 10 cm, distance from the nearest sample in y axis; 30° inclination with respect to the xy plane) (Figure 2), was selected to assess the desired spray width and the actual coverage area relevant to all the samples during rotation. The spray gun was connected to a peristaltic pump and to an air inlet, the rotation rate and pressure of which could be fine-tuned by the operator. Spray rate and pattern of either solutions or suspensions could be customized. While the control port of the gun just opened and closed the access for liquid, the atomizer allowed to adjust the drop dimensions and the geometry of the spray pattern. For instance, the ability to direct the spray cone towards samples was improved by changing the pattern pressure parameter, thus ovalizing the spray pattern. In the case of the 50 mm-long rod-shaped prototypes with one dimension bigger than the others, this led to a more targeted spraying over the entire length of the samples.
The rotating mechanism relied on an epicyclic planetary gearbox. Each part of the latter was designed and printed in-house (Figure 3).
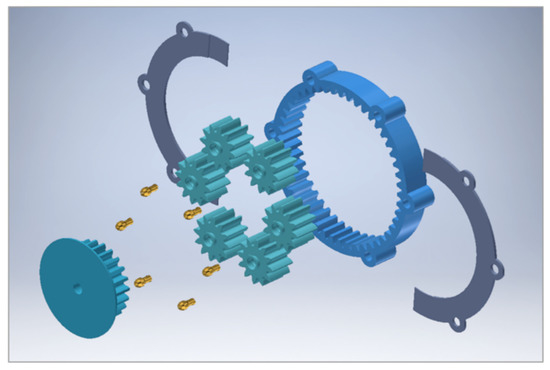
Figure 3.
Virtual models with dimensional details of the 3D printed parts of the equipment.
More into detail, the gearbox was composed of two different sets of gears (i.e., the sungear and the satellite gears) purposely assembled. The sungear was directly kept in motion by a rotor and transmitted combined movements to the six satellite gears attached to it. These were in-built with the sungear and were devised with a central hole containing mandrels, which were included to enable precise arrangement and secure fixing of samples. The presence of mandrels increased the equipment versatility, by allowing specimens with different shape and dimensions to be housed. While the sungear moved around its own axis only, the satellites also rotated around the sun (i.e., revolution movement). This way, the samples could periodically face the spray pattern, being able to dry during the rest of the rotation/revolution movement.
During the coating processes, the equipment was placed into an isolated chamber, provided with aspiration, control of temperature and of relative humidity, as well as a device for hot-air circulation to be activated when needed.
3.2. Process Setup
Liquid formulations of methacrylic copolymers, i.e., plasticized ethanolic solution of Eudragit® RS and RL and ready-to-use aqueous suspension of Eudragit® NE, were selected for the coating process. Preliminary tests based on a trial and error approach were carried out in order to identify suitable process parameters for coating PVA-based rod-shaped prototypes of 50 mm in length, fabricated by HME and FDM having circular or squared cross-section, respectively. This step was particularly challenging with the aqueous suspension in view of the high water solubility of PVA, which might compromise the device integrity during the process. In Table 2, the final coating conditions identified as suitable are reported.

Table 2.
Coating process conditions.
When dealing with the aqueous suspension, the early formation of a gel layer on the sample surface was observed by increasing the spraying rate. Therefore, this parameter was maintained lower compared to the ethanolic solution, and the drying conditions were consistently adapted, i.e., by increasing the air temperature and its flow. In order to attain a comparable spraying cone with either the solution or the suspension, the pattern pressure needed to be reduced as well. Overall, the selected spraying and drying conditions gave rise to alternating spraying passages occurring at constant intervals. Therefore, the rotation speed of the rod-shaped specimens needed to be fine-tuned, also taking spacing among samples into account, in order to lead to an effective coating, i.e., passage of samples inside the spray cone exactly at each spraying occurrence.
3.3. Evaluation of Coated Prototypes
Samples with increasing weight gain and coating thickness were obtained at successive time points of the coating processes (i.e., 4, 8 and 16 min) (Table 3). All samples underwent a curing process (i.e., 2 h in a ventilated oven set at 40 °C), which turned out to be essential to attain a continuous smooth film. By way of example, photographs of extruded cylindrical prototypes and printed squared ones coated with the Eudragit® RS/RL ethanolic solution for increasing process times are reported in Figure 4.

