The Mechanisms of Degradation of Titanium Dental Implants
Abstract
1. Introduction
2. Materials and Methods
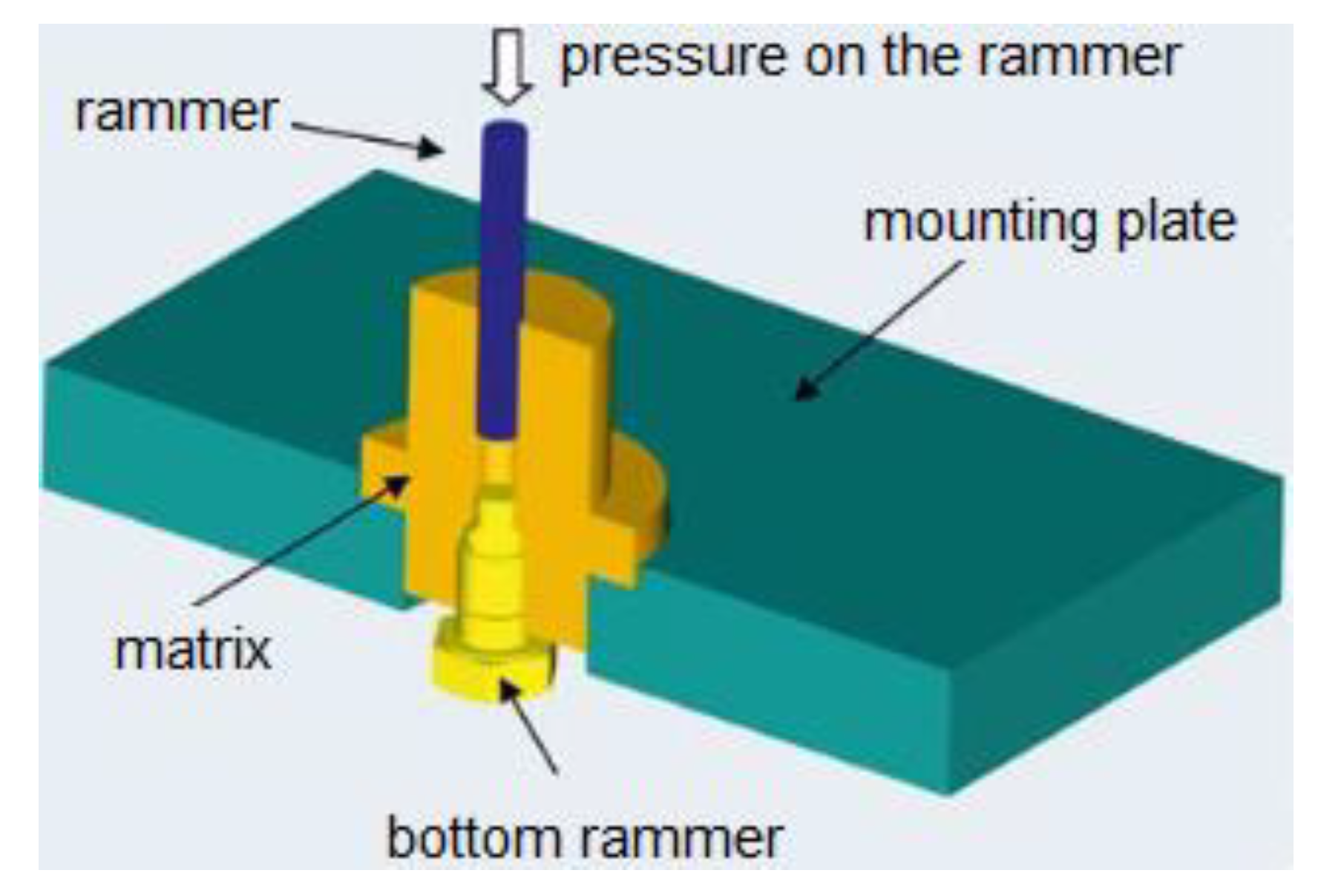
2.1. Microstructural Characterization of Surfaces of Implants
- new dental implants in number of four, made of the Ti6Al4V alloy by four different companies (called as A, B, C, and D);
- used dental implants in number of fourteen, removed at the Warsaw Medical Academy, from the patients, no more than half a year after implantation, made of the Ti6Al4V alloy by four different companies (called as above).
