Tunable Microstructure and Morphology of the Self-Assembly Hydroxyapatite Coatings on ZK60 Magnesium Alloy Substrates Using Hydrothermal Methods
Abstract
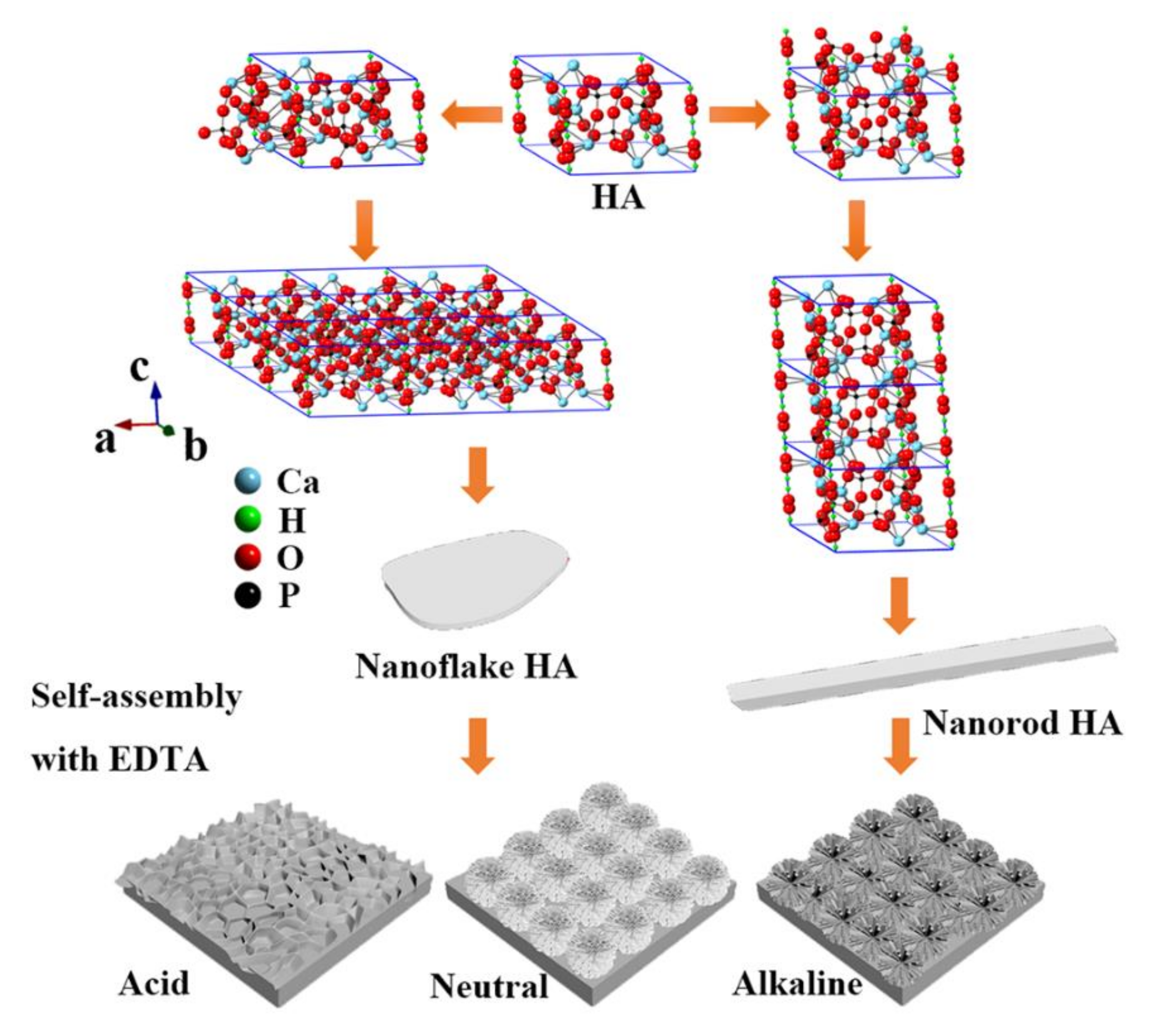
:1. Introduction
2. Materials and Methods
2.1. Preparation of ZK60 Magnesium Alloy Substrates
2.2. Preparation of the HA Coatings
2.3. Materials Characterization
2.4. Immersion Tests
2.5. Electrochemical Test
2.6. Cell Culture Preparation
2.7. Cell Live/Dead Assay
2.8. Statistical Analysis
3. Results
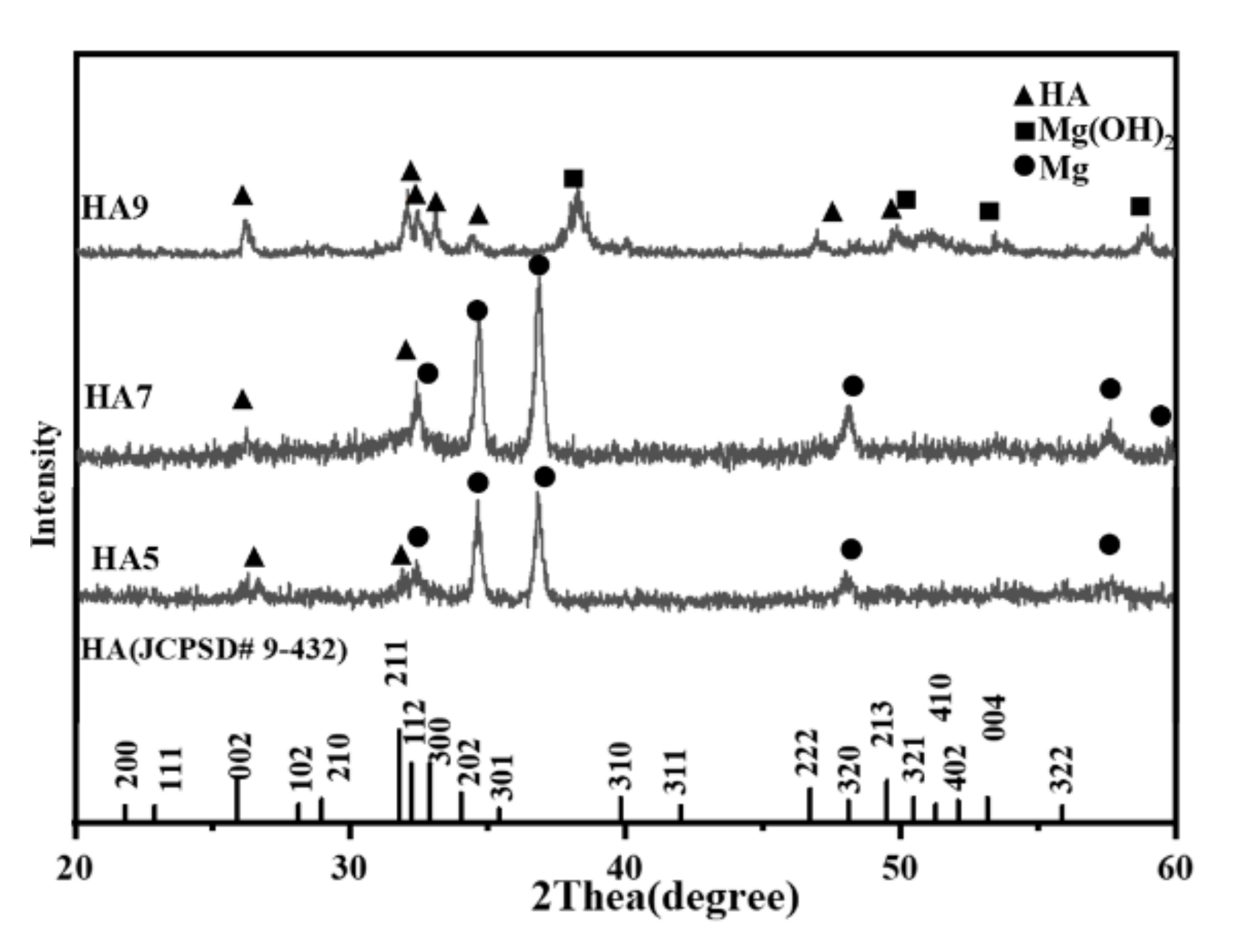
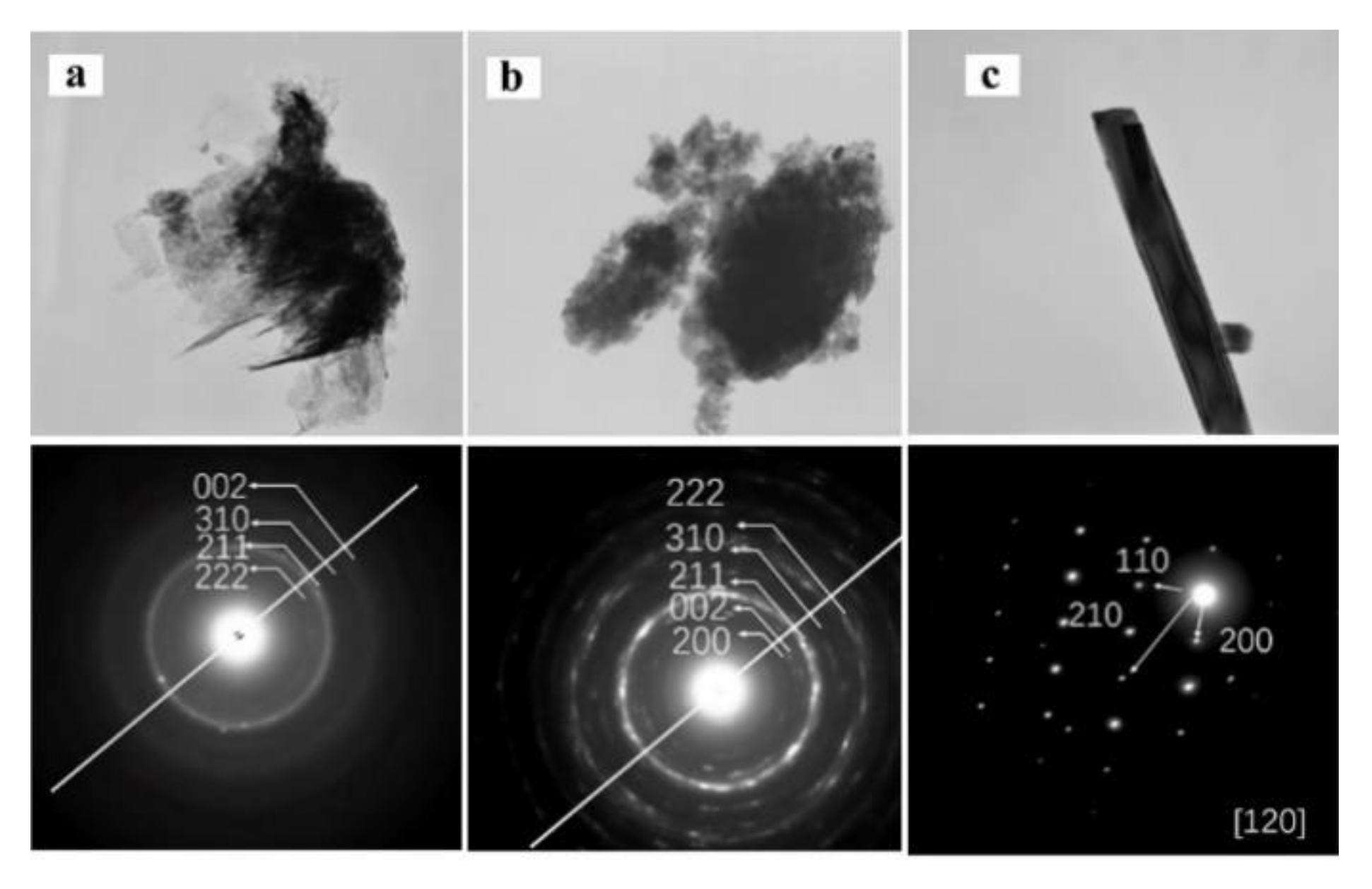
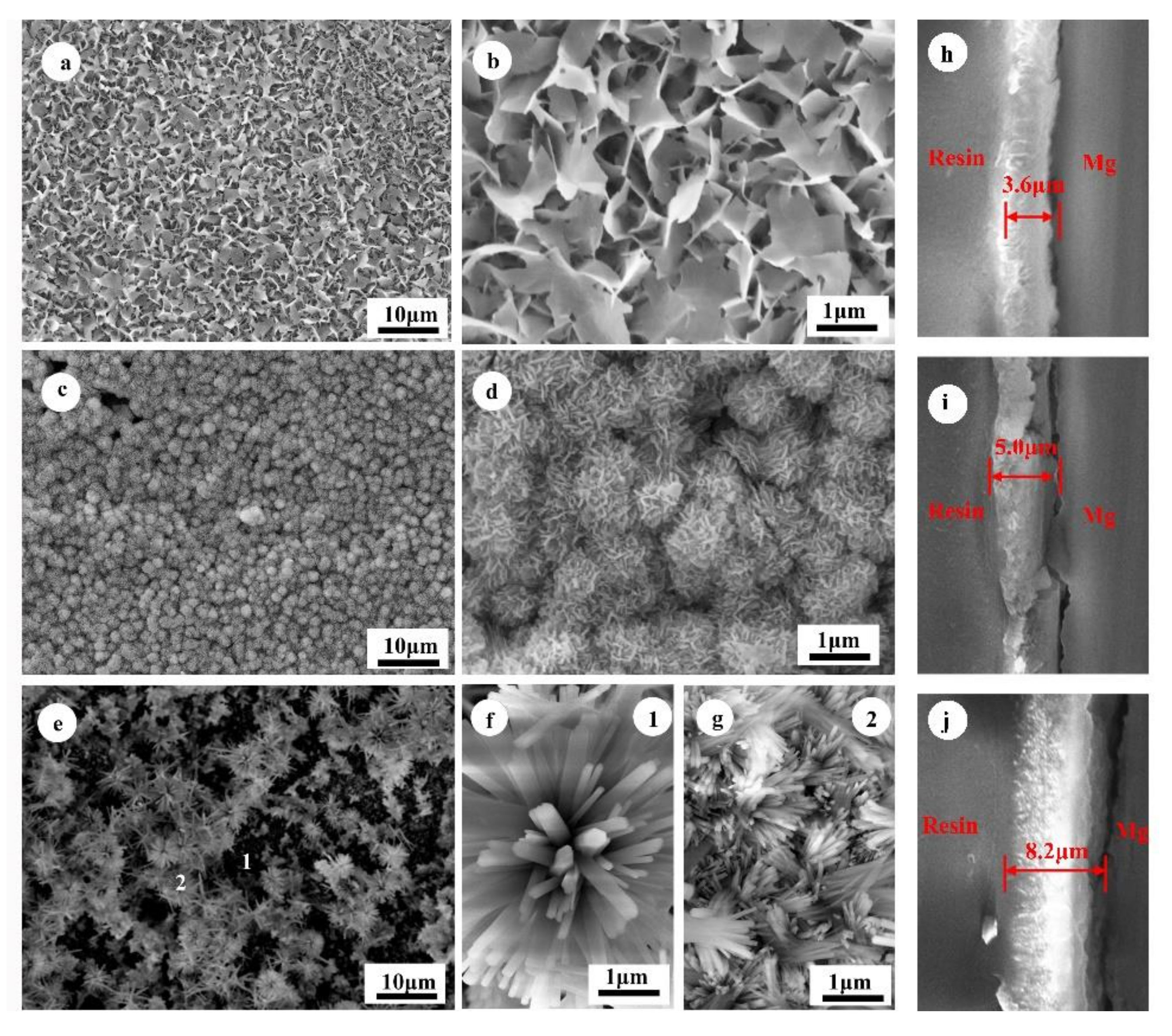
3.1. Characterization of the HA Coatings
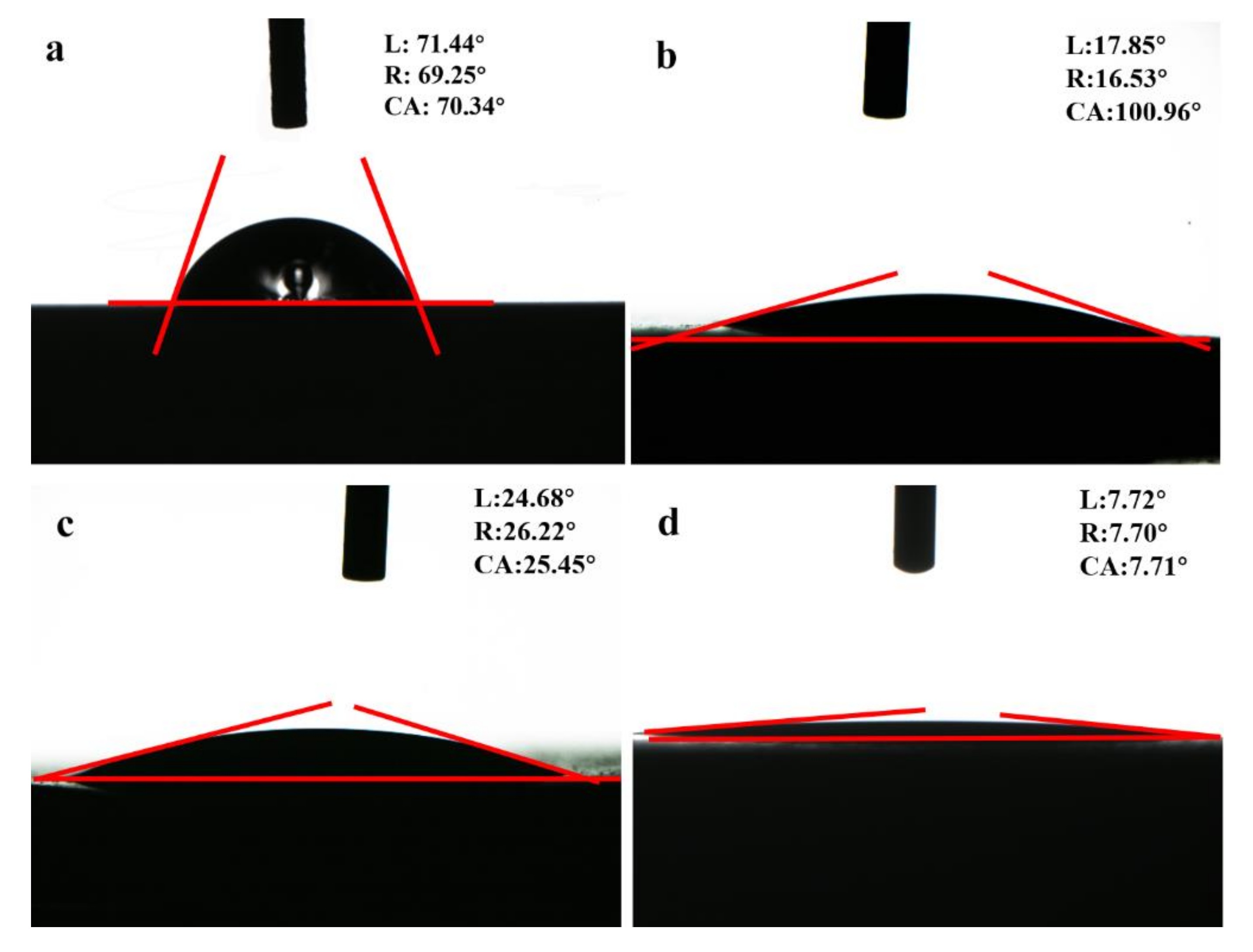
3.2. Wettability of HA Coatings
3.3. Electrochemical Tests of the HA-Coated Samples
3.4. Immersion Tests
3.5. Cell Culture of BMSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanchez, A.H.M.; Luthringer, B.J.; Feyerabend, F.; Willumeit, R. Mg and Mg alloys: How Comparable are In Vitro and In Vivo Corrosion Rates? A Review. Acta Biomater. 2015, 13, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Gu, X.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Liu, C.; Ren, Z.; Xu, Y.; Pang, S.; Zhao, X.; Zhao, Y. Biodegradable Magnesium Alloys Developed as Bone Repair Materials: A Review. Scanning 2018, 2018, 9216314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Zhang, M.; Li, Y.; Zhao, J.; Qin, L.; Lai, Y. Corrosion and biocompatibility improvement of magnesium-based alloys as bone implant materials: A review. Regen. Biomater. 