Interactions in Composite Film Formation of Mefp-1/graphene on Carbon Steel
Abstract
1. Introduction
2. Experimental
2.1. Mefp-1/graphene Film Preparation
2.2. Corrosion Inhibition Measurement
2.3. Mechanical Adhesion of the Film
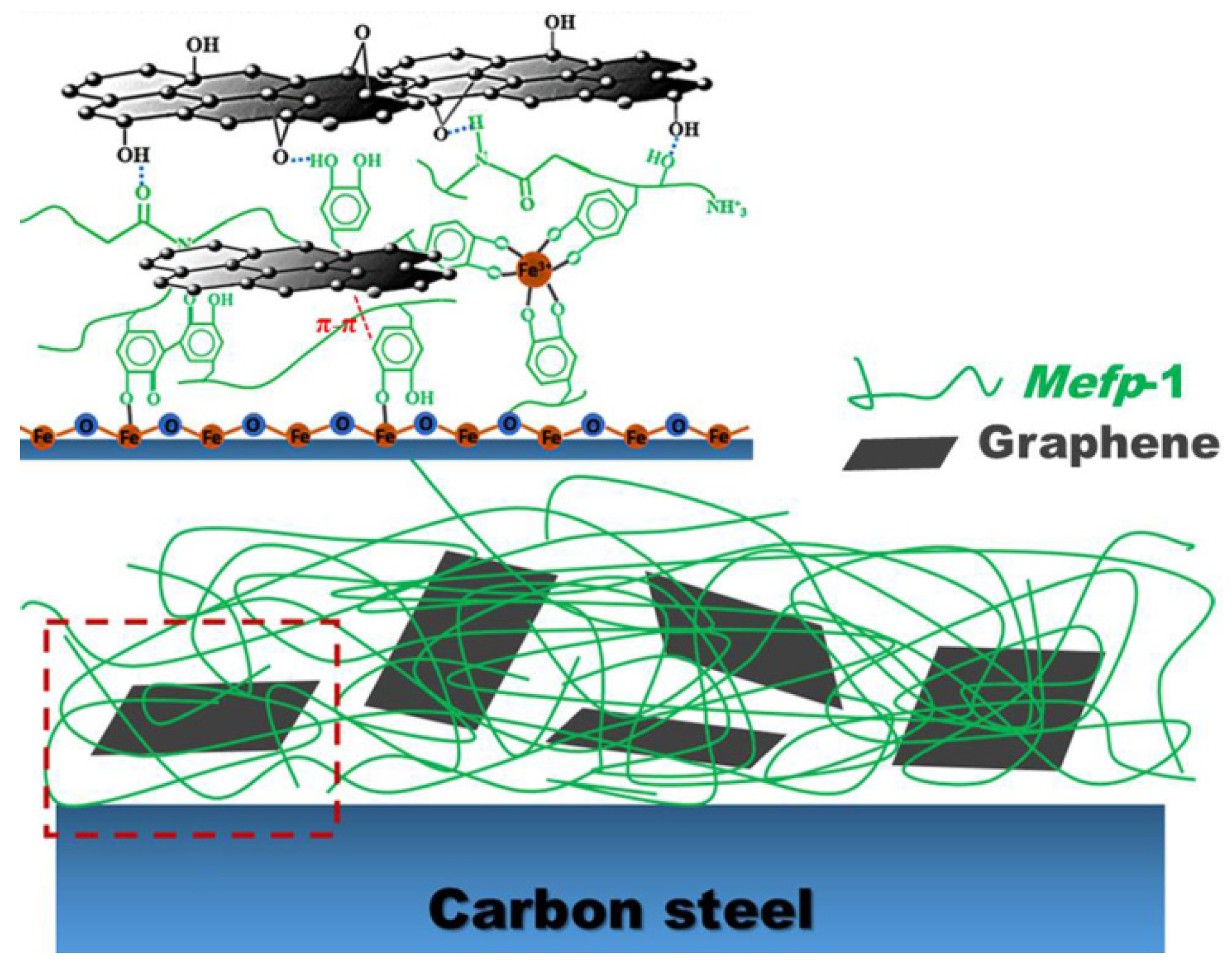
2.4. Adsorption and Interactions during Film Forming
3. Results and Discussion
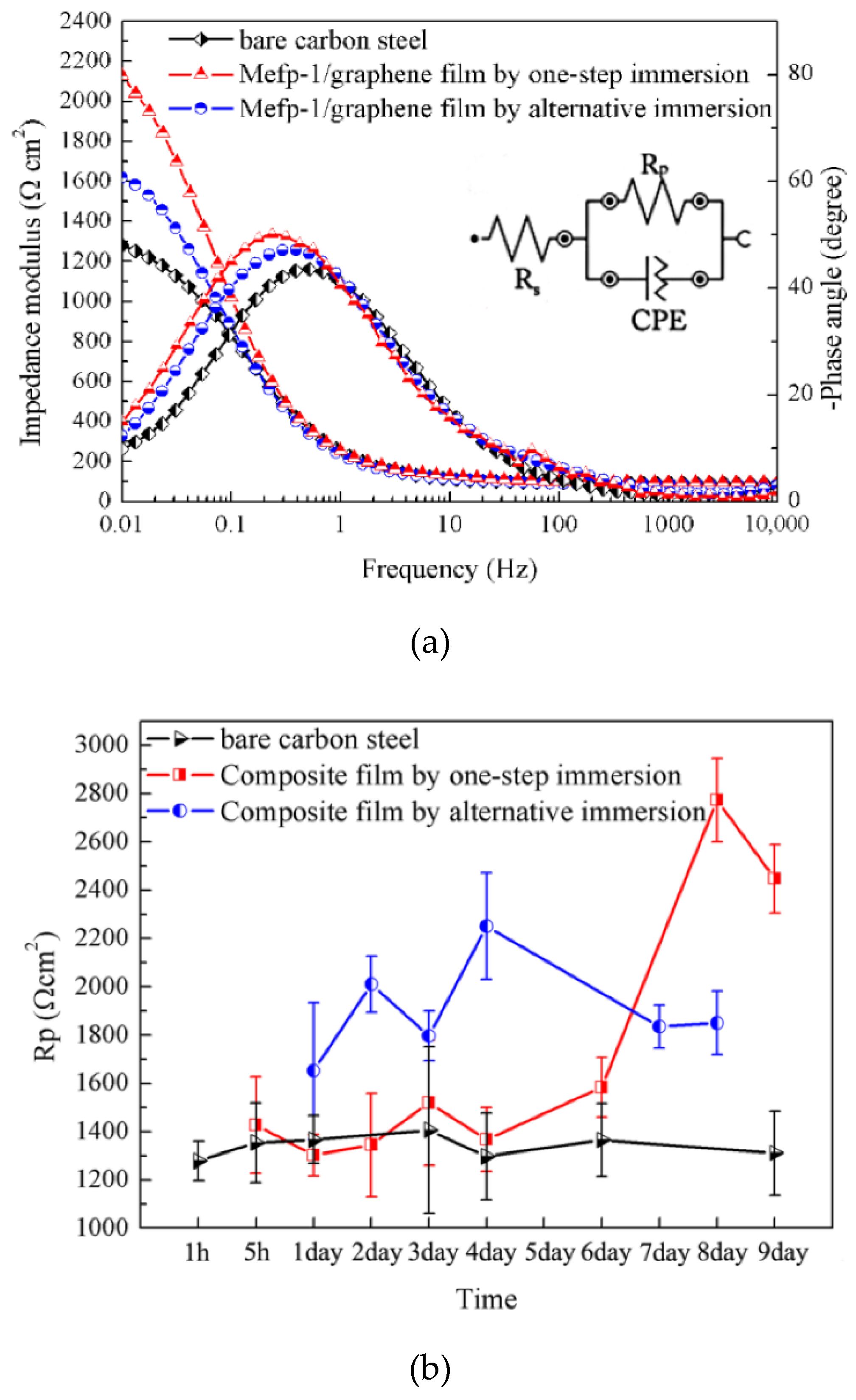
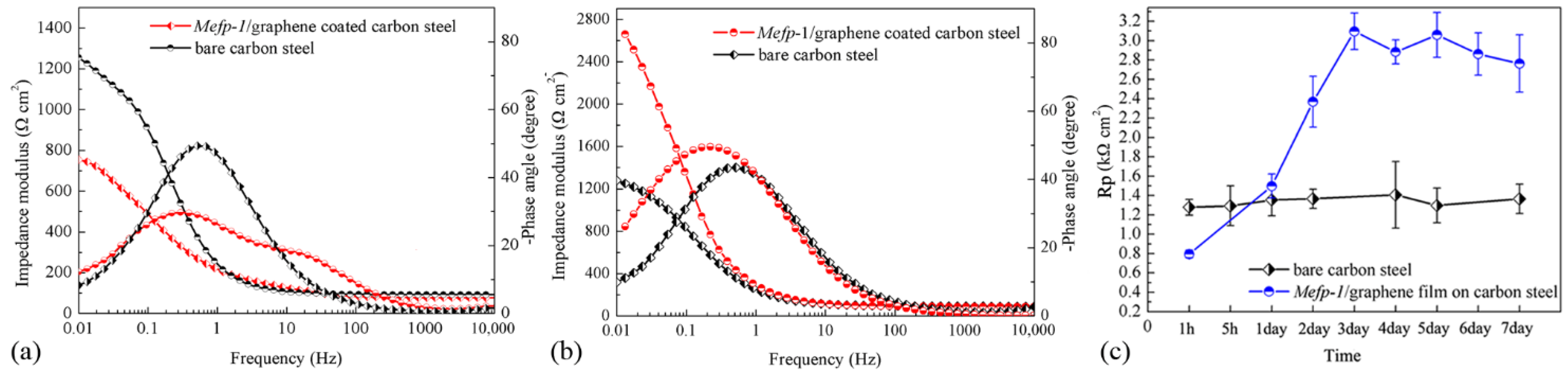
3.1. The Effect of Film Preparation Methods on Corrosion Protection Performance
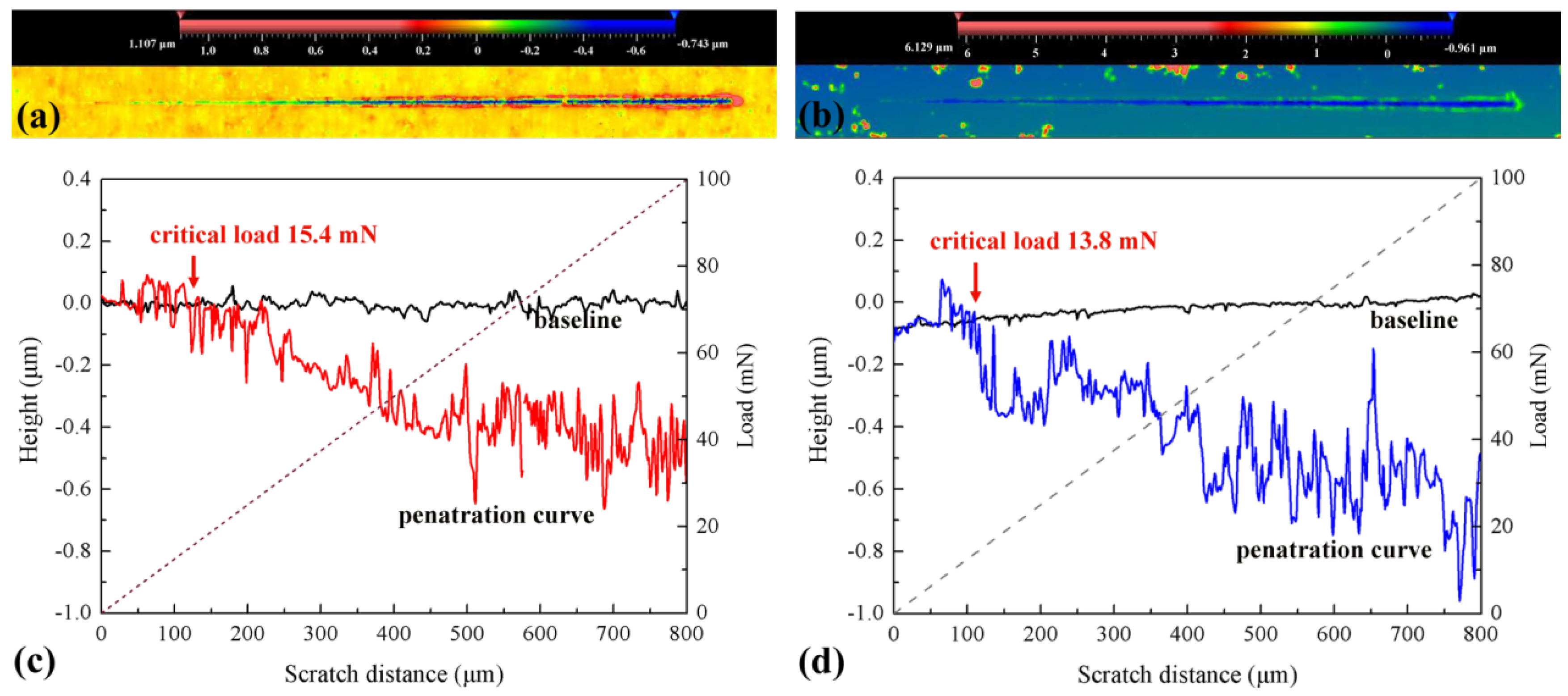
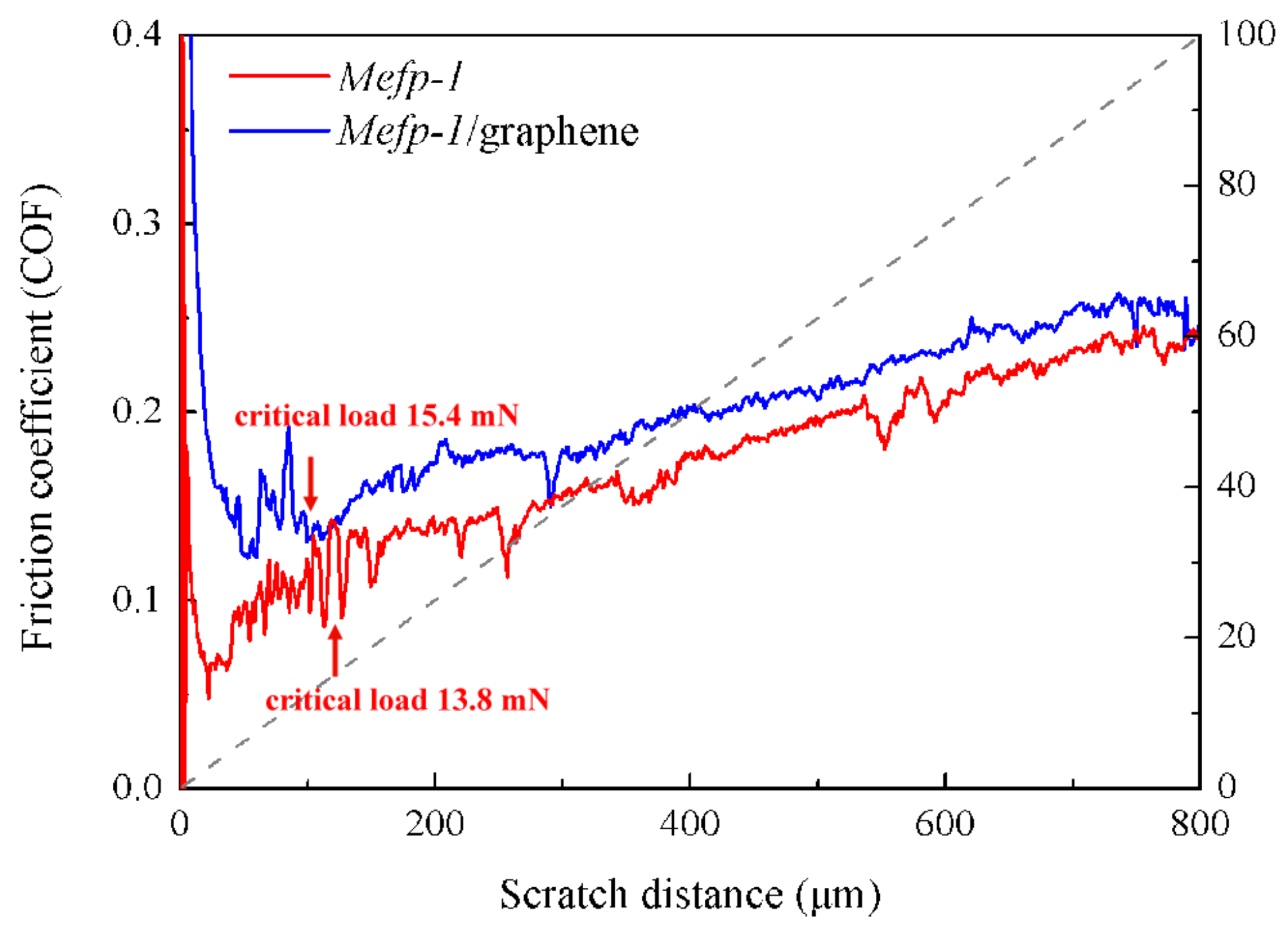
3.2. Interfacial Adhesion of the Composite Film
3.3. Infrared Spectra of Mefp-1/graphene Composite Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Waite, J.H.; Qin, X. Polyphosphoprotein from the Adhesive Pads of Mytilus edulis†. Biochemistry 2001, 40, 2887–2893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xie, G.; Pan, J. Tunable Adsorption and Film Formation of Mussel Adhesive Protein by Potential Control. Langmuir 2017, 33, 8749–8756. [Google Scholar] [CrossRef] [PubMed]
- Harrington, M.J.; Masic, A.; Holten-Andersen, N.; Waite, J.H.; Fratzl, P. Iron-Clad Fibers: A Metal-Based Biological Strategy for Hard Flexible Coatings. Science 2010, 328, 216–220. [Google Scholar] [CrossRef]