2.2. Investigation of Dissolution Rate of Oxide Coatings
2.3. Calculations of Diffusion of Titanium Ions through Oxide Coatings
3. Results
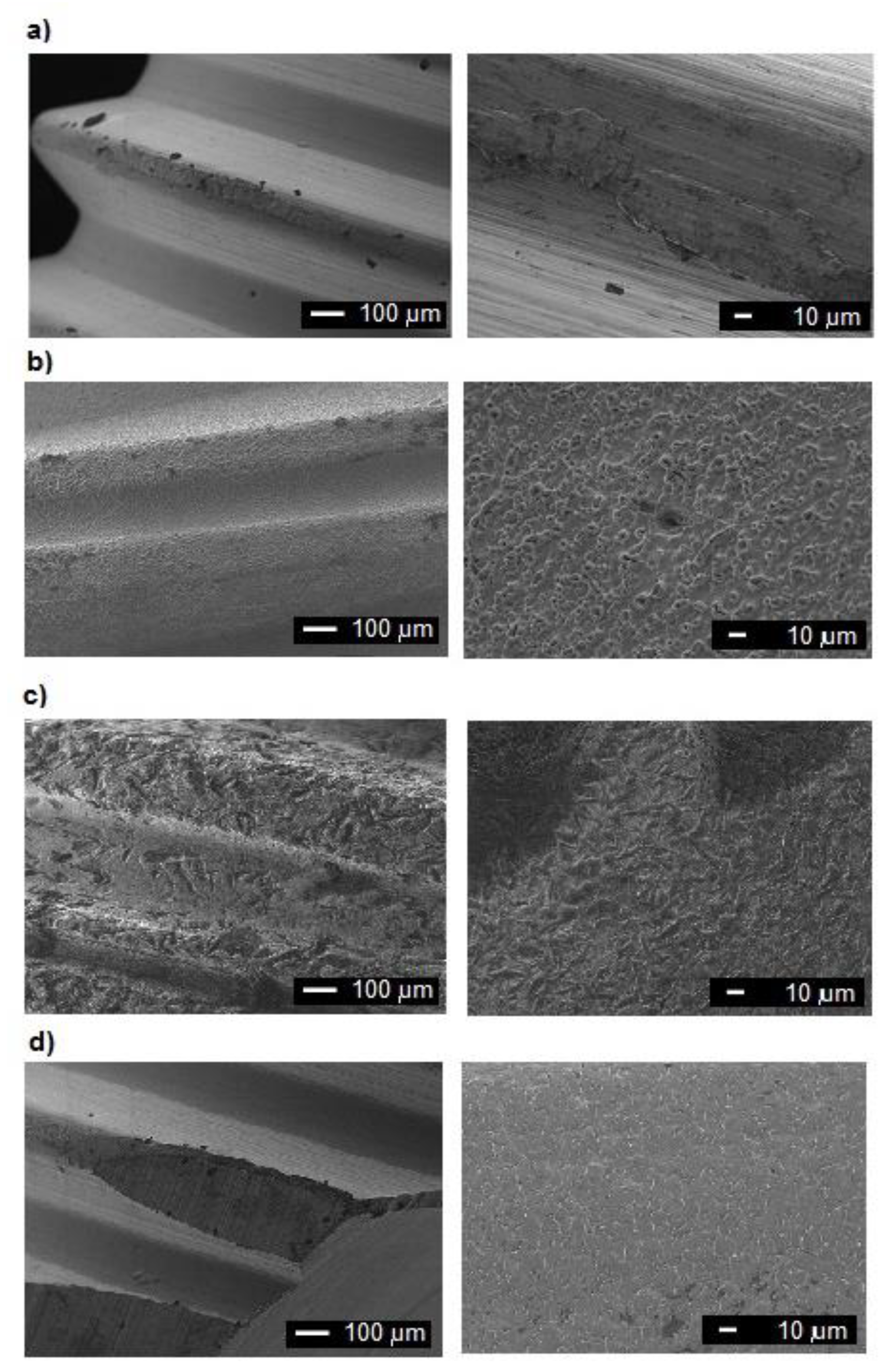
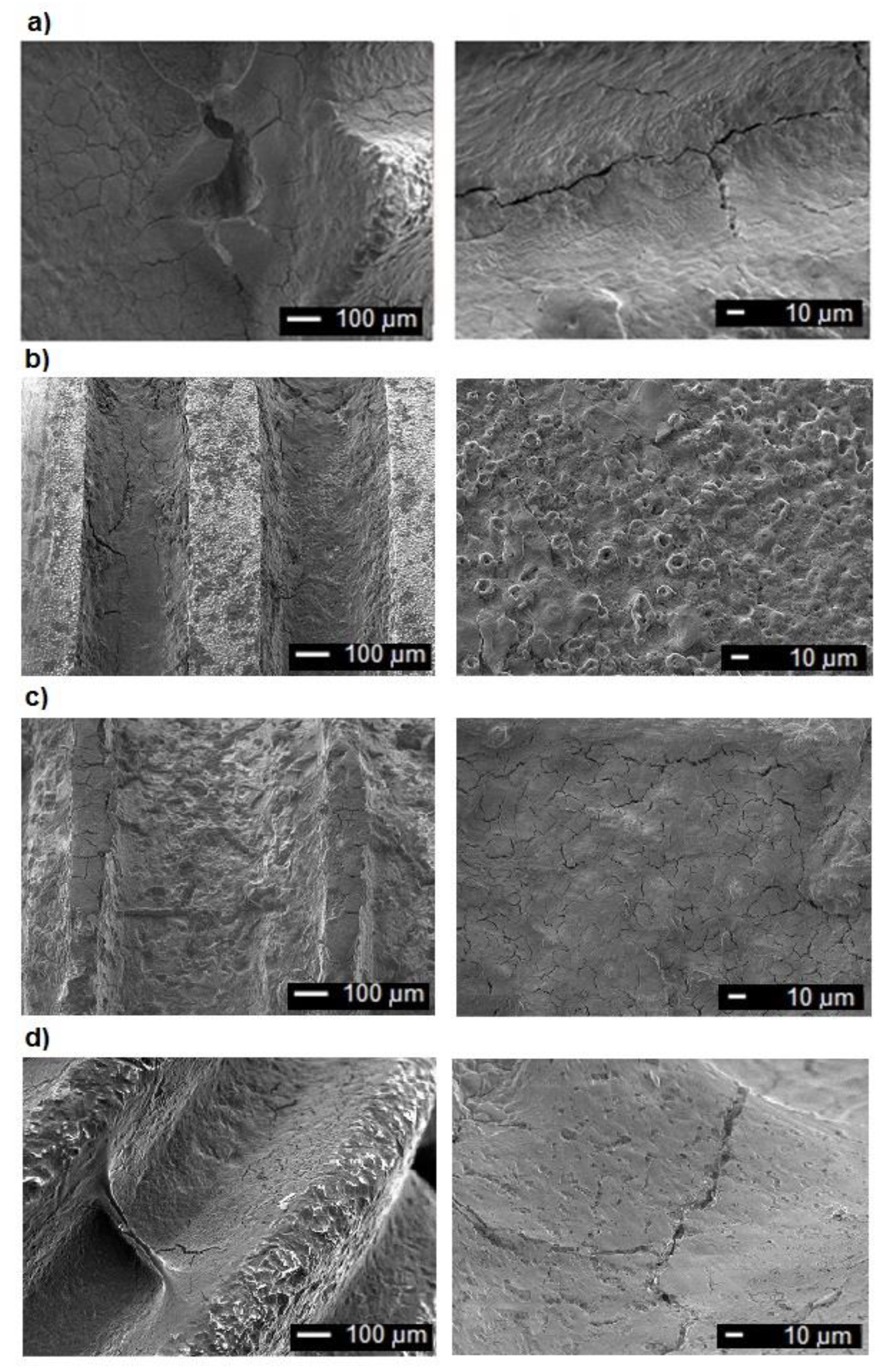
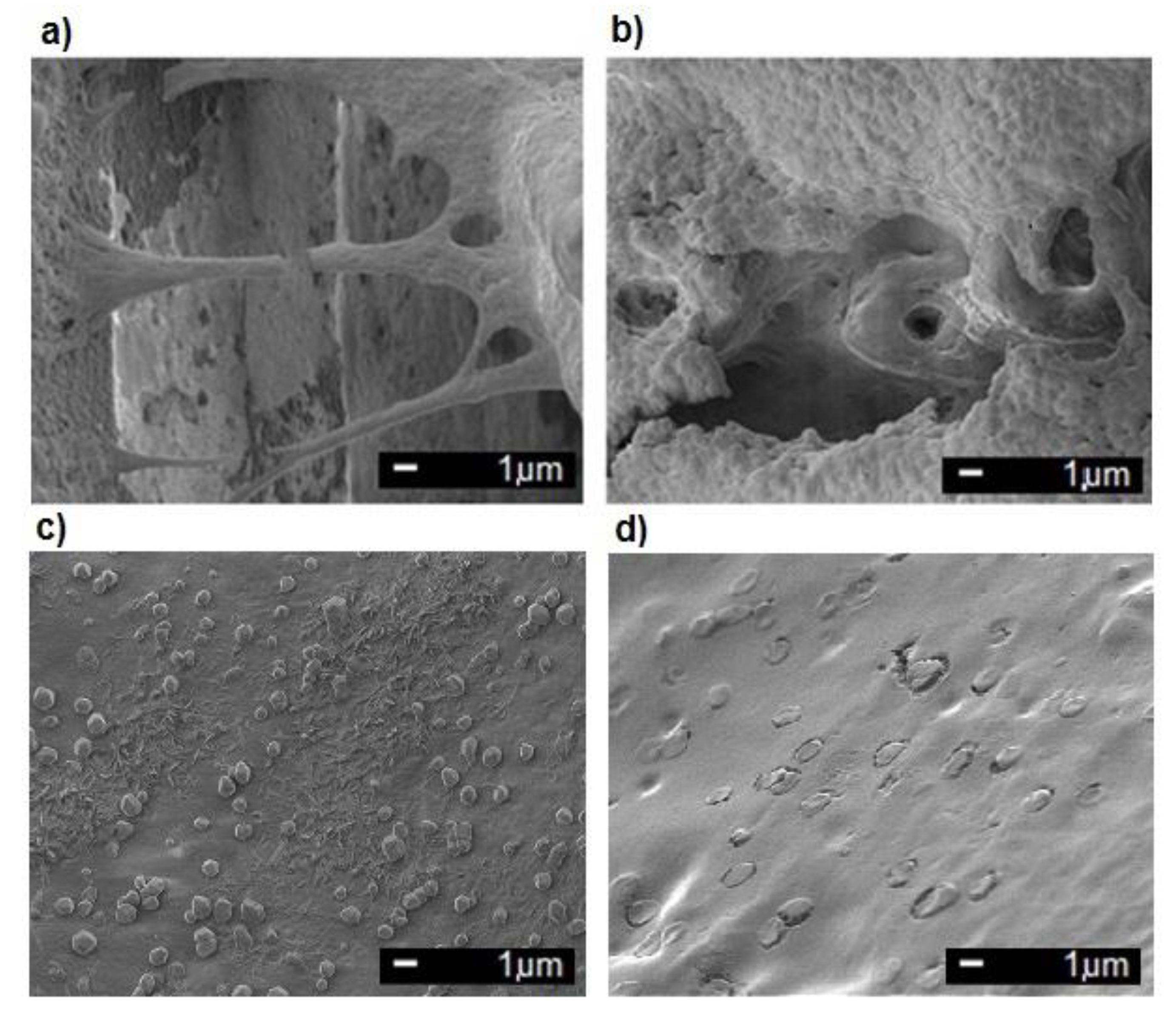
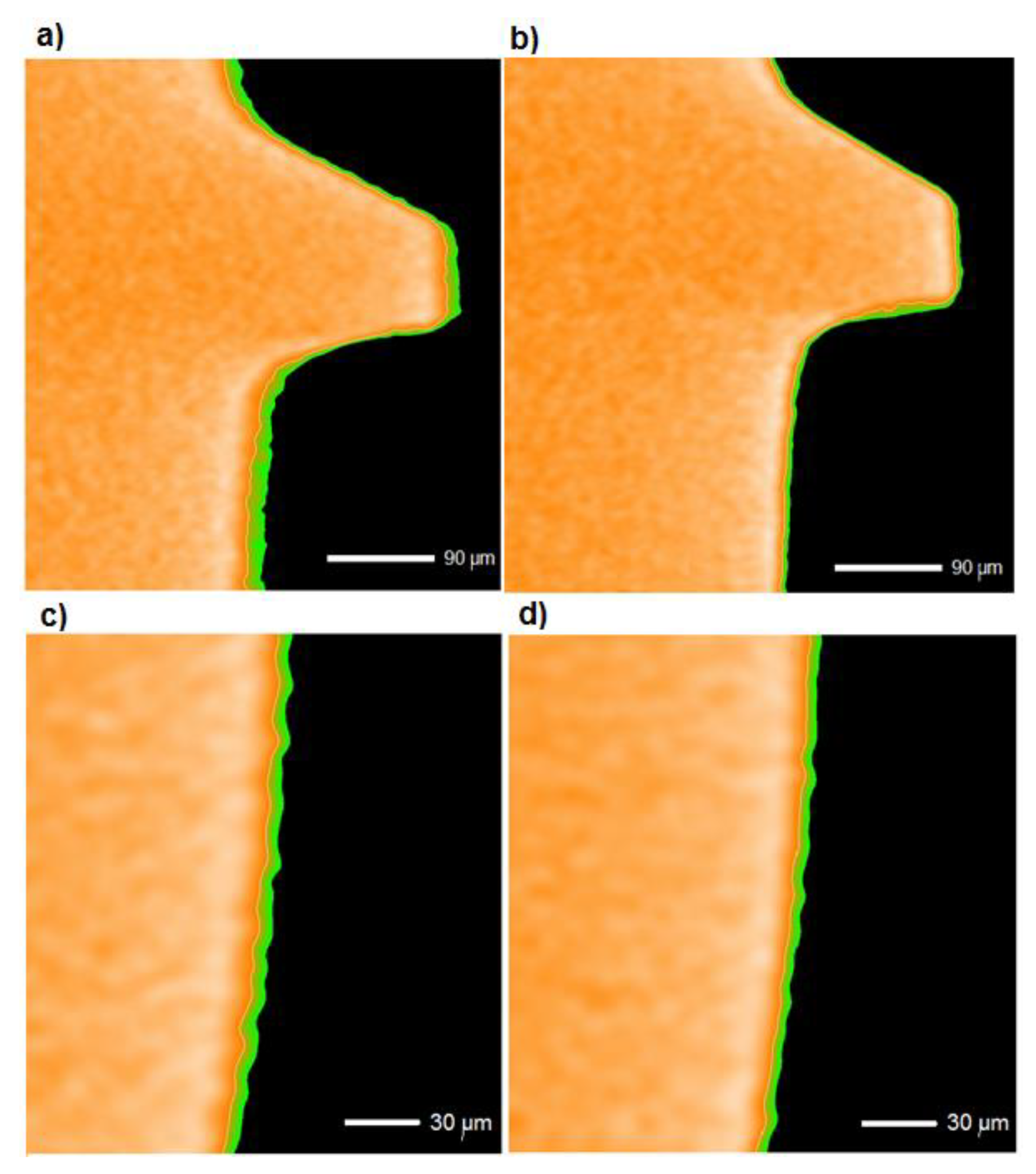
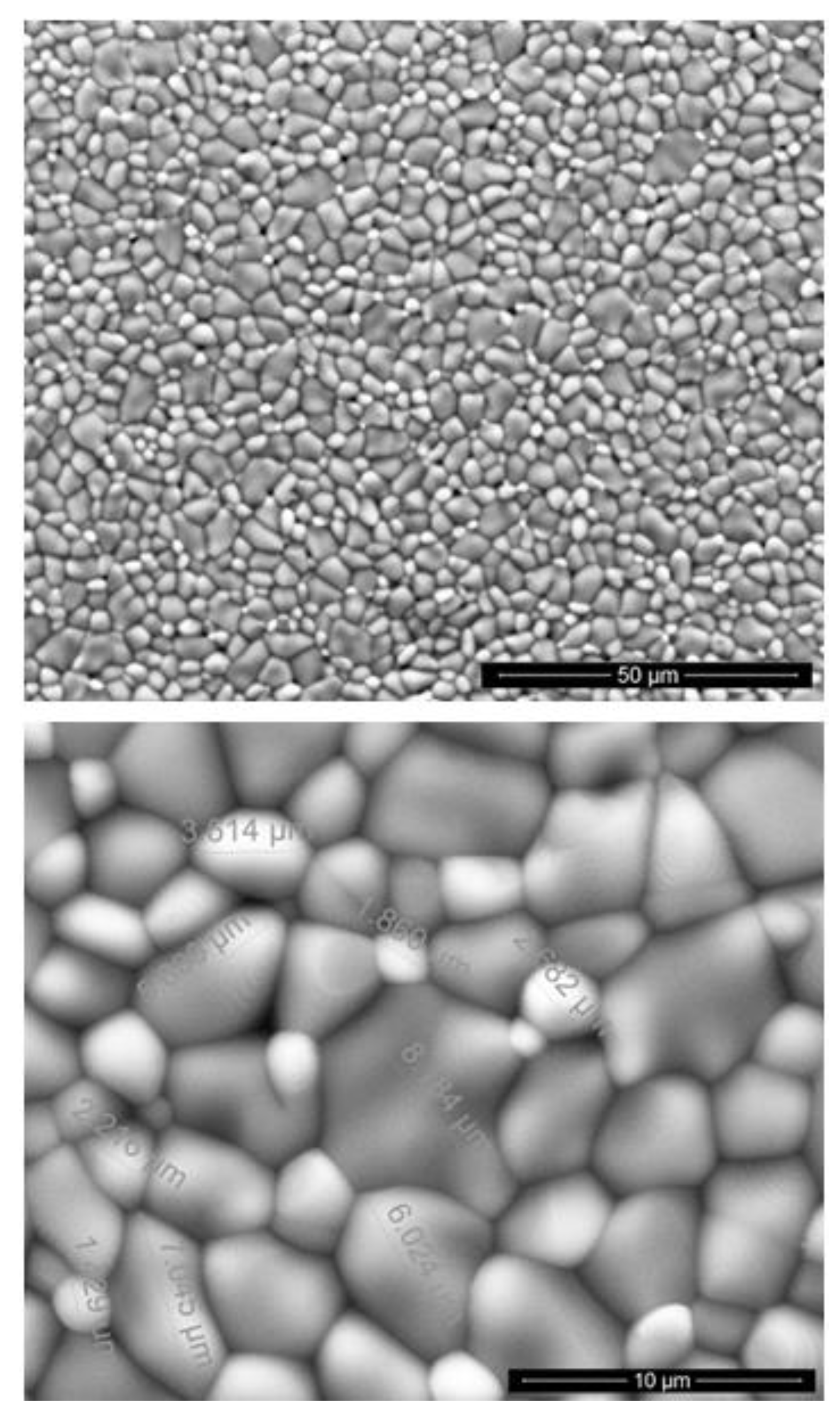
3.1. Examinations of Implants
3.2. The Dissolution of Rutile
3.3. Diffusion of Titanium Ions in The Oxide Layer
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, H.H. Effects of fluoride concentration and elastic tensile strain on the corrosion resistance of commercially pure titanium. Biomaterials 2002, 23, 59–63. [Google Scholar] [CrossRef]
- Toumelin-Chemla, F.; Rouellet, F.; Burdairon, G. Corrosive properties of fluoride containing odontologic gels against titanium. J. Dent. 1996, 24, 109–115. [Google Scholar] [CrossRef]
- Vargas, E.; Baier, R.; Meyer, A. Reduce corrosion of cp Ti and Ti–6Al–4V alloy endosseous dental implant after glow discharge treatment: A preliminary report. Int. J. Oral Maxillofac. Implants 1992, 7, 338–344. [Google Scholar] [PubMed]
- Agarwal, A.; Tyagi, A.; Ahuja, A.; Kumar, N.; De, N.; Bhutani, H. Corrosion aspect of dental implants—An overview and literature review. Open J. Stomatol. 2014, 4, 56–60. [Google Scholar] [CrossRef][Green Version]
- Lavos-Valereto, I.C.; Wolynec, S.; Ramires, I.; Guastaldi, A.C.; Costa, I. Electrochemical impedance spectroscopy characterization of passive film formed on implant Ti–6Al–7Nb alloy in Hank’s solution. J. Mater. Sci. Mater. Med. 2004, 15, 55–59. [Google Scholar] [CrossRef]
