Low-Cost Deposition of Antibacterial Ion-Substituted Hydroxyapatite Coatings onto 316L Stainless Steel for Biomedical and Dental Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Substituted HA
2.2. Deposition Procedure Used to Prepare Substituted HA Coatings
2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.4. X-ray Diffraction (XRD)
2.5. Scanning Electron Microscopic Analysis
2.6. Preparation of Controlled Discs of Substituted Has for Antibacterial Investigations
2.7. Bacterial Adhesion Testing
3. Results
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.2. X-ray Diffraction (XRD)
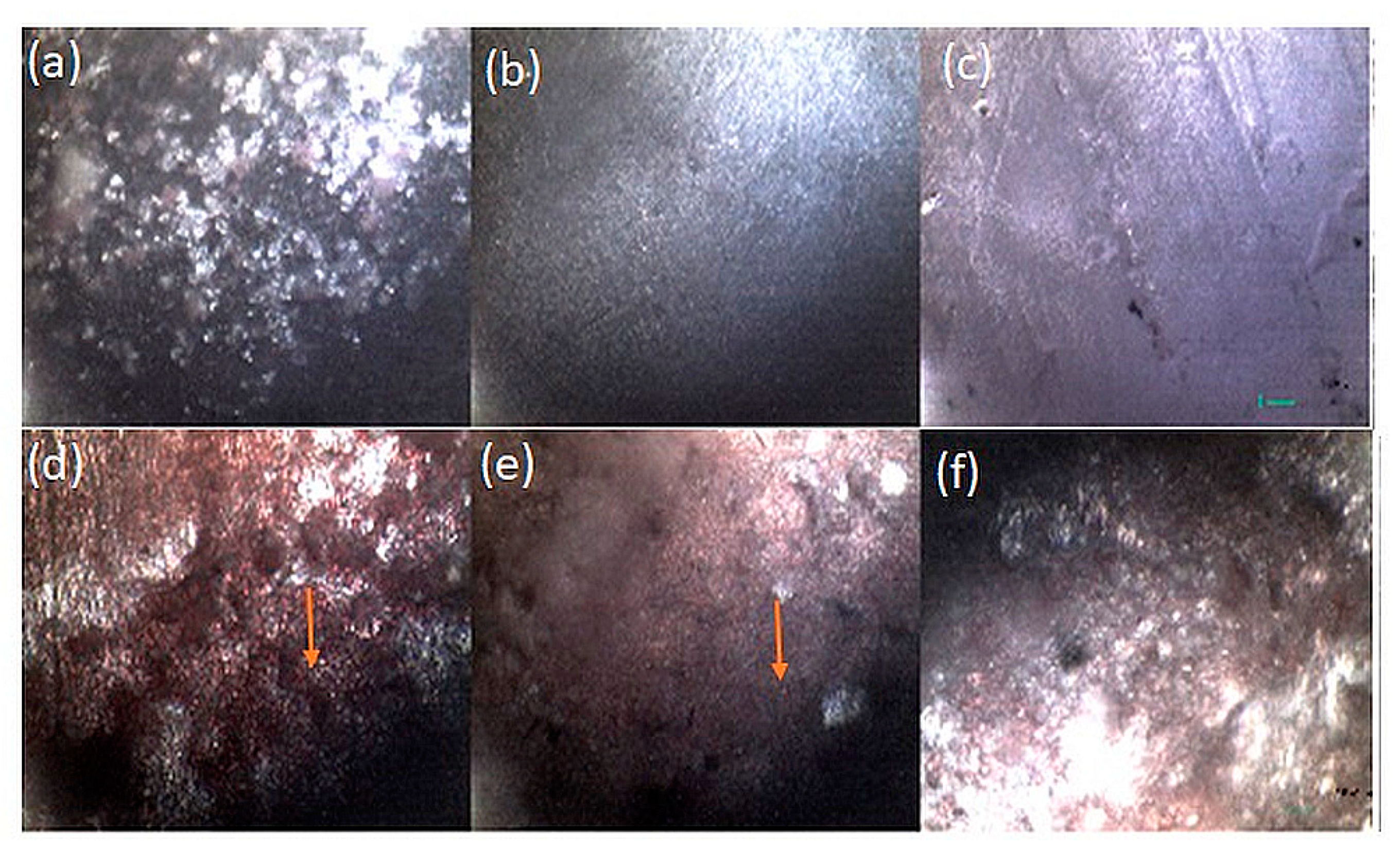
3.3. Scanning Electron Microscopic Analysis
3.4. Antibacterial Adhesion Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Gritsch, K.; Laroche, N.; Bonnet, J.-M.; Exbrayat, P.; Morgon, L.; Rabilloud, M.; Grosgogeat, B. In vivo evaluation of immediately loaded stainless steel and titanium orthodontic screws in a growing bone. PLoS ONE 2013, 8, e76223. [Google Scholar] [CrossRef] [PubMed]
- Salehi, P.; Babanouri, N.; Roein-Peikar, M.; Zare, F. Long-term antimicrobial assessment of orthodontic brackets coated with nitrogen-doped titanium dioxide against Streptococcus mutans. Prog. Orthod. 2018, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Hosman, A.H.; van der Mei, H.C.; Bulstra, S.K.; Kuijer, R.; Busscher, H.J.; Neut, D. Influence of Co-Cr particles and Co-Cr ions on the growth of staphylococcal biofilms. Int. J. Artif. Organs 2011, 34, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Tiller, J.C.; Liao, C.J.; Lewis, K.; Klibanov, A.M. Designing surfaces that kill bacteria on contact. Proc. Natl. Acad. Sci. USA 2001, 98, 5981–5985. [Google Scholar] [CrossRef]
- Cen, L.; Neoh, K.G.; Kang, E.T. Antibacterial activity of cloth functionalized with N-alkylated poly(4-vinylpyridine). J. Biomed. Mater. Res. Part A 2004, 71A, 70–80. [Google Scholar] [CrossRef]
- Shi, Z.; Neoh, K.G.; Kang, E.T.; Wang, W. Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles. Biomaterials 2006, 27, 2440–2449. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Lucke, M.; Wildemann, B.; Haas, N.P.; Raschke, M. Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: A review. Injury 2006, 37, S105–S112. [Google Scholar] [CrossRef]
- Van de Belt, H.; Neut, D.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Infection of orthopedic implants and the use of antibiotic-loaded bone cements—A review. Acta Orthop. Scand. 2001, 72, 557–571. [Google Scholar] [CrossRef]
- Yorganci, K.Y.; Krepel, C.K.; Weigelt, J.W.; Edmiston, C.E. In vitro evaluation of the antibacterial activity of three different central venous catheters against gram-positive bacteria. Eur. J. Clin. Microbiol. Infect. Dis. 2002, 21, 379–384. [Google Scholar] [CrossRef]
- Nohr, R.S.; Gavin Macdonald, J. New biomaterials through surface segregation phenomenon: New quaternary ammonium compounds as antibacterial agents. J. Biomater. Sci. Polym. Ed. 1994, 5, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, M.; Singh, H. Preparation and antibacterial evaluation of urinary balloon catheter. Biomed. Sci. Instrument 1997, 33, 240–245. [Google Scholar]
