Application and Development Progress of Cr-Based Surface Coating in Nuclear Fuel Elements: II. Current Status and Shortcomings of Performance Studies
Abstract
1. Introduction
2. Performance under Normal Operating Conditions
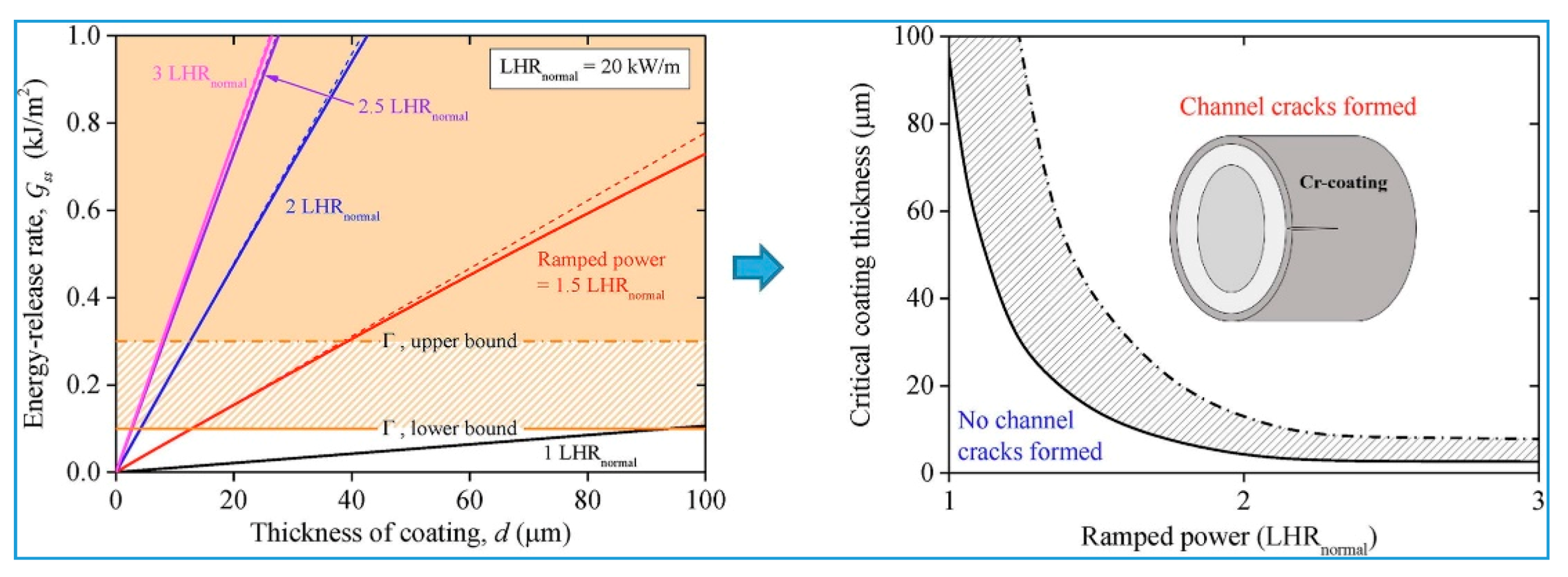
2.1. Mechanical Behavior
2.2. Corrosion Behavior
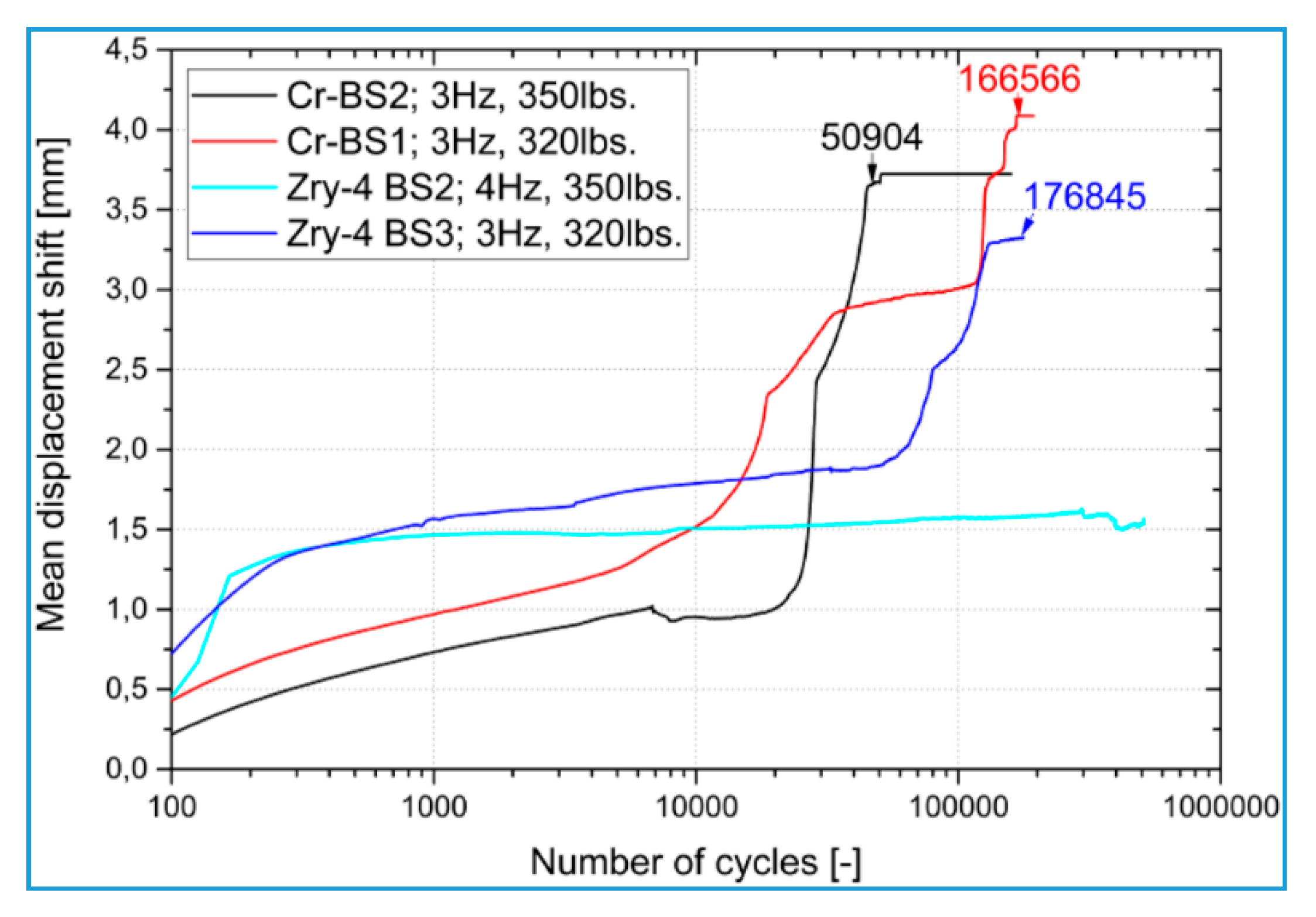
2.3. Fretting Wear Behavior
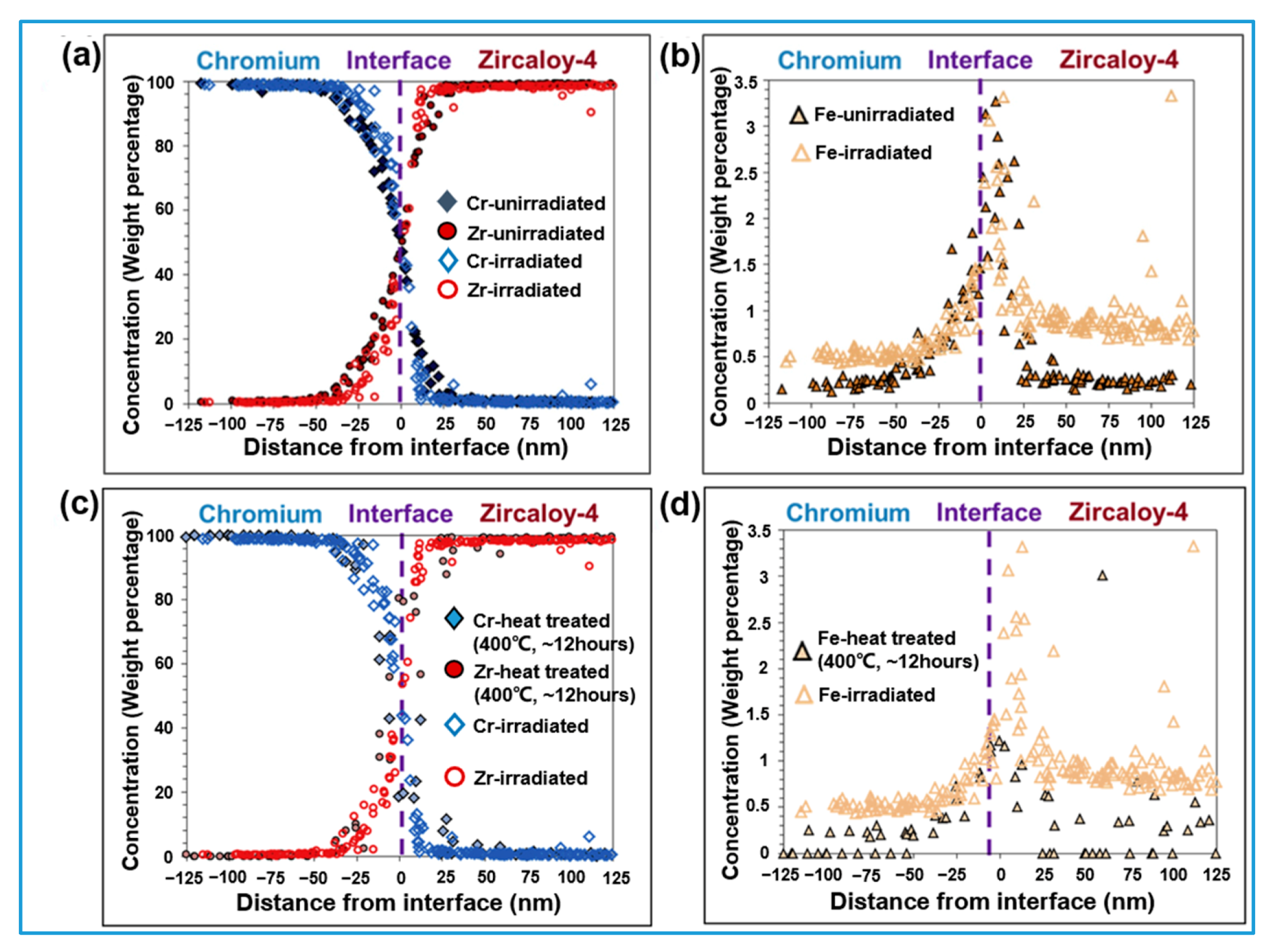
