Influence of Material Composition on Structure, Surface Properties and Biological Activity of Nanocrystalline Coatings Based on Cu and Ti
Abstract
1. Introduction
2. Materials and Methods
2.1. Thin Films Manufacturing
2.2. Characterization of Physicochemical Properties
2.3. Measurements of Antimicrobial Activity of Thin Films
2.4. Studies of Thin Films Cytotoxicity
2.4.1. Extracts from Tested Films
2.4.2. MTT Test
2.4.3. Clonogenic Test
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wilson, J. Metallic biomaterials: State of the art and new challenges. In Fundamental Biomaterials: Metals; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Shaston, UK, 2018; pp. 1–33. [Google Scholar]
- Murr, L.E. Strategies for creating living, additively manufactured, open-cellular metal and alloy implants by promoting osseointegration, osteoinduction and vascularization: An overview. J. Mater. Sci. Technol. 2019, 35, 231–241. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Ducheyne, P. Comprehensive Biomaterials; Elsevier Science: Amsterdam, The Netherlands, 2011; ISBN 978-0-08-055294-1. [Google Scholar]
- Gupta, S.; Sharma, A.; Verma, R.S. Polymers in biosensor devices for cardiovascular applications. Curr. Opin. Biomed. Eng. 2020, 13, 69–75. [Google Scholar] [CrossRef]
- Zhai, L.; Narkar, A.; Ahn, K. Self-healing polymers with nanomaterials and nanostructures. Nano Today 2020, 30, 100826. [Google Scholar] [CrossRef]
- Massera, J. 10: Bioactive glass-ceramics: From macro to nano. In Nanostructured Biomaterials for Regenerative Medicine; Woodhead Publishing: Shaston, UK, 2020; pp. 275–292. [Google Scholar]
- Galante, R.; Figueiredo-Pina, C.D.; Serro, A.P. Additive manufacturing of ceramics for dental applications: A review. Dent. Mater. 2019, 35, 825–846. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Salinas, A.J. 6: Ceramics as bone repair materials. In Bone Repair Biomaterials, 2nd ed.; Woodhead Publishing: Shaston, UK, 2019; pp. 141–178. [Google Scholar]
- Ragunathan, S.; Govindasamy, G.; Raghul, D.R.; Karuppaswamy, M.; Togo, R.K.V. Hydroxyapatite reinforced natural polymer scaffold for bone tissue regeneration. Mater. Today Proc. 2020, 23, 111–118. [Google Scholar] [CrossRef]
- Harun, W.S.W.; Asri, R.I.M.; Alias, J.; Zulkifli, F.H.; Shariffuddin, J.H.M. A comprehensive review of hydroxyapatite-based coatings adhesion on metallic biomaterials. Ceram. Int. 2018, 44, 1250–1268. [Google Scholar] [CrossRef]
- Chernozem, R.V.; Surmeneva, M.A.; Krause, B.; Baumbach, T.; Ignatov, P.; Prymak, O.; Loza, K.; Epple, M.; Ennen-Roth, F.; Wittmar, A.; et al. Functionalization of titania nanotubes with electrophoretically deposited silver and calcium phosphate nanoparticles: Structure, composition and antibacterial assay. Mater. Sci. Eng. C 2018, 97, 420–430. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Surmeneva, M.A.; Tyurin, A.I.; Surmenev, R.A. Correlation between structural and mechanical properties of RF magnetron sputter deposited hydroxyapatite coating. Mater. Charact. 2018, 142, 261–269. [Google Scholar] [CrossRef]
- Narayana, P.S.V.V.S.; Srihari, P.S.V.V. Biofilm resistant surfaces and coatings on implants: A review. Mater. Today Proc. 2019, 18, 4847–4853. [Google Scholar] [CrossRef]
- Silva-Bermudez, P.; Rodil, S.E. An overview of protein adsorption on metal oxide coatings for biomedical implants. Surf. Coat. Technol. 2013, 233, 147–158. [Google Scholar] [CrossRef]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef]
- Bordji, K.; Jouzeau, J.Y.; Mainard, D.; Payan, E.; Netter, P.; Rie, K.T.; Stucky, T.; Hage-Ali, M. Cytocompatibility of Ti-6Al-4V and Ti-5Al-2.5Fe alloys according to three surface treatments, using human fibroblasts and osteoblasts. Biomaterials 1996, 17, 929–940. [Google Scholar] [CrossRef]
- Brunette, D.M.; Tengvall, P.; Textor, M.; Thomsen, P. Titanium in Medicine; Springer: Berlin, Germany, 2001. [Google Scholar]
- Wang, K. The use of titanium for medical applications in the USA. Mater. Sci. Eng. A 1996, 213, 134–137. [Google Scholar] [CrossRef]
- Sittig, C.; Textor, M.; Spencer, N.D.; Wieland, M.; Vallotton, P.H. Surface characterization of implant materials c.p. Ti, Ti-6Al-7Nb and Ti-6Al-4V with different pretreatments. J. Mater. Sci. Mater. Med. 1999, 10, 35–46. [Google Scholar] [CrossRef]
- Hayama, A.O.F.; Andrade, P.N.; Cremasco, A.; Contieri, R.J.; Afonso, C.R.M.; Caram, R. Effects of composition and heat treatment on the mechanical behavior of Ti–Cu alloys. Mater. Des. 2014, 55, 1006–1013. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants-a review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Niinomi, M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. A 2002, 33, 477–486. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, J.; Scheideler, L.; Reichl, R.; Geis-Gerstorfer, J. Effects of topography and composition of titanium surface oxides on osteoblast responses. Biomaterials 2004, 25, 4087–4103. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Shirai, T.; Tsuchiya, H.; Shimizu, T.; Ohtani, K.; Zen, Y.; Tomita, K. Prevention of pin tract infection with titanium-copper alloys. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 373–380. [Google Scholar] [CrossRef]
- Oberg, E.; Jones, F.D.; Horton, H.L. Machinery’s Handbook, 24th ed.; Industrial Press Inc.: New York, NY, USA, 1992; p. 501. [Google Scholar]
- Breasted, J.H. The Edwin Smith Surgical Papyrus: Published in Facsimile and Hieroglyphic Transliteration with Translation and Commentary in Two Volumes; University of Chicago Press: Chicago, IL, USA, 1991; pp. 3–4. [Google Scholar]
- Dollwet, H.H.A.; Sorenson, J.R.J. Historic uses of copper compounds in medicine. In Trace Elements in Medicine, 2nd ed.; The Humana Press Inc.: Totowa, NJ, USA, 2001; pp. 80–87. [Google Scholar]
- Park, Y.J.; Song, Y.H.; An, J.H.; Song, H.J.; Anusavice, K.J. Cytocompatibility of pure metals and experimental binary titanium alloys for implant materials. J. Dent. 2013, 41, 1251–1258. [Google Scholar] [CrossRef]