- Zhang, F.; Sababi, M.; Brinck, T.; Persson, D.; Pan, J.; Claesson, P.M. In situ investigations of Fe3+ induced complexation of adsorbed Mefp-1 protein film on iron substrate. J. Colloid Interface Sci. 2013, 404, 62–71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burzio, L.A.; Waite, J.H. Cross-Linking in Adhesive Quinoproteins: Studies with Model Decapeptides. Biochemistry 2000, 39, 11147–11153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Pan, J.; Claesson, P.M. Electrochemical and AFM studies of mussel adhesive protein (Mefp-1) as corrosion inhibitor for carbon steel. Electroch. Acta 2011, 56, 1636–1645. [Google Scholar] [CrossRef]
- Hou, R.-Q.; Zhang, F.; Jiang, P.-L.; Dong, S.-G.; Pan, J.-S.; Lin, C.-J. Corrosion inhibition of pre-formed mussel adhesive protein (Mefp-1) film to magnesium alloy. Corros. Sci. 2019, 164, 108309. [Google Scholar] [CrossRef]
- Jiang, P.-L.; Hou, R.-Q.; Chen, C.-D.; Sun, L.; Dong, S.-G.; Pan, J.-S.; Lin, C.-J. Controllable degradation of medical magnesium by electrodeposited composite films of mussel adhesive protein (Mefp-1) and chitosan. J. Colloid Interface Sci. 2016, 478, 246–255. [Google Scholar] [CrossRef]
- Zhang, F.; Pan, J. Recent Development of Corrosion Protection Strategy Based on Mussel Adhesive Protein. Front. Mater. 2019, 6, 207. [Google Scholar] [CrossRef]