- Ewald, A.; Glückermann, S.K.; Thull, R.; Gbureck, U. Antimicrobial titanium/silver PVD coatings on titanium. Biomed. Eng. Online 2006, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.; Khan, M.; Khan, A.S. Bioceramics: Types and clinical applications. In Handbook of Ionic Substituted Hydroxyapatites; Elsevier: Amsterdam, The Netherlands, 2020; pp. 53–83. [Google Scholar]
- Capuccini, C.; Torricelli, P.; Sima, F.; Boanini, E.; Ristoscu, C.; Bracci, B.; Socol, G.; Fini, M.; Mihailescu, I.N.; Bigi, A. Strontium-substituted hydroxyapatite coatings synthesized by pulsed-laser deposition: In vitro osteoblast and osteoclast response. Acta Biomater. 2008, 4, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Moore, J.L.; Hosick, H.L.; Bose, S.; Bandyopadhyay, A.; Lu, W.W.; Cheung, K.M.C.; Luk, K.D.K. Osteoprecursor cell response to strontium-containing hydroxyapatite ceramics. J. Biomed. Mater. Res. Part A 2006, 79A, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Massa-Schlueter, E.A.; Smith, J.L.; Slamovich, E.B. Osteoblast response to hydroxyapatite doped with divalent and trivalent cations. Biomaterials 2004, 25, 2111–2121. [Google Scholar] [CrossRef]
- Thian, E.S.; Huang, J.; Vickers, M.E.; Best, S.M.; Barber, Z.H.; Bonfield, W. Silicon-substituted hydroxyapatite (SiHA): A novel calcium phosphate coating for biomedical applications. J. Mater. Sci. 2006, 41, 709–717. [Google Scholar] [CrossRef]
- Kalita, S.J.; Bhatt, H.A. Nanocrystalline hydroxyapatite doped with magnesium and zinc: Synthesis and characterization. Mater. Sci. Eng. C 2007, 27, 837–848. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Yang, H.; Guo, Q.; Zhou, W.; Tao, M. Large-area self assembled monolayers of silica microspheres formed by dip coating. Mater. Sci. 2010, 28, 467–478. [Google Scholar]
- Paital, S.; He, W.; Dahotre, N. Laser pulse dependent micro textured calcium phosphate coatings for improved wettability and cell compatibility. J. Mater. Sci. Mater. Med. 2010, 21, 2187–2200. [Google Scholar] [CrossRef]
- Yang, G.-L.; He, F.-M.; Hu, J.-A.; Wang, X.-X.; Zhao, S.-F. Biomechanical comparison of biomimetically and electrochemically deposited hydroxyapatite-coated porous titanium implants. J. Oral Maxillofac. Surg. 2010, 68, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xu, J.; Wang, F.; Zhao, L.; Shimizu, T. Biomimetic deposition of apatite coatings on micro-arc oxidation treated biomedical NiTi alloy. Surf. Coat. Technol. 2010, 204, 3294–3299. [Google Scholar] [CrossRef]
- Dey, A.; Mukhopadhyay, A.K.; Gangadharan, S.; Sinha, M.K.; Basu, D. Development of hydroxyapatite coating by microplasma spraying. Mater. Manufac. Proc. 2009, 24, 1321–1330. [Google Scholar] [CrossRef]
- Stoch, A.; Brozek, A.; Kmita, G.; Stoch, J.; Jastrzebski, W.; Rakowska, A. Electrophoretic coating of hydroxyapatite on titanium implants. J. Mol. Struct. 2001, 596, 191–200. [Google Scholar] [CrossRef]
- Ellies, L.G.; Nelson, D.G.A.; Featherstone, J.D.B. Crystallographic changes in calcium phosphates during plasma-spraying. Biomaterials 1992, 13, 313–316. [Google Scholar] [CrossRef]
