Application of Fragrance Microcapsules onto Cotton Fabric after Treatment with Oxygen and Nitrogen Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
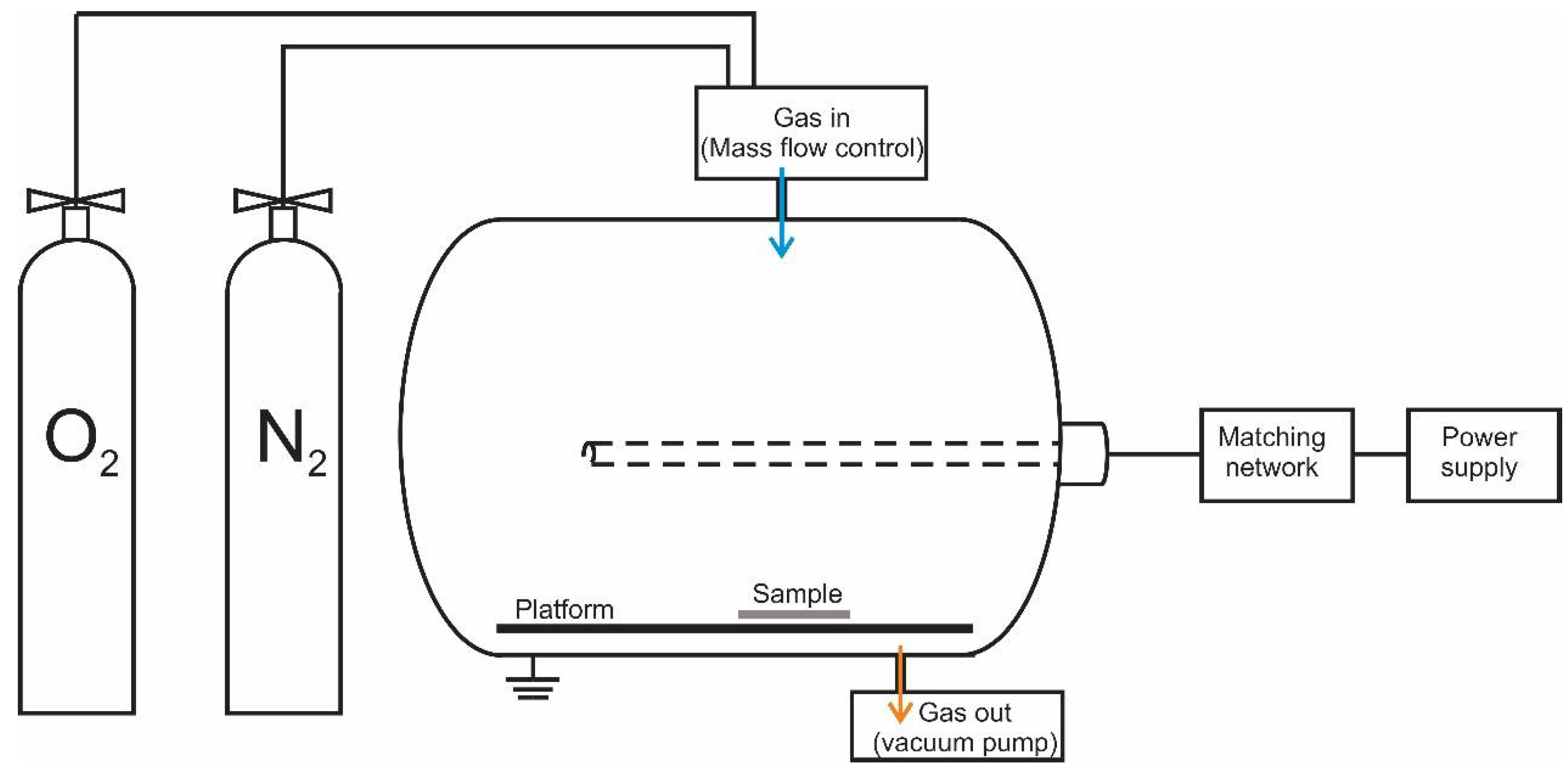
2.2. Treatment of Cotton in Plasma
2.3. Application of Microcapsules
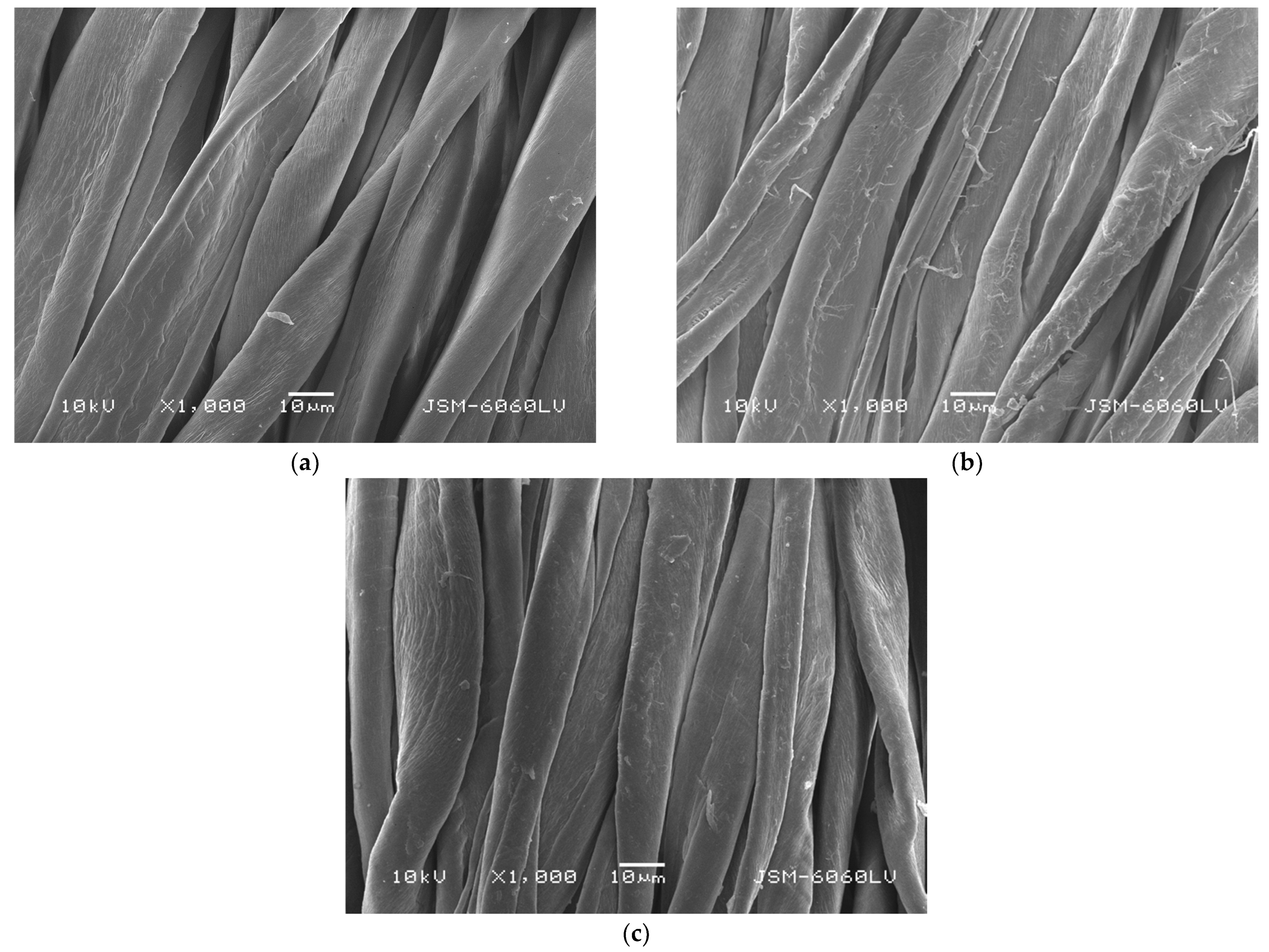
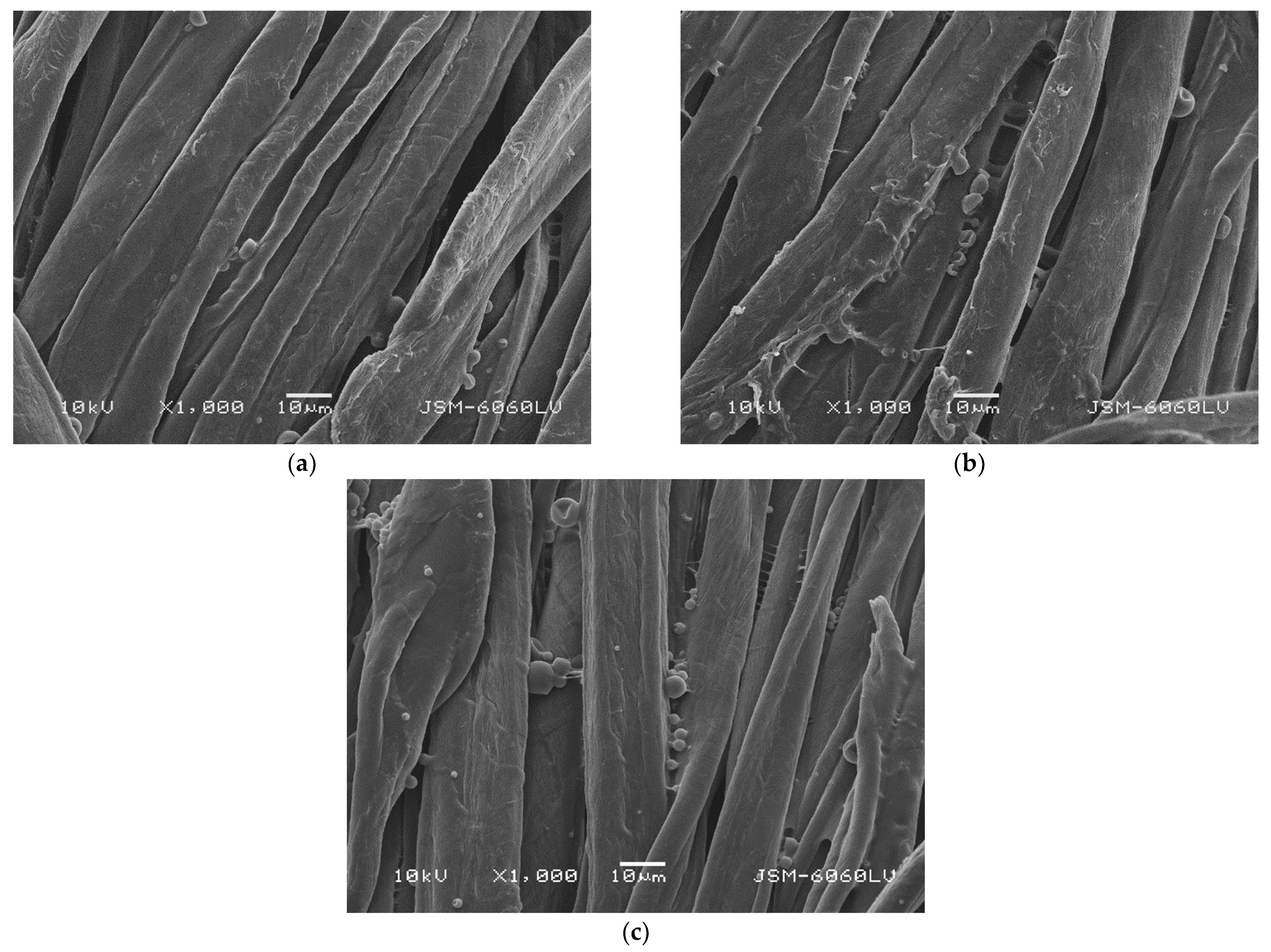
2.4. Scanning Electron Microscopy
2.5. Tensile Property of the Fabrics
2.6. Fabrics Stiffness
2.7. Absorptiveness of Fabrics
2.8. Fragrance Evaluation
2.9. Air Permeability of the Fabrics
2.10. Fastness of the Coated Fabric to Washing
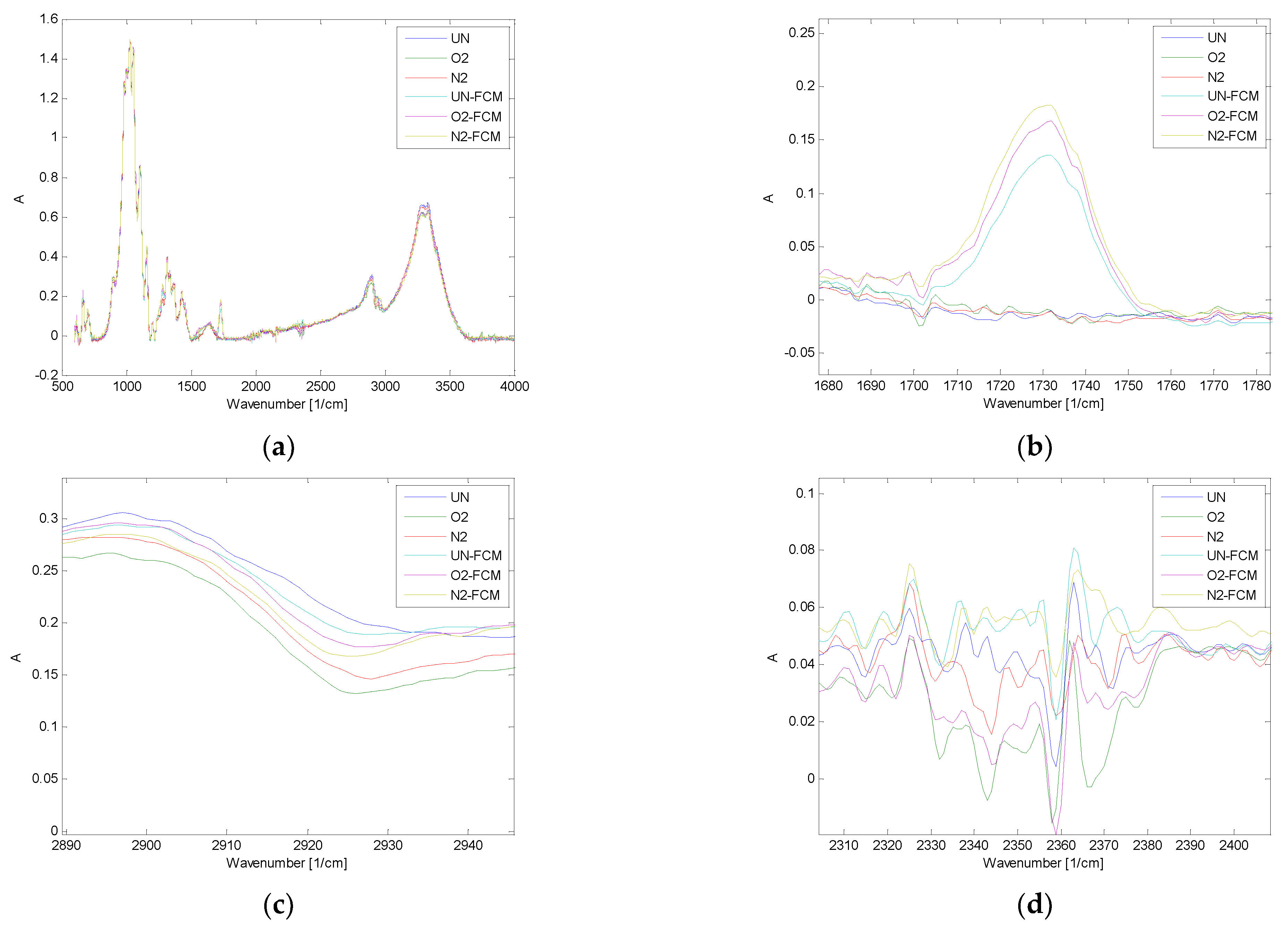
2.11. Fourier-Transform Infrared Spectroscopy (FTIR)
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cerempei, A. Aromatherapeutic textiles. In Active Ingredients from Aromatic and Medicinal Plants; El-Shemy, H.A., Ed.; InTech: Rijeka, Croatia, 2017; pp. 87–106. [Google Scholar]
- Singh, N.; Sheikh, J. Microencapsulation and its application in production of functional textiles. Indian J. Fibre Text 2020, 45, 495–509. [Google Scholar]
- Ghayempour, S.; Montazer, M. Micro/nanoencapsulation of essential oils and fragrances: Focus on perfumed, antimicrobial, mosquito-repellent and medical textiles. J. Microencapsul. 2016, 33, 497–510. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, W.; Zhang, X.; Long, G.; Fan, J.; Chen, H.; Deng, L. A novel fractal solution for permeability and Kozeny-Carman constant of fibrous porous media made up of solid particles and porous fibers. Powder Technol. 2019, 349, 92–98. [Google Scholar] [CrossRef]
- Cristina, E.D. Understanding true aromatherapy: Understanding essential oils. Home Health Care Manag. Pract. 2004, 16, 474–479. [Google Scholar] [CrossRef]
- Bonet-Aracil, M.; Monllor, P.; Capablanca, L.; Gisbert, J.; Díaz, P.; Montava, I. A comparison between padding and bath exhaustion to apply microcapsules onto cotton. Cellulose 2015, 22, 2117–2127. [Google Scholar] [CrossRef] [Green Version]
