1. Introduction
Nowadays, composite is the most commonly used material for dental repair that simultaneously meets mechanical, strength and esthetic requirements. However, in order for it to connect to the tissues of the tooth permanently and tightly, a bonding system must be used before its application. Dentin adhesives are divided into three groups, according to the type of adhesion to dentine. The first group is composed of adhesives that modify the smear layer, which is a kind of natural base; its dental tubules are closed. In the second group, the smear layer is partly dissolved and modified; the compounds of the adhesive system do not cause the tissue’s demineralization. A newly created, impregnated layer closes the dental tubules. The third group is composed of systems that require complete smear layer removal and etching with the use of acids or with chelation. In this, the dental tubules are opened. We can observe demineralized intertubular dentine with the matrix of collagen opened and dental tubules enlarged [
1]. Resin tags with a hybrid layer provide a strong connection to tooth tissues and protection against bacteria and other irritants [
2]. This is why these adhesive agents are used as an alternative to calcium hydroxide for biologic treatment of pulp [
3,
4,
5]. As compounds with both hydrophobic and hydrophilic groups it is possible for adhesive systems to connect to organic and non-organic elements of dentine—as well as react with composite material. Further developments have led to the creation of the seventh generation of bonding systems, and the next step is to use compobonds, composite materials that already include an integral bonding system. Bonding systems contained within the composite material were introduced in 2009. Their distinctive feature is that they do not require etching or the use of any bonding system [
6]. The elimination of etching with the use of orthophosphoric acid—and the absence of complicated bonding procedures—are expected to bring tangible benefits, namely, the shortening and simplification of the whole procedure, as well as the absence of postoperative hypersensitivity. This could mark the beginning of an eighth generation of bonding systems. ‘Compobonds’ is one of the proposed names for materials that combine the properties of self-etching bonding systems and nanofiller resins [
7,
8]. Their ability to connect to tooth tissues chemically and micromechanically is attributed to acidic monomers that are included in the flow composite [
9,
10,
11]. Products from this group include Vertise Flow (Kerr) and Fusio Liquid Dentin (Pentron). From the available articles, we could not find a simple answer to the question of the durability and quality of adhesion of dental materials belonging to this group [
12,
13]. In his research, Hamdy did not assess self-adhering flowable resin composite positively with regard to microleakage [
13]. On the other hand, the results of Oz et al. showed positive results from 5-year clinical observations of 47 I Class fillings. Oz et al. compared Vertise Flow with the conventional flowable resin composite used with an etch-and-rinse system. They assessed overall retention, marginal adaptation, marginal staining, surface luster and color match. They observed similar degradation of fillings when marginal adaptation and marginal discoloration were taken into consideration [
14]. These promising results have led the authors to carry out research with the use of more combinations of bonding systems and the Ryge scale, as well [
14]. The aim of the present research was to perform a comparative clinical study of a self-adhesive, light-curing composite material called Vertise Flow and a traditional flow material called Premise flowable used in combination with dedicated bonding systems. In order to standardize the clinical environment, the stability of the oral hygiene (analyzed with oral hygiene index and approximal plaque index) was taken into consideration, because insufficient oral cavity hygiene promotes degradation of all Black’s class fillings [
15]. In clinical trials, it is difficult to maintain constant conditions in long-term observations, so oral hygiene becomes important.
2. Materials and Methods
Clinical examination included adults of both genders who had no health contraindications that could have prevented their participation in conservative treatment. Before research started, all patients were informed about its aim and the risks it could carry. They were acquainted with the information for the patient and gave written approval for taking part in the research (in accordance with the proposal required by the Bioethical Commission of Silesian Medical University (KNW/0022/KB1/19a/12 of 06.03.2012).
2.1. Inclusion Criteria
Cavities were required to be classified as Black’s Class I caries affecting pits and fissures on the occlusal surfaces of molars and on lingual surface maxillary incisors in adult patients of Medical Center Reden in Dąbrowa Górnicza, Poland (not more than two in one patient). Included patients had no contraindications that would prevent the conservative treatment from taking place and were able to give their own consent for the examination.