- Sui, J.-L.; Li, M.-S.; Lu, Y.-P.; Bai, Y.-Q. The effect of plasma spraying power on the structure and mechanical properties of hydroxyapatite deposited onto carbon/carbon composites. Surf. Coat. Technol. 2005, 190, 287–292. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Rodriguez, H.H.; Vargas, G.; Cortes, D.A. Electrophoretic deposition of bioactive wollastonite and porcelain-wollastonite coatings on 316L stainless steel. Ceram. Int. 2008, 34, 1303–1307. [Google Scholar] [CrossRef]
- Sharma, S.; Soni, V.; Bellare, J. Chitosan reinforced apatite–wollastonite coating by electrophoretic deposition on titanium implants. J. Mater. Sci. Mater. Med. 2009, 20, 1427–1436. [Google Scholar] [CrossRef]
- Corni, I.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition: From traditional ceramics to nanotechnology. J. Eur. Ceram. Soc. 2008, 28, 1353–1367. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, F.; Huang, L.; Lin, C. Electrophoretic deposition and characterization of nano-sized hydroxyapatite particles. J. Mater. Sci. 2005, 40, 4955–4957. [Google Scholar] [CrossRef]
- Iqbal, N.; Nazir, R.; Asif, A.; Chaudhry, A.A.; Akram, M.; Fan, G.Y.; Akram, A.; Amin, R.; Park, S.H.; Hussain, R. Electrophoretic deposition of PVA coated hydroxyapatite on 316L stainless steel. Cur. Appl. Phys. 2012, 12, 755–759. [Google Scholar] [CrossRef]
- Wei, M.; Ruys, A.; Swain, M.; Kim, S.; Milthorpe, B.; Sorrell, C. Interfacial bond strength of electrophoretically deposited hydroxyapatite coatings on metals. J. Mater. Sci. Mater. Med. 1999, 10, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, M.S.; Cheng, M.H.W.; Tang, S.M.; Yu, S.C.; Liao, K.; Tan, C.T.; Khor, K.A.; Cheang, P. Tensile properties, tension-tension fatigue and biological response of polyetheretherketone-hydroxyapatite composites for load-bearing orthopedic implants. Biomaterials 2003, 24, 2245–2250. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regí, M. Substituted hydroxyapatite coatings of bone implants. J. Mater. Chem. B 2020, 8, 1781–1800. [Google Scholar] [CrossRef]
- Gibson, I.R.; Best, S.M.; Bonfield, W. Chemical characterization of silicon-substituted hydroxyapatite. J. Biomed. Mater. Res. 1999, 44, 422–428. [Google Scholar] [CrossRef]
- Thian, E.; Konishi, T.; Kawanobe, Y.; Lim, P.; Choong, C.; Ho, B.; Aizawa, M. Zinc-substituted hydroxyapatite: A biomaterial with enhanced bioactivity and antibacterial properties. J. Mater. Sci Mater. Med. 2013, 24, 437–445. [Google Scholar] [CrossRef]
- Omema, U.; Khalid, H.; Chaudhry, A.A. Magnesium-substituted hydroxyapatite. In Handbook of Ionic Substituted Hydroxyapatites; Elsevier: Amsterdam, The Netherlands, 2020; pp. 197–216. [Google Scholar]
- Nazir, R.; Iqbal, N.; Khan, A.S.; Akram, A.; Asif, A.; Chaudhry, A.A.; Rehman, I.U.; Hussain, R. Rapid synthesis of thermally stable hydroxyapaptite. Ceram. Int. 2012, 38, 457–462. [Google Scholar] [CrossRef]
- Mehboob, H.; Awais, M.; Khalid, H.; Ch, A.A.; Siddiqi, S.A.; Rehman, I. Polymer-assisted deposition of hydroxyapatite coatings using electrophoretic technique. Biomed. Eng. Appl. Basis Commun. 2014, 26, 1450073. [Google Scholar] [CrossRef]
- ASTM D6132–13 Standard Test Method for Nondestructive Measurement of Dry Film Thickness of Applied Organic Coatings Using an Ultrasonic Coating Thickness Gage; ASTM International: West Conshohocken, PA, USA, 2017.