- Mehtar, S.; Wiid, I.; Todorov, S.D. The antimicrobial activity of copper and copper alloys against nosocomial pathogens and Mycobacterium tuberculosis isolated from healthcare facilities in the Western Cape: An in-vitro study. J. Hosp. Infect. 2008, 68, 45–51. [Google Scholar] [CrossRef]
- Noyce, J.O.; Michels, H.; Keevil, W. Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare environment. J. Hosp. Infect. 2006, 63, 289–297. [Google Scholar] [CrossRef]
- Noyce, J.O.; Michels, H.; Keevil, W. Use of copper cast alloys to control Escherichia coli O157 cross-contamination during food processing. Appl. Environ. Microbiol. 2006, 72, 4239–4244. [Google Scholar] [CrossRef]
- Ramyadevi, J.; Jeyasubramanian, K.; Marikani, A.; Rajakumar, G.; Rahuman, A.A.; Santhoshkumar, T.; Kirthi, A.V.; Jayaseelan, C.; Marimuthu, S. Copper nanoparticles synthesized by polyol process used to control hematophagous parasite. Parasitol. Res. 2011, 109, 1403–1415. [Google Scholar] [CrossRef]
- Schrand, A.M.; Rahman, M.F.; Hussain, S.M.; Schlager, J.J.; Smith, D.A.; Syed, A.F. Metal-based nanoparticles and their toxicity assessment. Wiley Interdiscip. Rev. 2010, 2, 554–568. [Google Scholar] [CrossRef]
- Fujimori, Y.; Sato, T.; Hayata, T.; Nagao, T.; Nakayama, M.; Nakayama, T.; Sugamata, R.; Suzuki, K. Novel antiviral characteristics of nanosized copper (I) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Appl. Environ. Microbiol. 2012, 78, 951–955. [Google Scholar] [CrossRef]
- Noyce, J.O.; Michels, H.; Keevil, C.W. Inactivation of influenza a virus on copper versus stainless steel surfaces. Appl. Environ. Microbiol. 2007, 73, 2748–2750. [Google Scholar] [CrossRef]
- Shionoiri, N.; Sato, T.; Fujimori, Y.; Nakayama, T.; Nemoto, M.; Matsunaga, T.; Tanaka, T. Investigation of the antiviral properties of copper iodide nanoparticles against feline calicivirus. J. Biosci. Bioeng. 2012, 113, 580–586. [Google Scholar] [CrossRef]
- Anyaogu, K.C.; Fedorov, A.V.; Neckers, D.C. Synthesis, characterization, and antifouling potential of functionalized copper nanoparticles. Langmuir 2008, 24, 4340–4346. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, N.; Torsi, L.; Ditaranto, N.; Tantillo, G.; Ghibelli, L.; Sabbatini, L. Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties. Chem. Mater. 2005, 17, 5255–5262. [Google Scholar] [CrossRef]
- Mahapatra, S.S.; Karak, N. Hyperbranched polyamine/Cu nanoparticles for epoxy thermoset. J. Macromol. Sci. A 2009, 46, 296–303. [Google Scholar] [CrossRef]
- Weaver, L.; Noyce, J.O.; Michels, H.T.; Keevil, C.W. Potential action of copper surfaces on methicillin-resistant Staphylococcus aureus. J. Appl. Microbiol. 2010, 109, 2200–2205. [Google Scholar] [CrossRef]
- Weaver, L.; Michels, T.; Keevil, W. Survival of Clostridium difficile on copper and steel: Futuristic options for hospital hygiene. J. Hosp. Infect. 2008, 68, 145–151. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Cantini, F.; Ciofi-Baffoni, S. Cellular copper distribution: A mechanistic systems biology approach. Cell. Mol. Life Sci. 2010, 67, 2563–2589. [Google Scholar] [CrossRef]
- Prohaska, J.R.; Heller, L.J. Mechanical properties of the copper-deficient rat heart. J. Nutr. 1982, 112, 2042–2050. [Google Scholar]
- Arredondo, M.; Gonzalez, M.; Olivares, M.; Pizarro, F.; Araya, M. Ceruloplasmin, an indicator of copper status. Biol. Trace Elem. Res. 2008, 123, 261–269. [Google Scholar] [CrossRef]
- Chambers, A.; Krewski, D.; Birkett, N.; Plunkett, L.; Hertzberg, R.; Danzeisen, R.; Aggett, P.J.; Starr, T.B.; Baker, S.; Dourson, M.; et al. An exposure-response curve for copper excess and deficiency. J. Toxicol. Environ. Health B Crit. Rev. 2010, 13, 546–578. [Google Scholar] [CrossRef]
- Borkow, G. Copper’s role in wound healing. Biomaterials 2004, 20, 201–209. [Google Scholar]
- Failla, M.L. Roles of trace metals in the maturation, activation and effector functions of immune cells. Bibl. Nutr. Dieta 1998, 54, 103–111. [Google Scholar]
- Sen, C.K.; Khanna, S.; Venojarvi, M.; Trikha, P.; Ellison, E.C.; Hunt, T.K.; Roy, S. Copper-induced vascular endothelial growth factor expression and wound healing. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1821–H1827. [Google Scholar] [CrossRef]
- Santo, C.E.; Taudte, N.; Nies, D.H.; Grass, G. Contribution of copper ion resistance to survival of Escherichia coli on metallic copper surfaces. Appl. Environ. Microbiol. 2008, 74, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Tkeshelashvili, L.K.; McBride, T.; Spence, K.; Loeb, L.A. Mutation spectrum of copper-induced DNA damage. J. Biol. Chem. 1991, 266, 6401–6406. [Google Scholar] [PubMed]
- Gaetke, M.L.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef]
- Linder, M.C.; Wooten, L.; Cerveza, P.; Cotton, S.; Shulze, R.; Lomeli, N. Copper transport. Am. J. Clin. Nutr. 1998, 67, 965S–971S. [Google Scholar] [CrossRef]
- Sadhra, S.S.; Wheatley, A.D.; Cross, H.J. Dietary exposure to copper in the European Union and its assessment for EU regulatory risk assessment. Sci. Total Environ. 2007, 374, 223–234. [Google Scholar] [CrossRef]
- Turnlund, J.R.; Keyes, W.R.; Kim, S.K.; Domek, J.M. Long-term high copper intake: Effects on copper absorption, retention, and homeostasis in men. Am. J. Clin. Nutr. 2005, 81, 822–828. [Google Scholar] [CrossRef]
- Yamamoto, A.; Honma, R.; Sumita, M. Cytotoxicity evaluation of 43 metal salts using murine fibroblasts and osteoblastic cells. J. Biomed. Mater. Res. 1998, 39, 331–340. [Google Scholar] [CrossRef]
- Shedle, A.; Samorapoompichit, P.; Rausch-Fan, X.H.; Franz, A.; Fureder, W.; Sperr, W.R.; Sperr, W.; Ellinger, A.; Slavicek, R.; Boltz-Nitulescu, G.; et al. Response of L-929 fibroblasts, human gingival fibroblasts, and human tissue mast cells to various metal cations. J. Dent. Res. 1995, 74, 1420–1513. [Google Scholar] [CrossRef]