- González, J.E.G.; Mirza-Rosca, J.C. Study of the corrosion behavior of titanium alloys for biomedical and dental implants applications. J. Electroanal. Chem. 1999, 471, 109–115. [Google Scholar] [CrossRef]
- Cigada, A.; Cabrini, M.; Pedeferri, P. Increasing of the corrosion resistance of the Ti6Al4V alloy by high thickness anodic oxidation. J. Mat. Sci. Mat. Med. 1992, 3, 408–412. [Google Scholar] [CrossRef]
- Fathi, M.H.; Salehi, M.; Saatchi, A.; Mortazavi, V.; Moosavi, S.B. In vitro corrosion behavior of bioceramic, metallic, and bioceramic-metallic coated stainless steel dental implants. Dent. Mat. 2003, 19, 188–198. [Google Scholar] [CrossRef]
- Kasemo, B.; Lausmaa, J. Biomaterial and implant surfaces: A surface science approach. Int. J. Oral Maxillofac. Implants 1988, 3, 247–259. [Google Scholar]
- Aziz-Kerrzo, M.; Conroy, R.G.; Fenelon, A.M.; Farrell, S.T.; Breslin, C.B. Electrochemical studies on the stability and corrosion resistance of titanium-based implants materials. Biomaterials 2001, 22, 1531–1539. [Google Scholar] [CrossRef]
- Hanawa, T. Metals ions released from metal implants. Mat. Sci. Eng. C 2004, 24, 745–752. [Google Scholar] [CrossRef]
- Uo, M.; Watari, F.; Yokoyama, A.; Matsuno, H.; Kawasaki, T. Visualization and detectability of rarely contained elements in soft tissue by X-ray scanning analytical microscopy and electron probe micro analysis. Biomaterials 2001, 22, 1787–1794. [Google Scholar] [CrossRef]