- Khan, N.I.; Ijaz, K.; Zahid, M.; Khan, A.S.; Abdul Kadir, M.R.; Hussain, R.; Rehman, A.; Darr, J.A.; Rehman, I.U.; Chaudhry, A.A. Microwave assisted synthesis and characterization of magnesium substituted calcium phosphate bioceramics. Mater. Sci. Eng. C 2015, 56, 286–293. [Google Scholar] [CrossRef]
- Aminian, A.; Solati-Hashjin, M.; Samadikuchaksaraei, A.; Bakhshi, F.; Gorjipour, F.; Farzadi, A.; Moztarzadeh, F.; Schmücker, M. Synthesis of silicon-substituted hydroxyapatite by a hydrothermal method with two different phosphorous sources. Ceram. Int. 2011, 37, 1219–1229. [Google Scholar] [CrossRef]
- Selvaraju, S.; Ramalingam, S.; Rao, J.R. Inorganic apatite nanomaterial: Modified surface phenomena and its role in developing collagen based polymeric bio-composite (Coll-PLGA/HAp) for biological applications. Coll. Surfaces B Biointerfaces 2018, 172, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Khalajabadi, S.Z.; Abu, A.B.H.; Ahmad, N.; Kadir, M.R.A.; Ismail, A.F.; Nasiri, R.; Haider, W.; Redzuan, N.B.H. Biodegradable Mg/HA/TiO2 nanocomposites coated with MgO and Si/MgO for orthopedic applications: A study on the corrosion, surface characterization, and biocompatability. Coatings 2017, 7, 154. [Google Scholar] [CrossRef]
- Qasim, I.; Mumtaz, M.; Nadeem, K.; Abbas, S.Q. Zinc Nanoparticles at Intercrystallite Sites of (Cu0.5Tl0.5) Ba2Ca3Cu4O12−δ Superconductor. J. Nanomater. 2016, 2016, 9781790. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Blanco, I.; Piccolella, S.; Pacifico, S. Use of the sol–gel method for the preparation of coatings of titanium substrates with hydroxyapatite for biomedical application. Coatings 2020, 10, 203. [Google Scholar] [CrossRef]