2017, 4, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 948–963. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Li, S.-S.; Li, X.-M.; Fan, Y.-B. Magnesium based degradable biomaterials: A review. Front. Mater. Sci. 2014, 8, 200–218. [Google Scholar] [CrossRef]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Husak, Y.; Solodovnik, A.; Yanovska, A.; Kozik, Y.; Liubchak, I.; Ivchenko, V.; Mishchenko, O.; Zinchenko, Y.; Kuznetsov, V.; Pogorielov, M. Degradation and In Vivo Response of Hydroxyapatite-Coated Mg Alloy. Coatings 2018, 8, 375. [Google Scholar] [CrossRef] [Green Version]
- Özarslan, S.; Şevik, H.; Sorar, I. Microstructure, mechanical and corrosion properties of novel Mg-Sn-Ce alloys produced by high pressure die casting. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110064. [Google Scholar] [CrossRef]
- Li, L.-Y.; Cui, L.-Y.; Zeng, R.-C.; Li, S.-Q.; Chen, X.-B.; Zheng, Y.; Kannan, M.B. Advances in functionalized polymer coatings on biodegradable magnesium alloys—A review. Acta Biomater. 2018, 79, 23–36. [Google Scholar] [CrossRef]
- Wei, Z.; Tian, P.; Liu, X.; Zhou, B. Hemocompatibility and selective cell fate of polydopamine-assisted heparinized PEO/PLLA composite coating on biodegradable AZ31 alloy. Colloids Surf. B Biointerfaces 2014, 121, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Xiong, C.; Jiang, L.; Li, H.; Yuan, M. The Preparation, Characterization, Mechanical and Antibacterial Properties of GO-ZnO Nanocomposites with a Poly(l-lactide)-Modified Surface. Materials 2018, 11, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.; Wang, C.; Shi, X.; Zhang, G.; Chen, P.; Wang, J. Crystallization, rheology behavior, and antibacterial ap-plication of graphene oxide-graft-poly(l-lactide)/poly (l-lactide) nanocomposites. Appl. Surf. Sci. 2018, 451, 315–324. [Google Scholar] [CrossRef]
- Mukhametkaliyev, T.M.; Surmeneva, M.A.; Vladescu, A.; Cotrut, C.M.; Braic, M.; Dinu, M.; Vranceanu, D.M.; Pana, I.; Mueller, M.; Surmenev, R.A. A biodegradable AZ91 magnesium alloy coated with a thin nanostructured hydroxyapatite for improving the corrosion resistance. Mater. Sci. Eng. C 2017, 75, 95–103. [Google Scholar] [CrossRef]
- Xia, K.; Pan, H.; Wang, T.; Ma, S.; Niu, J.; Xiang, Z.; Song, Y.; Yang, H.; Tang, X.; Lu, W. Effect of Ca/P ratio on the structural and corrosion properties of biomimetic CaP coatings on ZK60 magnesium alloy. Mater. Sci. Eng. C 2017, 72, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ma, X.; Chang, L.; Zhu, S.; Guan, S. Characterization and cytocompatibility of polydopamine on MAO-HA coating supported on Mg-Zn-Ca alloy. Surf. Interface Anal. 2017, 49, 1115–1123. [Google Scholar] [CrossRef]
- Khalajabadi, S.Z.; Abu, A.B.H.; Ahmad, N.; Yajid, M.A.M.; Hj Redzuan, N.B.; Nasiri, R.; Haider, W.; Noshadi, I. Bio-corrosion behavior and mechanical characteristics of magnesium-titania-hydroxyapatite nanocomposites coated by magnesi-um-oxide flakes and silicon for use as resorbable bone fixation material. J. Mech. Behav. Biomed. Mater. 2018, 77, 360–374. [Google Scholar] [CrossRef]