2.2. Exclusion Criteria
Cavities diagnosed on the lingual surface of proximal surfaces of molars and premolars (Class II), the proximal surfaces of anterior teeth without/with involvement of the incisal angle (Class III, Class IV) or at the gingival third of the facial and lingual surfaces of anterior and posterior teeth (Class V) were excluded.
The study involved 37 patients with 64 fillings. The average age of patients was 33.09 (SD = 12.21). Eight women and 14 men were examined in Group I. The average age was 34.54 years (SD = 10.70) (women: 40.75 ± 11.98; men: 31.00 ± 7.96). In Group II, 10 women and 12 men were examined. The average age was 34.13 years (SD = 13.62) (women: 37.30 ± 11.99; men: 31.50 ± 14.33). In Group III, 6 women and 14 men were examined. The average age was 30.35 years (SD = 11.64) (women: 32.33 ± 8.47; men: 29.50 ± 12.67). Patient qualification and the application of 64 fillings on the occlusal surfaces of 60 molar teeth and in the blind holes of four incisors were performed. This amounted to 22 fillings in Group I, 22 fillings in Group II and 20 fillings in Group III. There was at least one filling in cavities on lingual surface maxillary incisors in each group.
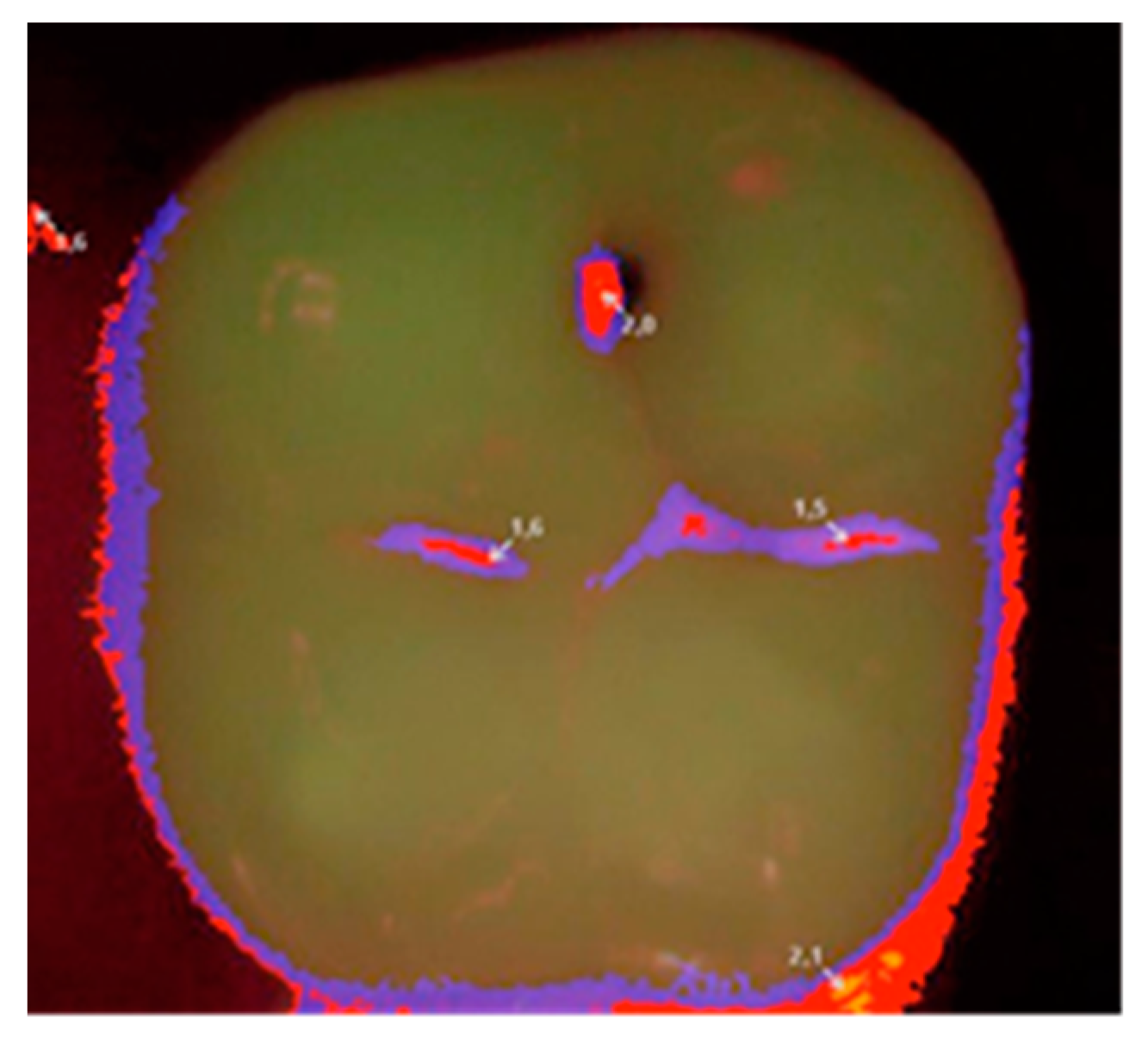
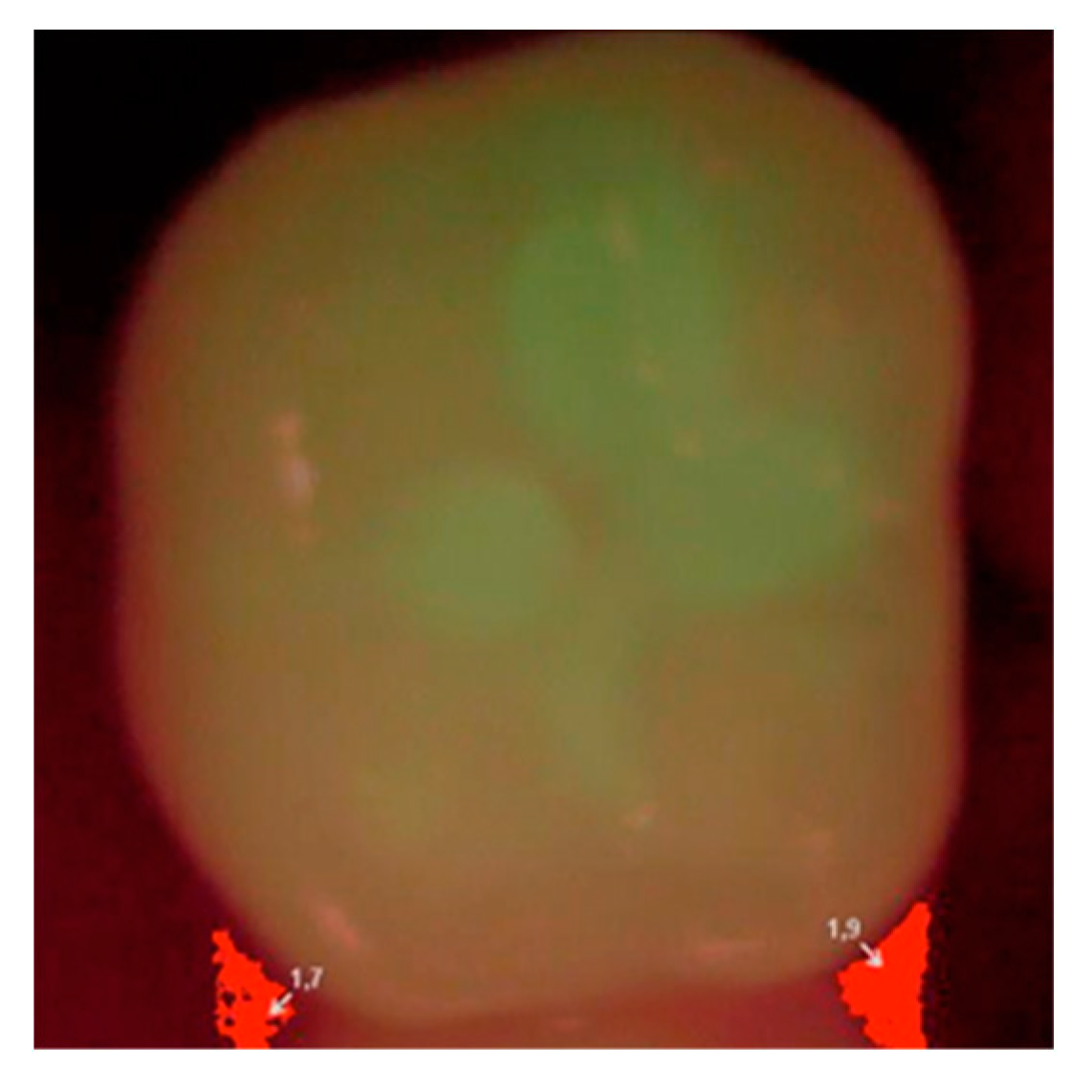
Each patient was qualified on the basis of a clinical statement that confirmed the presence of a small carious lesion, further validated by diagnostic results of Diagnodent (KaVo, Biberach, Germany) and a Vista Proof (Dürr Dental, Bietigheim-Bissingen, Germany) camera. An initial Vista Proof camera image (