- Hansen, D.C.; McCafferty, E. The Effect of Various Naturally Occurring Metal-Binding Compounds on the Electrochemical Behavior of Aluminum. J. Electrochem. Soc. 1996, 143, 114–119. [Google Scholar] [CrossRef]
- Sababi, M.; Zhang, F.; Krivosheeva, O.; Forslund, M.; Pan, J.; Claesson, P.M.; Dedinaite, A. Thin Composite Films of Mussel Adhesive Proteins and Ceria Nanoparticles on Carbon Steel for Corrosion Protection. J. Electrochem. Soc. 2012, 159, C364–C371. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, C.; Hou, R.; Li, J.; Cao, Y.; Dong, S.; Lin, C.; Pan, J. Investigation and application of mussel adhesive protein nanocomposite film-forming inhibitor for reinforced concrete engineering. Corros. Sci. 2019, 153, 333–340. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Graphene: A new emerging lubricant. Mater. Today 2014, 17, 31–42. [Google Scholar] [CrossRef]
- Berman, D.; Deshmukh, S.A.; Sankaranarayanan, S.K.R.S.; Erdemir, A.; Sumant, A.V. Extraordinary Macroscale Wear Resistance of One Atom Thick Graphene Layer. Adv. Funct. Mater. 2014, 24, 6640–6646. [Google Scholar] [CrossRef]
- Khan, Z.H.; Kermany, A.R.; Öchsner, A.; Iacopi, F. Mechanical and electromechanical properties of graphene and their potential application in MEMS. J. Phys. D Appl. Phys. 2017, 50, 53003. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Kim, J.-H.; Kang, H.; Bae, D.; Chang, H.; Choi, H. Reduced graphene oxide as a protection layer for Al. Appl. Surf. Sci. 2017, 407, 1–7. [Google Scholar] [CrossRef]