- Przybyszewska-Doroś, I.; Okrój, W.; Walkowiak, B. Surface modifications of metallic implants. Eng. Biomater. 2005, 43–44, 52–62. [Google Scholar]
- De Sena, L.A.; Rocha, N.C.C.; Andrade, M.C.; Soares, G.A. Bioactivity assessment of titanium sheets electrochemically coated with thick oxide film. Surf. Coat. Technol. 2003, 166, 254–258. [Google Scholar] [CrossRef]
- Lavos-Valereto, I.C.; König, B.; Rossa, C., Jr.; Marcantonio, E.; Zavaglia, A.C. A study of historical responses from Ti–6Al–7Nb alloy dental implants with and without plasma-sprayed hydroxyapatite coatings in dogs. J. Mat. Sci. Mat. Med. 2001, 12, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Uchida, M.; Kim, H.-M.; Zhang, X.; Kokubo, T. Preparation of bioactive titanium metal via anodic oxidation treatment. Biomaterials 2004, 25, 1003–1010. [Google Scholar] [CrossRef]
- Zieliński, A.; Sobieszczyk, S. Corrosion of titanium biomaterials, mechanisms, effects and modelisation. Corros. Rev. 2008, 26, 1–22. [Google Scholar] [CrossRef]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Veys-Renaux, D.; Ait El Haj, Z.; Rocca, E. Corrosion resistance in artificial saliva of titanium anodized by plasma electrolytic oxidation in Na3PO4. Surf. Coat. Techn. 2016, 285, 214–219. [Google Scholar] [CrossRef]
- Revathi, A.; Borrás, A.D.; Muñoz, A.I.; Richard, C.; Manivasagam, G. Degradation mechanisms and future challenges of titanium and its alloys for dental implant applications in oral environment. Mat. Sci. Eng. C 2017, 76, 1354–1368. [Google Scholar] [CrossRef]
