Abstract
Superhydrophobic coatings have a huge impact in various applications due to their extreme water-repellent properties. The main novelty of the current research work lies in the development of cheap, stable, superhydrophobic and self-cleaning coatings with extreme water-repellency. In this work, a composite of hydrothermally synthesized alumina (Al2O3), polymethylhydrosiloxane (PMHS) and polystyrene (PS) was deposited on a glass surface by a dip-coating technique. The Al2O3 nanoparticles form a rough structure, and low-surface-energy PHMS enhances the water-repellent properties. The composite coating revealed a water contact angle (WCA) of 171 ± 2° and a sliding angle (SA) of 3°. In the chemical analysis, Al2p, Si2p, O1s, and C1s elements were detected in the XPS survey. The prepared coating showed a self-cleaning property through the rolling action of water drops. Such a type of coating could have various industrial applications in the future.
1. Introduction
Superhydrophobic surfaces have earned much attention from researchers in the last two decades due to their excellent water repellent behavior and the high mobility of water, which can be used to avoid accumulating dirt, fouling, fogging, and icing [1,2,3,4]. Natural leaf surfaces, such as that of a lotus leaf, possess micro-scale papillae and nano-scale epicuticular wax crystals on their surface, forming a hierarchical surface morphology, which is responsible for their self-cleaning superhydrophobic properties by quickly removing the dirt particles from the surface by rolling water drops [5]. To date, many efforts have been devoted to the development of superhydrophobic coatings by forming a rough structure and/or reducing the surface energy by using low surface energy materials [6,7,8,9,10]. The use of low surface energy-based materials on the rough hierarchical structure may create a thin hydrophobic layer that could resist the adherence of water droplets [8,11,12]. The presence of a hierarchical surface with a thin layer of hydrophobic materials may also be another reason for the superhydrophobic property [13]. Similarly, different kinds of bio-mimicking surfaces were developed using natural or synthetic materials to achieve a micro-nano hierarchical surface structure with an extreme water repellent coating for self-cleaning, as well as oil–water sorption and separation applications [14,15]. Several studies were studied a mechanism of superhydrophobic as well as photocatalytic superhydrophilic surfaces in self-cleaning applications [16,17]. Photocatalytic superhydrophilic surfaces have also attracted considerable attention in terms of self-cleaning coatings due to the complete wettability of their substrates, which can easily remove an organic pollutant from the surfaces by the action of the flow of a water film [16,17]. Esmeryan et al. developed a novel soot-inspired superhydrophobic surface containing a quartz crystal microbalance (QCM)-based biosensor for the detection of human semen, and human spermatozoa quality assessment [18]. Similarly, the authors also studied the effect of soot-inspired superhydrophobic surfaces for human urine detection, as well as improving the success rate of the cryopreservation of human spermatozoa [19,20]. Here, we only focused on the fabrication of superhydrophobic coatings for self-cleaning applications.
Aluminium oxide (Al2O3) is one of the cheapest materials, with excellent usability in various applications. The use of Al2O3 in coatings also attracted significant attention in recent years due to its antibacterial property; excellent mechanical, electrical insulation, high-temperature properties; and first-rate impact, abrasion, and chemical resistance [21]. Several studies have focused on developing superhydrophobic surfaces using Al2O3 particles or on the Al2O3 surface [22,23,24,25]. Sutha et al. fabricated an optically transparent, anti-reflective, and self-cleaning superhydrophobic Al2O3 coating on a glass substrate [25]. In this process, the authors first prepared Al2O3 sol by mixing aluminium nitrate nonahydrate with 2-methoxyethanol solution by magnetic stirring in a monoethanolamine stabilizer at room temperature. Multiple layers of Al2O3 nanoparticles were applied onto a glass substrate by a spin-coating method. After annealing, the film was immersed in hot water to obtain a porous structure. Finally, low surface energy 1H,1H,2H,2H–perfluorooctyltrichlorosilane was coated onto a porous Al2O3 film by spin coating. On the other hand, Karapanagiotis et al. dispersed different-sized hydrophilic alumina nanoparticles (25, 35, and 150 nm) in different concentrations in solutions of a hydrophobic poly(alkyl siloxane), and the prepared suspensions were sprayed onto a glass surface [26]. They stated that the wettability of the composite film is independent of the size, but is affected by the concentration of the particles. However, Richard et al. dispersed stearic acid modified Al2O3 particles in ethanol, and sprayed them onto a glass slide in order to attain a superhydrophobic surface [27]. Tie et al. prepared a superhydrophobic and underwater superoleophobic surface by an aqueous mixture of hydrophilic nanoparticles (TiO2, SiO2, and Al2O3) and fluorocarbon surfactants through dip, brush, or spray coating on various substrates, such as fabric, sponge, cotton, nickel foam, stainless steel mesh, copper sheet, glass, and ceramics [28]. Byun et al. prepared a superhydrophobic surface by spraying phosphonic acid-functionalized Al2O3 nanoparticles onto glass, paper, cotton fabric, and flexible plastic substrates [29]. Several studies are available on the fabrication of superhydrophobic surfaces with extreme wettability by different techniques [13,14].