2.4. Irradiation Behavior
3. Performance under Accident Conditions
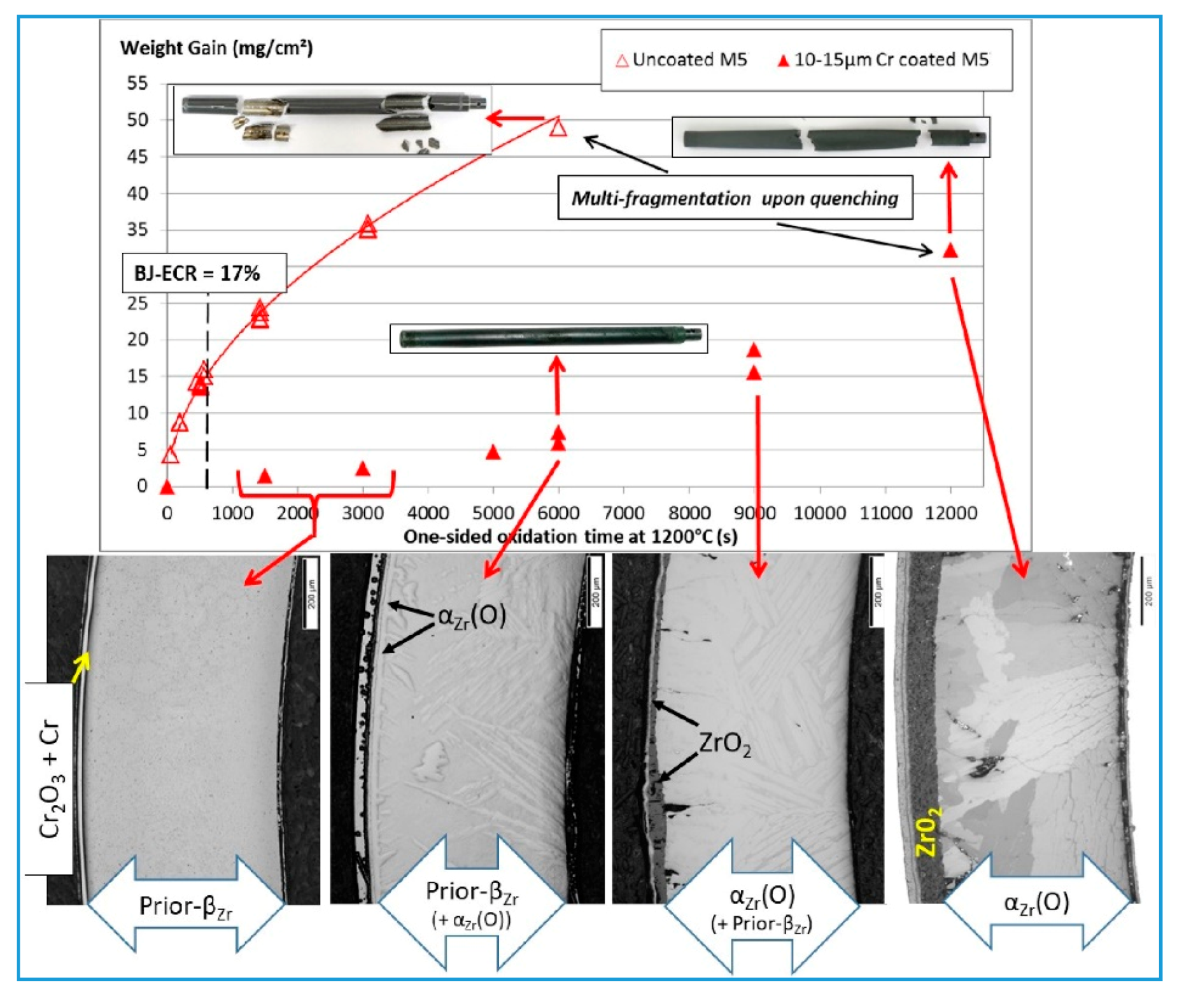
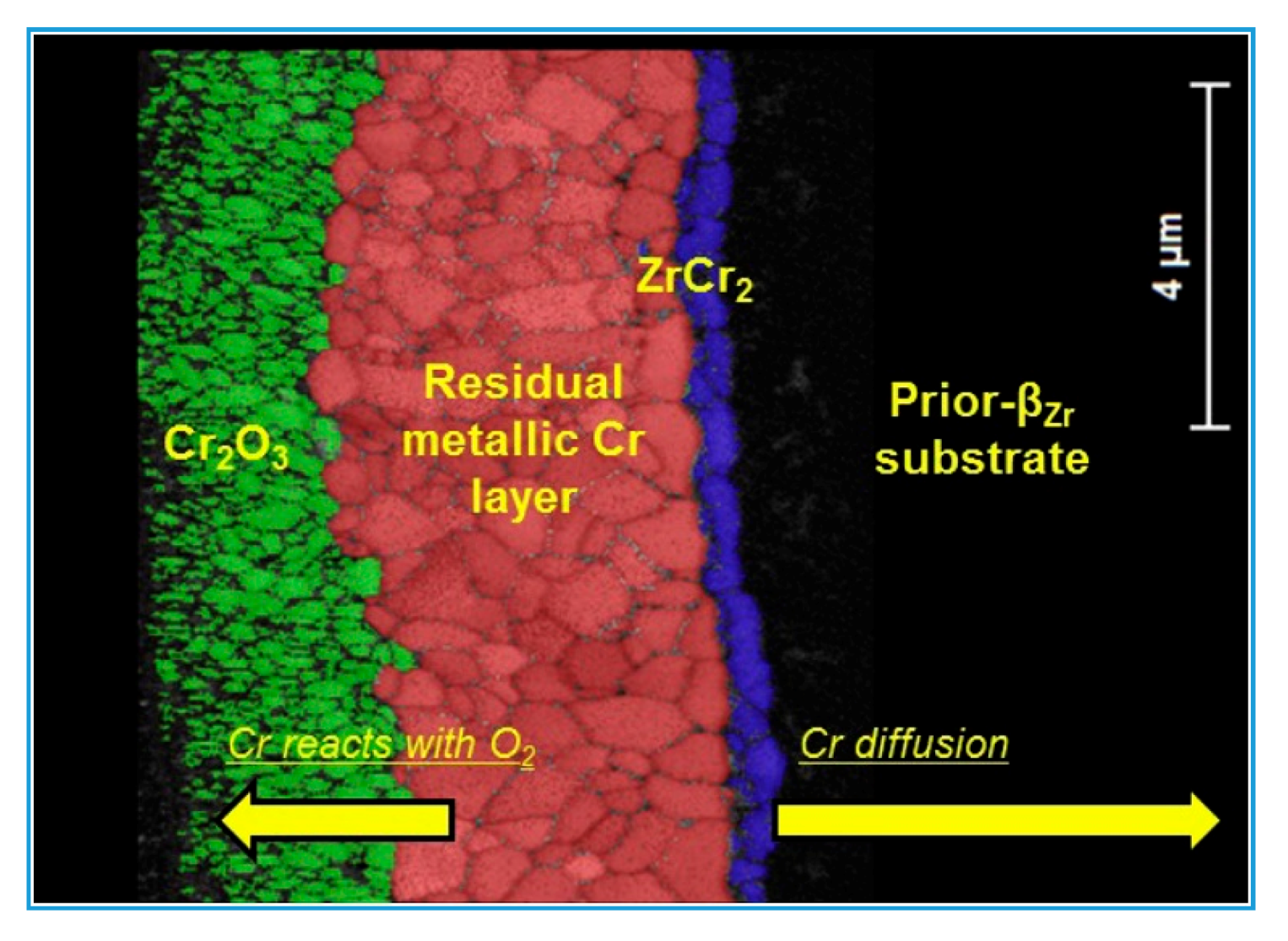
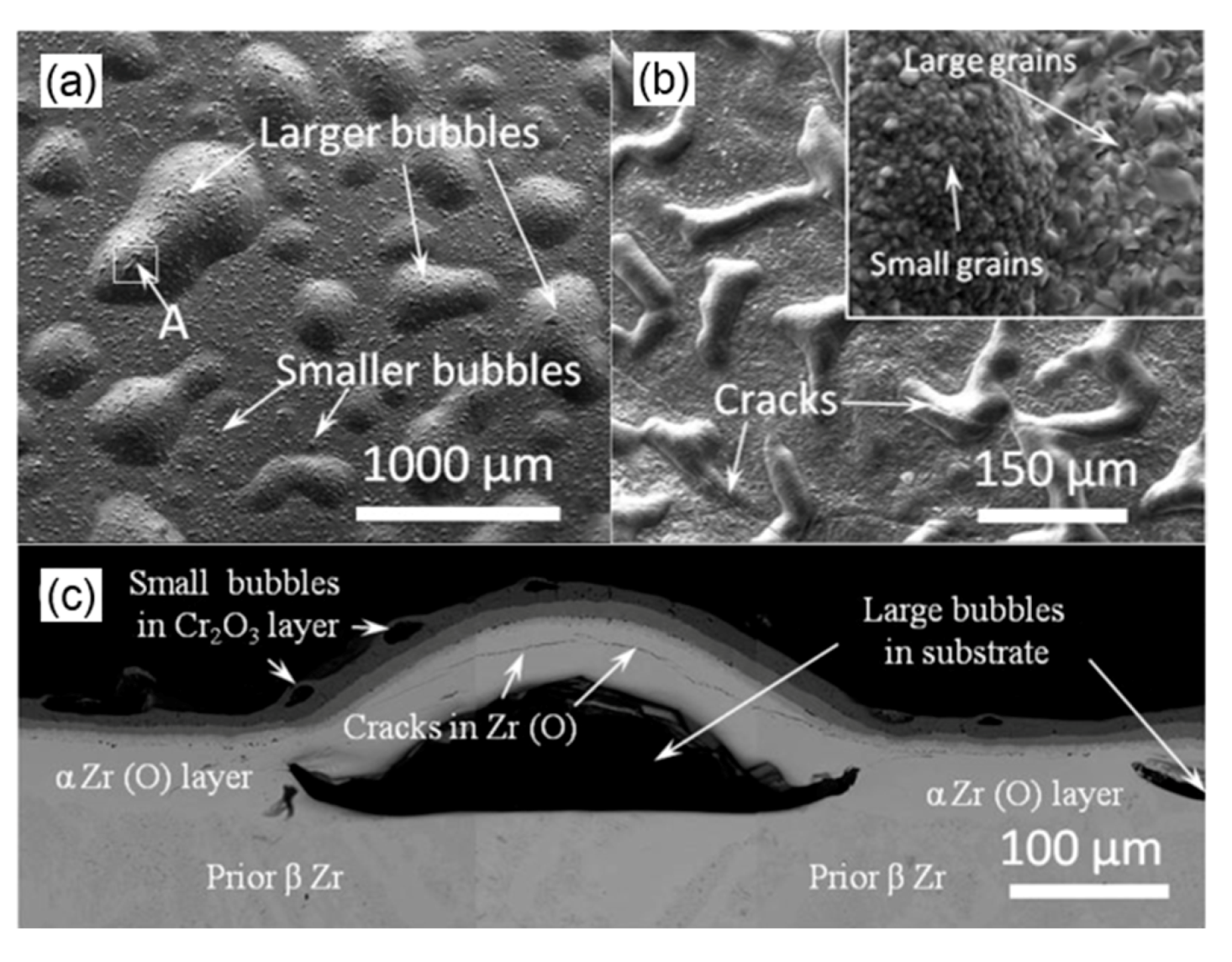
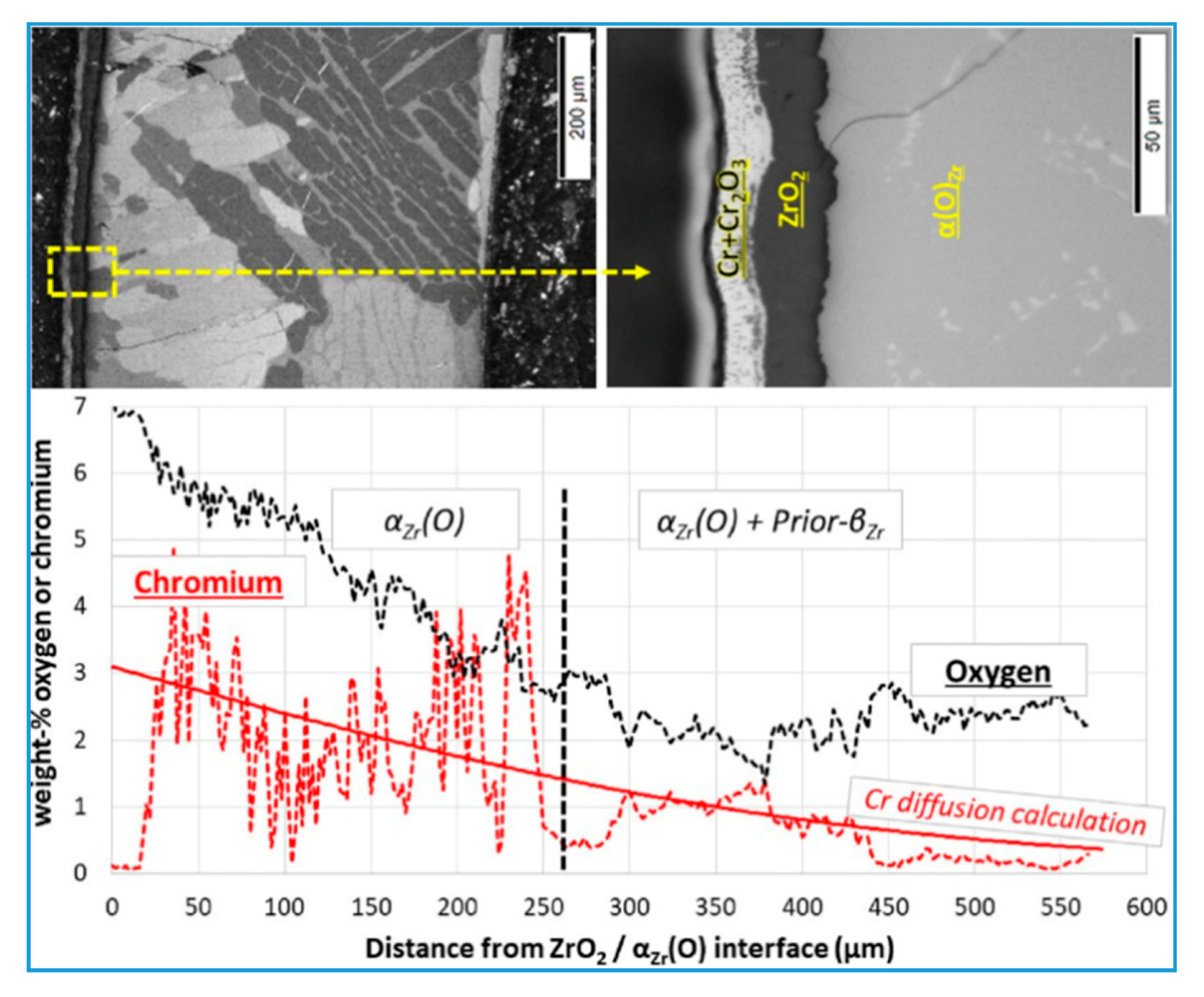
3.1. High-Temperature Oxidation Behavior
3.2. Embrittlement Behavior upon Quenching
- In the four-point bending tests, the failure of all samples (including coated samples) occurred near the center of the rupture region. The authors concluded that the failure position in the rupture region is determined by the high-degree local oxidation and the decrease in the wall thickness caused by ballooning. On the other hand, the steam that remained in the ballooned zone caused severe hydrogen absorption near the rupture open, transforming these brittle regions with high hydrogen contents into failure positions.