Figure 1) showing the extent and intensity of caries was recorded, and the corresponding numeric values (maximum) were saved.
Qualification of patients, cavity preparation and filling as well as clinical assessment were performed by one operator. The operator was trained before he started the procedures, and he was supervised by two independent, trained researchers with regard to strict adherence to the clinical management scheme. The method of randomization and qualification of the patients to allocate them to a given clinical group, which determined the choice of the applied material, was based on the drawing of cards by the patients from a limited poll.
Previously prepared cavities of Black’s Class I were filled with materials applied in accordance with the manufacturer’s recommendations in three configurations:
Group I (G I): Vertise Flow material without the use of an etching agent or a bonding system;
Group II (G II): Premise flowable material without the use of an etching agent, but with the use of the OptiBond All-In-One seventh-generation bonding system;
Group III (G III): Premise flowable material after etching and treatment of the hard tooth tissues using a fifth-generation OptiBond Solo Plus bonding system.
The composition and application mode of materials used in the study are presented in
Table 1 [
16,
17].
Group I: 22 Black’s Class I cavities were prepared traditionally, rinsed with water and dried with a maximum airstream for 5 s. Subsequently, the Vertise Flow material (without an etching agent or a bonding system) in the shade chosen by means of the colorant was applied to the cavities with the use of an application tip and distributed with a moderate pressure using a supplied brush for 15–20 s. Any excess of the composite was removed.
Group II: 22 Black’s Class I cavities were prepared traditionally, rinsed with a stream of water and dried (not overdried: the state of drying was controlled by leaving a shiny surface). The OptiBond All-In-One bonding system was shaken vigorously before application, and then 2–3 drops were transferred to a clean container. A large amount of the bonding system (without prior use of an earlier application) was applied to the enamel/dentine surface with the use of a disposable applicator. The preparation was rubbed into the surface by performing brushing movements for 20 s. OptiBond All-In-One was applied again and rubbed in for 20 s. It was dried first with a gentle stream of air and then with a medium force jet for at least 5 s. Finally, it was hardened with lamp light for 10 s. Premise flowable in the shade chosen by means of the colorant was applied to the cavity prepared in this way along the axial and gingival margins. It was distributed with a brushing motion in such a way that it filled the margins of the cavities. Any excess of the composite was removed.