- Wee, N.K.Y.; Weinstein, D.C.; Fraser, S.T.; Assinder, S.J. The mammalian copper transporters CTR1 and CTR2 and their roles in development and disease. Int. J. Biochem. Cell Biol. 2013, 45, 960–963. [Google Scholar] [CrossRef]
- Adelstein, S.J.; Vallee, B.L. Copper metabolism in man. N. Engl. J. Med. 1961, 265, 892–897. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1997. [Google Scholar]
- McHenry, R.; Charles, M. The New Encyclopedia Britannica 3, 15th ed.; Encyclopedia Britannica, Inc.: Chicago, IL, USA, 1992. [Google Scholar]
- Smith, W.F.; Hashemi, J. Foundations of Materials Science and Engineering; McGraw-Hill Professional: New York, NY, USA, 2003. [Google Scholar]
- Kikuchi, M.; Takahashi, M.; Okabe, T.; Okuno, O. Grindability of dental cast Ti-Ag and Ti-Cu alloys. Dent. Mater. J. 2003, 22, 191–205. [Google Scholar] [CrossRef]
- Takahashi, M.; Kikuchi, M.; Takada, Y.; Okuno, O. Grindability and mechanical properties of experimental Ti-Au, Ti-Ag and Ti-Cu alloys. Int. Congr. Ser. 2005, 1284, 326–327. [Google Scholar] [CrossRef]
- Holden, F.C.; Watts, A.A.; Ogden, H.R.; Jaffee, R.I. Heat treatment and mechanical properties of Ti-Cu alloys. Trans. Am. Inst. Min. Metall. Pet. Eng. Inc. 1955, 203, 117–225. [Google Scholar] [CrossRef]
- Andrade, P.N.; Coelho, A.A.; Afonso, C.R.M.; Contieri, R.J.; Robert, M.H.; Caram, R. Effects of composition on solidification microstructure of cast titanium alloys. Mater. Sci. Forum 2010, 646, 183–188. [Google Scholar] [CrossRef]
- Finke, B.; Polak, M.; Hempel, F.; Rebl, H.; Zietz, C.; Stranak, V.; Lukowski, G.; Hippler, R.; Bader, R.; Nebe, J.B.; et al. Antimicrobial potential of copper-containing titanium surfaces generated by ion implantation and dual high power impulse magnetron sputtering. Adv. Eng. Mater. 2012, 14, 224–230. [Google Scholar] [CrossRef]
- Patenge, N.; Arndt, K.; Eggert, T.; Zietz, C.; Kreikemeyer, B.; Bader, R.; Nebe, B.; Stranak, V.; Hippler, R.; Podbielski, A. Evaluation of antimicrobial effects of novel implant materials by testing the prevention of biofilm formation using a simple small scale medium-throughput growth inhibition assay. Biofouling 2012, 28, 267. [Google Scholar] [CrossRef]
- Stranak, V.; Wulff, H.; Rebl, H.; Zietz, C.; Arndt, K.; Bogdanowicz, R.; Nebe, B.; Bader, R.; Podbielski, A.; Hubicka, Z.; et al. Deposition of thin titanium-copper films with antimicrobial effect by advanced magnetron sputtering methods. Mater. Sci. Eng. C 2011, 31, 1512–1519. [Google Scholar] [CrossRef]
- Stranak, V.; Wulff, H.; Ksirova, P.; Zietz, C.; Drache, S.; Cada, M.; Hubicka, Z.; Bader, R.; Tichy, M.; Helm, C.A.; et al. Ionized vapor deposition of antimicrobial Ti-Cu films with controlled copper release. Thin Solid Film. 2014, 550, 389–394. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Kaczmarek, D.; Antosiak, A.; Mazur, M.; Rybak, Z.; Rusak, A.; Osekowska, M.; Poniedzialek, A.; Gamian, A.; Szponar, B. Influence of Cu-Ti thin film surface properties on antimicrobial activity and viability of living cells. Mater. Sci. Eng. C 2015, 56, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Domaradzki, J.; Wojcieszak, D.; Kaczmarek, D.; Mazur, P. Investigation of physicochemical properties of (Ti-V)Ox (4.3at.% of V) functional thin films and their possible application in the field of transparent electronics. Appl. Surf. Sci. 2014, 304, 73–80. [Google Scholar] [CrossRef]
- Grobelny, M.; Kalisz, M.; Mazur, M.; Wojcieszak, D.; Kaczmarek, D.; Domaradzki, J.; Świniarski, M.; Mazur, P. Functional Nb2O5 film and Nb2O5+CuO, Nb2O5+Graphene, Nb2O5+CuO+Graphene composite films to modify the properties of Ti6Al4V titanium alloy. Thin Solid Film. 2016, 616, 64–72. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Mazur, M.; Kaczmarek, D.; Mazur, P.; Szponar, B.; Domaradzki, J.; Kepinski, L. Influence of the surface properties on bactericidal and fungicidal activity of magnetron sputtered Ti-Ag and Nb-Ag thin films. Mater. Sci. Eng. C 2016, 62, 86–95. [Google Scholar] [CrossRef]
- Wasielewski, R.; Domaradzki, J.; Wojcieszak, D.; Kaczmarek, D.; Borkowska, A.; Prociow, E.L.; Ciszewski, A. Surface characterization of TiO2 thin films obtained by high-energy reactive magnetron sputtering. Appl. Surf. Sci. 2008, 254, 4396–4400. [Google Scholar] [CrossRef]
- Mazur, M.; Wojcieszak, D.; Domaradzki, J.; Kaczmarek, D.; Poniedziałek, A.; Domanowski, P. Investigation of microstructure, micro-mechanical and optical properties of HfTiO4 thin films prepared by magnetron co-sputtering. Mater. Res. Bull. 2015, 72, 116–122. [Google Scholar] [CrossRef]
- Mazur, M.; Howind, T.; Gibson, D.; Kaczmarek, D.; Morgiel, J.; Wojcieszak, D.; Zhu, W.; Mazur, P. Modification of various properties of HfO2 thin films obtained by changing magnetron sputtering conditions. Surf. Coat. Technol. 2017, 320, 426–431. [Google Scholar] [CrossRef]
- Mazur, M.; Domaradzki, J.; Wojcieszak, D.; Kaczmarek, D. Investigations of elemental composition and structure evolution in (Ti,Cu)-oxide gradient thin films prepared using (multi)magnetron co-sputtering. Surf. Coat. Technol. 2018, 334, 150–157. [Google Scholar] [CrossRef]
- Adamiak, B.; Wiatrowski, A.; Domaradzki, J.; Kaczmarek, D.; Wojcieszak, D.; Mazur, M. Preparation of multicomponent thin films by magnetron co-sputtering method: The Cu-Ti case study. Vacuum 2019, 161, 419–428. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Mazur, M.; Kaczmarek, D.; Szponar, B.; Grobelny, M.; Kalisz, M.; Pelczarska, A.; Szczygiel, I.; Poniedzialek, A.; Osekowska, M. Structural and surface properties of semitransparent and antibacterial (Cu,Ti,Nb)Ox coating. Appl. Surf. Sci. 2016, 380, 159–164. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Obstarczyk, A.; Domaradzki, J.; Kaczmarek, D.; Zakrzewska, K.; Pastuszek, R. Investigations of structure and electrical properties of TiO2/CuO thin film heterostructures. Thin Solid Films 2019, 690, 137538.