- Bezerra, F.M.; Carmona, O.G.; Carmona, C.G.; Lis, M.J.; de Moraes, F.F. Controlled release of microencapsulated citronella essential oil on cotton and polyester matrices. Cellulose 2016, 23, 1459–1470. [Google Scholar] [CrossRef]
- Boh, B.; Knez, E. Microencapsulation of essential oils and phase change materials for applications in textile products. Indian J. Fibre Text. Res. 2006, 31, 72–82. [Google Scholar]
- Bonet-Aracil, M.; Capablanca, L.; Monllor, P.; Díaz, P.; Montava, I. Studying bath exhaustion as a method to apply microcapsules on fabrics. J. Text. Inst. 2012, 103, 629–635. [Google Scholar] [CrossRef]
- Golja, B.; Šumiga, B.; Tavčer, P.F. Fragrant finishing of cotton with microcapsules: Comparison between printing and impregnation. Color. Technol. 2013, 129, 338–346. [Google Scholar] [CrossRef]
- Li, S.; Boyter, H.; Qian, L. UV curing for encapsulated aroma finish on cotton. J. Text. Inst. 2005, 96, 407–411. [Google Scholar] [CrossRef]
- Li, S.; Lewis, J.E.; Stewart, N.M.; Qian, L.; Boyter, H. Effect of finishing methods on washing durability of microencapsulated aroma finishing. J. Text. Inst. 2008, 99, 177–183. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Fernandes, M.; Carneiro, N.; Souto, A.P. Functionalization of wool fabric with phase-change materials microcapsules after plasma surface modification. J. Appl. Polym. Sci. 2013, 128, 2638–2647. [Google Scholar] [CrossRef] [Green Version]
- Ghayempour, S.; Mortazavi, S.M. Microwave curing for applying polymeric nanocapsules containing essential oils on cotton fabric to produce antimicrobial and fragrant textiles. Cellulose 2015, 22, 4065–4075. [Google Scholar] [CrossRef]
- Carmo, S.N.D.; Zille, A.; Souto, A.P. Plasma-assisted deposition of microcapsule containing Aloe vera extract for cosmeto-textiles. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 122007. [Google Scholar] [CrossRef] [Green Version]