- Strietzel, R.; Hösch, A.; Kalbfleisch, H.; Buch, D. In vitro corrosion of titanium. Biomaterials 1998, 19, 1495–1499. [Google Scholar] [CrossRef]
- Koike, M.; Fujii, H. The corrosion resistance of pure titanium in organic acids. Biomaterials 2001, 22, 2931–2936. [Google Scholar] [CrossRef]
- Wierzchoń, T.; Czarnowska, E.; Krupa, D. Surface Engineering in the Production of Titanium Biomaterials; Printing House PW: Warsaw, Poland, 2004. [Google Scholar]
- Haynes, D.R.; Rogers, S.D.; Hay, S. The differences in toxicity and release of bone-resorbing mediators induced by titanium and cobalt-chromium-alloy wear particles. J. Bone Jt. Surg. Am. 1993, 75, 825–834. [Google Scholar] [CrossRef]
- Wu, H.H.; Trinkle, D.R. Direct diffusion through interpenetrating networks: Oxygen in titanium. Phys. Rev. Lett. 2011, 107, 045504. [Google Scholar] [CrossRef]
- Sefer, B. Oxidation and Alpha-Case Phenomena in Titanium Alloys Used in Aerospace Industy: Ti-6Al-2Sn-4Zr-2Mo and Ti-6Al-4V. Bachelor’s Thesis, Luleå University of Technology, Luleå, Sweden, September 2014. [Google Scholar]
- Aerospace Series Test Method Titanium and Titanium Alloys Part 009-Determinantion of Surface Contaminantion; Swedish Standards Institute: Stockholm, Sweden, 2007.
- Hanawa, T.; Asami, K.; Asaoka, K. Repassivation of titanium and surface oxide film regenerated in simulated bioliquid. J. Biomed. Mat. Res. 1998, 40, 530–538. [Google Scholar] [CrossRef]