- Chen, S.; Shen, B.; Zhang, F.; Hong, H.; Pan, J. Mussel-Inspired Graphene Film with Enhanced Durability as a Macroscale Solid Lubricant. ACS Appl. Mater. Interfaces 2019, 11, 31386–31392. [Google Scholar] [CrossRef]
- Cheng, J.; Chen, S.; Zhang, F.; Shen, B.; Lu, X.; Pan, J. Corrosion- and wear-resistant composite film of graphene and mussel adhesive proteins on carbon steel. Corros. Sci. 2020, 164, 108351. [Google Scholar] [CrossRef]
- Suci, P.A.; Geesey, G.G. Use of Attenuated Total Internal Reflection Fourier Transform Infrared Spectroscopy To Investigate Interactions between Mytilus edulis Foot Proteins at a Surface. Langmuir 2001, 17, 2538–2540. [Google Scholar] [CrossRef]
- Fant, C.; Hedlund, J.; Höök, F.; Berglin, M.; Fridell, E.; Elwing, H. Investigation of Adsorption and Cross-Linking of a Mussel Adhesive Protein Using Attenuated Total Internal Reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR). J. Adhes. 2010, 86, 25–38. [Google Scholar] [CrossRef]
- Chalker, P.; Bull, S.; Rickerby, D. A review of the methods for the evaluation of coating-substrate adhesion. Mater. Sci. Eng. A 1991, 140, 583–592. [Google Scholar] [CrossRef]
- Zaffora, A.; Di Quarto, F.; Kura, C.; Sato, Y.; Aoki, Y.; Habazaki, H.; Santamaria, M. Electrochemical Oxidation of Hf-Nb Alloys as a Valuable Route to Prepare Mixed Oxides of Tailored Dielectric Properties. Adv. Electron. Mater. 2018, 4, 1800006. [Google Scholar] [CrossRef]