- Su, Y.; Li, D.; Su, Y.; Lu, C.; Niu, L.; Lian, J.; Li, G. Improvement of the Biodegradation Property and Biomineraliza-tion Ability of Magnesium–Hydroxyapatite Composites with Dicalcium Phosphate Dihydrate and Hydroxyapatite Coatings. ACS Biomater. Sci. Eng. 2016, 2, 818–828. [Google Scholar] [CrossRef]
- Perez, J.R.; Kouroupis, D.; Li, D.J.; Best, T.M.; Kaplan, L.; Correa, D. Tissue Engineering and Cell-Based Therapies for Fractures and Bone Defects. Front. Bioeng. Biotechnol. 2018, 6, 105. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.-L.; Huo, Y.-F.; Li, C.-Y.; Kannan, M.B.; Chen, X.-B.; Guan, S.-K.; Zeng, R.-C.; Ma, Q.-L. Corrosion resistance of nanostructured magnesium hydroxide coating on magnesium alloy AZ31: Influence of EDTA. Rare Met. 2019, 38, 520–531. [Google Scholar] [CrossRef]
- Dou, J.; Chen, Y.; Yu, H.; Chen, C. Research status of magnesium alloys by micro-arc oxidation: A review. Surf. Eng. 2017, 33, 731–738. [Google Scholar] [CrossRef]
- Zhuang, J.J.; Song, R.; Xiang, N.; Xiong, Y.; Hu, Q. Effect of current density on microstructure and properties of PEO ceramic coatings on magnesium alloy. Surf. Eng. 2016, 33, 744–752. [Google Scholar] [CrossRef]
- Mehrjou, B.; Soltani, R.; Sohi, M.H.; Torkamany, M.J.; Valefi, Z.; Ghorbani, H. Laser surface treatment of AZ91 magnesium alloy presprayed with WC–Co. Surf. Eng. 2016, 32, 893–901. [Google Scholar] [CrossRef]
- Hassan, M.N.; Mahmoud, M.M.; El-Fattah, A.A.; Kandil, S. Microwave-assisted preparation of Nano-hydroxyapatite for bone substitutes. Ceram. Int. 2016, 42, 3725–3744. [Google Scholar] [CrossRef]