- The maximum load of the Cr-coated Zr alloy cladding is higher than that of the uncoated sample. This may originate from a higher average wall thickness of the Cr-coated sample, where the oxidation of the outer surface does not occur to a significant extent.
- The wall thickness of the middle-plane sections within the ballooned zone greatly varies, yielding different oxidation rates at different circumferential positions in the same moment of the oxidation process. The position edge of the rupture has the lowest wall thickness and the highest oxidation degree. The cracking starts from the end of the brittle rupture opening and quickly expands through the ballooned zone. Therefore, the bending strength of the bursting tube mainly depends on the thickness of the load-bearing Zr matrix in the cladding wall section opposite to the rupture.
3.3. Ballooning/Bursting Behavior during LOCA Transient
- Under the given internal pressure, both Cr-coated Zr-4 and M5 claddings exhibit the creep rupture time 2 to 3 times higher than that of the uncoated samples.
- When the creep temperature is below 850 °C (α-phase region), the average and maximum circumferential strains of the Cr-coated claddings are generally lower than of the uncoated samples; above 850 °C (α + β phase mixed area and β-phase area), although the Cr-coated cladding expanded significantly, the rupture size is very small (approximately 1 mm2).
- Under the test conditions, the Cr coating always fully adheres to the substrate after ballooning/bursting (including the region in the vicinity of highly-deformed ruptures).
- For all samples, the bursting temperatures under high internal pressure (100 bar) and low internal pressure (10 bar) conditions are within the range of 700 to 800 °C (αZr phase area) and above 1000 °C (βZr phase area). Besides, the bursting and temperature of the Cr-coated Zr alloy cladding are, under most circumstances, higher than those of the corresponding uncoated samples tested at a similar heating rate.
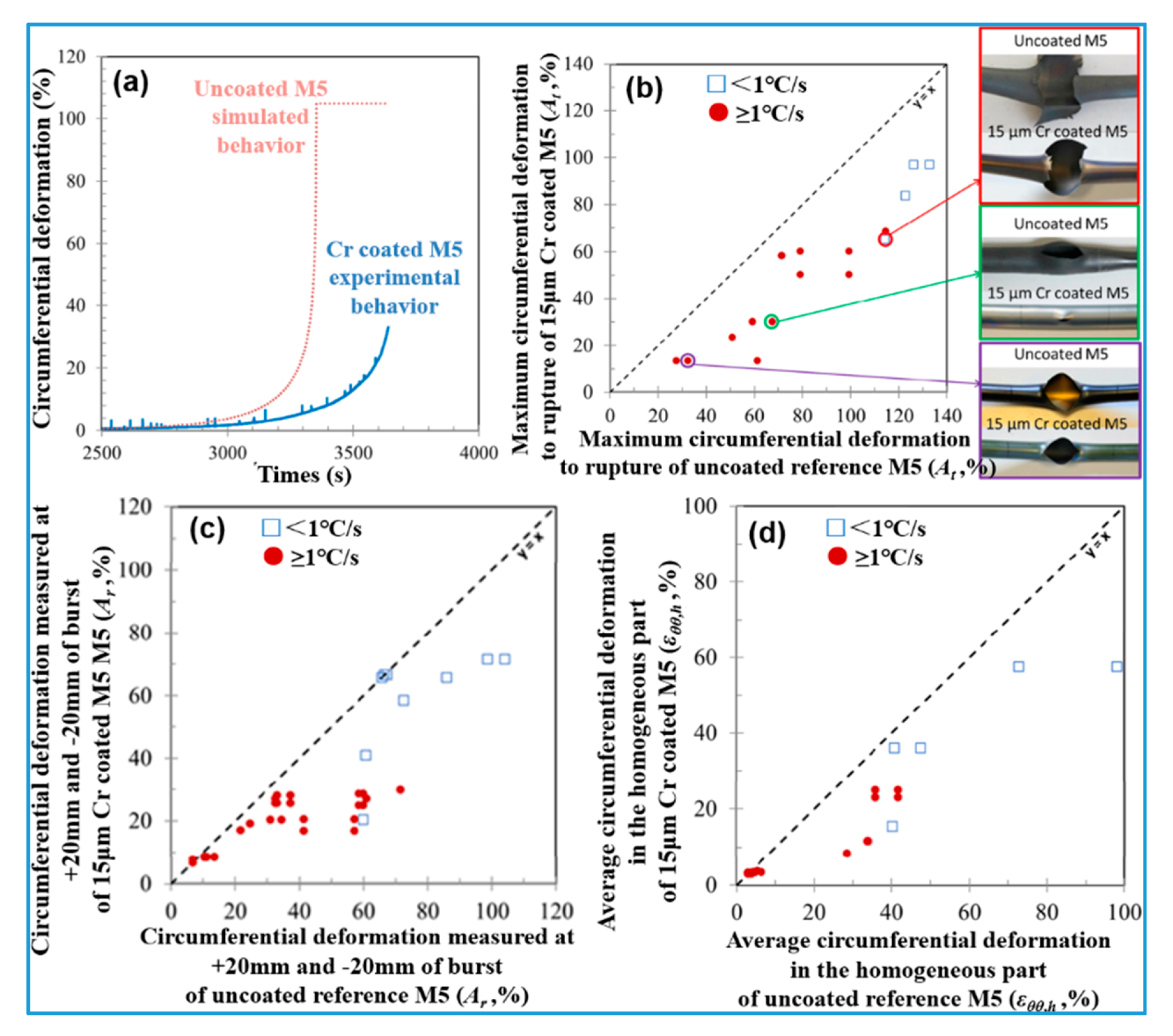
- Under the special conditions of low internal pressure (10 bar) and a high heating rate (25 °C·s−1), only limited creep deformation without bursting was observed on the Cr-coated M5 cladding when the temperature reached 1133 °C; the bursting temperature of the uncoated sample is in the range of 1030 to 1090 °C (β phase region). This indicates that the introduction of Cr coating increases the bursting temperature by at least 50 °C.
- The axial ballooning range and circumferential elongation of the Cr-coated M5 cladding are substantially lower than those of uncoated M5.
- When the heating rate is higher or equal to 1 °C·s−1 (representing most typical LOCA transients), the “average” circumferential elongation(εθθ, h) of the Cr-coated M5 cladding is consistently lower than 30%, while the maximum circumferential elongation (At) at the bursting position is lower than 70%. The authors recommended that these measured values should be employed as the upper threshold of the cladding’s behavior under LOCA conditions.
- The bursting temperature of the Cr-coated and uncoated Zr claddings are 839.5 and 754.8 °C, respectively.
- The balloon degree and the rupture size of the uncoated sample are larger than those of the Cr-coated sample.
- The circumferential strain of the middle-plane bursting of the Cr-coated and uncoated Zr cladding samples are 115.91% and 123.18%, respectively. The higher the circumferential strain, the lower the wall thickness after the bursting failure, and the wall has the lowest thickness near the bursting opening. Besides, oxidation occurs only locally on the inner surface of the ballooned and ruptured region, while the entire outer surface of the uncoated sample was oxidized. The former has a higher residual Zr matrix thickness (521 μm) than the latter (431 μm).
- Both samples exhibit a higher outer diameter strain along the direction parallel to the rupture than along the direction vertical to the rupture, while the Cr-coated sample has a much smaller maximal outer diameter strain and axial expansion zone.
- Although there are small axial surface cracks near the expansion section of the Cr-coated tube, cracking or spalling does not occur in the Cr coating to a significant extent. This indicates that despite the difference between the CTE of Cr and Zr, the rapid temperature change during the LOCA test still has a little effect on the bonding force between the Cr coating and the Zr matrix.