Although several techniques were used to fabricate superhydrophobic surfaces with self-cleaning behavior using various materials, only a few works were reported using Al2O3 nanoparticle-based nanocomposites for superhydrophobic and self-cleaning coatings [22,30,31]. Al2O3 based composites are highly useful in coating applications due to the abundant availability of the Al source and its antibacterial characteristics [32]. In the present work, we exclusively focused on a facile dip-coating method to coat an Al2O3–PMHS–PS hybrid system onto glass substrates. First, hydrothermally synthesized hydrophilic Al2O3 nanoparticles were modified with low-surface-energy PMHS. The flake-shaped Al2O3 nanoparticles were agglomerated during the deposition, forming a rough hierarchical structure and attaining a superhydrophobic surface. The chemical analysis of the prepared coating was also performed. Water jet impact, adhesive tape peeling and sandpaper abrasion tests were performed in order to evaluate the mechanical durability of the coating. Additionally, self-cleaning tests were conducted on the prepared superhydrophobic coatings. The coated substrates exhibited extreme water-repellent behavior as well as excellent mechanical stability.
2. Experiment
2.1. Materials
Aluminum nitrate nonahydrate [Al(NO3)3·9H2O], polystyrene (PS; 192,000 g/mol) and polymethylhydrosiloxane (PMHS, average Mn 1700-3200) were procured from Sigma-Aldrich (St. Louis, MO, USA). Dextrose [O(CHOH)4CHCH2OH] and urea [NH2CONH2] were secured from Thomas Baker (Mumbai, India). Ethanol and chloroform were bought from Spectrochem PVT. LTD (Mumbai, India). The micro-Glass substrates (75 × 25 × 1.35 mm3) were obtained from Blue star, Polar Industrial Corporation, Mumbai, India.
2.2. Synthesis of the Al2O3 Nanoparticles
Aluminum nitrate nonahydrate has been used to synthesize Al2O3 nanoparticles via a hydrothermal method [33,34]. In the synthesis process, 3.6 g dextrose, 3.75 g aluminum nitrate nonahydrate, and 3 g urea were dissolved in 50 mL distilled water under vigorous stirring for 30 min. The prepared homogeneous transparent solution was transferred to a 100 mL Teflon-lined stainless-steel autoclave and kept in an oven at 170 °C for 6 h for the hydrothermal process. A black powder of hydrated alumina was collected by filtration and washed with distilled water and ethanol several times, and was dried at 80 °C for 8 h. The dried black powder of hydrated alumina was placed in a silica crucible and kept in a muffle furnace at 1000 °C for 3 h, with a heating rate of 4 °C min−1 in air atmosphere. Finally, the collected Al2O3 particles were stored in the bottle for further use.
2.3. Preparation of the Superhydrophobic Coating
The micro-glass slides were washed using tap water and laboratory detergent (Molyclean 02 Neutral, Molychem, Mumbai, India) and, afterwards, cleaned ultrasonically using distilled water and ethanol for 10 min. A coating solution was prepared by the following process: 0.15 mL PMHS was mixed with 20 mL chloroform in a beaker, and kept on magnetic stirrer at 100 rpm. After 20 min of stirring, 400 mg Al2O3 was added and stirred continuously for another 1 h. Meanwhile, in a second beaker, 10 mg/mL PS solution in 20 mL of chloroform was prepared. This PS solution was poured into the beaker containing PMHS-Al2O3 and stirred further for 30 min.
The cleaned glass slide was dipped in a suspension of Al2O3–PMHS–PS for 10 s, with a controlled dip and withdrawal rate of 50 mm/s using a dip coating machine (Delta Scientific Equipment Pvt. Ltd., Kolkata, India); a coated sample was dried at room temperature (~25 °C). This is considered as one deposition layer of the coating solution. The coatings were repeated by applying 2, 4, and 6 deposition layers, and finally dried at 100 °C in an oven for 1 h. The samples with two, four, and six coating layers were labelled as the AD-1, AD-2 and AD-3 coating, respectively. In this work, we only focused on the study of the effect of dip-coating cycles on the superhydrophobic coating. Of course, changing the chemical compositions would alter the surface properties either partially or completely based on the formation of a hierarchical surface morphology, with more hydrophobic or hydrophilic characteristics based on the combinations of material.