Group III: 20 Black’s Class I cavities were prepared traditionally, rinsed with a stream of water and dried (not overdried). Enamel was etched with 37% orthophosphoric acid. Then, the cavity was thoroughly rinsed so that the etcher was removed completely. The OptiBond Solo Plus bonding system was vigorously shaken before application, and then 2–3 drops were transferred to a clean container. After drying (not overdrying), the bonding system was then applied to the enamel/dentine surface with a disposable applicator. Then, it was dried with a gentle stream of air for 3 s and hardened with lamp light for 20 s. Premise flowable material in the shade chosen by means of the colorant was applied to the cavity prepared in this way along the axial and gingival margins. It was distributed with a brushing motion in such a way that it filled the margins of the cavity. Any excess of the composite was removed.
In all groups, the single layers of materials did not exceed 2 mm, and each layer was hardened with lamp light for 20 s. The light intensity of the lamp was maintained at 800 MW/cm2 and was regularly checked with a radiometer. If A3.5 shade or universal opaque was used, the polymerization time was extended to 40 s. During the material’s polymerization, protective glasses were used, and the purity of the optical fiber was controlled. The end of the light-guide was placed at a distance of about 2–5 mm from the exposed material, and after starting the light exposure, it was close to it for 1–2 s. The fillings in all groups (I, II, and III) were finished and polished with KerrHawe OptiDisc, Occlubrusch or the HiLuster system (Kerr) and rinsed with a stream of water.
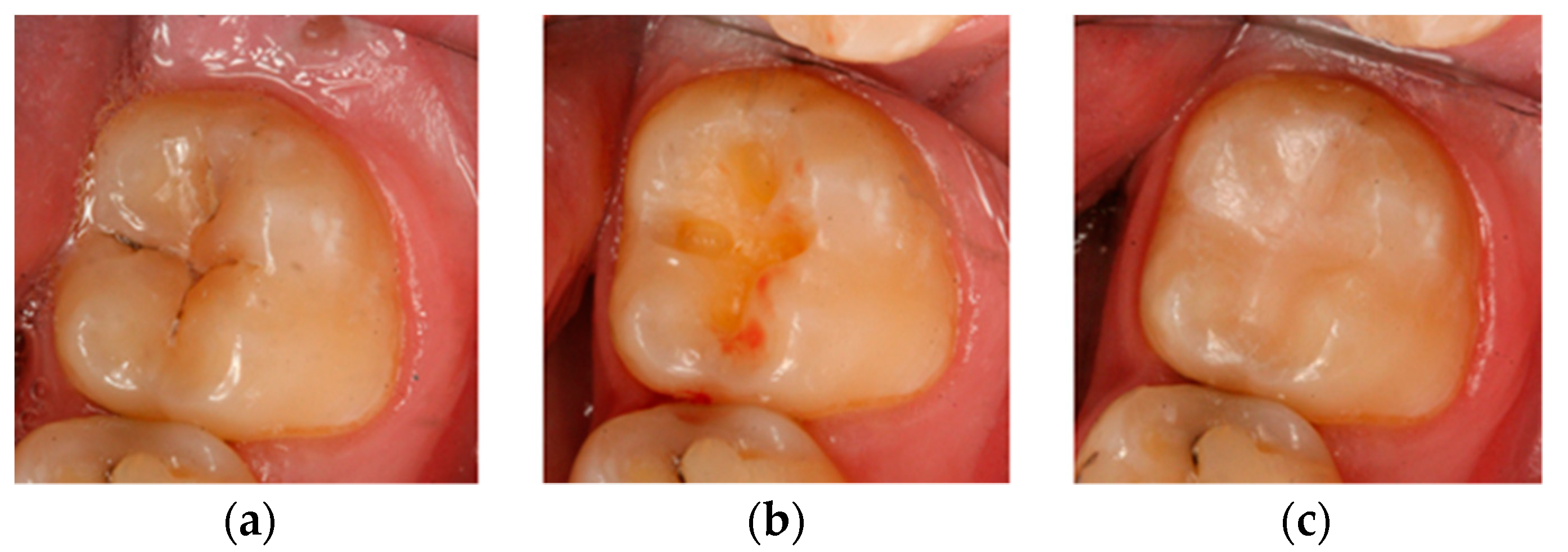
Then, at appropriate time intervals (0, i.e., right after filling, and after 6, 12, and 24 months), the patients were subjected to clinical examination, and the fillings were evaluated according to the Ryge scale criteria [
14] with the use of additional visual registration by means of a Vista Proof camera (
Figure 2). During each follow-up visit, an intraoral photograph was taken for the record with a Canon EOS 30D digital camera with a Canon EF 100 mm f/2.8 L Macro IS USM lens and a Canon Macro Ring Lite MR-14EX. The photographs were taken before and after the cavities were prepared, as well as after filling each cavity with a given material (
Figure 3). Control tests were complemented by a plaque/calculus evaluation using Greene and Vermilion’s oral hygiene index (OHI) index and Lange’s approximal plaque index (API) [
18].