- Sun, R.X.; Liu, P.; Zhang, R.X.; Lv, Y.P.; Chen, K.Z. Hydrothermal synthesis of microstructured fluoridated hydrox-yapatite coating on magnesium alloy. Surf. Eng. 2016, 32, 879–884. [Google Scholar] [CrossRef]
- He, D.; Du, J.; Liu, P.; Liu, X.; Chen, X.; Li, W.; Zhang, K.; Ma, F. Influence of EDTA-2Na on the hydroxyapatite coating deposited by hydrothermal-electrochemical method on Ti6Al4V surface. Surf. Coat. Technol. 2019, 365, 242–247. [Google Scholar] [CrossRef]
- Yu, W.; Sun, R.; Guo, Z.; Wang, Z.; He, Y.; Lu, G.; Chen, P.; Chen, K. Novel fluoridated hydroxyapatite/MAO composite coating on AZ31B magnesium alloy for biomedical application. Appl. Surf. Sci. 2019, 464, 708–715. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, Z.; Wang, T.; Yang, G.; Xi, W.; Gan, Y.; Lu, W.; Hu, J. Enhanced corrosion resistance and bioactivity of Mg alloy modified by Zn-doped nanowhisker hydroxyapatite coatings. Colloids Surf. B Biointerfaces 2020, 186, 110710. [Google Scholar] [CrossRef]
- Chen, W.; Tian, B.; Lei, Y.; Ke, Q.-F.; Zhu, Z.; Guo, Y.-P. Hydroxyapatite coatings with oriented nanoplate and nanorod arrays: Fabrication, morphology, cytocompatibility and osteogenic differentiation. Mater. Sci. Eng. C 2016, 67, 395–408. [Google Scholar] [CrossRef]
- Gokcekaya, O.; Ueda, K.; Narushima, T.; Ergun, C. Synthesis and characterization of Ag-containing calcium phosphates with various Ca/P ratios. Mater. Sci. Eng. C 2015, 53, 111–119. [Google Scholar] [CrossRef]