- Khalid, H.; Suhaib, F.; Zahid, S.; Ahmed, S.; Jamal, A.; Kaleem, M.; Khan, A.S. Microwave-assisted synthesis and in vitro osteogenic analysis of novel bioactive glass fibers for biomedical and dental applications. Biomed. Mater. 2018, 14, 015005. [Google Scholar] [CrossRef]
- Hughes, J.M.; Rakovan, J. The crystal structure of apatite, Ca5(PO4)3(F,OH,Cl). Rev. Mineral. Geochem. 2002, 48, 1–12. [Google Scholar] [CrossRef]
- Bertoni, E.; Bigi, A.; Cojazzi, G.; Gandolfi, M.; Panzavolta, S.; Roveri, N. Nanocrystals of magnesium and fluoride substituted hydroxyapatite. J. Inorg. Biochem. 1998, 72, 29–35. [Google Scholar] [CrossRef]
- Laurencin, D.; Almora-Barrios, N.; de Leeuw, N.H.; Gervais, C.; Bonhomme, C.; Mauri, F.; Chrzanowski, W.; Knowles, J.C.; Newport, R.J.; Wong, A. Magnesium incorporation into hydroxyapatite. Biomaterials 2011, 32, 1826–1837. [Google Scholar] [CrossRef]
- Ma, X.; Ellis, D.E. Initial stages of hydration and Zn substitution/occupation on hydroxyapatite (0001) surfaces. Biomaterials 2008, 29, 257–265. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Arcos, D. Silicon substituted hydroxyapatites. A method to upgrade calcium phosphate based implants. J. Mater. Chem. 2005, 15, 1509–1516. [Google Scholar] [CrossRef]
- Gasqueres, G.; Bonhomme, C.; Maquet, J.; Babonneau, F.; Hayakawa, S.; Kanaya, T.; Osaka, A. Revisiting silicate substituted hydroxyapatite by solid-state NMR. Magn. Reason. Chem. 2008, 46, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Ofudje, E.A.; Adeogun, A.I.; Idowu, M.A.; Kareem, S.O. Synthesis and characterization of Zn-Doped hydroxyapatite: Scaffold application, antibacterial and bioactivity studies. Heliyon 2019, 5, e01716. [Google Scholar] [CrossRef]