- Wojcieszak, D.; Mazur, M.; Kalisz, M.; Grobelny, M. Influence of Cu, Au and Ag on structural and surface properties of bioactive coatings based on titanium. Mater. Sci. Eng. C 2017, 71, 1115–1121. [Google Scholar] [CrossRef]
- Mazur, M.; Wojcieszak, D.; Kaczmarek, D.; Domaradzki, J.; Song, S.; Gibson, D.; Placido, F.; Mazur, P.; Kalisz, M.; Poniedzialek, A. Functional photocatalytically active and scratch resistant antireflective coating based on TiO2 and SiO2. Appl. Surf. Sci. 2016, 380, 165–171. [Google Scholar] [CrossRef]
- Domaradzki, J.; Kaczmarek, D.; Wojcieszak, D.; Mazur, M. Investigations of reversible optical transmission in gasochromic (Ti-V-Ta)Ox thin film for gas sensing applications. Sens. Actuators B Chem. 2014, 201, 420–425. [Google Scholar] [CrossRef]
- Domaradzki, J.; Wiatrowski, A.; Kotwica, T.; Mazur, M. Analysis of electrical properties of forward-to-open (Ti,Cu)Ox memristor rectifier with elemental gradient distribution prepared using (multi)magnetron co-sputtering process. Mater. Sci. Semicond. Process. 2019, 94, 9–14. [Google Scholar] [CrossRef]
- PN-EN ISO 10993-12: 2009. Biological Evaluation of Medical Devices-Part 12: Sample Preparation and Reference Materials; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- PN-EN ISO 10993-5: 2009. Biological Evaluation of Medical Devices-Part 5: In Vitro Cytotoxicity Studies; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Saphier, M.; Silberstein, E.; Shotland, Y.; Popov, S.; Saphier, O. Prevalence of monovalent copper over divalent in killing Escherichia coli and Staphylococcus aureus. Curr. Microbiol. 