- Guignard, M.I.; Campagne, C.; Giraud, S.; Brebu, M.; Vrinceanu, N.; Cioca, L.-I. Functionalization of a bamboo knitted fabric using air plasma treatment for the improvement of microcapsules embedding. J. Text. Inst. 2014, 106, 119–132. [Google Scholar] [CrossRef]
- Shin, S.; Shin, J.E.; Yoo, Y.J. Attachment of alginate microcapsules onto plasma-treated PDMS sheet for retrieval after transplantation. Biotechnol. Appl. Biochem. 2013, 60, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.R.; Silva, E.A.A.; Carmo, S.N.D.; Steffens, F.; Souto, A.P.G.D.V. Functionalization of natural cork composite with microcapsules after plasma treatment. Adv. Mater. Sci. Eng. 2014, 2014, 685829. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-J.; Kim, K.-S. Effect of oxygen plasma treatment on the release behaviors of poly (ɛ-caprolactone) microcapsules containing tocopherol. Colloids Surf. B Biointerfaces 2005, 43, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Gorjanc, M.; Mozetič, M.; Vesel, A.; Zaplotnik, R. Natural dyeing and UV protection of plasma treated cotton. Eur. Phys. J. D 2018, 72, 41. [Google Scholar] [CrossRef]
- Štular, D.; Primc, G.; Mozetič, M.; Jerman, I.; Mihelčič, M.; Ruiz-Zepeda, F.; Tomšič, B.; Simončič, B.; Gorjanc, M. Influence of non-thermal plasma treatement on the adsorption of a stimuli-responsive nanogel onto polyethylene terephthalate fabric. Prog. Org. Coat. 2018, 120, 198–207. [Google Scholar] [CrossRef]
- Gorjanc, M.; Savić, A.; Topalić-Trivunović, L.; Mozetič, M.; Zaplotnik, R.; Vesel, A.; Grujić, D. Dyeing of plasma treated cotton and bamboo rayon with Fallopia japonica extract. Cellulose 2016, 23, 2221–2228. [Google Scholar] [CrossRef]