- Ijaz, K.; Khalid, H.; Chaudhry, A.A. Zinc-substituted hydroxyapatite. In Handbook of Ionic Substituted Hydroxyapatites; Elsevier: Amsterdam, The Netherlands, 2020; pp. 217–236. [Google Scholar]
- Moreno-Perez, B.; Matamoros-Veloza, Z.; Rendon-Angeles, J.C.; Yanagisawa, K.; Onda, A.; Pérez-Terrazas, J.E.; Mejia-Martínez, E.E.; Burciaga Díaz, O.; Rodríguez-Reyes, M. Synthesis of silicon-substituted hydroxyapatite using hydrothermal process. Boletín de la Sociedad Española de Cerámica y Vidrio 2020, 59, 50–64. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Dal Poggetto, G.; Crescente, G.; Piccolella, S.; Pacifico, S. New SiO2/caffeic acid hybrid materials: Synthesis, spectroscopic characterization, and bioactivity. Materials 2020, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- El Yacoubi, A.; Massit, A.; Fathi, M.; El Idrissi, B.C.; Yamni, K. Characterization of silicon-substituted hydroxyapatite powders synthesized by a wet precipitation method. IOSR J. Appl. Chem. 2014, 7, 24–29. [Google Scholar] [CrossRef]
- An, Y.H.; Friedman, R.J. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J. Biomed. Mater. Res. 1998, 43, 338–348. [Google Scholar] [CrossRef]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Adv. Coll. Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef]