- Grant, D.M.; Lo, W.J.; Parker, K.H.; Parker, T.L. Biocompatible and mechanical properties of low temperature deposited quaternary (Ti, Al, V) N coatings on Ti6Al4V titanium alloy substrates. J. Mat. Sci. Mat. Med. 1996, 7, 576–584. [Google Scholar] [CrossRef]
- Li, J.; Jansen, J.A.; Walboomers, X.F.; van den Beucken, J.J.P. Mechanical aspects of dental implants and osseointegration: A narrative review. J. Mech. Behav. Biomed. Mat. 2020, 103, 103574. [Google Scholar] [CrossRef]
- Cheung, K.H.; Pabbruwe, M.B.; Chen, W.-F.; Koshy, P.; Sorrell, C.C. Effects of substrate preparation on TiO2 morphology and topography during anodization of biomedical Ti6Al4V. Mater. Chem. Phys. 2020, 252, 123224. [Google Scholar] [CrossRef]
- Kurup, A.; Dhatrak, P.; Khasnis, N. Surface modification techniques of titanium and titanium alloys for biomedical dental applications: A review. Mat. Today Proc. 2020. [Google Scholar] [CrossRef]
- Alves, A.C.; Wenger, F.; Ponthiaux, P.; Celis, J.-P.; Pinto, A.M.; Rocha, L.A.; Fernandes, J.C.S. Corrosion mechanisms in titanium oxide-based films produced by anodic treatment. Electrochim. Acta 2017, 234, 16–27. [Google Scholar] [CrossRef]
- Cui, W.F.; Jin, L.; Zhou, L. Surface characteristics and electrochemical corrosion behavior of a pre-anodized microarc oxidation coating on titanium alloy. Mater. Sci. Eng. C 2013, 33, 3775–3779. [Google Scholar] [CrossRef]
- Shokouhfar, M.; Allahkaram, S.R. Effect of incorporation of nanoparticles with different composition on wear and corrosion behavior of ceramic coatings developed on pure titanium by micro arc oxidation. Surf. Coat. Techn. 2017, 309, 767–778. [Google Scholar] [CrossRef]