2018, 75, 426–430. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef]
- Rouch, D.A.; Lee, B.T.; Morby, A.P. Understanding cellular responses to toxic agents: A model for mechanism choice in bacterial metal resistance. J. Ind. Microbiol. 1995, 14, 132–141. [Google Scholar] [CrossRef]
- Heidenau, F.; Mittelmeier, W.; Detsch, R.; Haenle, M.; Stenzel, F.; Ziegler, G.; Gollwitzer, H. A novel antibacterial titania coating: Metal ion toxicity and in vitro surface colonization. J. Mater. Sci. Mater. Med. 2005, 16, 883–888. [Google Scholar] [CrossRef]
- Ma, Z.; Ren, L.; Liu, R.; Yang, K.; Zhang, Y.; Liao, Z.; Liu, W.; Qi, M.; Misra, R.D.K. Effect of heat treatment on Cu distribution, antibacterial performance and cytotoxicity of Ti-6Al-4V-5Cu Alloy. J. Mater. Sci. Technol. 2015, 31, 723–732. [Google Scholar] [CrossRef]
- Zhang, E.; Lia, F.; Wang, H.; Liu, J.; Wang, C.; Li, M.; Yang, K. A new antibacterial titanium-copper sintered alloy: Preparation and antibacterial property. Mater. Sci. Eng. C 2013, 33, 4280–4287. [Google Scholar] [CrossRef]
- Lüthen, F.; Bergemann, C.; Bulnheim, U.; Prinz, C.; Neumann, H.; Podbielski, A.; Bader, R.; Rychly, J. A dual role of copper on the surface of bone implants. Mater. Sci. Forum 2010, 638–642, 600–605. [Google Scholar]