2.4. Characterizations
A Scanning Electron Microscope (SEM, JEOL, JSM-7610F, Tokyo, Japan) was used to investigate the surface micro/nanostructure of the prepared coatings. The surface roughness was calculated using a Stylus profiler (Mitutoyo, SJ 210, Sakado, Japan). The water contact angle (WCA) and sliding angle (SA) were measured on at least three places on the samples using a contact angle meter (HO-IAD-CAM-01, Holmarc Opto-Mechatronics Pvt. Ltd., Kochi, India). The average value of the WCA and SA of samples were noted. The chemical composition of the coating was analyzed by X-ray photoelectron spectroscopy (XPS, PHI Quantera-II, Tokyo, Japan). The mechanical stability of the coatings was examined using a water jet created by a syringe on the coating. The mechanical sustainability of the coatings was evaluated further by an adhesive tape test and a sandpaper abrasion test using commercial adhesive tape and sandpaper. The self-cleaning performance of the coatings was observed by scattering fine particles of chalk as a dust contaminant onto the coating.
3. Results and Discussion
3.1. Surface Morphology, Roughness and Wettability of the Prepared Coatings
The surface micro/nanostructure has been given much attention in the definition of the wetting property of the coating surface. Mostly, micro- and nano-scale hierarchical surface structures with low surface energy are responsible for superhydrophobicity. The surface morphologies of the prepared coatings was analyzed by SEM, and are given in Figure 1a–f. The flake-shaped alumina nanoparticles formed during the hydrothermal synthesis are visible in the SEM images [35]. The addition of PMHS and PS molecules to the flake-shaped nanoparticles can form an aggregated micro–nano-sized random particles during the deposition process. Moreover, the agglomerated particles provided micro- and nano-sized hierarchical rough structures on the glass surface. The active Si-H bond and methyl groups present in the PMHS were utilized in the surface modification of hydrophilic alumina nanoparticles. The formation of a thin layer of hydrophobic PMHS on Al2O3 would facilitate the enhancement of the hydrophobic property on the modified Al2O3–PMHS surface. Radwan et al. reported that the mixture of PS and Al2O3 can deliver the superhydrophobic property while forming three dimensional nanofibers by an electrospinning technique [22]. PS nanofibers can display an excellent hydrophobic property, which becomes superhydrophobic through the introduction of Al2O3 to the PS due to the formation of a multiscale hierarchical rough structure [22]. Likewise, the modification of PS with various hydrophobic agents can also develop a superhydrophobic surface property [36,37,38]. As such, the addition of a PS solution to Al2O3–PMHS would also help to form the multiscale hierarchical roughness by aggregation, as well as the formation of closely packed particles on the coated substrate.
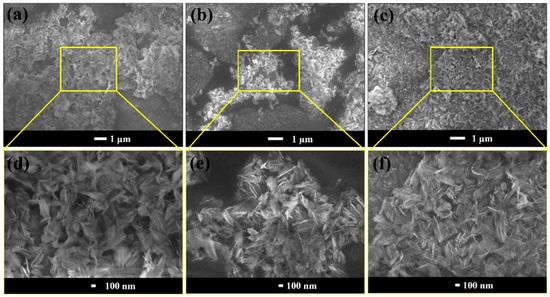
Figure 1.
(a–c) Low-magnified and (d–f) high-magnified FE-SEM images of the AD-1, AD-2 and AD-3 samples, respectively.
At two layers (AD-1) of deposition, the particles were agglomerated and uniformly distributed on the glass surface, as shown in Figure 1a,d. The AD-1 coating showed a surface roughness of 0.019 µm with WCA 120 ± 2°, and a water drop becomes stuck on the surface. The size of the agglomerated particles increased with increasing numbers of layers, up to four (AD-2), resulting in a highly rough structure with a roughness value of 0.038 µm, which is similar to a Cassie-Baxter surface. The developed micro/nano-sized rough structure of the coating is clearly seen in Figure 1b,e. In such a hierarchical surface structure, air pockets are trapped; consequently, WCA increased to 171 ± 2°, and water drops roll off at an inclination angle of 3 ± 0.5°. Based on the formation of a multiscale micro-nano hierarchical structure as well as the formation of thin layer of low surface energy hydrophobic PMHS, this provides an extreme superhydrophobic property on a dip-coated substrate [39]. Further increasing the number of deposition layers to six, we noticed that more agglomerated particles are formed on the glass surface, and the surface roughness decreased partially to 0.034 µm (Figure 1c,f). At the same time, the six-layer coated sample also exhibited WCA 170 ± 2°, with no changes in sliding angles. The AD-3 coated substrate can also deliver good mechanical durability when it forms densely packed particles on the coated surface, whereas the loosely packed particles on the surface mean that it can easily come out from the substrate under adhesive tape peeling and sandpaper abrasion tests [40]. As such, we further studied the effect of an AD-2-coated superhydrophobic substrate for the rest of the studies, because at four layers of coating, the fabricated substrate can exhibit the maximum contact angle, as well as a surface roughness which was reduced by the further increase of coating layers. As such, considering the practical point of view as well industrial applications, four layers of coating would be the optimum in order to minimize the time consumption of the coated solution.