- Primc, G.; Tomšič, B.; Vesel, A.; Mozetič, M.; Ražić, S.E.; Gorjanc, M. Biodegradability of oxygen-plasma treated cellulose textile functionalized with ZnO nanoparticles as antibacterial treatment. J. Phys. D Appl. Phys. 2016, 49, 324002. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, Y.; Li, W.; Chen, S.; You, S.; Ma, J. Preparation of silver-plated para-aramid fiber by employing low-temperature oxygen plasma treatment and dopamine functionalization. Coatings 2019, 9, 599. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.I.; Senturk, D.; Silva, K.K.; Modic, M.; Cvelbar, U.; Dinescu, G.; Mitu, B.; Nikiforov, A.; Leys, C.; Kuchakova, I.; et al. Antimicrobial efficacy of low concentration PVP-silver nanoparticles deposited on DBD plasma-treated polyamide 6,6 fabric. Coatings 2019, 9, 581. [Google Scholar] [CrossRef] [Green Version]
- Radetić, M.; Jocić, D.; Jovančić, P.; Rajaković, L.; Thomas, H.; Petrović, Z.L. Recycled-wool-based nonwoven material as a sorbent for lead cations. J. Appl. Polym. Sci. 2003, 90, 379–386. [Google Scholar] [CrossRef]
- Radetić, M.M.; Jocić, D.M.; Jovančić, P.; Petrović, Z.; Thomas, H.F. Recycled wool-based nonwoven material as an oil sorbent. Environ. Sci. Technol. 2003, 37, 1008–1012. [Google Scholar] [CrossRef]
- Mihailović, D.; Saponjic, Z.; Molina, R.; Puac, N.; Jovančić, P.; Nedeljkovic, J.; Radetić, M. Improved properties of oxygen and argon RF plasma-activated polyester fabrics loaded with TiO2 nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 1700–1706. [Google Scholar] [CrossRef]
- Makabe, T.; Petrović, Z.L. Plasma Electronics: Applications in Microelectronic Device Fabrication, 2nd ed.; CRC Press: New York, NY, USA, 2015; 360p. [Google Scholar]
- Lieberman, M.A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Chabert, P.; Braithwaite, N. Physics of Radio-Frequency Plasmas; Cambridge University Press: Cambridge, UK, 2011; p. 394. [Google Scholar]
- Gorjanc, M.; Mozetič, M.; Primc, G.; Vesel, A.; Spasić, K.; Puač, N.; Petrović, Z.L.; Kert, M. Plasma treated polyethylene terephthalate for increased embedment of UV-responsive microcapsules. Appl. Surf. Sci. 2017, 419, 224–234. [Google Scholar] [CrossRef]