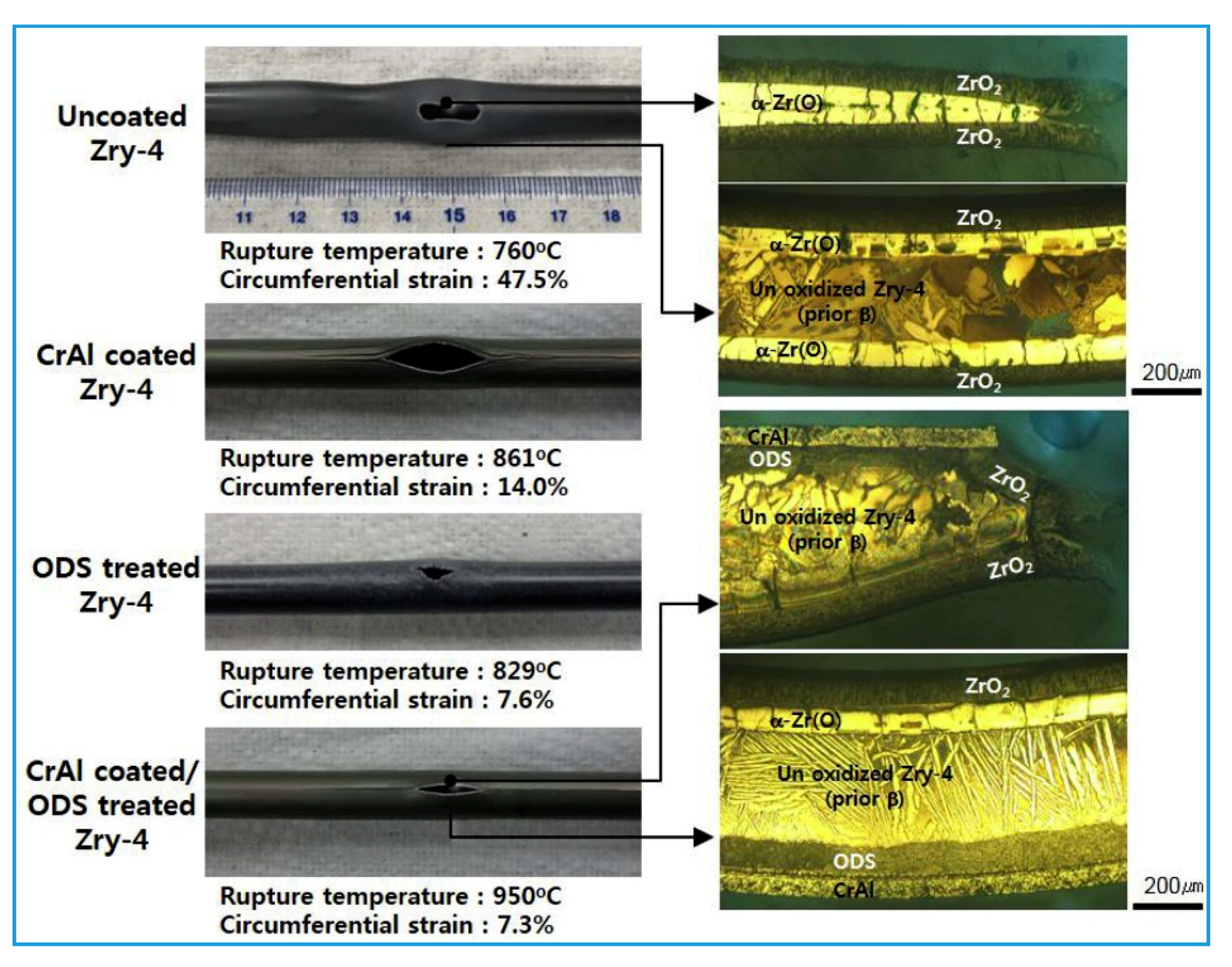
- Compared to the uncoated Zr-4 cladding, the bursting temperature of the CrAl-coated cladding (coating thickness of 50 μm) or the sample with the ODS layer (thickness of 100 μm) was improved. Besides, the sample coated with both the CrAl layer and the ODS layer had the highest bursting temperature.
- The application of the CrAl coating greatly reduced the circumferential deformation of the cladding, and this was even further reduced by the ODS treatment. However, these techniques still failed to completely prevent the cladding from the bursting.
- No severe oxidation of the CrAl coating occurred on the outer surface of the Zr-4 cladding coated with the CrAl and the ODS layers, and the Zr matrix with the ODS layer did not react with the CrAl coating. Besides, no severe oxidation of the CrAl coating or ODS treatment layer occurred in the ruptured section.
- The bursting time and temperature of all coated samples were higher than those of the uncoated samples.
- The maximal deformation and axial deformation of all coated samples were significantly lower than those of the uncoated samples. Among the coated samples, Cr-coated cladding exhibited the highest deformation decrease, and the axial deformation of the cladding coated with multicomponent CrN + Cr is close to that of the uncoated cladding.
- At 800 °C, the rupture size of most coated samples was larger than that of the uncoated samples, which is contrary to the above-mentioned experimental results.
- Two failure mechanisms were observed near the ruptures of the coated samples: sparsely distributed long cracks and a large rupture appeared on the Cr-coated sample, while the CrN + Cr-coated sample exhibited densely distributed cracks and a smaller rupture.
- At 800 °C, the creep rate shifts rapidly and discontinuously with the change of ring hoop stress. When the ring hoop stress exceeds 40 MPa, the bursting time decreases, and a part of the Zr alloy remains as the α-phase, resulting in a low creep rate; when the ring hoop stress is lower than 40 MPa, the bursting time prolongs, and the zirconium alloy is converted into the β-phase, yielding a high creep rate.
4. Discuss and Comment
5. Summary and Prospect
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, H.; Wang, X.; Zhang, R. Application and development progress of Cr-based surface coatings in nuclear fuel element: I. selection, preparation, and characteristics of coating materials. Coatings 2020, 10, 808. [Google Scholar] [CrossRef]
- Brachet, J.C.; Idarraga-Trujillo, I.; Flem, M.L.; Saux, M.L.; Vandenberghe, V.; Urvoy, S.; Rouesne, E.; Guilbert, T.; Toffolon-Masclet, V.; Tupin, M.; et al. Early studies on Cr-Coated Zircaloy-4 as enhanced accident tolerant nuclear fuel claddings for light water reactors. J. Nucl. Mater. 2019, 517, 268–285. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, I.H.; Jung, Y.I.; Park, D.J.; Park, J.Y.; Koo, Y.H. Adhesion property and High-temperature oxidation behavior of Cr-coated Zircaloy-4 cladding tube prepared by 3D laser coating. J. Nucl. Mater. 2015, 465, 531–539. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, H.S.; Kim, H.C.; Yang, Y.S. Preliminary study of mechanical behavior for Cr coated Zr-4 fuel cladding. In Proceedings of the Transactions of the Korean Nuclear Society Spring Meeting, Jeju, Korea, 7–8 May 2015. [Google Scholar]
- Yang, W.D. Materials for Nuclear Reactors; Atomic Energy Press: Beijing, China, 2000. [Google Scholar]
- Shah, H.; Romero, J.; Xu, P.; Maier, B.; Johnson, G.; Walters, J.; Dabney, T.; Yeom, H.; Sridharan, K. Development of surface coatings for enhanced accident tolerant fuel. In Proceedings of the 2017 Water Reactor Fuel Performance Meeting, Ramada Plaza, Jeju, Jeju Island, Korea, 10–14 September 2017; Available online: https://www.researchgate.net/publication/320190475 (accessed on 3 October 2017).
- Ribis, J.; Wu, A.; Brachet, J.C.; Barcelo, F.; Arnal, B. Atomic-scale interface structure of a Cr-coated Zircaloy-4 material. J. Mater. Sci. 2018, 53, 9879–9895. [Google Scholar] [CrossRef]
- NRC. Standard Review Plan—4.2 Fuel System Design; NUREG-0800; Nuclear Regulatory Commission: Washington, DC, USA, 2007.