The typical photographs of Al2O3–PMHS–PS composite-coated substrates are shown in Figure 2. A coated substrate has a translucent or opaque color due to the deposition of white Al2O3 and PS on the glass substrate. The PMHS solution was transparent, and its addition was helpful for a stronger adhesion of the composite coating the substrate. The extreme superhydrophobic property of the AD-2 sample was confirmed by placing water droplets on the coated substrate. Figure 2a shows spherical-shaped color-dyed water drops, which explain the exceptional water-repellent behavior of the coated substrate. The inset of Figure 2a reveals an optical image of the water drop (approximately 10 µL volume) on a superhydrophobic AD-2 coating, which is obtained from a contact-angle meter. A water drop rolled off when the substrate was inclined by nearly 3° with the help of the stage of the contact-angle meter. Figure 2(c1–c3) shows the rolling action of a water drop on a superhydrophobic coating. The water drops quickly rolled down from an inclined surface, as shown in the inset of Figure 2(c3). The stability of the coating against running water was checked using a water jet hitting test. The water jet was formed using a 10 mL syringe, hitting a specific place on the coating for more than one minute (Figure 2b). The continuous reflecting water jet from the superhydrophobic surface confirmed that the prepared coating is highly stable. More results on the mechanical durability of the coating are provided in Section 3.3. Unfortunately, we are unable to provide an alternative image to showcase the sliding angle. Because of the extreme water repellent behavior of the coated superhydrophobic surface, as well as the very low sliding angle, a droplet can easily run away after contacting the substrate. Moreover, the inset image of c3 also clearly suggests the non-adherence of the water droplet after the transportation of the water droplet from the substrate. As such, we hope this image is enough to illustrate the low sliding angle and extreme water-repellency of the superhydrophobic substrate.
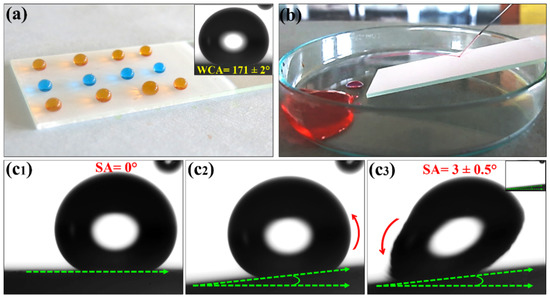
Figure 2.
(a) Photograph of the color-dyed water drops on the superhydrophobic AD–2 coating. (b) The screenshot of the water jet hitting the superhydrophobic AD–2 coating. (c1–c3) The screenshots of the water droplet rolling on the superhydrophobic AD–2 coating.
3.2. XPS Study
The surface elemental compositions of the prepared superhydrophobic Al2O3-PMHS-PS composite AD-2 coating were analyzed using XPS studies (Figure 3a). Four peaks were observed at 74.85 eV, 102.61 eV, 284.8 eV and 532.11 eV, and are related to Al2p, Si2p, C1s, and O1s, respectively. The presence of Al, Si, C and O elements confirms that the Al2O3-PMHS-PS composite exists on the glass substrate. In Al2p scan spectra (Figure 3b), the peaks correspond to Al-O (74.6 eV) and Al-O-Si (75.5 eV) bonds. In Figure 3c, the Si2p peak at 102.4 eV and 103.7 eV corresponds to the Si-O-Si and Si-O-Al bonds [41]. In the O1s scan (Figure 3d), the highest peak is associated to Al-O-Si (532.7 eV) and Al-O-Al (531.1 eV) bonds, corresponding to the PMHS chain and Al2O3 nanoparticles [42,43]. In the high-resolution C1s XPS spectrum (Figure 3e), the BEs of 284.2 to 286.1 eV are related C–C/C–H and C=O, respectively.
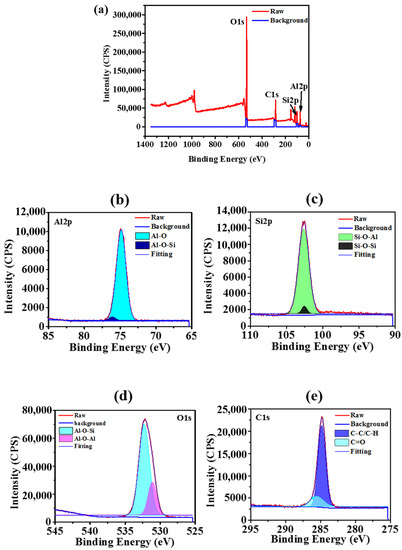
Figure 3.
(a) XPS survey scan spectra of the superhydrophobic AD-2 coating. (b) Al2p peak, (c) Si2p peak, (d) C1s peak, and (e) O1 peak, respectively.
3.3. Mechanical Durability Tests
The mechanical durability of the prepared superhydrophobic coating is highly important for commercial applications. A fragile hierarchical structure of superhydrophobic coatings can be ruined when exposed to outdoor applications. The preparation of a robust superhydrophobic property is always a challenging issue because the superhydrophobic surface property can be damaged under severe mechanical stress, as well as under hot water or acidic and basic conditions. Several studies focused on improving the robustness of superhydrophobic surfaces by introducing highly strong adhesives.