- Vesel, A.; Primc, G.; Zaplotnik, R.; Mozetič, M. Applications of highly non-equilibrium low-pressure oxygen plasma for treatment of polymers and polymer composites on an industrial scale. Plasma Phys. Control. Fusion 2020, 62, 024008. [Google Scholar] [CrossRef]
- Primc, G.; Mozetič, M. Neutral reactive gaseous species in reactors suitable for plasma surface engineering. Surf. Coat. Technol. 2019, 376, 15–20. [Google Scholar] [CrossRef]
- Golja, B.; Tavčer, P.F. Textile functionalisation by printing fragrant, antimicrobial and flame-retardant microcapsules. Tekstilec 2016, 59, 278–288. [Google Scholar] [CrossRef]
- Golja, B.; Forte-Tavčer, P. Behaviour of printed melamine-formaldehyde microcapsules exposed to different mechanical impacts. In Magic World of Textiles: Book of Proceedings, Proceeding of the 5th International Textile, Clothing & Design Conference, Dubrovnik, Croatia, 3–6 October 2010; Dragčević, Z., Ed.; University of Zagreb: Zagreb, Croatia, 2010; pp. 360–364. [Google Scholar]
- Golja, B. Funkcionalizacija Tekstilij z Nanosom Mikrokapsul. Ph.D. Thesis, University of Ljubljana, Ljubljana, Slovenia, 2013. [Google Scholar]
- Puač, N.; Petrović, Z.L.; Živković, S.; Giba, Z.; Grubišić, D.; Ðorđević, A.R. Low temperature plasma treatment of dry empress-tree seeds. In Plasma Processes and Polymers; D’Agostino, R., Favia, P., Oehr, C., Wertheimer, M.R., Eds.; Whiley: Weinheim, Germany, 2005; pp. 193–203. [Google Scholar]
- Gorenšek, M.; Gorjanc, M.; Bukošek, V.; Kovač, J.; Petrović, Z.; Puac, N. Functionalization of polyester fabric by Ar/N2 plasma and silver. Text. Res. J. 2010, 80, 1633–1642. [Google Scholar] [CrossRef]
- ISO 13934-1:2013 Textiles—Tensile Properties of Fabrics—Part 1: Determination of Maximum Force and Elongation at Maximum Force Using the Strip Method; International Organization for Standardization: Geneva, Switzerland, 2013.