- Gokcekaya, O.; Webster, T.J.; Ueda, K.; Narushima, T.; Ergun, C. In vitro performance of Ag-incorporated hydroxy-apatite and its adhesive porous coatings deposited by electrostatic spraying. Mater. Sci. Eng. C 2017, 77, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Gokcekaya, O.; Ueda, K.; Ogasawara, K.; Kanetaka, H.; Narushima, T. In vitro evaluation of Ag-containing calcium phosphates: Effectiveness of Ag-incorporated beta-tricalcium phosphate. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Tomozawa, M.; Hiromoto, S. Microstructure of hydroxyapatite- and octacalcium phosphate-coatings formed on magnesium by a hydrothermal treatment at various pH values. Acta Mater. 2011, 59, 355–363. [Google Scholar] [CrossRef]
- Chen, J.; Tan, L.; Yang, K. Recent advances on the development of biodegradable magnesium alloys: A review. Mater. Technol. 2016, 31, 681–688. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Kleinhans, C.; Vacun, G.; Kluger, P.J.; Schönhaar, V.; Müller, M.; Hein, S.B.; Wittmar, A.; Ulbricht, M.; Prymak, O.; et al. Nano-hydroxyapatite-coated metal-ceramic composite of iron-tricalcium phosphate: Improving the surface wettability, adhesion and proliferation of mesenchymal stem cells in vitro. Colloids Surf. B Biointerfaces 2015, 135, 386–393. [Google Scholar] [CrossRef]
- Qu, H.; Wei, M. The effect of fluoride contents in fluoridated hydroxyapatite on osteoblast behavior. Acta Biomater. 2006, 2, 113–119. [Google Scholar] [CrossRef]
- Zhao, Y.-B.; Liu, H.-P.; Li, C.-Y.; Chen, Y.; Zeng, R.-C.; Zeng, R.-C.; Wang, Z.-L. Corrosion resistance and adhesion strength of a spin-assisted layer-by-layer assembled coating on AZ31 magnesium alloy. Appl. Surf. Sci. 2018, 434, 787–795. [Google Scholar] [CrossRef]