- Zhang, F.; Brinck, T.; Brandner, B.D.; Claesson, P.M.; Dedinaite, A.; Pan, J. In situ confocal Raman micro-spectroscopy and electrochemical studies of mussel adhesive protein and ceria composite film on carbon steel in salt solutions. Electroch. Acta 2013, 107, 276–291. [Google Scholar] [CrossRef]
- Butz, F.; Aita, H.; Takeuchi, K.; Ogawa, T. Enhanced mineralized tissue adhesion to titanium over polystyrene assessed by the nano-scratch test. J. Biomed. Mater. Res. Part A 2005, 74, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Hang, Y.; Liu, G.; Huang, K.; Jin, W. Mechanical properties and interfacial adhesion of composite membranes probed by in-situ nano-indentation/scratch technique. J. Membr. Sci. 2015, 494, 205–215. [Google Scholar] [CrossRef]
- Ashraf, A.; Wu, Y.; Wang, M.C.; Yong, K.; Sun, T.; Jing, Y.; Haasch, R.T.; Aluru, N.R.; Nam, S. Doping-Induced Tunable Wettability and Adhesion of Graphene. Nano Lett. 2016, 16, 4708–4712. [Google Scholar] [CrossRef] [PubMed]
- Danner, E.; Kan, Y.; Hammer, M.U.; Israelachvili, J.N.; Waite, J.H. Adhesion of Mussel Foot Protein Mefp-5 to Mica: An Underwater Superglue. Biochemistry 2012, 51, 6511–6518. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Yang, X.; Ji, Z.; Zhu, L.; Ma, N.; Chen, D.; Jia, X.; Tang, J.; Cao, Y. DFT-Calculated IR Spectrum Amide I, II, and III Band Contributions of N-Methylacetamide Fine Components. ACS Omega 2020, 5, 8572–8578. [Google Scholar] [CrossRef]
- Chen, C.; Hou, R.; Zhang, F.; Dong, S.; Claesson, P.M.; Lin, C.; Pan, J. Heating-Induced Enhancement of Corrosion Protection of Carbon Steel by a Nanocomposite Film Containing Mussel Adhesive Protein. J. Electrochem. Soc. 2017, 164, C188–C193. [Google Scholar] [CrossRef]
- Mallamace, F.; Corsaro, C.; Mallamace, D.; Vasi, S.; Vasi, C.; Dugo, G. The role of water in protein’s behavior: The two dynamical crossovers studied by NMR and FTIR techniques. Comput. Struct. Biotechnol. 2015, 13, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Sever, M.J.; Weisser, J.T.; Monahan, J.; Srinivasan, S.; Wilker, J.J. Metal-Mediated Cross-Linking in the Generation of a Marine-Mussel Adhesive. Angew. Chem. Int. Ed. 2004, 43, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Matin, M.A.; Islam, M.M.; Bredow, T.; Aziz, M.A. The Effects of Oxidation States, Spin States and Solvents on Molecular Structure, Stability and Spectroscopic Properties of Fe-Catechol Complexes: A Theoretical Study. Adv. Chem. Eng. Sci. 2017, 7, 137–153. [Google Scholar] [CrossRef]