- Wagih, M.; Spencer, B.; Hales, J.; Shirvan, K. Fuel performance of chromium-coated zirconium alloy and silicon carbide accident tolerant fuel claddings. Ann. Nucl. Energy 2018, 120, 304–318. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.I.; No, H.C. Mechanical analysis of surface-coated zircaloy cladding. Nucl. Eng. Technol. 2017, 49, 1031–1043. [Google Scholar] [CrossRef]
- Ševecek, M.; Shirvan, K.; Ballinger, R.G. Study of thermal creep of coated cladding materials. Acta Polytech. CTU Proc. 2018, 19, 22–29. [Google Scholar] [CrossRef]
- Ševecek, M.; Krejcí, J.; Shahin, M.; Shahin, M.H.; Petrik, J.; Ballinger, R.G.; Shirvan, K. Fatigue behavior of cold spray-coated accident tolerant cladding. In Proceedings of the TopFuel 2018, Prague, Czech Republic, 30 September–4 October 2018; Available online: https://www.researchgate.net/publication/328066444 (accessed on 4 October 2018).
- Hong, K.; Barber, J.R.; Thouless, M.D.; Lu, W. Cracking of Cr-coated accident-tolerant fuel during normal operation and under power-ramping conditions. Nucl. Eng. Des. 2019, 353, 110275. [Google Scholar] [CrossRef]
- Brachet, J.C.; Lorrette, C.; Michaux, A.; Sauder, C.; Idarraga-Trujillo, I.; Le Saux, M.; Le Flem, M.; Schuster, F.; Billard, A.; Monsifrot, E.; et al. CEA studies on advanced nuclear fuel claddings for enhanced Accident Tolerant LWRs Fuel (LOCA and beyond LOCA conditions). In Proceedings of the Fontevraud 8: Conference on Contribution of Materials Investigations and Operating Experience to LWRs’ Safety, Performance and Reliability, Avignon, France, 15–18 September 2014; Available online: https://www.researchgate.net/publication/268444279 (accessed on 18 November 2014).
- Brachet, J.C.; Le Saux, M.; Le Flem, M.; Urvoy, S.; Rouesne, E.; Guilbert, T.; Cobac, C.; Lahogue, F.; Rousselot, J.; Tupin, M. On-going studies at CEA on chromium coated zirconium based nuclear fuel claddings for enhanced accident tolerant LWRs fuel. In Proceedings of the TopFuel 2015, Zurich, Switzerland, 13–17 September 2015; Available online: https://www.researchgate.net/publication/283446965 (accessed on 3 November 2015).
- Bischoff, J.; Delafoy, C.; Vauglin, C.; Barberis, P.; Roubeyrie, C.; Perche, D.; Duthoo, D.; Schuster, F.; Brachet, J.-C.; Schweitzer, E.W.; et al. AREVA NP’s enhanced accident-tolerant fuel developments: Focus on Cr-coated M5 cladding. Nucl. Eng. Technol. 2018, 50, 223–228. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, R.; Yang, H.; Liu, H.; Qiu, S.; Wang, Y.; Du, P.; He, K.; Hu, X.; Dong, C. Microstructure, corrosion resistance and oxidation behavior of Cr-coatings on Zircaloy-4 prepared by vacuum arc plasma deposition. Corros. Sci. 2019, 158, 108077. [Google Scholar] [CrossRef]
- Krejčí, J.; Kabátová, J.; Manoch, F.; Kočí, J.; Cvrček, L.; Málek, J.; Krum, S.; Šutta, P.; Bublíková, P.; Halodová, P.; et al. Development and testing of multicomponent fuel cladding with enhanced accidental performance. Nucl. Eng. Technol. 2020, 52, 597–609. [Google Scholar] [CrossRef]
- Delafoy, C.; Bischoff, J.; Larocque, J.; Attal, P.; Gerken, L.; Nimishakavi, K. Benefits of Framatome’s E-ATF evolutionary solution: Cr-coated cladding with Cr2O3-doped fuel. In Proceedings of the TopFuel 2018, Prague, Czech Republic, 30 September–4 October 2018. Paper A0149. [Google Scholar]
- Bischoff, J.; Delafoy, C.; Chaari, N.; Vauglin, C.; Buchanan, K.; Barberis, P.; Monsifort, E.; Schuster, F.; Brachet, J.C.; Nimishakavi, K. Cr-coated cladding development at framatome. In Proceedings of the Top Fuel 2018, Prague, Czech Republic, 30 September–4 October 2018. Paper A0152. [Google Scholar]
- Wen, Q.L. Fretting wear test of Cr coated zirconium tube. In Proceedings of the ATF Fuel Technology Forum, Chengdu, China, 27–28 November 2019. [Google Scholar]
- Maier, B.R.; Yeom, H.; Johnson, G.; Dabney, T.; Hu, J.; Baldo, P.; Li, M.M.; Sridharan, K. In situ TEM investigation of irradiation-induced defect formation in cold spray Cr coatings for accident tolerant fuel applications. J. Nucl. Mater. 2018, 512, 320–323. [Google Scholar] [CrossRef]
- Kuprin, A.S.; Belous, V.A.; Voyevodin, V.N.; Vasilenko, R.L.; Ovcharenko, V.D.; Tolstolutskaya, G.D.; Kopanets, I.E.; Kolodiy, I. Irradiation resistance of vacuum arc chromium coatings for zirconium alloy fuel claddings. J. Nucl. Mater. 2018, 510, 163–167. [Google Scholar] [CrossRef]
- Wu, A.; Ribis, J.; Brachet, J.C.; Clouet, E.; Lepretre, F.; Bordas, E.; Arnal, B. HRTEM and chemical study of an ion-irradiated chromium/zircaloy-4 interface. J. Nucl. Mater. 2018, 504, 289–299. [Google Scholar] [CrossRef]
- Huang, M.; Li, Y.; Ran, G.; Yang, Z.; Wang, P. Cr-coated Zr-4 alloy prepared by electroplating and its in situ He+ irradiation behavior. J. Nucl. Mater. 2020, 538, 152240. [Google Scholar] [CrossRef]
- Jiang, L.; Peng, Q.; Xiu, P.; Yan, Y.; Jiao, Z.; Lu, C.; Liu, T.; Ye, C.; Shu, R.; Liao, Y.; et al. Elucidating He-H assisted cavity evolution in alpha Cr under multiple ion beam irradiation. Scr. Mater. 2020, 187, 291–295. [Google Scholar] [CrossRef]
- Imtyazuddin, M.; Mir, A.H.; Tunes, M.A.; Vishnyakov, V.M. Radiation resistance and mechanical properties of magnetron-sputtered Cr2AlC thin films. J. Nucl. Mater. 2019, 526, 151742. [Google Scholar] [CrossRef]
- Wang, C.; Han, Z.; Su, R.; Gao, J.; Shi, L. Effects of irradiation damage on the structure in Cr2AlC thin film. Nucl. Inst. Methods Phys. Res. B 2019, 450, 286–290. [Google Scholar] [CrossRef]
- Daghbouj, N.; Li, B.S.; Callisti, M.; Sen, H.S.; Karlik, M.; Polcar, T. Microstructural evolution of helium-irradiated 6H–SiC subjected to different irradiation conditions and annealing temperatures: A multiple characterization study. Acta Mater. 2019, 181, 160–172. [Google Scholar] [CrossRef]
- Daghbouj, N.; Li, B.S.; Callisti, M.; Sen, H.S.; Lin, J.; Ou, X.; Karlik, M.; Polcar, T. The structural evolution of light-ion implanted 6H-SiC single crystal: Comparison of the effect of helium and hydrogen. Acta Mater. 2020, 188, 609–622. [Google Scholar] [CrossRef]
- Terrani, K.A. Accident tolerant fuel cladding development: Promise, status, and challenges. J. Nucl. Mater. 2018, 501, 13–30. [Google Scholar] [CrossRef]
- Young, D.J. High Temperature Oxidation and Corrosion of Metals, 2nd ed.; Elsevier: San Francisco, CA, USA, 2016. [Google Scholar]