- Freedman, J.H.; Weiner, R.J.; Peisach, J. Resistance to copper toxicity of cultured hepatoma cells, Characterization of resistant cell lines. J. Biol. Chem. 1986, 261, 11840–11848. [Google Scholar] [PubMed]
- Cao, B.; Zheng, Y.; Xi, T.; Zhang, C.; Song, W.; Burugapalli, K.; Yang, H.; Ma, Y. Concentration-dependent cytotoxicity of copper ions on mouse fibroblasts in vitro: Effects of copper ion release from TCu380A vs TCu220C intra-uterine devices. Biomed. Microdevices 2012, 14, 709–720. [Google Scholar] [CrossRef]
- Huang, D.; Ma, K.; Cai, X.; Yang, X.; Hu, Y.; Huang, P.; Wang, F.; Jiang, T.; Wang, Y. Evaluation of antibacterial, angiogenic, and osteogenic activities of green synthesized gap-bridging copper-doped nanocomposite coatings. Int. J. Nanomed. 2017, 12, 7483–7500. [Google Scholar] [CrossRef]
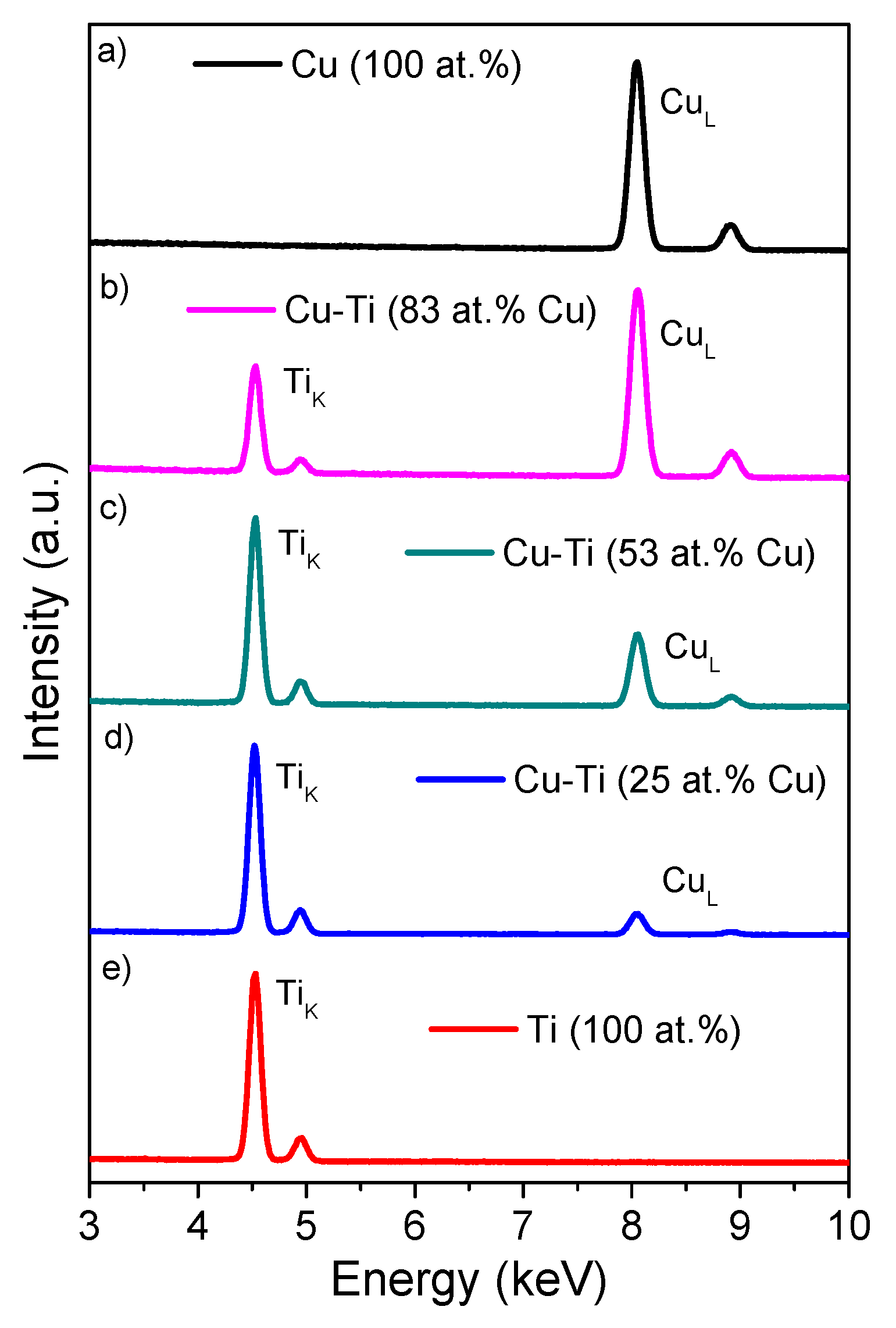




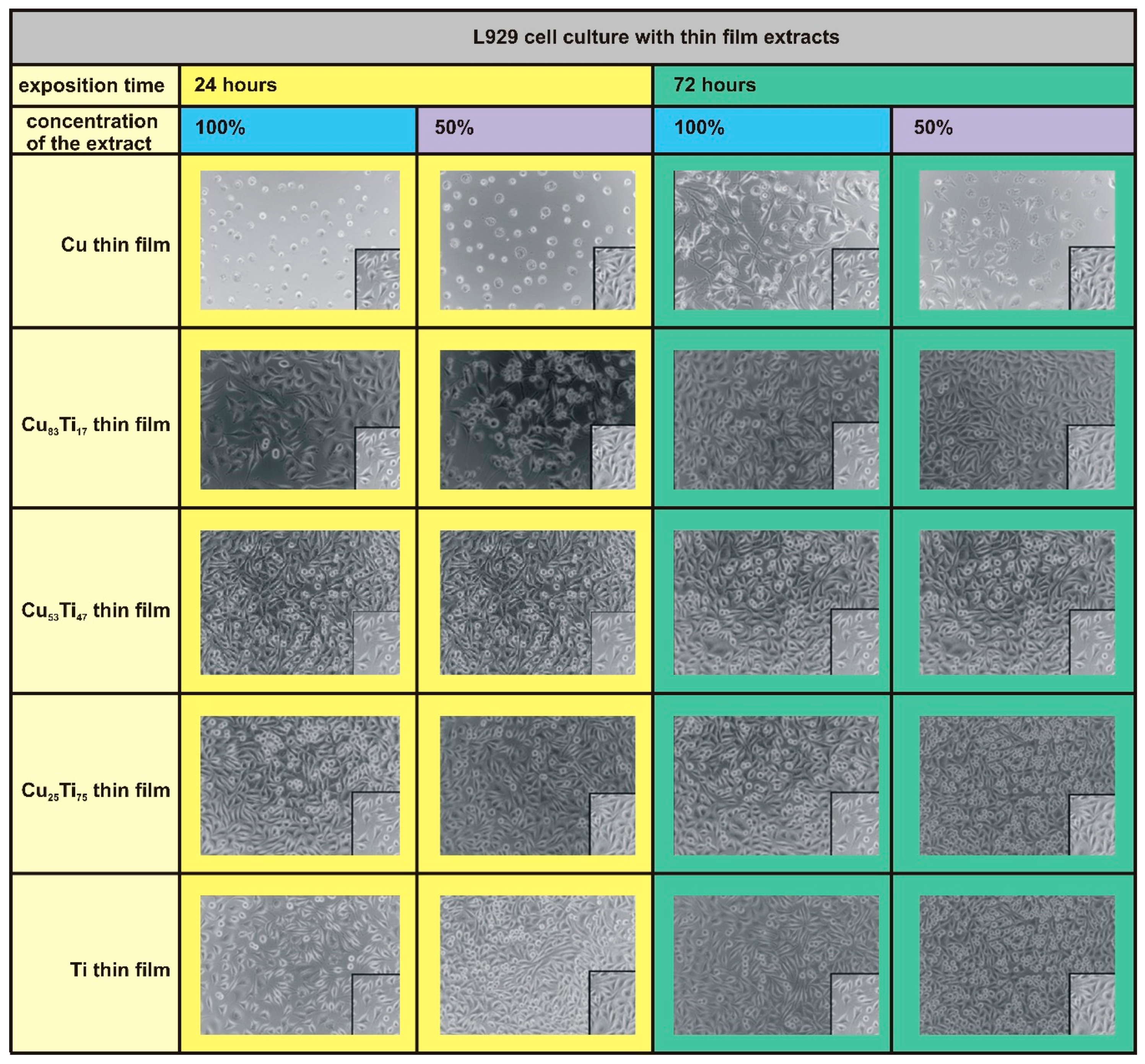
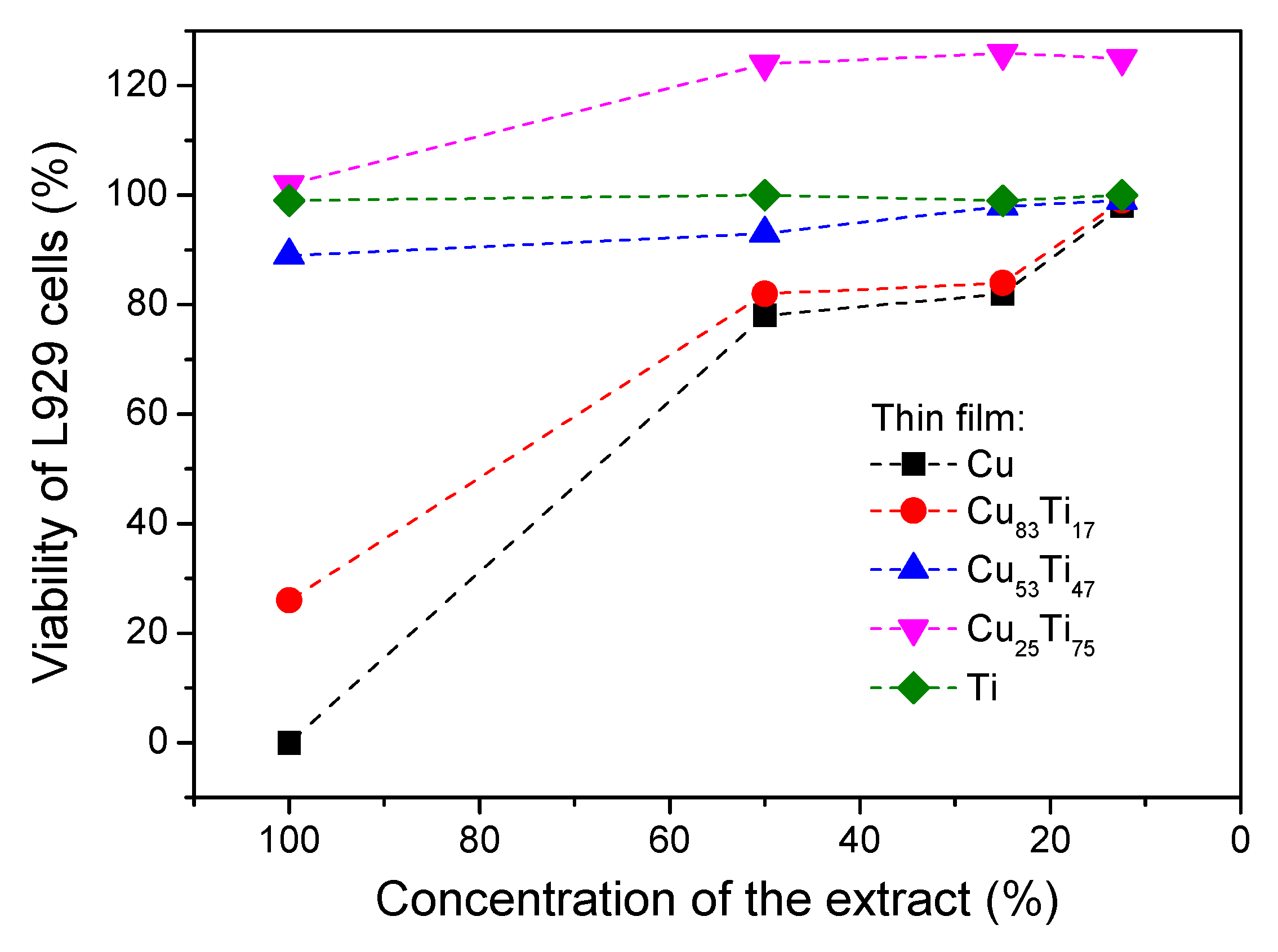
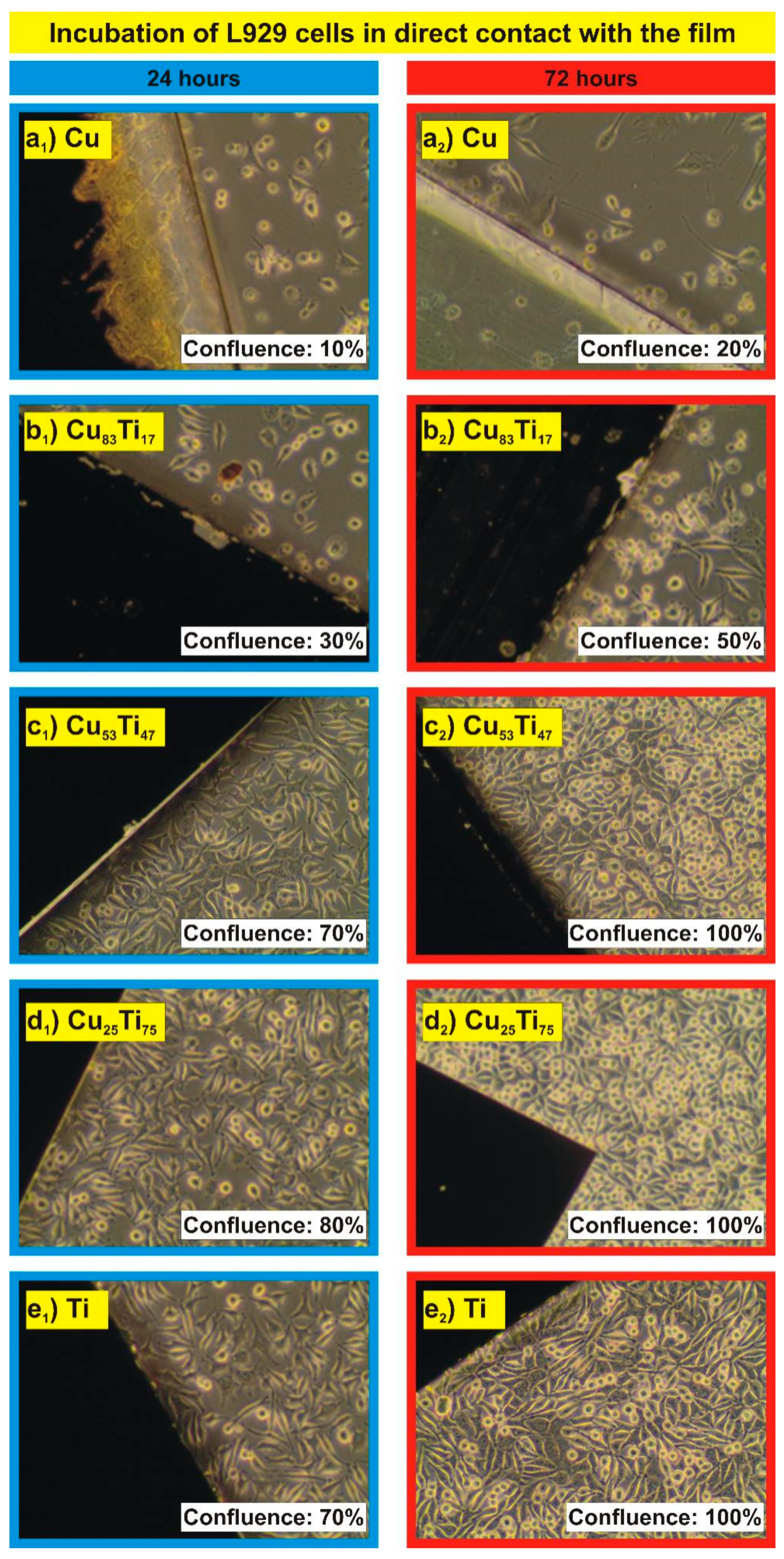
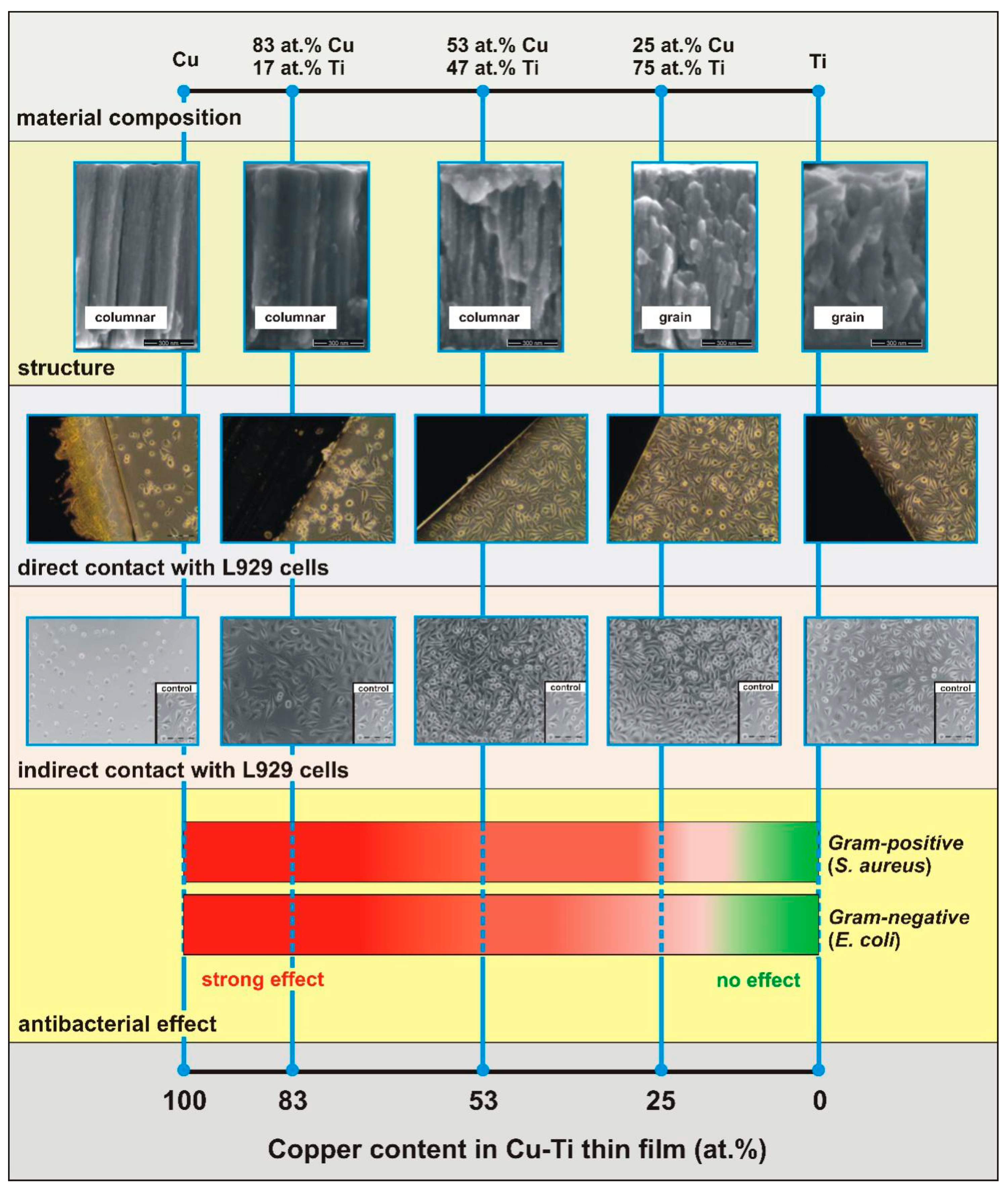
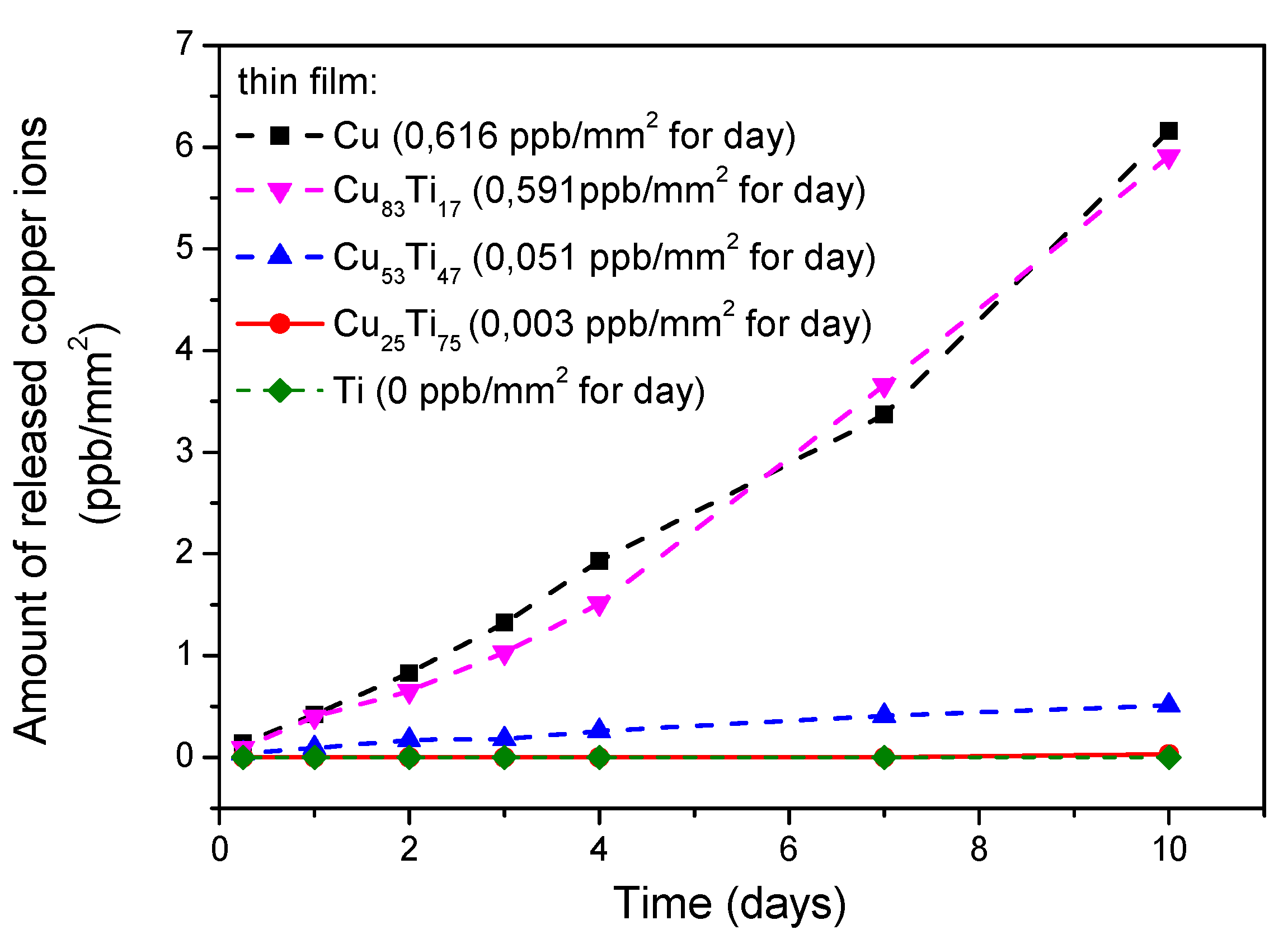
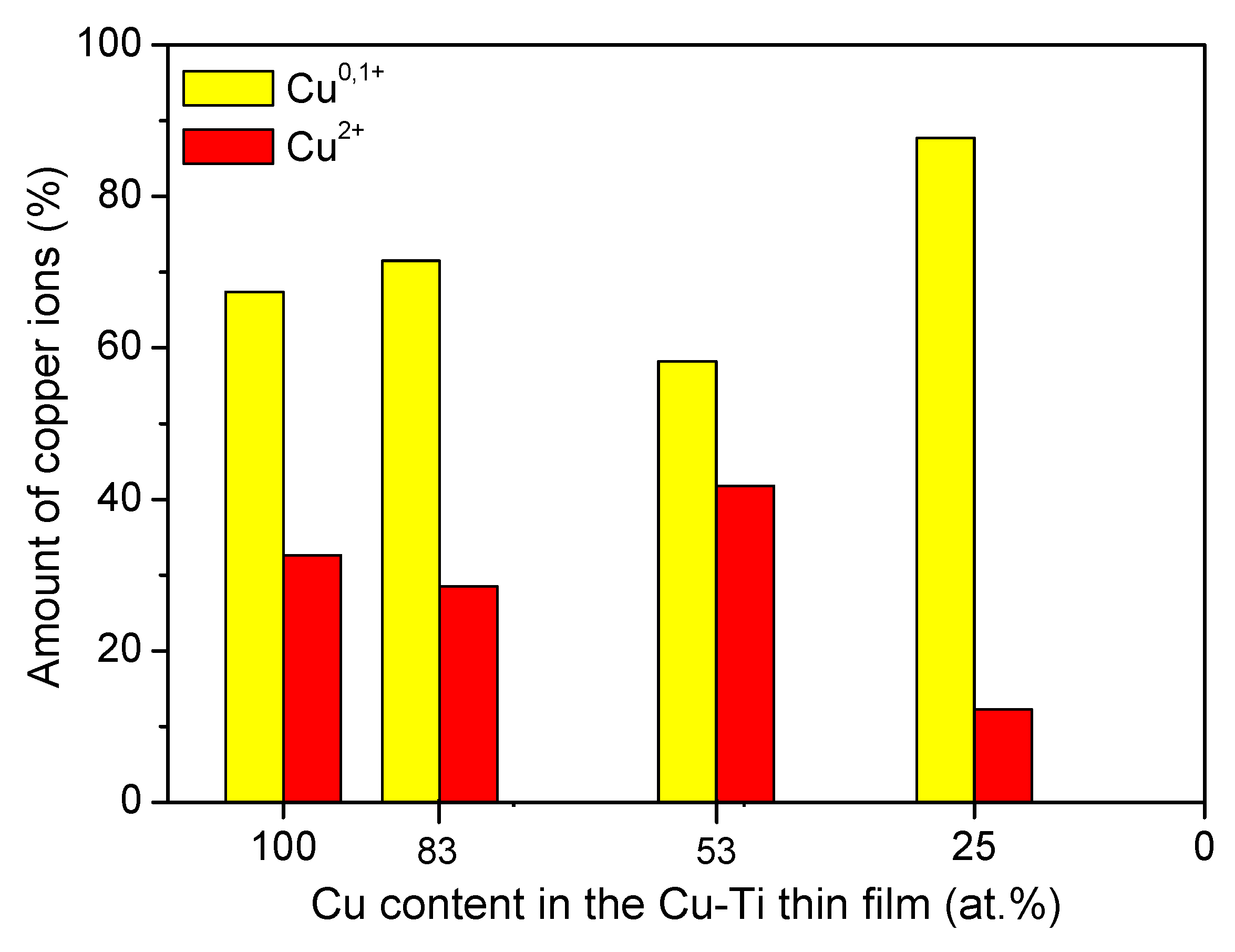

| Thin Film | Composition [at. %] | PAr [mbar] | Sputtering Power [W] | t [nm] | |||
|---|---|---|---|---|---|---|---|
| Cu | Ti | Magnetron 1-target Ti | Magnetron 2-target Cu | Magnetron 3-target Ti | |||
| Cu | 100 | - | 3 × 10−2 | - | 300 | - | 880 |