- Al-Mashta, F.; Sheppard, N.; Lorenzelli, V.; Busca, G. Infrared study of adsorption on oxygen-covered α-Fe2O3: Bands due to adsorbed oxygen and their modification by co-adsorbed hydrogen or water. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1982, 78, 979–989. [Google Scholar] [CrossRef]
- Cai, S.; Singh, B.R. Identification of β-turn and random coil amide III infrared bands for secondary structure estimation of proteins. Biophys. Chem. 1999, 80, 7–20. [Google Scholar] [CrossRef]
- Witkowski, A.; Wójcik, M. Infrared spectra of hydrogen bond a general theoretical model. Chem. Phys. 1973, 1, 9–16. [Google Scholar] [CrossRef]
- Mostad, A.; Ottersen, T.; Rømming, C.; Hammarström, S.; Lousberg, R.J.J.C.; Weiss, U. On the Structure of L-DOPA (2S-3-(3,4-Dihydroxyphenyl)alanine). Acta Chem. Scand. 1971, 25, 3549–3560. [Google Scholar] [CrossRef][Green Version]
- Leng, C.; Liu, Y.; Jenkins, C.; Meredith, H.; Wilker, J.J.; Chen, Z. Interfacial Structure of a DOPA-Inspired Adhesive Polymer Studied by Sum Frequency Generation Vibrational Spectroscopy. Langmuir 2013, 29, 6659–6664. [Google Scholar] [CrossRef]
- Guo, H.; Sun, Y.; Niu, X.; Wei, N.; Pan, C.; Wang, G.; Zhang, H.; Chen, H.; Yi, T.; Chen, X. The preparation of poly-levodopa coated capillary column for capillary electrochromatography enantioseparation. J. Chromatogr. A 2018, 1578, 91–98. [Google Scholar] [CrossRef]
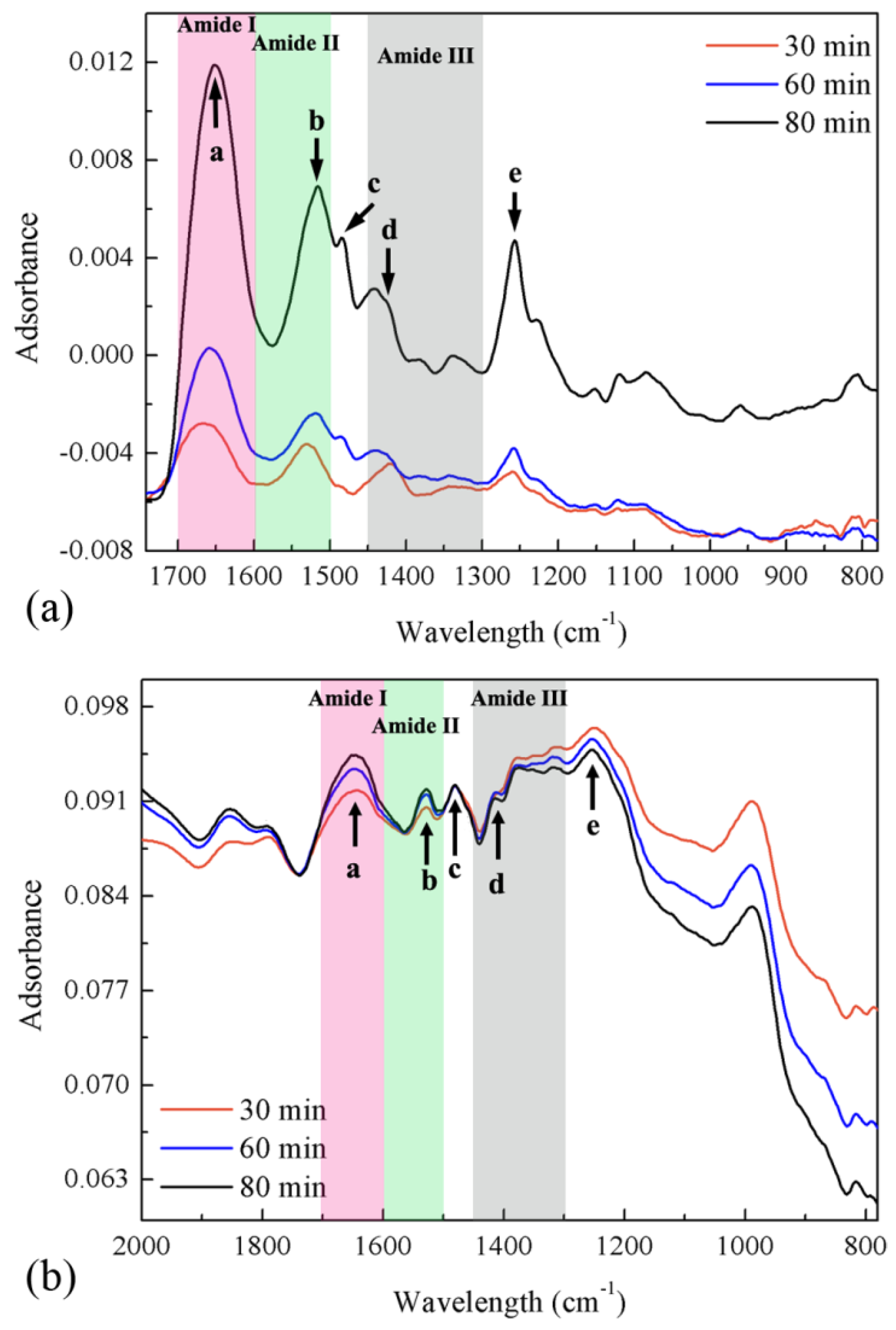