All obtained results on a four-level Ryge scale (values from 0 to 3) were grouped in a table. The Ryge scale is presented in
Table 2.
2.3. OHI-S (Oral Hygiene Index-Simplified) by Green and Vermillion
The debris index and the calculus index are two components of this index. Each of these indices is based on numeric determinations representing the amount of debris or calculus found on the preselected six tooth surfaces.
The examination was performed with the use of a dental mirror. Six teeth were examined, including 16 and 26 on the buccal surfaces, 26 and 46 on the lingual surfaces and 11 and 31 on the labial surfaces. The result of the examination was assigned a value between 0 and 3:
- 0-
No debris or calculus;
- 1-
Soft debris or supragingival calculus, covering not more than one-third of the exposed surface;
- 2-
Soft debris or supragingival calculus, covering not more than two-thirds of the exposed tooth surface or presence of flecks of subgingival calculus around the cervical portion of the tooth or both;
- 3-
Soft debris or supragingival calculus covering more than two-thirds of the exposed tooth surface or a continuous heavy band of subgingival calculus around the cervical portion of the tooth or both.
The results obtained for particular teeth (surfaces) were added and then divided by the number of examined teeth. The possible results vary between 0 and 3 for the debris and calculus indices and between 0 and 6 for the OHI-S values.
2.4. API (Approximal Plaque Index) by Lange
A yes/no decision was made with regard to whether the examined interproximal surfaces are covered by plaque (+) or not (−). The index is calculated as per the following formula:
According to the results, the oral hygiene conditions of individuals can be determined as follows:
100–70%: poor oral hygiene;
69–40%: insufficient oral hygiene;
39–25%: pretty good oral hygiene;
<25%: optimum hygiene.
2.5. Statistical Analysis
In order to check the differences in API and OHI across the groups over time, an analysis of variance for mixed samples was performed. To check the exact differences between measurements in groups, a series of Bonferroni post hoc comparisons was performed. In order to check the differences in the status of the fillings on the Ryge scale after 6, 12, and 24 months, an analysis with the Friedman test was performed. To check the differences in fillings with regard to the groups, an analysis with the Kruskal–Wallis test was conducted. To check the exact differences between the results in groups, the Mann–Whitney U-test was performed.
4. Discussion
The development of successive generations of bonding systems with improved adhesion and affinity to tooth tissues, which we have been observing over the years, mainly boils down to the simplification of the bonding procedure itself. The number of components of the bonding system is reduced, the use of etching is a thing of the past owing to the presence of acid monomers in the primer, and finally, the binding system is included in the composite material itself. It appears that such progress could eliminate errors made during the application of bonding systems and, as a consequence, protect the dentine–pulp complex against excessive mechanical and chemical interference. The exact and meticulous procedure of using a bonding agent in compliance with the recommended time to perform each stage of the procedure, while simultaneously paying close attention so as not to dry the dentin, may actually seem demanding and prone to mistakes [
19].