- Gorjanc, M.; Bukošek, V.; Gorenšek, M.; Mozetič, M. CF4 plasma and silver functionalized cotton. Text. Res. J. 2010, 80, 2204–2213. [Google Scholar] [CrossRef]
- ASTM D 1388-18 Standard Test Method for Stiffness of Fabrics; ASTM International: West Conshohocken, PA, USA, 2018; Volume 07.01. [CrossRef]
- Gorjanc, M.; Jazbec, K.; Maloprav, A.; Godec, M.; Forte-Tavčer, P.; Simončič, B. Creation of “lotus effect” on cotton fabric with use of plasma, enzymes and sol-gel finishing. Tekstilec 2012, 55, 206–214. [Google Scholar]
- DIN 53924 Testing of Textiles—Velocity of Soaking Water of Textile Fabrics (Method by Determining the Rising Height); German Institute for Standardization: Berlin, Germany, 2020.
- ISO 9237:1995 Textiles-Determination of Permeability of Fabrics to Air; International Organization for Standardization: Geneva, Switzerland, 1995.
- ISO 105-C06:2010 Textiles—Tests for Colour Fastness—Part C06: Colour Fastness to Domestic and Commercial Laundering; International Organization for Standardization: Geneva, Switzerland, 2010.
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer: New York, NY, USA, 2002; 487p. [Google Scholar]
- Strlic, M.; Thomas, J.; Trafela, T.; Cséfalvayová, L.; Cigić, I.K.; Kolar, J.; Cassar, M. Material degradomics: On the smell of old books. Anal. Chem. 2009, 81, 8617–8622. [Google Scholar] [CrossRef]
- Debeljak, M.; Hladnik, A.; Černe, L.; Gregor-Svetec, D. Use of effect pigments for quality enhancement of offset printed specialty papers. Color Res. Appl. 2013, 38, 168–176. [Google Scholar] [CrossRef]
- Mihailović, D.; Saponjic, Z.; Radoicic, M.; Lazovic, S.; Baily, C.J.; Jovančić, P.; Nedeljkovic, J.; Radetić, M. Functionalization of cotton fabrics with corona/air RF plasma and colloidal TiO2 nanoparticles. Cellulose 2011, 18, 811–825. [Google Scholar] [CrossRef]
- Flynn, C.; Byrne, C.; Meenan, B. Surface modification of cellulose via atmospheric pressure plasma processing in air and ammonia–nitrogen gas. Surf. Coat. Technol. 2013, 233, 108–118. [Google Scholar] [CrossRef]