Table 3.
Weight gain and coating thickness of extruded and printed samples coated for different times.
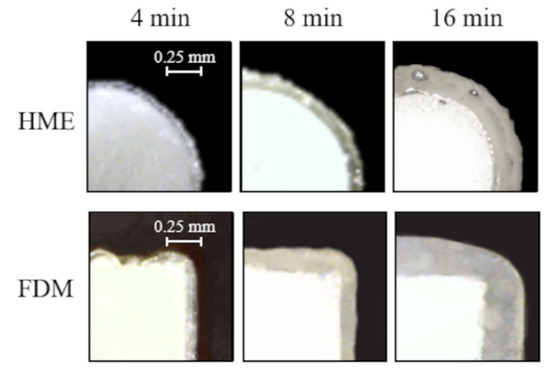
Figure 4.
Photographs of the cross-section of extruded and printed samples coated with the Eudragit® RS/RL ethanolic solution for different times.
The coating thickness was shown to increase with the process time and turned out to be uniform in all the positions over the different samples. At any process time considered, a lower thick film was found on Eudragit® NE-coated prototypes with respect to Eudragit® RS/RL-coated ones. This was consistent with the lower amount of water-based suspension sprayed for the same time interval.
In order to confirm the suitability of the coating process developed, reproducibility of the performance of coated rod-shaped prototypes, in terms of both shape memory effect and drug release, was evaluated. Shape memory behavior was tested according to a method previously developed, involving the programming of samples in a temporary U-shape [8]. The integrity of the film after programming was visually checked. No cracking phenomena were observed, regardless of the coating formulation and thickness considered. By way of example, photographs of extruded and printed samples coated with either Eudragit® NE or RS/RL formulations, before and after programming of the temporary shape, are reported in Figure 5.

Figure 5.
Photographs of extruded and printed samples coated with Eudragit® RS/RL ethanolic solution and Eudragit® NE water suspension (4 min), before and after programming of the temporary shape.
Recovery of the original rod-shape was tested following immersion of prototypes in aqueous fluids at 37 °C. Calculated recovery parameters, i.e., time to attain a recovery index equal to 50% and 80%, relevant to coated and uncoated samples used as reference, are reported in Table 4. On the other hand, the percentages of drug released after 0.5, 2 and 6 h from uncoated and coated rod-shaped prototypes are summarized in Table 5. The variability of data relevant to both shape recovery and release performance of coated samples, highlighted by the CV parameters, was shown analogous to that of the uncoated ones.

Table 4.
Recovery parameters (t50%RI and t80%RI) relevant to uncoated and coated samples.