The adhesive tape peeling and sandpaper abrasion tests are the most commonly used methods to evaluate the mechanical durability of superhydrophobic coatings [44]. A Cello tape no.405 (adhesiveness 3.93 N/10 mm) was placed on the AD-2 coating, and a metal disc of weight of 200 g was rolled on it to create good contact between the coating’s surface and the tape. The tape was peeled off slowly from the coating’s surface to check the adhesive tape peeling test performance, and this is considered one cycle of the tape-peeling test [44]. The WCA was also measured after test to check wetting property of the coating. The analysis of the WCA versus the number of tape peeling tests revealed that the superhydrophobicity remained stable for up to five cycles of tape peeling. After seven cycles, the WCA decreased to lower than ~140°. A variation of the WCA with an increasing number of tape-peeling cycles is shown in Figure 4a, and a photograph of the tape-peeling test is shown in the inset of Figure 4a.
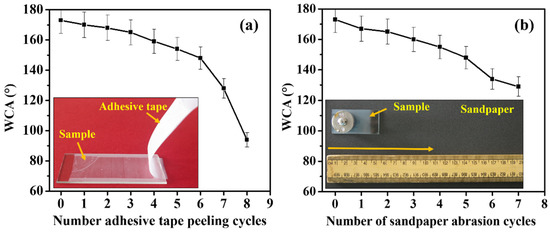
Figure 4.
(a) An adhesive tape peeling test and (b) a sandpaper abrasion test on the AD-2 coating.
The AD-2 sample was placed on sandpaper of grit no. 400, then a 50 g weight was loaded onto it, and subsequently the sample was rubbed with a speed of ~5 mm/s for 10 cm (one cycle of the sandpaper abrasion test). A WCA was recorded after every sandpaper abrasion cycle (Figure 4b). The experimental setup of the sandpaper abrasion test is shown in the inset of Figure 4b. The WCA was reduced to ~162°, and the SA slightly increased (7°) after the completion of three cycles of the sandpaper abrasion test. This result indicated that the prepared coating is highly stable. After five abrasion cycles, the WCA decreased to ~150°. Subsequently, at seven cycles, water drops start to spread on the coating surface, as the composite material might have been removed by abrasion. A weak van der Waals force of attraction and hydrogen bonding can occur between the Al2O3 and PMHS, whereas the addition of PS to the Al2O3-PMHS suspension can have a hydrophobic–hydrophobic interaction between the hydrophobic PMHS and PS. While coating Al2O3-PMHS-PS composites onto a hydrophilic glass substrate, it would show a stronger interaction with the hydrophilic Al2O3. At the same time, an aggregated hydrophobic micro–nano hierarchical structure was observed on the surface, which produced a highly stable superhydrophobic property on the coated substrate under the thermal curing at 100 °C. These results suggest that the prepared Al2O3–PMHS–PS composite-coated substrate has excellent mechanical durability and robustness, which are important for practical applications. The coated substrate can maintain the superhydrophobic property for up to 5 to 6 cycles of adhesive tape peeling and sandpaper abrasion tests. We hope this mechanical durability is quite enough for various applications. Most of the superhydrophobic coatings reported can last less than five cycles of adhesive tape peeling and sandpaper abrasion tests [45,46,47]. As such, we hope that our coated substrate is better than the reported superhydrophobic coatings. Of course, enhanced robustness with the maintenance of superhydrophobicity and self-cleaning properties over 10 cycles of adhesive tape peeling and sandpaper abrasion tests are particularly recommended for high-end product development.
3.4. Self-Cleaning Test
Self-cleaning is one of the most desirable properties of a superhydrophobic coating. Such coatings can easily clean dust particles from their surface by the action of rolling water drops or without external force. Figure 5a shows a spreading of fine particles of colored chalk that are randomly scattered on the prepared superhydrophobic AD-2 coating, which was kept at an inclination angle nearly 10°. When a water shower produced by the syringe was sprinkled on this coating surface, owing to the highly water repellent property, the water drops rolled down the surface by collecting dust particles from the coating’s surface (Figure 5b). Figure 5c illustrates that rolling water drops completely remove dust particles from the superhydrophobic surface, supporting the excellent self-cleaning property of the Al2O3–PMHS–PS composite coating. The superhydrophobic and self-cleaning properties are retained on the coated substrate by repeated wetting, and are also more durable in nature.

Figure 5.
Self-cleaning performance of the prepared superhydrophobic AD-2 coating. (a) Dust particles on the superhydrophobic coating, (b) water drops carrying the dust particles, and (c) the cleaned surface.