- Sharma, A.; Mcquillan, A.J.; Sharma, L.A.; Waddell, J.; Shibata, Y.; Duncan, W. Spark anodization of titanium–zirconium alloy: Surface characterization and 216 bioactivity assessment. J. Mater. Sci. Mater. Med. 2015, 26, 1–11. [Google Scholar] [CrossRef]
- Ossowska, A.; Zieliński, A.; Supernak, M. Electrochemical oxidation and corrosion resistance of the Ti13Nb13Zr alloy. Eng. Biomat. 2013, XVI, 4–6. [Google Scholar]
- Callister, W.D. Materials Science and Engineering—An Introduction; Wiley: New York, NY, USA, 2007. [Google Scholar]
- Venkatu, D.A.; Poteat, L.E. Diffusion of titanium of single crystal rutile. Mater. Sci. Eng. 1970, 5, 258–262. [Google Scholar] [CrossRef]
- Akse, J.R.; Whitehurst, H.B. Diffusion of titanium in slightly reduced rutile. J. Phys. Chem. Solids 1978, 39, 457–465. [Google Scholar] [CrossRef]
- Schwertmann, U. Solubility and dissolution of iron oxides. Plant Soil 1991, 130, 1–25. [Google Scholar] [CrossRef]
- Ko, C.K.; Lee, W.G. Effects of pH variation in aqueous solutions on dissolution of copper oxide. Surf. Interface Anal. 2010, 42, 1128–1130. [Google Scholar] [CrossRef]
- Uzbekov, A.A.; Klement’eva, V.S. Radiochemical investigation of the selectivity of dissolution of components of ruthenium-titanium oxide anodes (ORTA) in chloride solutions. Sov. Electrochem. 1985, 21, 758–763. [Google Scholar]