| Cu83Ti17 | 83 | 17 | 150 | 200 | 150 | 790 | |
| Cu53Ti47 | 53 | 47 | 400 | 100 | 400 | 650 | |
| Cu25Ti75 | 25 | 75 | 400 | 50 | 400 | 540 | |
| Ti | - | 100 | 400 | - | 400 | 420 | |
| Thin Film | 2θ (°) | Phase | d (nm) | D (nm) |
|---|---|---|---|---|
| Cu | 26.42 | Cu | 0.2203 | 45.4 |
| 43.31 | Cu | 0.1355 | 29.3 | |
| 44.33 | Cu | 0.1325 | 18.4 | |
| 50.44 | Cu | 0.1173 | 12.4 | |
| Cu83Ti17 | 26.25 | Cu | 0.2202 | 32.7 |
| 42.66 | Cu4Ti3 | 0.1374 | 4.8 | |
| 44.32 | Cu | 0.1326 | 9.9 | |
| Cu53Ti47 | 26.24 | Cu | 0.2203 | 36.6 |
| 42.36 | Cu4Ti3 | 0.1384 | 2.6 | |
| 44.34 | Cu | 0.1325 | 15.3 | |
| Cu25Ti75 | 26.24 | Cu | 0.2203 | 24.9 |
| 42.70 | Cu4Ti3 | 0.1373 | 4.8 | |
| 44.41 | Cu | 0.1323 | 10.2 | |
| Ti | 35.35 | Ti | 0.1647 | 10.5 |
| 38.27 | Ti | 0.1525 | 16.6 | |
| 44.21 | Ti | 0.1327 | 14.9 |
| Exposure Time (h) | Amount of Colony Forming Units per Milliliter (CFU/mL) | ||||
|---|---|---|---|---|---|
| Cu | Cu83Ti17 | Cu53Ti47 | Cu25Ti75 | Ti | |
| for E. coli | |||||
| 0 | 7.3 × 107 | 7.3 × 107 | 7.3 × 107 | 7.3 × 107 | 7.3 × 107 |
| 2 | 0 | 0 | 1.0 × 105 | 7.2 × 107 | 5.1 × 107 |
| 4 | 0 | 0 | 5.0 × 104 | 7.0 × 107 | 6.0 × 107 |
| 6 | 0 | 0 | 0 | 5.4 × 107 | 5.0 × 107 |
| 24 | 0 | 0 | 0 | 1.3 × 106 | 9.2 × 106 |
| for S. aureus | |||||
| 0 | 6.2 × 107 | 6.2 × 107 | 6.2 × 107 | 6.2 × 107 | 6.2 × 107 |
| 2 | 0 | 0 | 5.0 × 107 | 2.0 × 107 | 3.7 × 107 |
| 4 | 0 | 0 | 0 | 1.3 × 107 | 2,1 × 107 |