- Zmantar, T.; Bettaieb, F.; Chaieb, K.; Ezzili, B.; Mora-Ponsonnet, L.; Othmane, A.; Jaffrezic, N.; Bakhrouf, A. Atomic force microscopy and hydrodynamic characterization of the adhesion of staphylococcus aureus to hydrophilic and hydrophobic substrata at different pH values. World J. Microbiol. Biotechnol. 2011, 27, 887–896. [Google Scholar] [CrossRef]
- Van Loosdrecht, M.; Lyklema, J.; Norde, W.; Schraa, G.; Zehnder, A. The role of bacterial cell wall hydrophobicity in adhesion. Appl. Environ. Microbiol. 1987, 53, 1893–1897. [Google Scholar] [CrossRef]

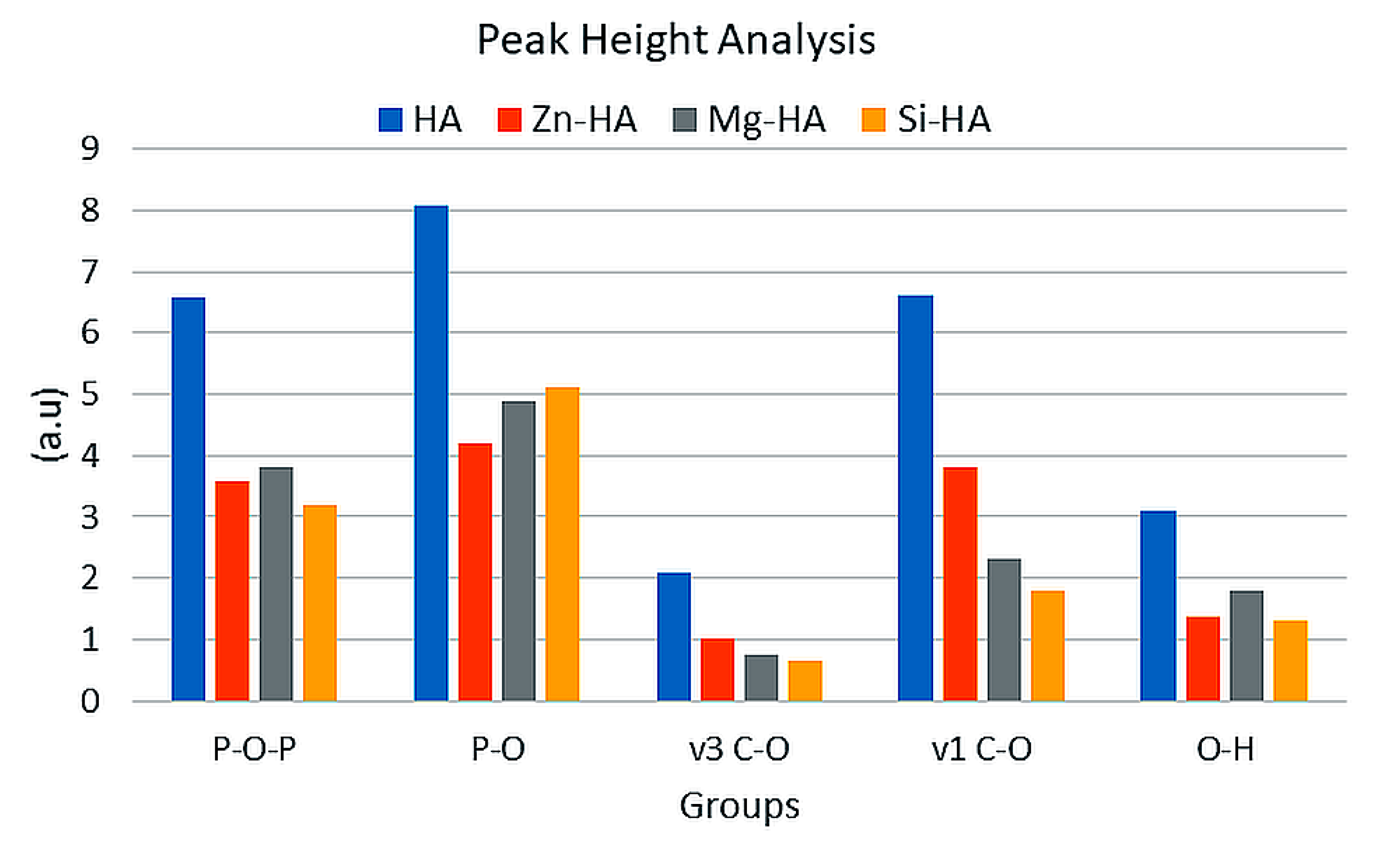
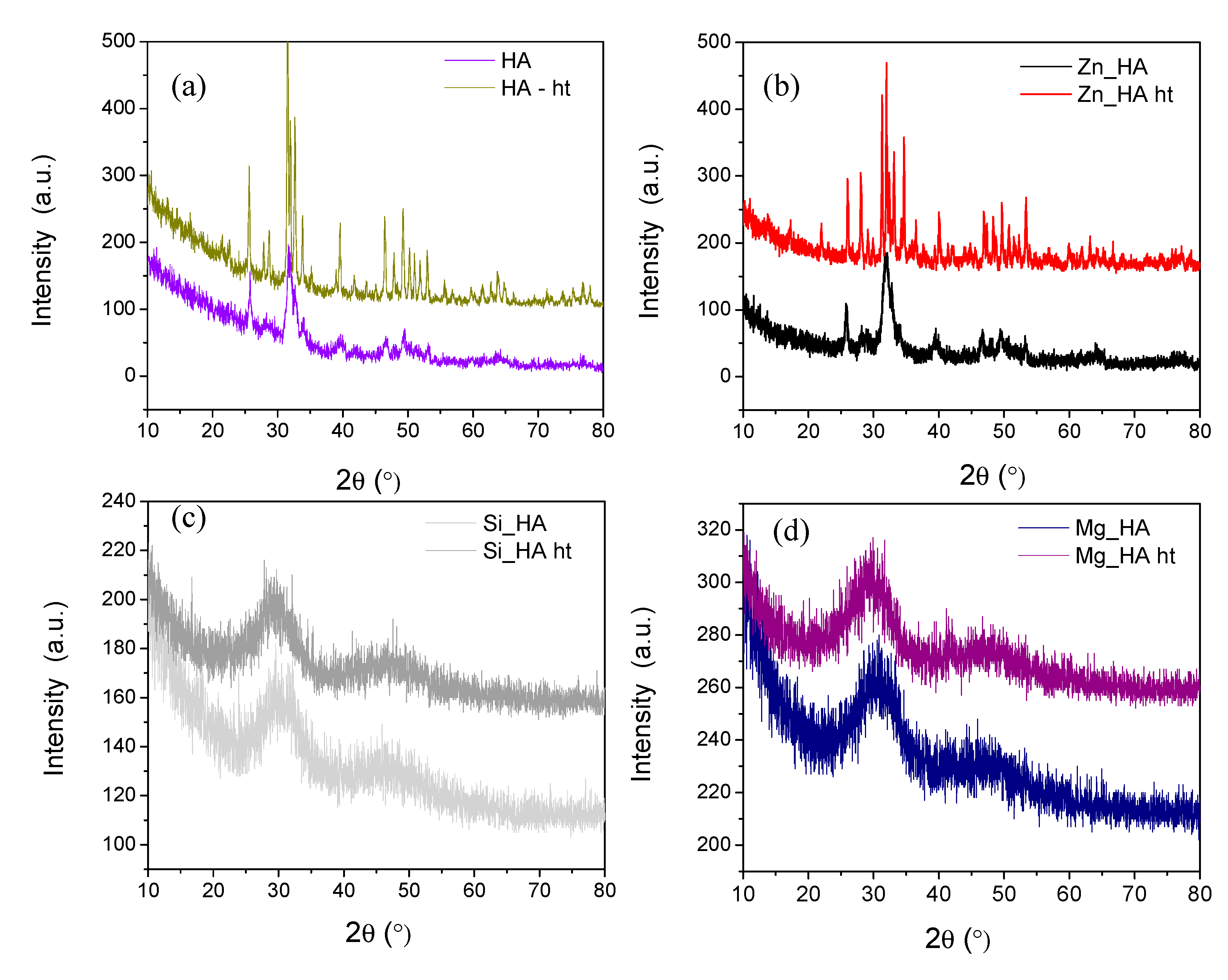
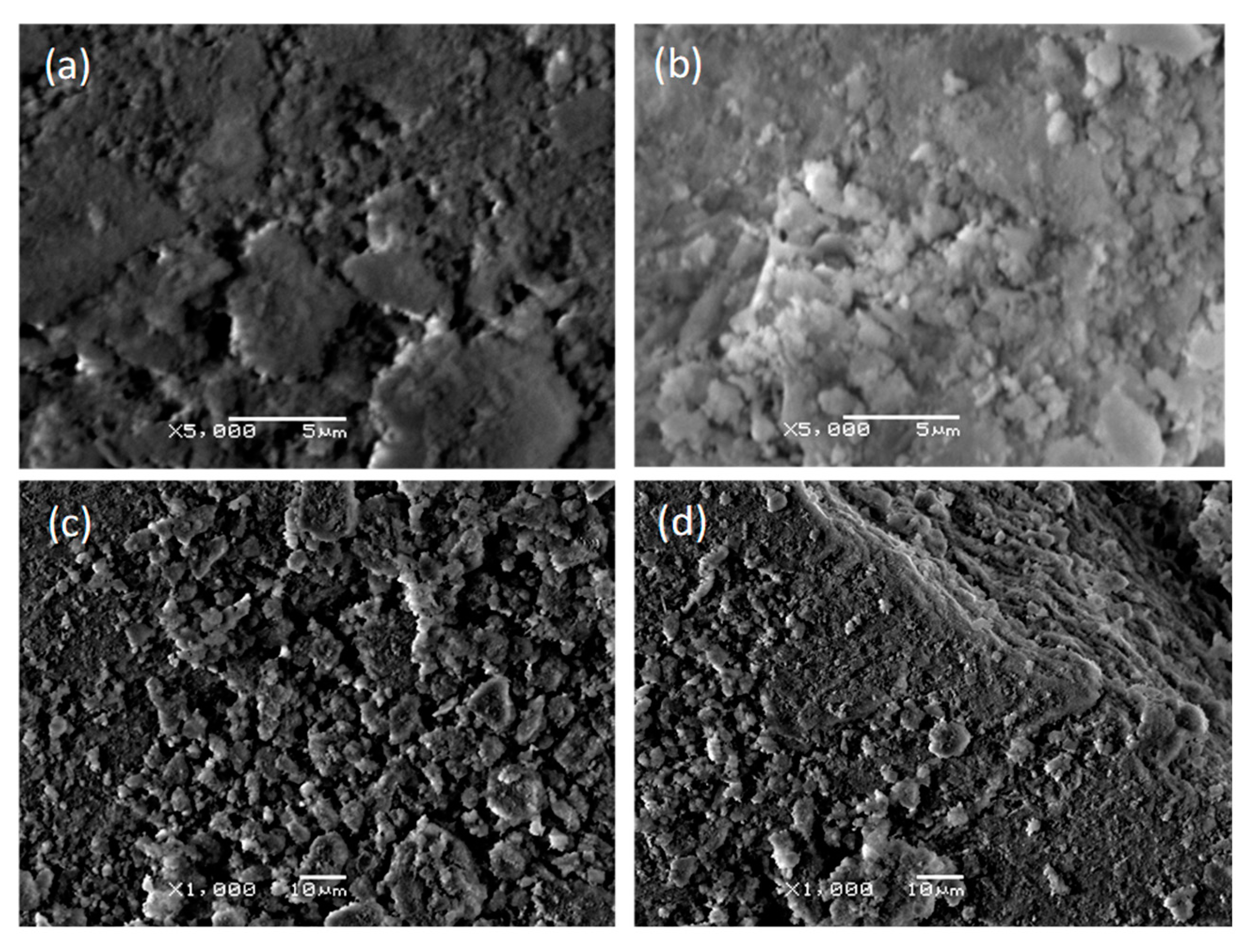
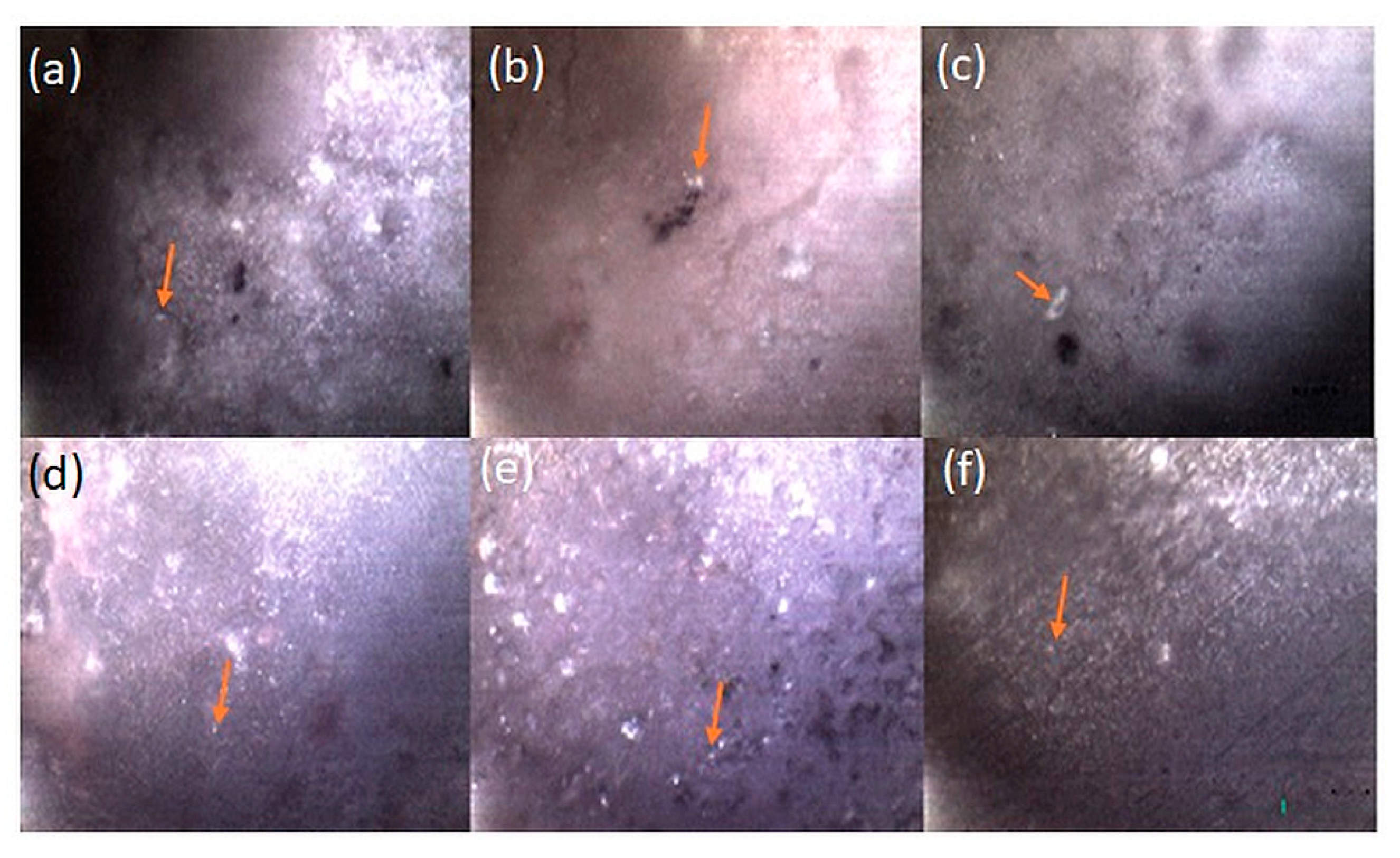

| Groups | Ca(NO3)2.4H2O Conc. (M) | (NH4)2HPO4 Conc. (M) | Conc. Of Substituted Salts (M) |
|---|---|---|---|
| HA | 0.105 | 0.062 | – |
| Zn–HA | 0.10 | 0.059 | Zn(NO3)2·6H2O = 0.008 |
| Mg–HA | 0.0823 | 0.063 | Mg(NO3)2·6H2O = 0.021 |
| Si–HA | 0.102 | 0.047 | TEOS = 0.018 |
| Peak Assignment | HA | Si–HA | Mg–HA | Zn–HA |
|---|---|---|---|---|
| Degenerated bending (v2) phosphate | 470 | – | – | – |
| Triply degenerated bending mode, v4, of the O–P–O bond | 564–604 | 500–650 (band shape) * | 500–650 (band shape) * | 564, 600 |
| Bending peak of C–O | 827 | 827 ** | 827 ** | 835 |
| Asymmetric stretching peak (v1) of PO43− | 963 | – | – | 941 *** |
| triply degenerated vibration,v3 peak of P–O | 1083–1035 | 1180–920 * | 1130–920 * | 1050–1110 |
| Stretching mode, v1, of the CO3 | 1343–1490 | 1500–1410 | 1440 | – |
| Bending peak of O–H | 1635 and 633 | 1640 | 1640 | 1642, 625 * |
| Si–O–Si | – | 1220–830, 430–470 | – | – |