- Chen, J.; Tan, L.; Yang, K. Effect of heat treatment on mechanical and biodegradable properties of an extruded ZK60 alloy. Bioact. Mater. 2017, 2, 19–26. [Google Scholar] [CrossRef]
- Shrestha, S. Magnesium and surface engineering. Surf. Eng. 2010, 26, 313–316. [Google Scholar] [CrossRef]
- Yang, H.; Xia, K.; Wang, T.; Niu, J.; Song, Y.; Xiong, Z.; Zheng, K.; Wei, S.; Lu, W. Growth, in vitro biodegradation and cytocompatibility properties of nano-hydroxyapatite coatings on biodegradable magnesium alloys. J. Alloy. Compd. 2016, 672, 366–373. [Google Scholar] [CrossRef]
- Wang, X.; Shi, J.; Li, Z.; Zhang, S.; Wu, H.; Jiang, Z.; Yang, C.; Tian, C. Facile one-pot preparation of chi-tosan/calcium pyrophosphate hybrid microflowers. ACS Appl. Mater. Interfaces 2014, 6, 14522–14532. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, L.; Zhang, L.; Zhao, Q.; Zhou, Y.; Yuan, S.; Tang, Z.; Liu, X. Peroxidase-Like Activity of Ethylene Diamine Tetraacetic Acid and Its Application for Ultrasensitive Detection of Tumor Biomarkers and Circular Tumor Cells. Anal. Chem. 2016, 89, 666–672. [Google Scholar] [CrossRef] [PubMed]
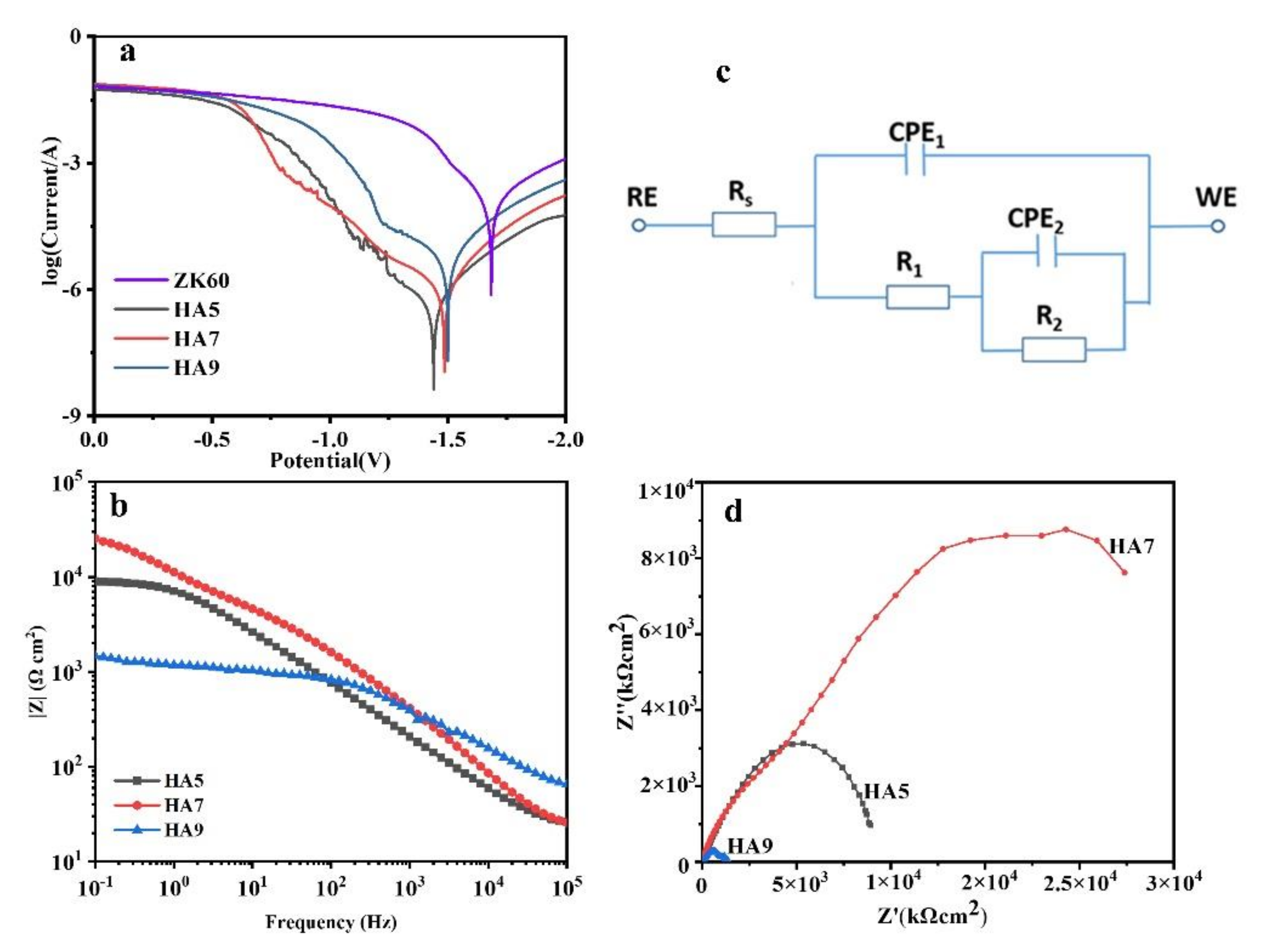
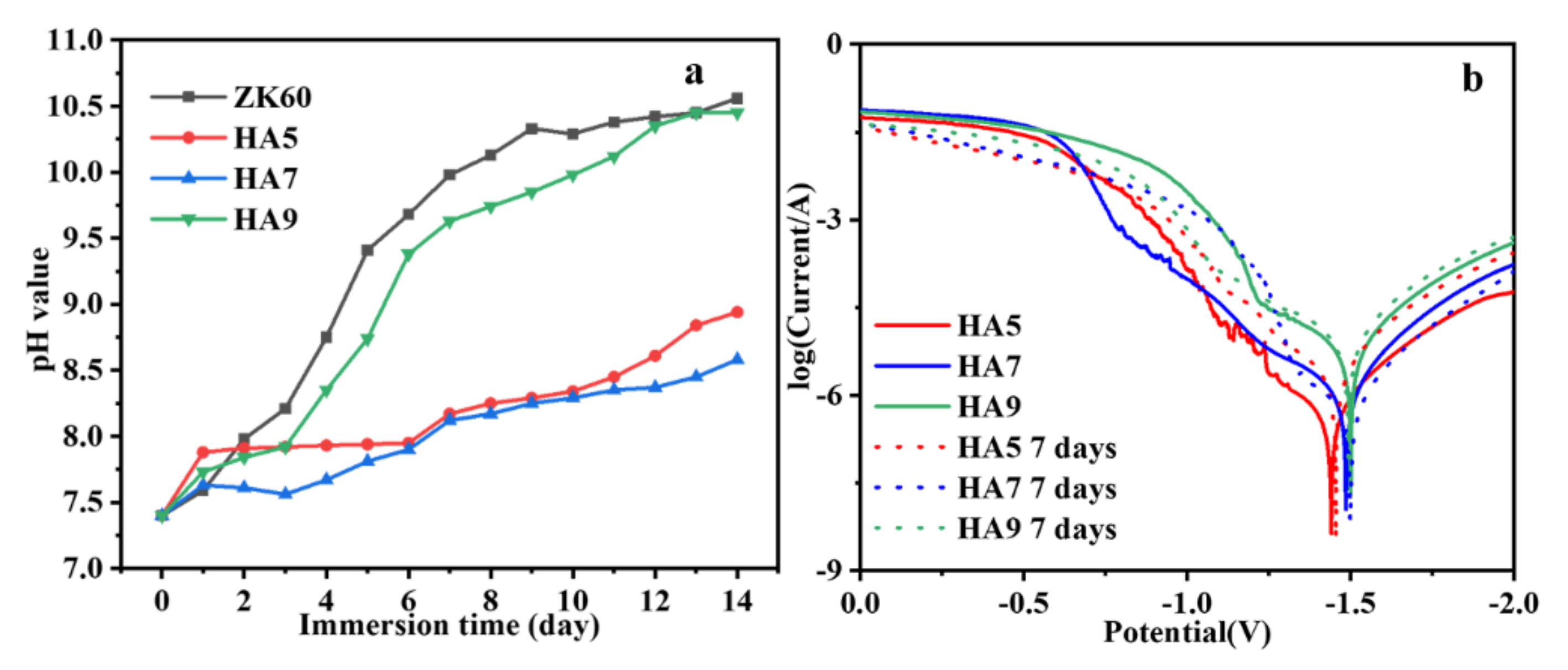


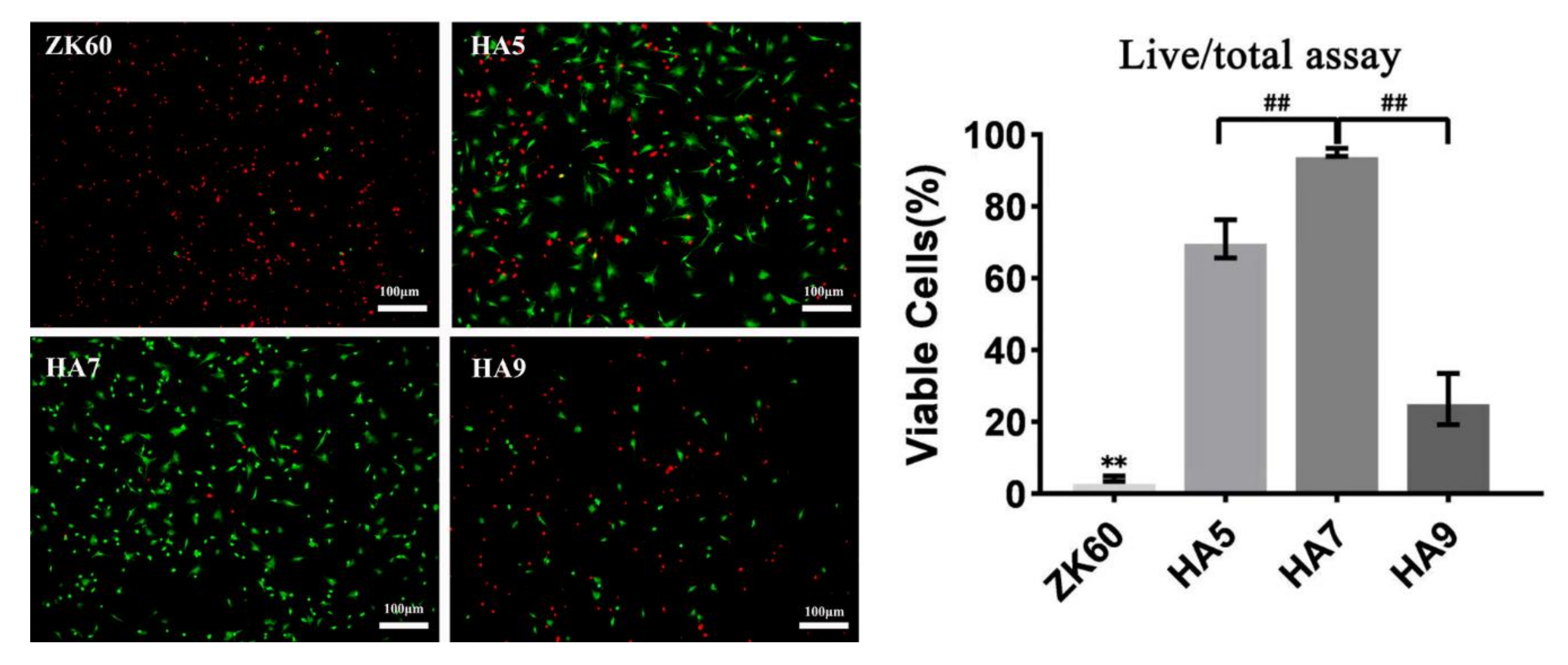
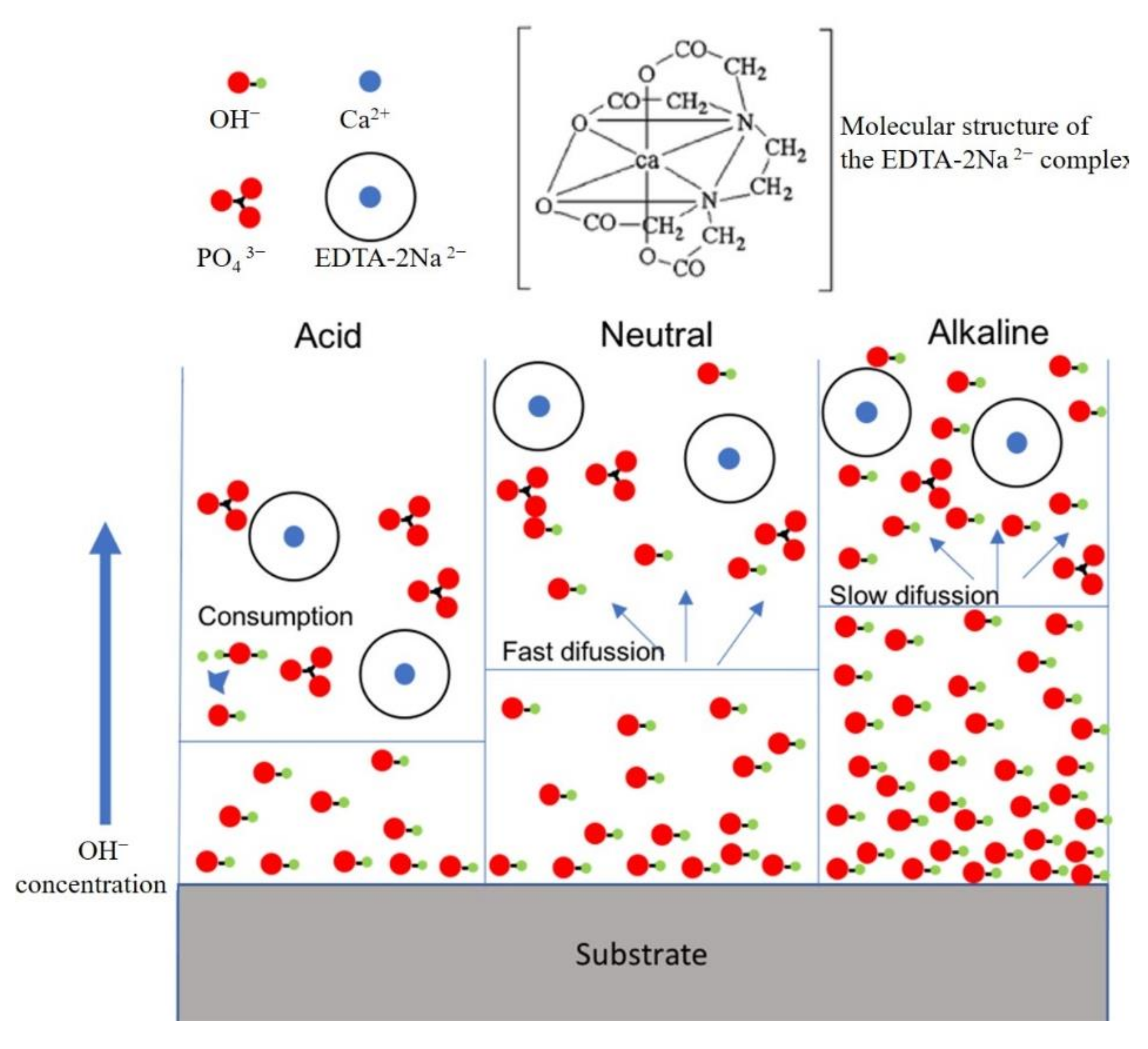
| Sample | Icorr (A·cm−2) | Ecorr (V) |
|---|---|---|
| ZK60 | 1.46·10−4 | −1.68 |
| HA5 | 5.28·10−7 | −1.44 |
| HA7 | 1.04·10−6 | −1.49 |
| HA9 | 5.09·10−6 | −1.50 |