4. Conclusions
We demonstrated a facile dip-coating method for the fabrication of a stable superhydrophobic coating. A stable superhydrophobic surface was achieved by the four-time dip coating of an Al2O3-PHMS-PS composite onto a glass substrate. The coated substrate presented a WCA ~171° and an SA ~3°. SEM micrographs of the coating showed flake-shaped alumina particles which were agglomerated and formed a rough microstructure. The XPS analysis revealed the co-existence of Al2O3, PHMS, and PS on the surface. The durability tests—such as the water jet impact, adhesive tape, and sandpaper abrasion test—displayed the high mechanical stability of the coating. In addition, the superhydrophobic Al2O3-PHMS-PS composite coating revealed excellent self-cleaning performance. The overall results suggest that the prepared Al2O3-PHMS-PS composite coating can be used to develop excellent superhydrophobic surfaces which might be potentially useful for various applications.
Author Contributions
Methodology and investigation—R.S.S.; review and modify the manuscript—S.N. and S.S.L.; supervision—A.K.B., K.K.S., K.-H.P., C.-S.H. and S.S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Department of Science and Technology (DST), Goernment of India. [DST/INSPIRE/04/2015/000281] and National Natural Science Foundation of China (21950410531).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was financially supported by the DST–INSPIRE Faculty Scheme, Department of Science and Technology (DST), Goernment of India. [DST/INSPIRE/04/2015/000281]. SSL acknowledges financial assistance from Henan University, Kaifeng, China. We greatly appreciate the support of the National Natural Science Foundation of China (21950410531).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Latthe, S.S.; Sutar, R.S.; Kodag, V.S.; Bhosale, A.K.; Kumar, A.M.; Kumar Sadasivuni, K.; Xing, R.; Liu, S. Self-cleaning su-perhydrophobic coatings: Potential industrial applications. Prog. Org. Coat. 2019, 128, 52–58. [Google Scholar] [CrossRef]
- Pan, R.; Zhang, H.; Zhong, M. Triple-Scale Superhydrophobic Surface with Excellent Anti-Icing and Icephobic Performance via Ultrafast Laser Hybrid Fabrication. ACS Appl. Mater. Interfaces 2021, 13, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Varshney, P.; Lomga, J.; Gupta, P.K.; Mohapatra, S.S.; Kumar, A. Durable and regenerable superhydrophobic coatings for aluminium surfaces with excellent self-cleaning and anti-fogging properties. Tribol. Int. 2018, 119, 38–44. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Q.; Hokkanen, M.J.; Zhang, C.; Lin, F.-Y.; Liu, Q.; Zhu, S.-P.; Zhou, T.; Chang, Q.; He, B. Design of robust su-perhydrophobic surfaces. Nature 2020, 582, 55–59. [Google Scholar] [CrossRef]
- Dalawai, S.P.; Saad Aly, M.A.; Latthe, S.S.; Xing, R.; Sutar, R.S.; Nagappan, S.; Ha, C.S.; Kumar Sadasivuni, K.; Liu, S. Recent Advances in durability of superhydrophobic self-cleaning technology: A critical review. Prog. Org. Coat. 2020, 138, 105381. [Google Scholar] [CrossRef]
- Geyer, F.; D’Acunzi, M.; Sharifi-Aghili, A.; Saal, A.; Gao, N.; Kaltbeitzel, A.; Sloot, T.-F.; Berger, R.; Butt, H.-J.; Vollmer, D. When and how self-cleaning of superhydrophobic surfaces works. Sci. Adv. 2020, 6, eaaw9727. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Ding, Y.; Zhang, M.; Gao, S.; Li, Y.; Huang, C.; Fu, G. Nature-inspired chemistry toward hierarchical superhydro-phobic, antibacterial and biocompatible nanofibrous membranes for effective UV-shielding, self-cleaning and oil-water separation. J. Hazard. Mater. 2020, 384, 121476. [Google Scholar] [CrossRef]
- Teisala, H.; Butt, H.-J. Hierarchical Structures for Superhydrophobic and Superoleophobic Surfaces. Langmuir 2018, 35, 10689–10703. [Google Scholar] [CrossRef]
- Wen, F.; Lei, C.; Chen, J.; Huang, Y.; Wang, B. Hierarchical superhydrophobic surfaces for oil–water separation via a gradi-ent of ammonia content controlling of dopamine oxidative self-polymerization. J. Appl. Polym. Sci. 2019, 136, 48044. [Google Scholar] [CrossRef]
- Wen, R.; Xu, S.; Zhao, D.; Lee, Y.-C.; Ma, X.; Yang, R. Hierarchical Superhydrophobic Surfaces with Micropatterned Nan-owire Arrays for High-Efficiency Jumping Droplet Condensation. ACS Appl. Mater. Interfaces 2017, 9, 44911–44921. [Google Scholar] [CrossRef]