| Si in Si–HA | – | 876 | – | – |
| Time | Control Discs | Coated Plates | ||||||
|---|---|---|---|---|---|---|---|---|
| HA | Zn–HA | Mg–HA | Si–HA | HA | Zn–HA | Mg–HA | Si–HA | |
| 30 min | 0.086 (0.008) | 0.000 | 0.015 (0.009) | 0.090 (0.006) | 0.09 (0.003) | 0.019 (0.008) | 0.006 (0.000) | 0.059 (0.002) |
| 2 h | 0.095 (0.002) | 0.026 (0.008) | 0.017 (0.009) | 0.11 (0.006) | 0.14 (0.003) | 0.063 (0.005) | 0.020 (0.001) | 0.162 (0.005) |
| 6 h | 0.18 (0.002) | 0.024 (0.004) | 0.042 (0.007) | 0.094 (0.005) | 0.15 (0.006) | 0.081 (0.009) | 0.086 (0.002) | 0.025 (0.007) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.S.; Awais, M. Low-Cost Deposition of Antibacterial Ion-Substituted Hydroxyapatite Coatings onto 316L Stainless Steel for Biomedical and Dental Applications. Coatings 2020, 10, 880. https://doi.org/10.3390/coatings10090880
Khan AS, Awais M. Low-Cost Deposition of Antibacterial Ion-Substituted Hydroxyapatite Coatings onto 316L Stainless Steel for Biomedical and Dental Applications. Coatings. 2020; 10(9):880. https://doi.org/10.3390/coatings10090880
Chicago/Turabian StyleKhan, Abdul Samad, and Muhammad Awais. 2020. "Low-Cost Deposition of Antibacterial Ion-Substituted Hydroxyapatite Coatings onto 316L Stainless Steel for Biomedical and Dental Applications" Coatings 10, no. 9: 880. https://doi.org/10.3390/coatings10090880
APA StyleKhan, A. S., & Awais, M. (2020). Low-Cost Deposition of Antibacterial Ion-Substituted Hydroxyapatite Coatings onto 316L Stainless Steel for Biomedical and Dental Applications. Coatings, 10(9), 880. https://doi.org/10.3390/coatings10090880