| Sample | Rs (Ω·cm2) | (CPE-T)1 (S−n·cm−2) | R1 (Ω·cm2) | (CPE-T)2 (S−n·cm−2) | R2 (Ω·cm2) |
|---|---|---|---|---|---|
| HA5 | 36.25 | 5.14·10−5 | 6.17·102 | 4.26·10−6 | 7.14·103 |
| HA7 | 32.90 | 2.65·10−7 | 2.69·103 | 6.39·10−6 | 1.76·103 |
| HA9 | 62.51 | 9.74·10−10 | 2.64·102 | 8.53·10−7 | 7.36·102 |
| Soaked Samples | Icorr (A·cm−2) | Ecorr (V) |
|---|---|---|
| HA5 | 1.44 | 1.45 |
| HA7 | 1.49 | 1.49 |
| HA9 | 1.50 | 1.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Lin, C.; Batalu, D.; Hu, J.; Lu, W. Tunable Microstructure and Morphology of the Self-Assembly Hydroxyapatite Coatings on ZK60 Magnesium Alloy Substrates Using Hydrothermal Methods. Coatings 2021, 11, 8. https://doi.org/10.3390/coatings11010008
Wang T, Lin C, Batalu D, Hu J, Lu W. Tunable Microstructure and Morphology of the Self-Assembly Hydroxyapatite Coatings on ZK60 Magnesium Alloy Substrates Using Hydrothermal Methods. Coatings. 2021; 11(1):8. https://doi.org/10.3390/coatings11010008
Chicago/Turabian StyleWang, Taolei, Chao Lin, Dan Batalu, Jingzhou Hu, and Wei Lu. 2021. "Tunable Microstructure and Morphology of the Self-Assembly Hydroxyapatite Coatings on ZK60 Magnesium Alloy Substrates Using Hydrothermal Methods" Coatings 11, no. 1: 8. https://doi.org/10.3390/coatings11010008
APA StyleWang, T., Lin, C., Batalu, D., Hu, J., & Lu, W. (2021). Tunable Microstructure and Morphology of the Self-Assembly Hydroxyapatite Coatings on ZK60 Magnesium Alloy Substrates Using Hydrothermal Methods. Coatings, 11(1), 8. https://doi.org/10.3390/coatings11010008