- Vesel, A.; Junkar, I.; Cvelbar, U.; Kovac, J.; Mozetic, M. Surface modification of polyester by oxygen- and nitrogen-plasma treatment. Surf. Interface Anal. 2008, 40, 1444–1453. [Google Scholar] [CrossRef]
- Pransilp, P.; Pruettiphap, M.; Bhanthumnavin, W.; Paosawatyanyong, B.; Kiatkamjornwong, S. Surface modification of cotton fabrics by gas plasmas for color strength and adhesion by inkjet ink printing. Appl. Surf. Sci. 2016, 364, 208–220. [Google Scholar] [CrossRef]
- Vallon, S.; Hofrichter, A.; Drévillon, B.; Klemberg-Sapieha, J.; Martinu, L.; Poncin-Epaillard, F. Improvement of the adhesion of silica layers to polypropylene induced by nitrogen plasma treatment. Thin Solid Film. 1996, 290–291, 68–73. [Google Scholar] [CrossRef]
- Ogawa, T.; Uematsu, S.; Gejyo, M. Surface composition and its stability of nitrogen corona treated polypropylene. Kobunshi Ronbunshu 2008, 65, 67–72. [Google Scholar] [CrossRef]
- Shahidi, S.; Ghoranneviss, M. Comparison between oxygen and nitrogen plasma treatment on adhesion properties and antibacterial activity of metal coated polypropylene fabrics. Fibers Polym. 2012, 13, 971–978. [Google Scholar] [CrossRef]
- Babaei, S.; Fekete, N.; Hoesli, C.A.; Girard-Lauriault, P.-L. Adhesion of human monocytes to oxygen- and nitrogen-containing plasma polymers: Effect of surface chemistry and protein adsorption. Colloids Surf. B Biointerfaces 2018, 162, 362–369. [Google Scholar] [CrossRef] [PubMed]




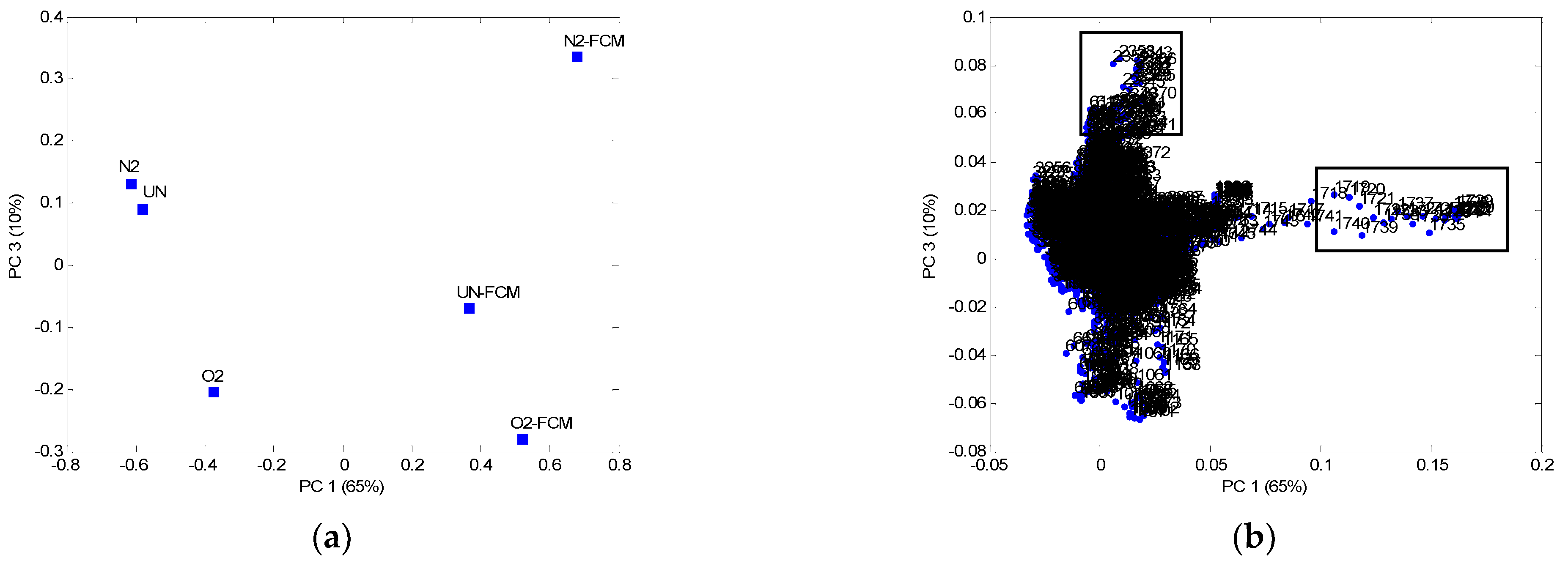
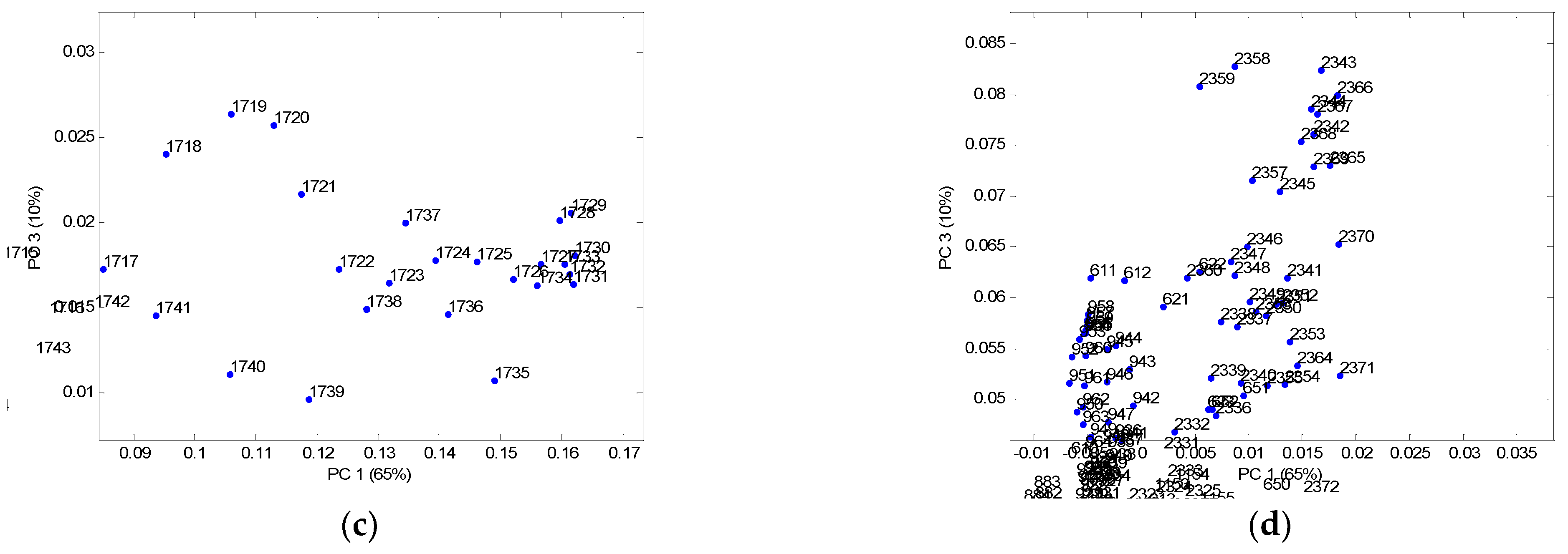
| Sample | Breaking Force (N) | Breaking Elongation (%) | Flexural Rigidity (mg·cm) |
|---|---|---|---|
| Untreated | 357.7 ± 13.0 | 14.9 ± 1.4 | 266.0 ± 18.6 |
| O2 plasma | 403.6 ± 9.1 | 16.4 ± 0.1 | 223.0 ± 3.7 |
| N2 plasma | 368.1 ± 26.3 | 15.7 ± 0.7 | 196.0 ± 9.1 |
| Sample | Wicking Height (mm) | ||
|---|---|---|---|
| 30 s | 60 s | 300 s | |
| Untreated | 33.5 ± 0.12 | 41.5 ± 0.13 | 65.0 ± 0.11 |
| O2 plasma | 38.5 ± 0.07 | 46.5 ± 0.12 | 74.0 ± 0.07 |
| N2 plasma | 38.0 ± 0.07 | 44.0 ± 0.11 | 73.5 ± 0.07 |