However, does easier bonding also mean an effective connection to tooth tissues? If we take into account the strength of such a connection, it is not necessarily true. On the other hand, we noted that the fourth-generation systems still dominate in terms of the durability of connection to tooth tissues [
20]. Nonetheless, when it comes to posttreatment hypersensitivity, leaving the hybrid layer to react only with the binding system may reduce the negative feelings associated with tooth filling [
21]. Giacomini sheds a different light on the above issue: depending on the type of dentin, she prefers other bonding systems. Her research shows that dentin with small tubules, which is located in the peripheral regions (tooth necks, incisal margins), acquires a greater strength in its connection to new universal bonding systems than to previously used ones [
22]. How that connection behaves over time is not without significance. The hybrid layer, which is largely responsible for the quality of the connection, should be of good quality at the time of its creation and, more important, should maintain these properties over time [
23]. Matrix metalloproteinases (MMPs) play an important role in this process: endogenous enzymes present in dentin cause degradation of the collagen matrix. This may be triggered by adhesive procedures that use simplified total-etch (TE) binding systems [
24] or strongly acidic self-etch (SE) systems [
25]. MMP activity and the lack or presence of MMP inhibitors have an impact not only on the appearance of secondary caries at the material–tooth tissue boundary, but also on the presence and activity of primary caries.
The Vertise Flow material—which was the inspiration behind the research in this study—is an innovative and one of the first composite materials that contain a functional GPDM monomer (glycerol phosphate dimethacrylate), which the procedure of applying classical etching and bonding to be omitted. For this reason, there are still only a few reports evaluating this material in the literature [
11,
12,
13]. It is even more difficult to find a study that compares the Vertise Flow material with other traditional materials and includes laboratory and clinical or clinical tests lasting for at least 24 months. Vichi et al. clinically studied Vertise Flow in vivo, applied in small Class I cavities in accordance with the manufacturer’s instructions. They then evaluated those defects using the Ryge scale after one day, seven days, one month, three months and six months. After three months, three out of 40 fillings already had the indication ‘1′ in the marginal adhesion zone (presence of discoloration, fissures), one of which was qualified for replacement within a year [
11]. This was considered to be satisfactory clinical behavior. The obtained result could be influenced by the fact that the researchers carefully checked the occlusion before and after the defect and eliminated the cases in which the extent of the filling was in the areas of occlusion and articulation points (functional areas). Nevertheless, it is similar to the fact that the Ryge scale indications already seem worse in the area of marginal adhesion in the 6th month. In clinical studies, Chałas et al. assessed Vertis Flow with the four-level Ryge scale immediately after performing 67 fillings in small Black’s Class I defects outside the occlusion zone and small Black’s Class V cervical lesions. They obtained very good results for all parameters of the analyzed categories [
26]. Similarly, after three months of observation of the Vertise Flow material in 30 Black class I defects assessed with the Ryge scale, Marzec et al. assessed 100% of the fillings to be clinically acceptable with 100% retention [
27]. The clinical study of Kucukyilmaza is also worth mentioning, as it compared, after 24 months, groove sealants, Vertise Flow and a traditional flow material, applied with the use of a bonding system. The retention of the Vertise Flow material after that time was the lowest and amounted to 62.9%. For comparison, the retention of groove sealants ranged between 80.6% and 73.1%, and a traditional flow with the bonding system was as high as 95.7% [
28]. In the face of a small number of clinical trials, it is worth paying attention to the works of Celik, who clinically tested another self-adhesive flow type compomer with the commercial name Fusio Liquid Dentine. Forty cervical cavities were filled with that material, while another 40 were filled with the G-aenial nanohybrid material using a fourth-generation binding system (OptiBond FL), which he treated as a control group. The condition of 27 out of 40 Fusio Liquid Dentine fillings after six months was assessed as clinically unacceptable, whereas in the control group, 40 out of 40 fillings were still satisfactory. The author defined the success rate as 33% and considered the clinical evaluation of the tested material to be unacceptable [
29].