- Streckbein, P.; Wilbrand, J.-F.; Kähling, C.; Pons-Kīühnemann, J.; Rehmann, P.; Wöstmann, B.; Howaldt, H.-P.; Möhlhenrich, S.C. Evaluation of the surface damage of dental implants caused by different surgical protocols: An in vitro study. Int. J. Oral Maxillofac. Surg. 2019, 48, 971–981. [Google Scholar] [CrossRef]
- Kowalski, K.; Bernasik, A.; Sadowski, A. Bulk and grain boundary diffusion of titanium in yttria-stabilized zirconia. J. Eur. Ceram. Soc. 2000, 20, 951–958. [Google Scholar] [CrossRef]

| Compound | Content (g/L) |
|---|---|
| (NH2)2CO | 0.13 |
| NaCl | 0.7 |
| NaHCO3 | 1.5 |
| Na3HPO4 | 0.26 |
| K2HPO4 | 0.2 |
| KSCN | 0.33 |
| KCl | 1.2 |
| Time | pH | Ringer’s Solution | Artificial Saliva |
|---|---|---|---|
| 3 months | 3 | <GO * | <GO * |
| 5 | <GO * | <GO * | |
| 7 | <GO * | <GO * | |
| 12 months | 3 | 0.093 | 0.136 |
| 5 | <GO * | 0.107 | |
| 7 | <GO * | 0.107 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ossowska, A.; Zieliński, A. The Mechanisms of Degradation of Titanium Dental Implants. Coatings 2020, 10, 836. https://doi.org/10.3390/coatings10090836
Ossowska A, Zieliński A. The Mechanisms of Degradation of Titanium Dental Implants. Coatings. 2020; 10(9):836. https://doi.org/10.3390/coatings10090836
Chicago/Turabian StyleOssowska, Agnieszka, and Andrzej Zieliński. 2020. "The Mechanisms of Degradation of Titanium Dental Implants" Coatings 10, no. 9: 836. https://doi.org/10.3390/coatings10090836
APA StyleOssowska, A., & Zieliński, A. (2020). The Mechanisms of Degradation of Titanium Dental Implants. Coatings, 10(9), 836. https://doi.org/10.3390/coatings10090836