Table 5.
Percentage of ALP released at different time points relevant to uncoated and coated samples.
Overall, the data obtained confirmed that the application of polymeric coatings is a suitable strategy to control the release rate of shape-memory drug delivery systems without affecting their smart performance. The coating process developed was successful for the attainment of systems intended for organ retention and based on pharmaceutical-grade hydrophilic swellable/soluble shape-memory polymers. In addition, the equipment proposed, aside from being scalable, would be particularly useful for pharmaceutical scientists who need to implement film-coating processes for non-traditional substrates (e.g., those having non-spherical or complex shapes with edges and folds, critical thermal and mechanical properties) at a small scale level.
Author Contributions
Conceptualization, M.U. and A.M.; methodology, A.M.; formal analysis, M.U.; investigation, M.U. and A.M.; resources, A.G. and L.Z.; data curation, M.U., A.M. and M.C.; writing—original draft preparation, M.U. and A.M.; writing—review and editing, M.C., L.Z. and A.G.; visualization, M.U. and S.M.; supervision, A.G. and L.Z.; project administration, L.Z.; funding acquisition, A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by: “Università degli Studi di Milano, Linea 3-Bando Straordinario per Progetti Interdipartimentali (Bando SEED2019)”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available with the article.
Acknowledgments
The authors would like to thank Rofarma Italia S.r.l. for kindly providing samples of Eudragit® NE, RS and RL.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Melocchi, A.; Uboldi, M.; Cerea, M.; Foppoli, A.; Maroni, A.; Moutaharrik, S.; Palugan, L.; Zema, L.; Gazzaniga, A. A graphical review on the escalation of fused deposition modeling (FDM) 3D printing in the pharmaceutical field. J. Pharm. Sci. 2020, 109, 2943–2957. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.V.; Patil, H.; Repka, M.A. Contribution of hot-melt extrusion technology to advance drug delivery in the 21st century. Expert Opin. Drug Deliv. 2016, 13, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Zema, L.; Loreti, G.; Melocchi, A.; Maroni, A.; Gazzaniga, A. Injection molding and its application to drug delivery. J. Control. Release 2012, 159, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Maroni, A.; Melocchi, A.; Zema, L.; Foppoli, A.; Gazzaniga, A. Retentive drug delivery systems based on shape memory. Mater. J. Appl. Polym. Sci. 2020, 137, 48798. [Google Scholar] [CrossRef]
- Melocchi, A.; Uboldi, M.; Cerea, M.; Foppoli, A.; Maroni, A.; Moutaharrik, S.; Palugan, L.; Zema, L.; Gazzaniga, A. Shape memory materials and 4D printing in pharmaceutics. Adv. Drug Deliv. Rev. 2021, 173, 216–237. [Google Scholar] [CrossRef] [PubMed]
- Alshebly, Y.S.; Nafea, M.; Mohamed Ali, M.S.; Almurib, H.A.F. Review on recent advances in 4D printing of shape memory polymers. Eur. Polym. J. 2021, 159, 110708. [Google Scholar] [CrossRef]
- Zema, L.; Melocchi, A.; Maroni, A.; Gazzaniga, A. Three-dimensional printing of medicinal products and the challenge of personalized therapy. J. Pharm. Sci. 2017, 106, 1697–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melocchi, A.; Inverardi, N.; Uboldi, M.; Baldi, F.; Maroni, A.; Pandini, S.; Briatico-Vangosa, F.; Zema, L.; Gazzaniga, A. Retentive device for intravesical drug delivery based on water-induced shape memory response of poly(vinyl alcohol): Design concept and 4D printing feasibility. Int. J. Pharm. 2019, 559, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Uboldi, M.; Inverardi, N.; Briatico-Vangosa, F.; Baldi, F.; Pandini, S.; Scalet, G.; Auricchio, F.; Cerea, M.; Foppoli, A.; et al. Expandable drug delivery system for gastric retention based on shape memory polymers: Development via 4D printing and extrusion. Int. J. Pharm. 2019, 571, 118700. [Google Scholar] [CrossRef] [PubMed]
- Inverardi, N.; Scaletb, G.; Melocchi, A.; Uboldi, M.; Maroni, A.; Zema, L.; Gazzaniga, A.; Auricchio, F.; Briatico-Vangosa, F.; Baldi, F.; et al. Experimental and computational analysis of a pharmaceutical-grade shape memory polymer applied to the development of gastroretentive drug delivery systems. J. Mech. Behav. Biomed. Mater. 2021, 124, 104814. [Google Scholar] [CrossRef] [PubMed]
- Felton, L.A. Characterization of coating systems. AAPS PharmSciTech 2007, 8, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Felton, L.A.; Porter, S.C. An update on pharmaceutical film coating for drug delivery. Expert Opin. Drug Deliv. 2013, 10, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.C.; Felton, L.A. Techniques to assess film coatings and evaluate film-coated products. Drug Dev. Ind. Pharm. 2010, 36, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Parietti, F.; Maroni, A.; Foppoli, A.; Gazzaniga, A.; Zema, L. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. Int. J. Pharm. 2016, 509, 255–263. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).