This study involved an assessment of fillings in small Black’s Class I cavities applied in accordance with the manufacturer’s recommendations in three configurations: the Vertise Flow material without an etching agent or a bonding system (G I), the Premise flowable material without the use of an etching agent but with the use of the OptiBond All-In-One binding system (G II) and the Premise flowable material after etching and treatment of hard tooth tissues with a fifth-generation OptiBond Solo Plus bonding system (G III). Then, at appropriate time intervals (0, i.e., right after filling and after 6, 12, and 24 months), these fillings were subjected to clinical evaluation performed according to the Ryge scale and with the use of a fluorescent high-intensity visible light beam produced by a camera (Vista Proof). Similar to Chałas’s study, very good results were obtained in all analyzed categories on the four-level Ryge scale immediately after the filling with the Vertise Flow material. After six months of observation, as many as 12 of the 22 fillings had the indication ‘1′ in the marginal adhesion zone (presence of discolorations, fissures). For comparison, Vichi obtained 3 out of 40 in the same area [
11]. In other material groups, the results were much better. Premise flowable with a seventh-generation bonding system scored 4 out of 22 and that with a fifth-generation bonding system scored 2 out of 20. After 24 months, as many as 20 fillings with Vertise Flow had indications in the marginal adhesion zone. For comparison, after 24 months, Premise flowable with a seventh-generation bonding system had a score of 10 and that with a fifth-generation bonding system scored only 5. Vertise Flow material used without an etching agent or a bonding system (G I) presented the weakest results with respect to marginal adaptation and smoothness among those evaluated in this study. The intensification of the degradation continued over time until the final clinical observation at 24 months. Kucukyilmaza made similar observations, as he reported that after 24 months, Vertise Flow had the worst retention among the materials examined [
26]. Therefore, the above assessments are in line with Celik’s study, which, after six months, showed that 27 out of 40 fillings applied with another material that was also self-adhesive, i.e., Fusio Liquid Dentine, were clinically unacceptable [
29].
In the case of small cavities, although the application of flow type materials is an acceptable solution [
30] due to their mechanical properties, they should not be used solo for molar reconstruction, especially in the areas of large forces (occlusal contacts) [
31]. In these areas, flow type materials should be used as liners, for extended sealing of fissures, for repairs of minor damage to the margins of fillings and for restorations in fissures and anatomic openings. Helvatjoglu-Antoniades et al. believe that their use as liners reduces the incidence of microleakage and the risk of debonding [
32]. Chałas et al. reported that flow type materials should be placed in class I and II cavities, but with a narrow outline, not exposed to large occlusion loads [
26]. In our own clinical and laboratory studies, it was found that the Vertise Flow material is the weakest when it comes to the strength of the connection to the tooth tissues as compared with other flow materials. However, the author of the study believes that poor results are not only caused by material properties, but also a consequence of abandoning selective enamel etching and, instead, bidding everything on the acid monomers included in the composition of the material itself. It is encouraging that companies are trying to introduce new technologies and facilitate the work of the dentist, but progress in general means that, sometimes, it takes two steps backward before taking one step forward. From the results of this study, the omission of the enamel etching procedure at the current stage of development of dental materials may be considered to be these ‘two steps backward’.
Oral hygiene indicators are widely used and included among the basic criteria for describing the current state of hygiene of the patient [
18]. However, it is interesting to observe how their values are shaped in the case of those patients who are constantly subjected to dental control. The authors of the study observed that both OHI and API improved their average values at the beginning of treatment (OHI = 0.58, API = 51.71) and after 24 months (OHI = 0.40 and API = 38.71). In her doctoral dissertation, Czaplińska showed that in orthodontic patients who were motivated every day and instructed to improve their toothbrushing, the API index was lower (more favorable) at the end of the treatment than at the beginning [
33]. The same was confirmed in the studies by Śmiech-Słomkowska, by Jabłońska-Zrobek and also by Bardal et al. [
34,
35]. It is difficult to maintain constant conditions in clinical trial observations of composite materials. Therefore, the analysis of the level of oral hygiene with the OHI and/or API index can be an extremely important element in standardizing the conditions of observation in compared groups of patients during long-term observation.