- Kota, A.K.; Kwon, G.; Tuteja, A. The design and applications of superomniphobic surfaces. NPG Asia Mater. 2014, 67, e109. [Google Scholar] [CrossRef]
- Si, Y.; Guo, Z. Superhydrophobic nanocoatings: From materials to fabrications and to applications. Nanoscale 2015, 7, 5922–5946. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; McHale, G.; Atherton, S.; Newton, M.I. An introduction to superhydrophobicity. Adv. Colloid Interface Sci. 2010, 161, 124–138. [Google Scholar] [CrossRef]
- Nagappan, S.; Ha, C.-S. Emerging trends in superhydrophobic surface based magnetic materials: Fabrications and their po-tential applications. J. Mater. Chem. A 2015, 3, 3224–3251. [Google Scholar] [CrossRef]
- Nagappan, S.; Park, J.J.; Park, S.S.; Lee, W.-K.; Ha, C.-S. Bio-inspired, multi-purpose and instant superhydrophobic–superoleophilic lotus leaf powder hybrid micro–nanocomposites for selective oil spill capture. J. Mater. Chem. A 2013, 1, 6761–6769. [Google Scholar] [CrossRef]
- Adachi, T.; Latthe, S.S.; Gosavi, S.W.; Roy, N.; Suzuki, N.; Ikari, H.; Kato, K.; Katsumata, K.-I.; Nakata, K.; Furudate, M.; et al. Photocatalytic, superhydrophilic, self-cleaning TiO2 coating on cheap, light-weight, flexible polycarbonate substrates. Appl. Surf. Sci. 2018, 458, 917–923. [Google Scholar] [CrossRef]
- Nundy, S.; Ghosh, A.; Mallick, T.K. Hydrophilic and Superhydrophilic Self-Cleaning Coatings by Morphologically Varying ZnO Microstructures for Photovoltaic and Glazing Applications. ACS Omega 2020, 5, 1033–1039. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Ganeva, R.R.; Stamenov, G.S.; Chaushev, T.A. Superhydrophobic Soot Coated Quartz Crystal Microbalanc-es: A Novel Platform for Human Spermatozoa Quality Assessment. Sensors 2019, 19, 123. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Chaushev, T.A. Complex characterization of human urine using super-nonwettable soot coated quartz crystal microbalance sensors. Sens. Actuators A Phys. 2021, 317, 112480. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Lazarov, Y.; Stamenov, G.S.; Chaushev, T.A. When condensed matter physics meets biology: Does super-hydrophobicity benefiting the cryopreservation of human spermatozoa? Cryobiology 2020, 92, 263–266. [Google Scholar] [CrossRef]
- Mallakpour, S.; Sirous, F.; Hussain, C.M. Green synthesis of nano-Al2O3, recent functionalization, and fabrication of syn-thetic or natural polymer nanocomposites: Various technological applications. New J. Chem. 2021, 45, 4885–4920. [Google Scholar] [CrossRef]
- Radwan, A.B.; Abdullah, A.M.; Mohamed, A.M.A.; Al-Maadeed, M.A. New Electrospun Polystyrene/Al2O3 Nanocomposite Superhydrophobic Coatings; Synthesis, Characterization, and Application. Coatings 2018, 8, 65. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, H.; Mao, P.; Wang, Y.; Ge, Y. Superhydrophobic alumina surface based on stearic acid modification. Appl. Surf. Sci. 2011, 257, 3959–3963. [Google Scholar] [CrossRef]
- Jagdheesh, R. Fabrication of a Superhydrophobic Al2O3 Surface Using Picosecond Laser Pulses. Langmuir 2014, 30, 12067–12073. [Google Scholar] [CrossRef] [PubMed]
- Sutha, S.; Suresh, S.; Raj, B.; Ravi, K.R. Transparent alumina based superhydrophobic self–cleaning coatings for solar cell cover glass applications. Sol. Energy Mater. Sol. Cells 2017, 165, 128–137. [Google Scholar] [CrossRef]
- Karapanagiotis, I.; Manoudis, P.N.; Savva, A.; Panayiotou, C. Superhydrophobic polymer-particle composite films produced using various particle sizes. Surf. Interface Anal. 2012, 44, 870–875. [Google Scholar] [CrossRef]
- Richard, E.; Aruna, S.T.; Basu, B.J. Superhydrophobic surfaces fabricated by surface modification of alumina particles. Appl. Surf. Sci. 2012, 258, 10199–10204. [Google Scholar] [CrossRef]
- Tie, L.; Li, J.; Liu, M.; Guo, Z.; Liang, Y.; Liu, W. Facile Fabrication of Superhydrophobic and Underwater Superoleophobic Coatings. ACS Appl. Nano Mater. 2018, 1, 4894–4899. [Google Scholar] [CrossRef]