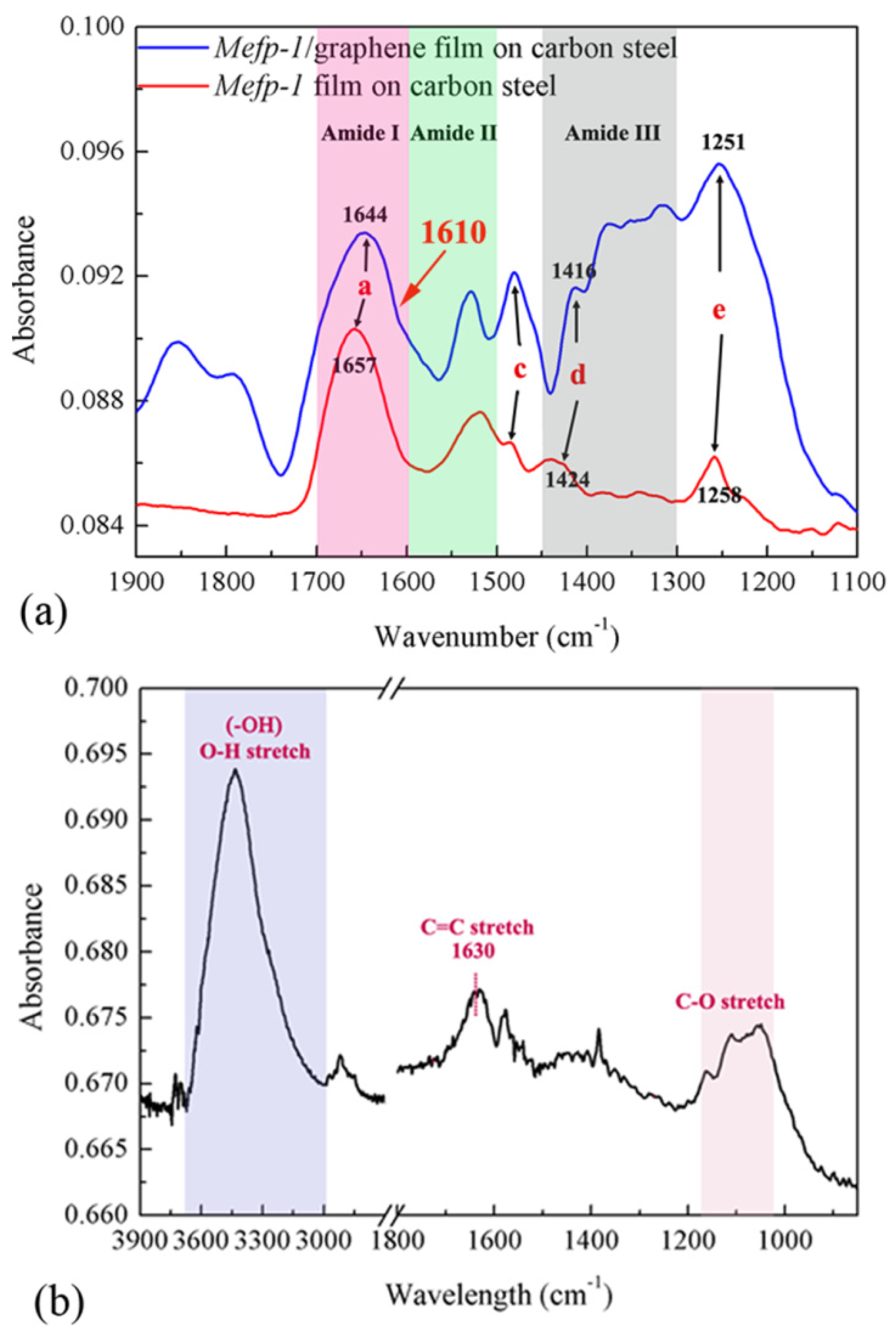
| Assignment and Remarks | Mefp-1 | Mefp-1/graphene |
|---|---|---|
| a: mainly C=O stretch in Amide I sensitive to hydrogen bond | 1657 | 1644 |
| b: mainly N–H bending in Amide II | 1525 | 1528 |
| c: C–C ring stretch in DOPA | 1484 | 1480 |
| d: C–C ring stretch in DOPA diagnostic signal of tris (DOPA) Fe(III) complex | 1423 | 1414 |
| e: C–O stretch in DOPA | 1258 | 1251 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Mei, N.; Chen, S.; Bai, P.; Shen, B.; Pan, J.; Zhang, F. Interactions in Composite Film Formation of Mefp-1/graphene on Carbon Steel. Coatings 2021, 11, 1161. https://doi.org/10.3390/coatings11101161
Cheng J, Mei N, Chen S, Bai P, Shen B, Pan J, Zhang F. Interactions in Composite Film Formation of Mefp-1/graphene on Carbon Steel. Coatings. 2021; 11(10):1161. https://doi.org/10.3390/coatings11101161
Chicago/Turabian StyleCheng, Jie, Nanxuan Mei, Sulin Chen, Pengpeng Bai, Bin Shen, Jinshan Pan, and Fan Zhang. 2021. "Interactions in Composite Film Formation of Mefp-1/graphene on Carbon Steel" Coatings 11, no. 10: 1161. https://doi.org/10.3390/coatings11101161
APA StyleCheng, J., Mei, N., Chen, S., Bai, P., Shen, B., Pan, J., & Zhang, F. (2021). Interactions in Composite Film Formation of Mefp-1/graphene on Carbon Steel. Coatings, 11(10), 1161. https://doi.org/10.3390/coatings11101161