| 6 | 0 | 0 | 0 | 4.8 × 106 | 7.0 × 106 |
| 24 | 0 | 0 | 0 | 0 | 6.0 × 106 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojcieszak, D.; Osekowska, M.; Kaczmarek, D.; Szponar, B.; Mazur, M.; Mazur, P.; Obstarczyk, A. Influence of Material Composition on Structure, Surface Properties and Biological Activity of Nanocrystalline Coatings Based on Cu and Ti. Coatings 2020, 10, 343. https://doi.org/10.3390/coatings10040343
Wojcieszak D, Osekowska M, Kaczmarek D, Szponar B, Mazur M, Mazur P, Obstarczyk A. Influence of Material Composition on Structure, Surface Properties and Biological Activity of Nanocrystalline Coatings Based on Cu and Ti. Coatings. 2020; 10(4):343. https://doi.org/10.3390/coatings10040343
Chicago/Turabian StyleWojcieszak, Damian, Malgorzata Osekowska, Danuta Kaczmarek, Bogumila Szponar, Michal Mazur, Piotr Mazur, and Agata Obstarczyk. 2020. "Influence of Material Composition on Structure, Surface Properties and Biological Activity of Nanocrystalline Coatings Based on Cu and Ti" Coatings 10, no. 4: 343. https://doi.org/10.3390/coatings10040343
APA StyleWojcieszak, D., Osekowska, M., Kaczmarek, D., Szponar, B., Mazur, M., Mazur, P., & Obstarczyk, A. (2020). Influence of Material Composition on Structure, Surface Properties and Biological Activity of Nanocrystalline Coatings Based on Cu and Ti. Coatings, 10(4), 343. https://doi.org/10.3390/coatings10040343