- Yeom, H.; Maier, B.; Johnson, G.; Dabney, T.; Lenling, M.; Sridharan, K. High temperature oxidation and microstructural evolution of cold spray chromium coatings on Zircaloy-4 in steam environments. J. Nucl. Mater. 2019, 526, 151737. [Google Scholar] [CrossRef]
- Brachet, J.C.; Rouesne, E.; Ribis, J.; Guilbert, T.; Urvoy, S.; Nony, G.; Toffolon-Masclet, C.; Le Saux, M.; Chaabane, N.; Palancher, H.; et al. High temperature steam oxidation of chromium-coated zirconium-based alloys: Kinetics and process. Corros. Sci. 2020, 167, 108537. [Google Scholar] [CrossRef]
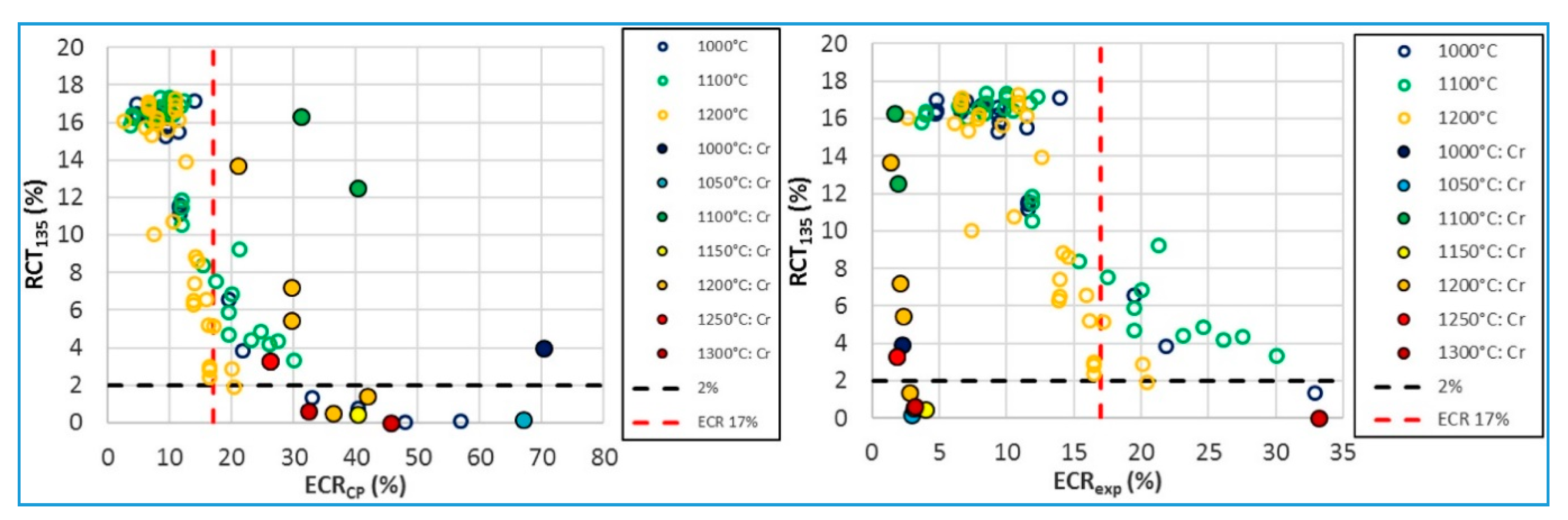
- Brachet, J.; Le Saux, M.; Bischoff, J.; Palancher, H.; Chosson, R.; Pouillier, E.; Guilbert, T.; Urvoy, S.; Nony, G.; Vandenberghe, T.; et al. Evaluation of equivalent cladding reacted parameters of Cr-coated claddings oxidized in steam at 1200 °C in relation with oxygen diffusion/partitioning and post-quench ductility. J. Nucl. Mater. 2020, 533, 152106. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Wen, Q.; Ruan, X.; Luo, F.; Bai, G.; Qing, Y.; Zhu, D.; Huang, Z.; Zhang, Y.; et al. Behavior of plasma sprayed Cr coatings and FeCrAl coatings on Zr fuel cladding under loss-of-coolant accident conditions. Surf. Coat. Technol. 2018, 344, 141–148. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, H.G.; Park, J.Y.; Jung, Y.I.; Park, D.J.; Koo, Y.H. High temperature steam-oxidation behavior of arc ion plated Cr coatings for accident tolerant fuel claddings. Surf. Coat. Technol. 2015, 280, 256–259. [Google Scholar] [CrossRef]
- Hu, X.; Dong, C.; Wang, Q.; Chen, B.; Yang, H.; Wei, T.; Zhang, R.; Gu, W.; Chen, D. High-temperature oxidation of thick Cr coating prepared by arc deposition for accident tolerant fuel claddings. J. Nucl. Mater. 2019, 519, 145–156. [Google Scholar] [CrossRef]
- Maier, B.; Yeom, H.; Johnson, G.; Dabney, T.; Walters, J.; Xu, P.; Romero, J.; Shah, H.; Sridharan, K. Development of cold spray chromium coatings for improved accident tolerant zirconium-alloy cladding. J. Nucl. Mater. 2019, 519, 247–254. [Google Scholar] [CrossRef]
- Michalik, M.; Hänsel, M.; Zurek, J.; Singheiser, L.; Quadakkers, W.J. Effect of water vapour on growth and adherence of chromia scales formed on Cr in high and low pO2-environments at 1000 and 1050 °C. Mater. High. Temp. 2005, 22, 213–221. [Google Scholar] [CrossRef]
- Michalik, M.; Hänsel, M.; Quadakkers, W.J. Effect of Water Vapour on Growth and Adherence of Chromia Scales on Pure Chromium. Master’s Thesis, Forschungszentrum Jülich, Jülich, Germany, 2007. [Google Scholar]
- Kofstad, P.; Lillerud, K. On high temperature oxidation of chromium II. Properties of and the oxidation mechanism of chromium. J. Electrochem. Soc. 1980, 127, 2410–2419. [Google Scholar] [CrossRef]
- Lillerud, K.; Kofstad, P. On high temperature oxidation of chromium I. Oxidation of annealed, thermally etched chromium at 800–1100 °C. J. Electrochem. Soc. 1980, 127, 2397–2410. [Google Scholar] [CrossRef]
- Hu, X.; Dong, C.; Chen, B.; Yang, H.; Zhang, R.; Gu, W.; Chen, D. Preparation and high temperature oxidation resistance of thick Cr coated on Zr-4 alloy by cathodic arc deposition for accident tolerant fuel claddings. Surf. Technol. 2019, 48, 207–219. [Google Scholar]
- Krejčí, J.; Ševecek, M.; Kabátová, J.; Manoch, F.; Kocí, J.; Cvrcek, L.; Málek, J.; Krum, S.; Šutta, P.; Bublíková, P.; et al. Experimental behavior of chromium-based coatings. In Proceedings of the TopFuel 2018, Prague, Czech Republic, 30 September–4 October 2018; Available online: https://www.researchgate.net/publication/328066345 (accessed on 4 October 2018).
- Brachet, J.C.; Guilbert, T.; Le Saux, M.; Rousselot, J.; Nony, G.; Toffolon-Masclet, C.; Michau, A.; Schuster, F.; Palancher, H.; Bischoff, J. Behavior of Cr-coated M5 claddings during and after high temperature steam oxidation from 800 °C up to 1500 °C (Loss-of-Coolant Accident & Design Extension Conditions). In Proceedings of the Topfuel 2018, Prague, Czech Republic, 30 September–4 October 2018; Available online: https://www.researchgate.net/publication/328095354 (accessed on 5 October 2018).
- Idarraga-Trujillo, I.; Le Flem, M.; Brachet, J.C.; Le Saux, M. Assessment at CEA of coated nuclear fuel cladding for LWRs with increasing margins in LOCA and beyond LOCA conditions. In Proceedings of the TopFuel 2013, Charlotte, NC, USA, 15–19 September 2013; pp. 860–867. [Google Scholar]
- Chaabane-Jebali, N.; Brachet, J.; Lesaux, M.; Hamon, D.; Rouesne, E.; Urvoy, S.; Tabarant, M.; Schiegel, M.; Lomello, F. Effect of chromium grain size and morphology on the HT oxidation behavivor of chromium coated Zr based alloys. In Proceedings of the 1st International Conference on Materials Science and Engineering (Mat Science 2019), San Francisco, CA, USA, 18–20 February 2019; Available online: https://hal.archives-ouvertes.fr/hal-02411074 (accessed on 14 December 2019).
- Kim, H.G.; Kim, I.H.; Jung, Y.I.; Park, D.J.; Yang, J.H.; Koo, Y.H. Development of surface modified Zr cladding by coating technology for ATF. In Proceedings of the Top Fuel 2016, Boise, ID, USA, 11–15 September 2016; pp. 1157–1163. [Google Scholar]
- Kim, H.G.; Kim, I.H.; Jung, Y.I.; Park, D.J.; Park, J.H.; Choi, B.K.; Lee, Y.H. Out-of-pile performance of surface-modified Zr cladding for accident tolerant fuel in LWRs. J. Nucl. Mater. 2018, 510, 93–99. [Google Scholar] [CrossRef]
- Zhong, W.; Mouche, P.A.; Heuser, B.J. Response of Cr and Cr-Al coatings on Zircaloy-2 to high temperature steam. J. Nucl. Mater. 2018, 498, 137–148. [Google Scholar] [CrossRef]
- Wei, D.; Wu, Y.; He, X.; Ma, X.; Wang, H.; Shi, H. High temperature oxidation and tensile behaviors of CrAl coating on zirconium alloy. China Surf. Eng. 2019, 32, 44–53. [Google Scholar]
- Dong, Y.; Ge, F.; Meng, F.; Zhao, G.; Zhou, J.; Deng, Z.; Huang, Q.; Huang, F. Improved oxidation resistance of zirconium at high-temperature steam by magnetron sputtered Cr-Al-Si ternary coatings. Surf. Coat. Technol. 2018, 350, 841–847. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, I.H.; Kim, H.G. Effects of Si addition on the microstructure and properties of Cr-Al alloy for high temperature coating. Korean J. Mater. Res. 2019, 29, 7–10. [Google Scholar] [CrossRef]
- US; NRC. Acceptance Criteria for Emergency Core Cooling Systems for Light Water Nuclear Power Reactors; 10 CFR 50.46; Nuclear Regulatory Commission: Washington, DC, USA, 2014.