| Sample | Air Permeability (mm/s) | |||
|---|---|---|---|---|
| 0 w | 1 w | 5 w | 10 w | |
| Untreated + FCM | 457.2 ± 12.3 | 508.9 ± 13.1 | 552.4 ± 10.6 | 605.8 ± 5.1 |
| O2 plasma + FCM | 444.2 ± 10.6 | 488.9 ± 12.5 | 545.7 ± 3.1 | 595.5 ± 6.12 |
| N2 plasma + FCM | 435.9 ± 8.5 | 475.5 ± 9.5 | 526.9 ± 2.23 | 566.0 ± 1.4 |
| Sample | Number of Wash Cycles | Fragrance Intensity | |||
|---|---|---|---|---|---|
| Strong | Medium | Weak | Absent | ||
| Control * | 0 | 0 | 0 | 30 | |
| Untreated | 0 | 30 | 0 | 0 | 0 |
| O2 plasma | 30 | 0 | 0 | 0 | |
| N2 plasma | 30 | 0 | 0 | 0 | |
| Untreated | 1 | 2 | 28 | 0 | 0 |
| O2 plasma | 3 | 27 | 0 | 0 | |
| N2 plasma | 29 | 1 | 0 | 0 | |
| Untreated | 5 | 0 | 5 | 25 | 0 |
| O2 plasma | 0 | 3 | 27 | 0 | |
| N2 plasma | 26 | 4 | 0 | 0 | |
| Untreated | 10 | 0 | 0 | 1 | 29 |
| O2 plasma | 0 | 0 | 2 | 28 | |
| N2 plasma | 1 | 27 | 2 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kert, M.; Forte Tavčer, P.; Hladnik, A.; Spasić, K.; Puač, N.; Petrović, Z.L.; Gorjanc, M. Application of Fragrance Microcapsules onto Cotton Fabric after Treatment with Oxygen and Nitrogen Plasma. Coatings 2021, 11, 1181. https://doi.org/10.3390/coatings11101181
Kert M, Forte Tavčer P, Hladnik A, Spasić K, Puač N, Petrović ZL, Gorjanc M. Application of Fragrance Microcapsules onto Cotton Fabric after Treatment with Oxygen and Nitrogen Plasma. Coatings. 2021; 11(10):1181. https://doi.org/10.3390/coatings11101181
Chicago/Turabian StyleKert, Mateja, Petra Forte Tavčer, Aleš Hladnik, Kosta Spasić, Nevena Puač, Zoran Lj. Petrović, and Marija Gorjanc. 2021. "Application of Fragrance Microcapsules onto Cotton Fabric after Treatment with Oxygen and Nitrogen Plasma" Coatings 11, no. 10: 1181. https://doi.org/10.3390/coatings11101181
APA StyleKert, M., Forte Tavčer, P., Hladnik, A., Spasić, K., Puač, N., Petrović, Z. L., & Gorjanc, M. (2021). Application of Fragrance Microcapsules onto Cotton Fabric after Treatment with Oxygen and Nitrogen Plasma. Coatings, 11(10), 1181. https://doi.org/10.3390/coatings11101181