- Byun, H.R.; Ha, Y.G. Non-wetting superhydrophobic surface enabled by one-step spray coating using molecular self-assembled nanoparticles. J. Nanosci. Nanotechnol. 2017, 17, 5515–5519. [Google Scholar] [CrossRef]
- Kim, I.-S.; Cho, M.-Y.; Jeong, Y.; Shin, Y.-C.; Lee, D.-W.; Park, C.; Koo, S.-M.; Shin, W.H.; Yang, W.-J.; Park, Y.; et al. Aero-sol-deposited Al2O3/PTFE hydrophobic coatings with adjustable transparency. J. Am. Ceram. Soc. 2021, 104, 1716–1725. [Google Scholar] [CrossRef]
- Na, M.J.; Yang, H.; Jung, H.J.; Park, S.D. Robust hydrophobic surface driven by Al2O3/glass composite coatings. Surf. Coat. Technol. 2019, 372, 134–139. [Google Scholar] [CrossRef]
- Jeon, Y.; Nagappan, S.; Li, X.-H.; Lee, J.-H.; Shi, L.; Yuan, S.; Lee, W.-K.; Ha, C.-S. Highly Transparent, Robust Hydrophobic, and Amphiphilic Organic–Inorganic Hybrid Coatings for Antifogging and Antibacterial Applications. ACS Appl. Mater. Interfaces 2021, 13, 6615–6630. [Google Scholar] [CrossRef] [PubMed]
- Naskar, M.K. Soft Solution Processing for the Synthesis of Alumina Nanoparticles in the Presence of Glucose. J. Am. Ceram. Soc. 2010, 93, 1260–1263. [Google Scholar] [CrossRef]
- Xue, G.; Huang, X.; Zhao, N.; Xiao, F.; Wei, W. Hollow Al2O3 spheres prepared by a simple and tunable hydrothermal method. RSC Adv. 2015, 5, 13385–13391. [Google Scholar] [CrossRef]
- Ghosh, S.; Dalapati, R.; Naskar, M.K. Understanding the role of tetramethyl urea for the synthesis of mesoporous alumina. J. Asian Ceram. Soc. 2014, 2, 380–386. [Google Scholar] [CrossRef][Green Version]
- Latthe, S.S.; Demirel, A.L. Polystyrene/octadecyltrichlorosilane superhydrophobic coatings with hierarchical morphology. Polym. Chem. 2012, 4, 246–249. [Google Scholar] [CrossRef]
- Pawar, P.G.; Xing, R.; Kambale, R.C.; Kumar, A.M.; Liu, S.; Latthe, S.S. Polystyrene assisted superhydrophobic silica coatings with surface protection and self-cleaning approach. Prog. Org. Coat. 2017, 105, 235–244. [Google Scholar] [CrossRef]
- Xue, C.-H.; Zhang, Z.-D.; Zhang, J.; Jia, S.-T. Lasting and self-healing superhydrophobic surfaces by coating of polysty-rene/SiO2 nanoparticles and polydimethylsiloxane. J. Mater. Chem. A 2014, 2, 15001–15007. [Google Scholar] [CrossRef]
- Nagappan, S.; Ha, C.S. In-situ addition of graphene oxide for improving the thermal stability of superhydrophobic hybrid materials. Polymer 2017, 116, 412–422. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Gong, X. Large-Scale Spraying Fabrication of Robust Fluorine-Free Superhydrophobic Coatings Based on Dual-Sized Silica Particles for Effective Antipollution and Strong Buoyancy. Langmuir 2021, 37, 6042–6051. [Google Scholar] [CrossRef]
- Zhang, C.; Huo, R.; Wang, X.; Zhang, J.; Cheng, J.; Shi, L. In-situ encapsulation of flaky aluminum pigment with poly(methylhydrosiloxane) anti-corrosion film for high-performance waterborne coatings. J. Ind. Eng. Chem. 2020, 89, 239–249. [Google Scholar] [CrossRef]
- Fang, C.; Pu, M.; Zhou, X.; Lei, W.; Pei, L.; Wang, C. Facile preparation of hydrophobic aluminum oxide film via sol-gel method. Front. Chem. 2018, 6, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Rodič, P.; Kapun, B.; Panjan, M.; Milošev, I. Easy and fast fabrication of self-cleaning and anti-icing perfluoroalkyl silane film on aluminium. Coatings 2020, 10, 234. [Google Scholar] [CrossRef]
- Tong, W.; Xiong, D.; Wang, N.; Wu, Z.; Zhou, H. Mechanically robust superhydrophobic coating for aeronautical composite against ice accretion and ice adhesion. Compos. Part B Eng. 2019, 176, 107267. [Google Scholar] [CrossRef]
- Cai, C.; Sang, N.; Teng, S.; Shen, Z.; Guo, J.; Zhao, X.; Guo, Z. Superhydrophobic surface fabricated by spraying hydrophobic R974 nanoparticles and the drag reduction in water. Surf. Coat. Technol. 2016, 307, 366–373. [Google Scholar] [CrossRef]
- Hill, D.; Barron, A.; Alexander, S. Controlling the wettability of plastic by thermally embedding coated aluminium oxide nanoparticles into the surface. J. Colloid Interface Sci. 2020, 567, 45–53. [Google Scholar] [CrossRef]
- Shah, S.; Zulfiqar, U.; Hussain, S.; Ahmad, I.; Hussain, I.; Subhani, T. A durable superhydrophobic coating for the protection of wood materials. Mater. Lett. 2017, 203, 17. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).