- Billone, M.; Yan, Y.; Burtseva, T.; Daum, R. Cladding Embrittlement during Postulated Loss-of-Coolant Accidents; NUREG/CR-6967; ANL-07/04; Nuclear Regulatory Commission: Washington, DC, USA, 2008.
- Baker, L., Jr.; Just, L.C. Studies of Netal-Water Reactions at High Temperatures. III. Experimental and Theoretical Studies of the Zirconium-Water Reaction; ANL-6548; Argonne National Labratory (ANL): Argonne, IL, USA, 1962.
- Cathcart, J.V.; Pawel, R.E.; Mckee, R.A.; Druschel, R.E.; Yurek, G.J.; Campbell, J.J.; Jury, S.H. Zirconium Metal-Water Oxidation Kinetics. IV. Reaction Rate Studies. [BWR:PWR]; ORNL/NUREG-17; Oak Ridge National Laboratory (ORNL): Oak Ridge, TN, USA, 1977.
- Alam, T.; Khan, M.K.; Pathak, M.; Ravi, K.; Singh, R.; Gupta, S.K. A review on the clad failure studies. Nucl. Eng. Des. 2011, 241, 3658–3677. [Google Scholar] [CrossRef]
- Erbacher, F.; Leistikow, S. Zircaloy fuel cladding behavior in a loss-of-coolant accident: A review. Zircon. Nucl. Ind. Astm Int. 1987, 939, 451. [Google Scholar]
- Namburi, H.K.; Ottazzi, L.; Chocholousek, M.; Lomonaco, G.; Gavelova, P.; Krejčí, J. Study hydrogen embrittlement and determination of E110 fuel cladding mechanical properties by ring compression testing. IOP Conf. Ser. Mater. Sci. Eng. 2019, 461, 012059. [Google Scholar] [CrossRef]
- Brachet, J.C.; Vandenberghe-Maillot, V.; Portier, L.; Gilbon, D.; Lesbros, A.; Waeckel, N.; Mardon, J.P. Hydrogen content, preoxidation, and cooling scenario effects on post-quench microstructure and mechanical properties of zircaloy-4 and M5® alloys in LOCA conditions. J. ASTM Inter. 2008, 5, 1–28. [Google Scholar] [CrossRef]
- Herb, J.; Sievers, J.; Sonnenburg, H.G. A new cladding embrittlement criterion derived from ring compression tests. Nucl. Eng. Des. 2014, 273, 615–630. [Google Scholar] [CrossRef]
- Park, D.J.; Kim, H.G.; Jung, Y.I.; Park, J.H.; Yang, J.H.; Koo, Y.H. Behavior of an improved Zr fuel cladding with oxidation resistant coating under loss-of-coolant accident conditions. J. Nucl. Mater. 2016, 482, 75–82. [Google Scholar] [CrossRef]
- Brachet, J.C.; Le Saux, M.; Lezaud-Chaillioux, V.; Dumerval, M.; Houmaire, Q.; Lomello, F.; Schuster, F.; Monsifrot, E.; Bischoff, J.; Pouillier, E. Behavior under LOCA conditions of enhanced accident tolerant chromium coated zircaloy-4 claddings. In Proceedings of the TopFuel 2016 Conference, Boise, ID, USA, 11–15 September 2016; pp. 1173–1178. [Google Scholar]
- Brachet, J.C.; Dumerval, M.; Lezaud-Chaillioux, V.; Le Saux, M.; Rouesne, E.; Hamon, D.; Urvoy, S.; Guilbert, T.; Houmaire, Q.; Cobac, C. Behavior of chromium coated M5TM claddings under LOCA conditions. In Proceedings of the 2017 Water Reactor Fuel Performance Meeting, Ramada Plaza Jeju, Jeju Island, Korea, 10–14 September 2017; Available online: https://www.researchgate.net/publication/319988324 (accessed on 22 September 2017).
- Dumerval, M.; Houmaire, Q.; Brachet, J.C.; Palancher, H.; Bischoff, J.; Pouillier, E. Behavior of Chromium Coated M5 Claddings upon Thermal Ramp Tests under Internal Pressure (Loss-of-Coolant Accident Conditions). In Proceedings of the TopFuel 2018, Prague, Czech Republic, 30 September–4 October 2018; Available online: https://www.researchgate.net/publication/328095355 (accessed on 5 October 2018).
- Chalupová, A.; Krejčí, J.; Cvrcek, L.; Ševecek, M.; Rozkošný, V.; Pribyl, A.; Halodová, P.; Gávelová, P. Coated cladding behavior during high-temperature transients. Acta Polytech. CTU Proc. 2019, 24, 9–14. [Google Scholar] [CrossRef]
- Zhong, W.; Mouche, P.A.; Han, X.; Heuser, B.J.; Mandapaka, K.K.; Was, G.S. Performance of iron-chromium-aluminum alloy surface coatings on Zircaloy 2 under high-temperature steam and normal BWR operating conditions. J. Nucl. Mater. 2016, 470, 327–338. [Google Scholar] [CrossRef]
- Danek, M.; Fernandes, F.; Cavaleiro, A.; Polcar, T. Influence of Cr additions on the strcture and oxidation resistance of multilayered TiAlCrN films. Surf. Coat. Technol. 2017, 313, 158–167. [Google Scholar] [CrossRef]
- Ma, X.F.; Wu, Y.W.; Tan, J.; Meng, C.Y.; Yang, L.; Dang, W.A.; He, X.J. Evaluation of corrosion and oxidation behaviors of TiAlCrN coatings for nuclear fuel cladding. Surf. Coat. Technol. 2019, 358, 521–530. [Google Scholar] [CrossRef]
- Ougier, M.; Michau, A.; Lomello, F.; Schuster, F.; Maskrot, H.; Schlegel, M.L. High-temperature oxidation behavior of HiPIMS as-deposited Cr–Al–C and annealed Cr2AlC coatings on Zr-based alloy. J. Nucl. Mater. 2020, 528, 151855. [Google Scholar] [CrossRef]
- Tang, C.; Steinbrueck, M.; Stueber, M.; Grosse, M.; Yu, X.; Ulrich, S.; Seifert, H.J. Deposition, characterization and high-temperature steam oxidation behavior of single-phase Ti2AlC-coated Zircaloy-4. Corrossion Sci. 2018, 135, 87–98. [Google Scholar] [CrossRef]
- Terrani, K.A.; Parish, C.M.; Shin, D.; Pint, B.A. Protection of zirconium by alumina- and chromia-forming iron alloys under High-temperature steam exposure. J. Nucl. Mater. 2013, 438, 64–71. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wang, X.; Zhang, R. Application and Development Progress of Cr-Based Surface Coating in Nuclear Fuel Elements: II. Current Status and Shortcomings of Performance Studies. Coatings 2020, 10, 835. https://doi.org/10.3390/coatings10090835
Chen H, Wang X, Zhang R. Application and Development Progress of Cr-Based Surface Coating in Nuclear Fuel Elements: II. Current Status and Shortcomings of Performance Studies. Coatings. 2020; 10(9):835. https://doi.org/10.3390/coatings10090835
Chicago/Turabian StyleChen, Huan, Xiaoming Wang, and Ruiqian Zhang. 2020. "Application and Development Progress of Cr-Based Surface Coating in Nuclear Fuel Elements: II. Current Status and Shortcomings of Performance Studies" Coatings 10, no. 9: 835. https://doi.org/10.3390/coatings10090835
APA StyleChen, H., Wang, X., & Zhang, R. (2020). Application and Development Progress of Cr-Based Surface Coating in Nuclear Fuel Elements: II. Current Status and Shortcomings of Performance Studies. Coatings, 10(9), 835. https://doi.org/10.3390/coatings10090835



